User login
› Consider continuous intrathecal (IT) analgesia for chronic pain patients with refractory symptoms or intolerance to systemic medication. B
› Explore the possibility of using an IT delivery system
to treat malignant pain syndrome, particularly for patients with a life expectancy of more than 6 months. A
› Do not rule out IT analgesia for patients with refractory nonmalignant pain; while considerations in such cases are more complex, benefits include the efficacy of lower doses and fewer adverse effects. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A switch to hydromorphone 20 mg/d—the physician used the 5:1 morphine-to-hydromorphone conversion ratio, then decreased the dose by 50% to account for incomplete cross-tolerance—left Ms. G lethargic. In addition, her pain score rose to 5, and she began having difficulty swallowing the medication. Prior to the drug rotation, she was able to perform light tasks and was alert enough to interact with her family.
If Ms. G were your patient, what would be your next step?
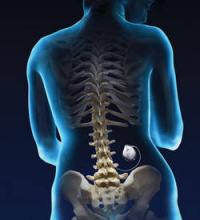
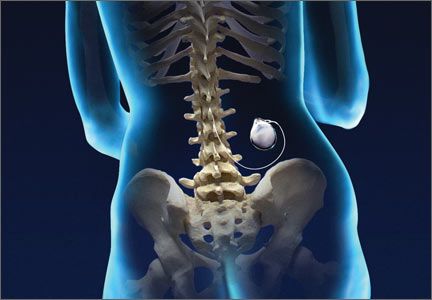
Continuous intrathecal (IT) drug delivery systems have been in use for more than 30 years.1 And, while IT administration of analgesia has become increasingly useful for patients with refractory chronic pain and spasticity, it remains an underutilized resource.2 Delivered directly into the pre- and post-synaptic opioid receptors in the dorsal horn of the spinal cord, IT analgesia bypasses first-pass metabolism. The result: a higher rate of efficacy, with smaller dosages and fewer adverse effects than systemic delivery.1
The drugs are delivered via a small battery-powered programmable pump that is implanted under the subcutaneous tissue of the abdomen and connected to a catheter tunneled to the site of spinal entry. The device must be refilled periodically—typically every one to 3 months—but this is not a difficult process. It can be done in an office setting or in the patient’s home by a specially trained visiting nurse.3
There is ample reason to consider this approach when systemic analgesics or antispasmodics fail to control pain or cause unacceptable adverse effects. So why isn’t it used more frequently? One factor may be that many primary care physicians—often the first practitioners called upon to manage these complicated cases—know too little about it.
Who is a potential candidate for IT analgesia? What medications can be administered via this route? What is the role of a family physician (FP) in coordinating and overseeing the care of a patient being treated with IT therapy? Our goals in writing this review are to address these questions.
Patient selection: Not just for cancer pain
FPs interested in referring patients for IT therapy have many factors to consider before consulting a pain specialist. Foremost among them are the different criteria for individuals with cancer-related pain and those with chronic nonmalignant pain.
IT analgesia for cancer pain has been shown to improve patients’ quality of life and potentially increase long-term survival due to a decrease in systemic toxicity.4-6 An appropriate candidate is an individual who, like Ms. G, was initially responsive to systemic opioids but later developed refractory symptoms or intolerance.7 Because of the invasive nature and high cost of implantation, subcutaneous IT pumps are typically reserved for patients with a life expectancy of more than 6 months.7 But implantation may be considered for those with a shorter life expectancy if they have severe pain or cannot tolerate the adverse effects of systemic analgesia.
Noncancer pain is more complex
The use of IT analgesia in patients with chronic nonmalignant pain, such as failed back surgery syndrome, spasticity associated with multiple sclerosis, or diabetic neuropathy, is both more controversial and more complex. It is important for FPs to recognize the multidimensional nature of this type of pain, which may be complicated by physical, psychological, and behavioral factors, including the possibility of addiction.8-11
Although IT analgesia is less subject to abuse and diversion than systemic opioids, the dependent relationship associated with a continuous delivery system makes risk stratification a necessity.12 Psychological testing is commonly used to evaluate potential candidates for long-term IT analgesia.
Prior to placement, patients must have had a failed course of conservative pain management and have no surgical options, no medical contraindications (eg, spinal pathology or susceptibility to infection), and no evidence of active addiction.12 A medication history is crucial, too, to identify use of anticoagulation therapy—a relative contraindication—as well as drug allergies and potential drug-drug interactions to guard against.3
An IT trial may be required
It is common practice for patients to undergo an IT analgesia trial prior to implantation of a subcutaneous pump. This involves using an external pump to infuse the selected medication intrathecally and slowly titrating it according to symptoms for 2 to 3 days. During this time frame, the patient records his or her response; a reduction by more than half in VAS pain score is considered a success, indicating that the patient is an appropriate candidate for placement of the device.3,13
Drug choices—a look at the evidence
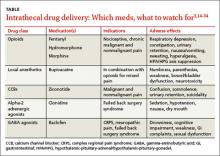
The US Food and Drug Administration (FDA) has approved 3 medications for continuous IT delivery: morphine, ziconotide, and baclofen. But it is common practice to use alternative agents, such as other opioids, local anesthetics, or alpha 2-adrenergic agonists (TABLE).3,14-34
CASE › Ms. G’s primary care physician referred her to a pain specialist, who thought she would benefit from IT analgesia. After a successful single-shot IT trial with 0.5 mg morphine, the patient underwent implantation. The specialist chose morphine as the IT agent because of Ms. G’s history of successful pain relief with it, and because such a low dose was unlikely to be a problem for a patient with renal failure.
A month later, when she returned to the specialist to have the pump refilled, Ms. G reported a pain score of 3.
Opioids such as morphine exhibit a wider spread of analgesia when administered intrathecally, resulting in fewer adverse effects than systemic opioids.13,35,36 The mu-opioid receptors in the dorsal horn of the spinal cord are the primary target of IT opioids.
