User login
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
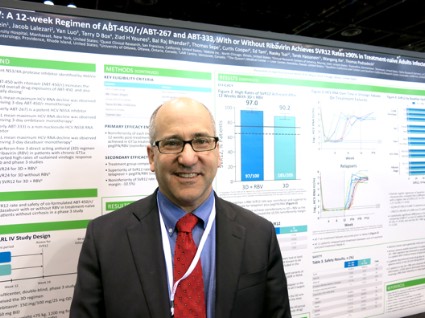
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
AT DDW 2014
Major finding: For patients with genotype 1a infection, SVR occurred in 97% with ribavirin and 90.2% without ribavirin. For patients with genotype 1b infection, rates were 99.5% and 99%, respectively.
Data source: Two randomized, phase III trials of previously untreated patients with hepatitis C and no cirrhosis who were treated with 12 weeks of ABT-450/r, ombitasvir, and dasabuvir with or without ribavirin.
Disclosures: AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.