In a multicenter randomized trial involving 200 cancer patients on opioids, Smith et al4 compared implantable IT drug delivery systems with comprehensive medical management. The mean VAS pain score in the IT group fell 52% vs a decline of 39% in the medical management group.
The evidence supporting IT opioids for nonmalignant pain is not as strong. This may be due to inherent differences in pain mechanisms. In cancer pain, between 75% and 90% of pain is either nociceptive or mixed nociceptive-neuropathic; the etiology of noncancer pain is more variable.37-39
Although IT opioid therapy is associated with a lower incidence of adverse effects than systemic therapy, this route is not devoid of adverse effects. Opioids delivered intrathecally may still be associated with respiratory depression, constipation, urinary retention, nausea/vomiting, sweating, and hyperalgesia.39 In addition, chronic opioid use suppresses the hypothalamic-pituitary-gonadal axis and the hypothalamic-pituitary-adrenal axis14,40,41—a risk with long-term IT as well as systemic administration.14 Respiratory depression most commonly results from accidental overdosing, and patients must be monitored during initiation and dose escalation of IT opioid therapy.15
Local anesthetics. Numerous studies have documented the favorable outcomes of combining local anesthetics with opioids for patients with cancer16-20 and noncancer pain.21,22 Local anesthetics work via the blockade of voltage-gated sodium channels, interfering with neuron depolarization.17
A retrospective study in which patients with malignant pain and those with failed back surgery syndrome had bupivacaine added to their IT opioid solution found that the combination led to lower pain scores and a 23% reduction in opioid dosage.20 In another retrospective review, researchers demonstrated that the coadministration of IT bupivacaine and an opioid decreased the rate of opioid dose escalation by 65% over the first year in patients with noncancer pain.23
However, a double-blind randomized, crossover multicenter study found that in patients with chronic nonmalignant pain, the addition of bupivacaine to IT opioids failed to produce significant improvement in pain control compared with opioid use alone. Quality of life scores did improve, however, in the group receiving combination therapy.24
Adverse effects of local anesthetics delivered intrathecally include numbness, paresthesias, weakness, bowel/bladder dysfunction, and neurotoxicity.17,19,25
Calcium channel blockers. Found in venom produced by the marine snail Conus magus, ziconotide blocks presynaptic N-type channels. It is the only calcium channel blocker used to manage chronic pain.26 Several trials in patients with malignant and nonmalignant pain have shown a significant decrease in VAS pain scores compared with placebo.25,26 In addition, a multicenter, double-blind placebo-controlled crossover study evaluating IT ziconotide for the treatment of refractory pain in 111 patients with cancer and AIDS found that the treatment group obtained significantly better pain relief than the controls (53% vs 17.5% using a VAS pain intensity score).25 However, 31% of those in the treatment group experienced adverse effects, the most common of which were confusion, somnolence, and urinary retention.
Ziconotide has FDA approval only as monotherapy. But because of its high cost and adverse effect profile, it is mainly used in combination with other IT drugs.27 Ziconotide increases the risk of suicide in patients with a history of depression.28 The prevalence of adverse effects correlates with a higher dose, faster titration rate, and older age.26,28
Alpha-2 adrenergic agonists. Clonidine is the only alpha-2 agonist with FDA approval for epidural use, with several studies supporting its off-label use in combination with IT therapy.22,29 In a prospective open-label study evaluating combination IT therapy in patients with failed back surgery syndrome, 73% reported subjective ratings of good or excellent at 2-year follow-up.22 The most common adverse effects were sedation, hypotension, nausea, and dry mouth.
Gamma-aminobutyric acid (GABA) agonists. Baclofen, a GABA agonist with FDA approval for the treatment of spasticity, has been used intrathecally since the mid-1980s.32 Several studies have supported its effectiveness for this purpose.30,42 Clinical studies have also found IT baclofen to be effective in treating conditions such as complex regional pain syndrome, central pain, and neuropathic pain secondary to failed back surgery syndrome.31,32 In one randomized double-blind crossover trial, 7 women with complex regional pain syndrome were given bolus injections of baclofen or saline. Those treated with baclofen experienced a reduction in pain and regained function.31
In another trial—a double-blind placebo-controlled study of patients with multiple sclerosis and spinal cord injury comparing baclofen with placebo—those treated with baclofen showed significant reductions in dysesthetic and spasm-related pain.32 The most common adverse effects of baclofen are drowsiness, cognitive impairment, weakness, gastrointestinal complaints, and sexual dysfunction.31
Which patients and which drugs? An expert consensus
Due to the potential for inconsistent patient management and the use of therapies with anecdotal evidence, the Polyanalgesic Consensus Conference (PACC)—a panel of experts in IT therapy—convened in 2000, 2003, 2007, and 2011 to develop recommendations for IT therapy and an algorithm for drug selection. PACC’s list of chronic conditions for which IT should be considered includes axial low back pain, postherpetic neuralgia, spinal cord injury, spinal stenosis, pancreatitis, osteoporosis, compression fracture, and phantom limb pain, among others.
The algorithm contains separate arms for neuropathic, nociceptive, and mixed pain states. First-line agents for neuropathic pain include morphine, alone or combined with bupivacaine, and ziconotide. For nociceptive pain, morphine, hydromorphone, fentanyl, and ziconotide are all first-line agents; for mixed pain states, the appropriate choice should be based on the clinical scenario.33
Overseeing IT pain management in primary care
Referring potential candidates for IT therapy to specialists in pain management is just the beginning. While patients typically return to the specialist for pump refills, it is important that they see their primary care physician regularly, as well. Vigilance is required of both the FP and the patient. Any sudden worsening in pain level or acute change in neurologic function must be reported to the pain specialist immediately.
Adverse effects of medications are the most common complications
Kamran and Wright43 performed a retrospective review of their practice’s Intrathecal Drug Delivery Systems database of 122 patients and found that adverse medication effects were most common, accounting for 77% of complications.
Catheter malfunctions were next, at 16%, followed by infections, at 5%.43 In other studies, catheter-related complications were found to have an incidence of 15% to 25%.44,45 Problems include kinking, breaking, leaking, and migration of the catheter. Advise patients to immediately contact their pain specialist for evaluation if they experience a sudden loss of, or change in, pain control.
Infectious complications, which occur infrequently, are usually limited to superficial wounds, although epidural abscesses and meningitis are possible.46 Standard perioperative antibiotic administration helps to minimize the risk of infection. If a patient presents with signs and symptoms of an epidural abscess—back pain, fever, and variable neurologic deficits—emergent initiation of intravenous antibiotics is needed. Magnetic resonance imaging (MRI) with and without gadolinium should be obtained, as well.22
Spinal damage. Although IT catheters are placed under fluoroscopic guidance, there is a risk of direct injury to the spinal cord; this is more common if the catheter is placed above the level of the conus medullaris. Damage to the spinal cord or exiting spinal nerves will manifest as pain, sensory loss, and/or weakness over a dermatomal distribution.43
Neurologic sequelae, ranging from mild symptoms to paraplegia, can result from the formation of a granuloma at the tip of the spinal catheter. A sudden increase in pain usually occurs prior to neurologic deterioration, thereby allowing for early detection and intervention.47 Development of a granuloma appears to be related to the long-term infusion of high-concentration opioids.34 The diagnosis is confirmed by MRI, but physical exam and history are imperative in making the initial diagnosis.
In cases of mild neurologic symptoms, a transition to saline infusion through the pump may allow the granuloma to absorb; more severe cases may require neurosurgical intervention.47
Is your patient scheduled for an IT drug trial?
If a patient of yours is scheduled for an IT drug trial, ideally followed by pump implantation, microdosing—the practice of weaning the individual from oral opioids prior to the procedure so that very low doses of IT opioids will suffice—may play a role.48,49 While this approach appears promising, however, there is little in the way of definitive evidence of efficacy.
CASE › Over time, Ms. G’s maintenance IT dose of morphine had to be slowly increased from 0.5 mg to 1 mg/d. At bimonthly visits with her FP, she consistently reports pain scores of 3 on a scale of 1 to 10. The patient’s function has returned to baseline, and she has minimal adverse effects.
CORRESPONDENCE
Jessica Tsukanov, DO, Montefiore Medical Center, 3347 Steuben Avenue, Bronx, NY 10467; [email protected]
1. Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149-151.
2. Hayek SM, Hanes MC. Intrathecal therapy for chronic pain: current trends and future needs. Curr Pain Headache Rep. 2014;18:338.
3. Krames ES. Intraspinal opioid therapy for chronic nonmalignant pain: current practice and clinical guidelines. J Pain Symptom Manage. 1996;11:333-352.
4. Smith TJ, Staats PS, Deer T, et al; Implantable Drug Delivery Systems Study Group. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20:4040-4049.
5. Rauck RL, Cherry D, Boyer MF, et al. Long-term intrathecal opioid therapy with a patient-activated, implanted delivery system for the treatment of refractory cancer pain. J Pain. 2003;4:441-447.
6. Burton AW, Rajagopal A, Shah HN, et al. Epidural and intrathecal analgesia is effective in treating refractory cancer pain. Pain Med. 2004;5:239-247.
7. Hassenbusch SJ. Cost modeling for alternate routes of administration of opioids for cancer pain. Oncology. 1999;13(5 suppl 2):S63-S67.
8. Thimineur MA, Kravitz E, Vodapally MS. Intrathecal opioid treatment for chronic non-malignant pain: a 3-year prospective study. Pain. 2004;109:242-249.
9. Gerber HR. Intrathecal morphine for chronic benign pain. Best Pract Res Clin Anesthesiol. 2003;17:429-442.
10. Tuner JA, Sears JM, Loeser JD. Programmable intrathecal opioid delivery systems for chronic noncancer pain: a systematic review of effectiveness and complications. Clin J Pain. 2007;23:180-195.
11. Brown J, Klapow J, Doleys D, et al. Disease-specific and generic health outcomes: a model for the evaluation of long-term intrathecal opioid therapy in noncancer low back pain patients. Clin J Pain. 1999;15:122-131.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2006;6:432-442.
13. Ahmed SU, Martin NM, Chang Y. Patient selection and trial methods for intraspinal drug delivery for chronic pain: a national survey. Neuromodulation. 2005;8:112-120.
14. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85:2215-2222.
15. Coffey RJ, Owens ML, Broste SK, et al. Mortality associated with implantation and management of intrathecal opioid drug infusion systems to treat noncancer pain. Anesthesiology. 2009;111:881-891.
16. Sjöberg M, Nitescu P, Appelgren L, et al. Long-term intrathecal morphine and bupivacaine in patients with refractory cancer pain. Results from a morphine:bupivacaine dose regimen of 0.5:4.75 mg/ml. Anesthesiology. 1994;80:284-297.
17. Sjöberg M, Appelgen L, Einarsson S, et al. Long-term intrathecal morphine and bupivacaine in “refractory” cancer pain. I. Results from the first series of 52 patients. Acta Anaesthsiol Scand. 1991;35:30-43.
18. Van Dongen RT, Crul BJ, De Bock M. Long-term intrathecal infusion of morphine and morphine/bupivacaine mixtures in the treatment of cancer pain: a retrospective analysis of 51 cases. Pain. 1993;55:119-123.
19. van Dongen RT, Crul BJ, van Egmond J. Intrathecal coadministration of bupivacaine diminishes morphine dose progression during long-term intrathecal infusion in cancer patients. Clin J Pain. 1999;15:166-172.
20. Deer TR, Caraway DL, Kim CK, et al. Clinical experience with intrathecal bupivacaine in combination with opioid for the treatment of chronic pain related to failed back surgery syndrome and metastatic cancer pain of the spine. Spine J. 2002;2:274-278.
21. Krames ES, Lanning RM. Intrathecal infusional analgesia for nonmalignant pain: analgesic efficacy of intrathecal opioid with or without bupivacaine. J Pain Symptom Manage. 1993;8:539-548.
22. Rainov NG, Heidecke V, Burkert W. Long-term intrathecal infusion of drug combinations for chronic back and leg pain. J Pain Symptom Manage. 2001;22:862-871.
23. Veizi IE, Hayek SM, Narouze S, et al. Combination of intrathecal opioids with bupivacaine attenuates opioid dose escalation in chronic noncancer pain patients. Pain Med. 2011;12:1481-1489.
24. Mironer YE, Haasis JC, Chapple I, et al. Efficacy and safety of intrathecal opioid/bupivacaine mixture in chronic nonmalignant pain: A double blind, randomized, crossover, multicenter study by the National Forum of Independent Pain Clinicians (NFIPC). Neuromodulation. 2002;5:208-213.
25. Staats PS, Yearwood T, Charapata SG, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291:63-70.
26. Rauck RL, Wallace MS, Leong MS, et al; Ziconotide 301 Study Group. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J Pain Symptom Manage. 2006;31:393-406.
27. Wallace MS, Rauck R, Fisher R, et al; Ziconotide 98-022 Study Group. Intrathecal ziconotide for severe chronic pain: safety and tolerability results of an open-label, long-term trial. Anesth Analg. 2008;106:628-637.
28. Maier C, Gockel HH, Gruhn K, et al. Increased risk of suicide under intrathecal ziconotide treatment? - a warning. Pain. 2011;152:235-237.
29. Ackerman LL, Follett KA, Rosenquist RW. Long-term outcomes during treatment of chronic pain with intrathecal clonidine or clonidine/opioid combinations. J Pain Symptom Manage. 2003;26:668-677.
30. Tarrico M, Adone R, Pagliacci C, et al. Pharmacological interventions for spasticity following spinal cord injury. Cochrane Database Syst Rev. 2000;(2):CD001131.
31. van Hilten BJ, van de Beek WT, Hoff JI, et al. Intrathecal baclofen for the treatment of dystonia in patients with reflex sympathetic dystrophy. N Engl J Med. 2000;343:625-630.
32. Herman RM, D’Luzansky SC, Ippolito R. Intrathecal baclofen suppresses central pain in patients with spinal lesions. A pilot study. Clin J Pain. 1992;8:338-345.
33. Deer T, Prager J, Levy R, et al. Polyanalgesic consensus conference 2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation. 2012;15:436-466.
34. Yaksh TL, Coffey RJ. Spinal opiate toxicity. In: Proceedings of American Society of Regional Anesthesia and Pain Medication Conference; November 18-21, 2004; Phoenix, AZ.
35. Levy MH. Pharmacologic management of cancer pain. Semin Oncol. 1994;21:718-739.
36. Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61:276-310.
37. Zeppetella G, O’Doherty CA, Collins S. Prevalence and characteristics of breakthrough pain in patients with non-malignant terminal disease admitted to a hospice. Palliat Med. 2001;15:243-246.
38. Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41:273-281.
39. Hanks GW, Forbes K. Opioid responsiveness. Acta Anaesthesiol Scan. 1997;41:154-158.
40. Paice JA, Penn RD, Ryan WG. Altered sexual function and decreased testosterone in patients receiving intraspinal opioids. J Pain Symptom Manage. 1994;9:126-131.
41. Brennan MJ. The effect of opioid therapy on endocrine function. Am J Med. 2013;126(3 suppl 1):S12-S18.
42. Beard S, Hunn A. Wight J. Treatments for spasticity and pain in multiple sclerosis: a systematic review. Health Technol Assess. 2003;7:iii,ix-x,1-111.
43. Kamran S, Wright BD. Complications of intrathecal drug delivery systems. Neuromodulation. 2001;4:111-115.
44. Follett KA, Naumann CP. A prospective study of catheter-related complications of intrathecal drug delivery systems. J Pain Symptom Manage. 2000;19:209-215.
45. Follett KA, Burchiel K, Deer T, et al. Prevention of intrathecal drug delivery catheter-related complications. Neuromodulation. 2003;6:32-41.
46. Paice JA, Penn RD, Shott S. Intraspinal morphine for chronic pain: a retrospective, multicenter study. J Pain Symptom Manage. 1996;11:71-80.
47. Miele VJ, Price KO, Bloomfield S, et al. A review of intrathecal morphine therapy related granulomas. Eur J Pain. 2006;10:251-261.
48. Hayek SM. Intrathecal “microdosing”: reality or artifact? Pain Med. 2012;13:1664-1665.
49. Grider JS, Harned ME, Etscheidt MA. Patient selection and outcomes using a low-dose intrathecal opioid trialing method for chronic nonmalignant pain. Pain Physician. 2011;14:343-351.
› Consider continuous intrathecal (IT) analgesia for chronic pain patients with refractory symptoms or intolerance to systemic medication. B
› Explore the possibility of using an IT delivery system
to treat malignant pain syndrome, particularly for patients with a life expectancy of more than 6 months. A
› Do not rule out IT analgesia for patients with refractory nonmalignant pain; while considerations in such cases are more complex, benefits include the efficacy of lower doses and fewer adverse effects. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A switch to hydromorphone 20 mg/d—the physician used the 5:1 morphine-to-hydromorphone conversion ratio, then decreased the dose by 50% to account for incomplete cross-tolerance—left Ms. G lethargic. In addition, her pain score rose to 5, and she began having difficulty swallowing the medication. Prior to the drug rotation, she was able to perform light tasks and was alert enough to interact with her family.
If Ms. G were your patient, what would be your next step?
Continuous intrathecal (IT) drug delivery systems have been in use for more than 30 years.1 And, while IT administration of analgesia has become increasingly useful for patients with refractory chronic pain and spasticity, it remains an underutilized resource.2 Delivered directly into the pre- and post-synaptic opioid receptors in the dorsal horn of the spinal cord, IT analgesia bypasses first-pass metabolism. The result: a higher rate of efficacy, with smaller dosages and fewer adverse effects than systemic delivery.1
The drugs are delivered via a small battery-powered programmable pump that is implanted under the subcutaneous tissue of the abdomen and connected to a catheter tunneled to the site of spinal entry. The device must be refilled periodically—typically every one to 3 months—but this is not a difficult process. It can be done in an office setting or in the patient’s home by a specially trained visiting nurse.3
There is ample reason to consider this approach when systemic analgesics or antispasmodics fail to control pain or cause unacceptable adverse effects. So why isn’t it used more frequently? One factor may be that many primary care physicians—often the first practitioners called upon to manage these complicated cases—know too little about it.
Who is a potential candidate for IT analgesia? What medications can be administered via this route? What is the role of a family physician (FP) in coordinating and overseeing the care of a patient being treated with IT therapy? Our goals in writing this review are to address these questions.
Patient selection: Not just for cancer pain
FPs interested in referring patients for IT therapy have many factors to consider before consulting a pain specialist. Foremost among them are the different criteria for individuals with cancer-related pain and those with chronic nonmalignant pain.
IT analgesia for cancer pain has been shown to improve patients’ quality of life and potentially increase long-term survival due to a decrease in systemic toxicity.4-6 An appropriate candidate is an individual who, like Ms. G, was initially responsive to systemic opioids but later developed refractory symptoms or intolerance.7 Because of the invasive nature and high cost of implantation, subcutaneous IT pumps are typically reserved for patients with a life expectancy of more than 6 months.7 But implantation may be considered for those with a shorter life expectancy if they have severe pain or cannot tolerate the adverse effects of systemic analgesia.
Noncancer pain is more complex
The use of IT analgesia in patients with chronic nonmalignant pain, such as failed back surgery syndrome, spasticity associated with multiple sclerosis, or diabetic neuropathy, is both more controversial and more complex. It is important for FPs to recognize the multidimensional nature of this type of pain, which may be complicated by physical, psychological, and behavioral factors, including the possibility of addiction.8-11
Although IT analgesia is less subject to abuse and diversion than systemic opioids, the dependent relationship associated with a continuous delivery system makes risk stratification a necessity.12 Psychological testing is commonly used to evaluate potential candidates for long-term IT analgesia.
Prior to placement, patients must have had a failed course of conservative pain management and have no surgical options, no medical contraindications (eg, spinal pathology or susceptibility to infection), and no evidence of active addiction.12 A medication history is crucial, too, to identify use of anticoagulation therapy—a relative contraindication—as well as drug allergies and potential drug-drug interactions to guard against.3
An IT trial may be required
It is common practice for patients to undergo an IT analgesia trial prior to implantation of a subcutaneous pump. This involves using an external pump to infuse the selected medication intrathecally and slowly titrating it according to symptoms for 2 to 3 days. During this time frame, the patient records his or her response; a reduction by more than half in VAS pain score is considered a success, indicating that the patient is an appropriate candidate for placement of the device.3,13
Drug choices—a look at the evidence
The US Food and Drug Administration (FDA) has approved 3 medications for continuous IT delivery: morphine, ziconotide, and baclofen. But it is common practice to use alternative agents, such as other opioids, local anesthetics, or alpha 2-adrenergic agonists (TABLE).3,14-34
CASE › Ms. G’s primary care physician referred her to a pain specialist, who thought she would benefit from IT analgesia. After a successful single-shot IT trial with 0.5 mg morphine, the patient underwent implantation. The specialist chose morphine as the IT agent because of Ms. G’s history of successful pain relief with it, and because such a low dose was unlikely to be a problem for a patient with renal failure.
A month later, when she returned to the specialist to have the pump refilled, Ms. G reported a pain score of 3.
Opioids such as morphine exhibit a wider spread of analgesia when administered intrathecally, resulting in fewer adverse effects than systemic opioids.13,35,36 The mu-opioid receptors in the dorsal horn of the spinal cord are the primary target of IT opioids.
In a multicenter randomized trial involving 200 cancer patients on opioids, Smith et al4 compared implantable IT drug delivery systems with comprehensive medical management. The mean VAS pain score in the IT group fell 52% vs a decline of 39% in the medical management group.
The evidence supporting IT opioids for nonmalignant pain is not as strong. This may be due to inherent differences in pain mechanisms. In cancer pain, between 75% and 90% of pain is either nociceptive or mixed nociceptive-neuropathic; the etiology of noncancer pain is more variable.37-39
Although IT opioid therapy is associated with a lower incidence of adverse effects than systemic therapy, this route is not devoid of adverse effects. Opioids delivered intrathecally may still be associated with respiratory depression, constipation, urinary retention, nausea/vomiting, sweating, and hyperalgesia.39 In addition, chronic opioid use suppresses the hypothalamic-pituitary-gonadal axis and the hypothalamic-pituitary-adrenal axis14,40,41—a risk with long-term IT as well as systemic administration.14 Respiratory depression most commonly results from accidental overdosing, and patients must be monitored during initiation and dose escalation of IT opioid therapy.15
Local anesthetics. Numerous studies have documented the favorable outcomes of combining local anesthetics with opioids for patients with cancer16-20 and noncancer pain.21,22 Local anesthetics work via the blockade of voltage-gated sodium channels, interfering with neuron depolarization.17
A retrospective study in which patients with malignant pain and those with failed back surgery syndrome had bupivacaine added to their IT opioid solution found that the combination led to lower pain scores and a 23% reduction in opioid dosage.20 In another retrospective review, researchers demonstrated that the coadministration of IT bupivacaine and an opioid decreased the rate of opioid dose escalation by 65% over the first year in patients with noncancer pain.23
However, a double-blind randomized, crossover multicenter study found that in patients with chronic nonmalignant pain, the addition of bupivacaine to IT opioids failed to produce significant improvement in pain control compared with opioid use alone. Quality of life scores did improve, however, in the group receiving combination therapy.24
Adverse effects of local anesthetics delivered intrathecally include numbness, paresthesias, weakness, bowel/bladder dysfunction, and neurotoxicity.17,19,25
Calcium channel blockers. Found in venom produced by the marine snail Conus magus, ziconotide blocks presynaptic N-type channels. It is the only calcium channel blocker used to manage chronic pain.26 Several trials in patients with malignant and nonmalignant pain have shown a significant decrease in VAS pain scores compared with placebo.25,26 In addition, a multicenter, double-blind placebo-controlled crossover study evaluating IT ziconotide for the treatment of refractory pain in 111 patients with cancer and AIDS found that the treatment group obtained significantly better pain relief than the controls (53% vs 17.5% using a VAS pain intensity score).25 However, 31% of those in the treatment group experienced adverse effects, the most common of which were confusion, somnolence, and urinary retention.
Ziconotide has FDA approval only as monotherapy. But because of its high cost and adverse effect profile, it is mainly used in combination with other IT drugs.27 Ziconotide increases the risk of suicide in patients with a history of depression.28 The prevalence of adverse effects correlates with a higher dose, faster titration rate, and older age.26,28
Alpha-2 adrenergic agonists. Clonidine is the only alpha-2 agonist with FDA approval for epidural use, with several studies supporting its off-label use in combination with IT therapy.22,29 In a prospective open-label study evaluating combination IT therapy in patients with failed back surgery syndrome, 73% reported subjective ratings of good or excellent at 2-year follow-up.22 The most common adverse effects were sedation, hypotension, nausea, and dry mouth.
Gamma-aminobutyric acid (GABA) agonists. Baclofen, a GABA agonist with FDA approval for the treatment of spasticity, has been used intrathecally since the mid-1980s.32 Several studies have supported its effectiveness for this purpose.30,42 Clinical studies have also found IT baclofen to be effective in treating conditions such as complex regional pain syndrome, central pain, and neuropathic pain secondary to failed back surgery syndrome.31,32 In one randomized double-blind crossover trial, 7 women with complex regional pain syndrome were given bolus injections of baclofen or saline. Those treated with baclofen experienced a reduction in pain and regained function.31
In another trial—a double-blind placebo-controlled study of patients with multiple sclerosis and spinal cord injury comparing baclofen with placebo—those treated with baclofen showed significant reductions in dysesthetic and spasm-related pain.32 The most common adverse effects of baclofen are drowsiness, cognitive impairment, weakness, gastrointestinal complaints, and sexual dysfunction.31
Which patients and which drugs? An expert consensus
Due to the potential for inconsistent patient management and the use of therapies with anecdotal evidence, the Polyanalgesic Consensus Conference (PACC)—a panel of experts in IT therapy—convened in 2000, 2003, 2007, and 2011 to develop recommendations for IT therapy and an algorithm for drug selection. PACC’s list of chronic conditions for which IT should be considered includes axial low back pain, postherpetic neuralgia, spinal cord injury, spinal stenosis, pancreatitis, osteoporosis, compression fracture, and phantom limb pain, among others.
The algorithm contains separate arms for neuropathic, nociceptive, and mixed pain states. First-line agents for neuropathic pain include morphine, alone or combined with bupivacaine, and ziconotide. For nociceptive pain, morphine, hydromorphone, fentanyl, and ziconotide are all first-line agents; for mixed pain states, the appropriate choice should be based on the clinical scenario.33
Overseeing IT pain management in primary care
Referring potential candidates for IT therapy to specialists in pain management is just the beginning. While patients typically return to the specialist for pump refills, it is important that they see their primary care physician regularly, as well. Vigilance is required of both the FP and the patient. Any sudden worsening in pain level or acute change in neurologic function must be reported to the pain specialist immediately.
Adverse effects of medications are the most common complications
Kamran and Wright43 performed a retrospective review of their practice’s Intrathecal Drug Delivery Systems database of 122 patients and found that adverse medication effects were most common, accounting for 77% of complications.
Catheter malfunctions were next, at 16%, followed by infections, at 5%.43 In other studies, catheter-related complications were found to have an incidence of 15% to 25%.44,45 Problems include kinking, breaking, leaking, and migration of the catheter. Advise patients to immediately contact their pain specialist for evaluation if they experience a sudden loss of, or change in, pain control.
Infectious complications, which occur infrequently, are usually limited to superficial wounds, although epidural abscesses and meningitis are possible.46 Standard perioperative antibiotic administration helps to minimize the risk of infection. If a patient presents with signs and symptoms of an epidural abscess—back pain, fever, and variable neurologic deficits—emergent initiation of intravenous antibiotics is needed. Magnetic resonance imaging (MRI) with and without gadolinium should be obtained, as well.22
Spinal damage. Although IT catheters are placed under fluoroscopic guidance, there is a risk of direct injury to the spinal cord; this is more common if the catheter is placed above the level of the conus medullaris. Damage to the spinal cord or exiting spinal nerves will manifest as pain, sensory loss, and/or weakness over a dermatomal distribution.43
Neurologic sequelae, ranging from mild symptoms to paraplegia, can result from the formation of a granuloma at the tip of the spinal catheter. A sudden increase in pain usually occurs prior to neurologic deterioration, thereby allowing for early detection and intervention.47 Development of a granuloma appears to be related to the long-term infusion of high-concentration opioids.34 The diagnosis is confirmed by MRI, but physical exam and history are imperative in making the initial diagnosis.
In cases of mild neurologic symptoms, a transition to saline infusion through the pump may allow the granuloma to absorb; more severe cases may require neurosurgical intervention.47
Is your patient scheduled for an IT drug trial?
If a patient of yours is scheduled for an IT drug trial, ideally followed by pump implantation, microdosing—the practice of weaning the individual from oral opioids prior to the procedure so that very low doses of IT opioids will suffice—may play a role.48,49 While this approach appears promising, however, there is little in the way of definitive evidence of efficacy.
CASE › Over time, Ms. G’s maintenance IT dose of morphine had to be slowly increased from 0.5 mg to 1 mg/d. At bimonthly visits with her FP, she consistently reports pain scores of 3 on a scale of 1 to 10. The patient’s function has returned to baseline, and she has minimal adverse effects.
CORRESPONDENCE
Jessica Tsukanov, DO, Montefiore Medical Center, 3347 Steuben Avenue, Bronx, NY 10467; [email protected]
› Consider continuous intrathecal (IT) analgesia for chronic pain patients with refractory symptoms or intolerance to systemic medication. B
› Explore the possibility of using an IT delivery system
to treat malignant pain syndrome, particularly for patients with a life expectancy of more than 6 months. A
› Do not rule out IT analgesia for patients with refractory nonmalignant pain; while considerations in such cases are more complex, benefits include the efficacy of lower doses and fewer adverse effects. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A switch to hydromorphone 20 mg/d—the physician used the 5:1 morphine-to-hydromorphone conversion ratio, then decreased the dose by 50% to account for incomplete cross-tolerance—left Ms. G lethargic. In addition, her pain score rose to 5, and she began having difficulty swallowing the medication. Prior to the drug rotation, she was able to perform light tasks and was alert enough to interact with her family.
If Ms. G were your patient, what would be your next step?
Continuous intrathecal (IT) drug delivery systems have been in use for more than 30 years.1 And, while IT administration of analgesia has become increasingly useful for patients with refractory chronic pain and spasticity, it remains an underutilized resource.2 Delivered directly into the pre- and post-synaptic opioid receptors in the dorsal horn of the spinal cord, IT analgesia bypasses first-pass metabolism. The result: a higher rate of efficacy, with smaller dosages and fewer adverse effects than systemic delivery.1
The drugs are delivered via a small battery-powered programmable pump that is implanted under the subcutaneous tissue of the abdomen and connected to a catheter tunneled to the site of spinal entry. The device must be refilled periodically—typically every one to 3 months—but this is not a difficult process. It can be done in an office setting or in the patient’s home by a specially trained visiting nurse.3
There is ample reason to consider this approach when systemic analgesics or antispasmodics fail to control pain or cause unacceptable adverse effects. So why isn’t it used more frequently? One factor may be that many primary care physicians—often the first practitioners called upon to manage these complicated cases—know too little about it.
Who is a potential candidate for IT analgesia? What medications can be administered via this route? What is the role of a family physician (FP) in coordinating and overseeing the care of a patient being treated with IT therapy? Our goals in writing this review are to address these questions.
Patient selection: Not just for cancer pain
FPs interested in referring patients for IT therapy have many factors to consider before consulting a pain specialist. Foremost among them are the different criteria for individuals with cancer-related pain and those with chronic nonmalignant pain.
IT analgesia for cancer pain has been shown to improve patients’ quality of life and potentially increase long-term survival due to a decrease in systemic toxicity.4-6 An appropriate candidate is an individual who, like Ms. G, was initially responsive to systemic opioids but later developed refractory symptoms or intolerance.7 Because of the invasive nature and high cost of implantation, subcutaneous IT pumps are typically reserved for patients with a life expectancy of more than 6 months.7 But implantation may be considered for those with a shorter life expectancy if they have severe pain or cannot tolerate the adverse effects of systemic analgesia.
Noncancer pain is more complex
The use of IT analgesia in patients with chronic nonmalignant pain, such as failed back surgery syndrome, spasticity associated with multiple sclerosis, or diabetic neuropathy, is both more controversial and more complex. It is important for FPs to recognize the multidimensional nature of this type of pain, which may be complicated by physical, psychological, and behavioral factors, including the possibility of addiction.8-11
Although IT analgesia is less subject to abuse and diversion than systemic opioids, the dependent relationship associated with a continuous delivery system makes risk stratification a necessity.12 Psychological testing is commonly used to evaluate potential candidates for long-term IT analgesia.
Prior to placement, patients must have had a failed course of conservative pain management and have no surgical options, no medical contraindications (eg, spinal pathology or susceptibility to infection), and no evidence of active addiction.12 A medication history is crucial, too, to identify use of anticoagulation therapy—a relative contraindication—as well as drug allergies and potential drug-drug interactions to guard against.3
An IT trial may be required
It is common practice for patients to undergo an IT analgesia trial prior to implantation of a subcutaneous pump. This involves using an external pump to infuse the selected medication intrathecally and slowly titrating it according to symptoms for 2 to 3 days. During this time frame, the patient records his or her response; a reduction by more than half in VAS pain score is considered a success, indicating that the patient is an appropriate candidate for placement of the device.3,13
Drug choices—a look at the evidence
The US Food and Drug Administration (FDA) has approved 3 medications for continuous IT delivery: morphine, ziconotide, and baclofen. But it is common practice to use alternative agents, such as other opioids, local anesthetics, or alpha 2-adrenergic agonists (TABLE).3,14-34
CASE › Ms. G’s primary care physician referred her to a pain specialist, who thought she would benefit from IT analgesia. After a successful single-shot IT trial with 0.5 mg morphine, the patient underwent implantation. The specialist chose morphine as the IT agent because of Ms. G’s history of successful pain relief with it, and because such a low dose was unlikely to be a problem for a patient with renal failure.
A month later, when she returned to the specialist to have the pump refilled, Ms. G reported a pain score of 3.
Opioids such as morphine exhibit a wider spread of analgesia when administered intrathecally, resulting in fewer adverse effects than systemic opioids.13,35,36 The mu-opioid receptors in the dorsal horn of the spinal cord are the primary target of IT opioids.
In a multicenter randomized trial involving 200 cancer patients on opioids, Smith et al4 compared implantable IT drug delivery systems with comprehensive medical management. The mean VAS pain score in the IT group fell 52% vs a decline of 39% in the medical management group.
The evidence supporting IT opioids for nonmalignant pain is not as strong. This may be due to inherent differences in pain mechanisms. In cancer pain, between 75% and 90% of pain is either nociceptive or mixed nociceptive-neuropathic; the etiology of noncancer pain is more variable.37-39
Although IT opioid therapy is associated with a lower incidence of adverse effects than systemic therapy, this route is not devoid of adverse effects. Opioids delivered intrathecally may still be associated with respiratory depression, constipation, urinary retention, nausea/vomiting, sweating, and hyperalgesia.39 In addition, chronic opioid use suppresses the hypothalamic-pituitary-gonadal axis and the hypothalamic-pituitary-adrenal axis14,40,41—a risk with long-term IT as well as systemic administration.14 Respiratory depression most commonly results from accidental overdosing, and patients must be monitored during initiation and dose escalation of IT opioid therapy.15
Local anesthetics. Numerous studies have documented the favorable outcomes of combining local anesthetics with opioids for patients with cancer16-20 and noncancer pain.21,22 Local anesthetics work via the blockade of voltage-gated sodium channels, interfering with neuron depolarization.17
A retrospective study in which patients with malignant pain and those with failed back surgery syndrome had bupivacaine added to their IT opioid solution found that the combination led to lower pain scores and a 23% reduction in opioid dosage.20 In another retrospective review, researchers demonstrated that the coadministration of IT bupivacaine and an opioid decreased the rate of opioid dose escalation by 65% over the first year in patients with noncancer pain.23
However, a double-blind randomized, crossover multicenter study found that in patients with chronic nonmalignant pain, the addition of bupivacaine to IT opioids failed to produce significant improvement in pain control compared with opioid use alone. Quality of life scores did improve, however, in the group receiving combination therapy.24
Adverse effects of local anesthetics delivered intrathecally include numbness, paresthesias, weakness, bowel/bladder dysfunction, and neurotoxicity.17,19,25
Calcium channel blockers. Found in venom produced by the marine snail Conus magus, ziconotide blocks presynaptic N-type channels. It is the only calcium channel blocker used to manage chronic pain.26 Several trials in patients with malignant and nonmalignant pain have shown a significant decrease in VAS pain scores compared with placebo.25,26 In addition, a multicenter, double-blind placebo-controlled crossover study evaluating IT ziconotide for the treatment of refractory pain in 111 patients with cancer and AIDS found that the treatment group obtained significantly better pain relief than the controls (53% vs 17.5% using a VAS pain intensity score).25 However, 31% of those in the treatment group experienced adverse effects, the most common of which were confusion, somnolence, and urinary retention.
Ziconotide has FDA approval only as monotherapy. But because of its high cost and adverse effect profile, it is mainly used in combination with other IT drugs.27 Ziconotide increases the risk of suicide in patients with a history of depression.28 The prevalence of adverse effects correlates with a higher dose, faster titration rate, and older age.26,28
Alpha-2 adrenergic agonists. Clonidine is the only alpha-2 agonist with FDA approval for epidural use, with several studies supporting its off-label use in combination with IT therapy.22,29 In a prospective open-label study evaluating combination IT therapy in patients with failed back surgery syndrome, 73% reported subjective ratings of good or excellent at 2-year follow-up.22 The most common adverse effects were sedation, hypotension, nausea, and dry mouth.
Gamma-aminobutyric acid (GABA) agonists. Baclofen, a GABA agonist with FDA approval for the treatment of spasticity, has been used intrathecally since the mid-1980s.32 Several studies have supported its effectiveness for this purpose.30,42 Clinical studies have also found IT baclofen to be effective in treating conditions such as complex regional pain syndrome, central pain, and neuropathic pain secondary to failed back surgery syndrome.31,32 In one randomized double-blind crossover trial, 7 women with complex regional pain syndrome were given bolus injections of baclofen or saline. Those treated with baclofen experienced a reduction in pain and regained function.31
In another trial—a double-blind placebo-controlled study of patients with multiple sclerosis and spinal cord injury comparing baclofen with placebo—those treated with baclofen showed significant reductions in dysesthetic and spasm-related pain.32 The most common adverse effects of baclofen are drowsiness, cognitive impairment, weakness, gastrointestinal complaints, and sexual dysfunction.31
Which patients and which drugs? An expert consensus
Due to the potential for inconsistent patient management and the use of therapies with anecdotal evidence, the Polyanalgesic Consensus Conference (PACC)—a panel of experts in IT therapy—convened in 2000, 2003, 2007, and 2011 to develop recommendations for IT therapy and an algorithm for drug selection. PACC’s list of chronic conditions for which IT should be considered includes axial low back pain, postherpetic neuralgia, spinal cord injury, spinal stenosis, pancreatitis, osteoporosis, compression fracture, and phantom limb pain, among others.
The algorithm contains separate arms for neuropathic, nociceptive, and mixed pain states. First-line agents for neuropathic pain include morphine, alone or combined with bupivacaine, and ziconotide. For nociceptive pain, morphine, hydromorphone, fentanyl, and ziconotide are all first-line agents; for mixed pain states, the appropriate choice should be based on the clinical scenario.33
Overseeing IT pain management in primary care
Referring potential candidates for IT therapy to specialists in pain management is just the beginning. While patients typically return to the specialist for pump refills, it is important that they see their primary care physician regularly, as well. Vigilance is required of both the FP and the patient. Any sudden worsening in pain level or acute change in neurologic function must be reported to the pain specialist immediately.
Adverse effects of medications are the most common complications
Kamran and Wright43 performed a retrospective review of their practice’s Intrathecal Drug Delivery Systems database of 122 patients and found that adverse medication effects were most common, accounting for 77% of complications.
Catheter malfunctions were next, at 16%, followed by infections, at 5%.43 In other studies, catheter-related complications were found to have an incidence of 15% to 25%.44,45 Problems include kinking, breaking, leaking, and migration of the catheter. Advise patients to immediately contact their pain specialist for evaluation if they experience a sudden loss of, or change in, pain control.
Infectious complications, which occur infrequently, are usually limited to superficial wounds, although epidural abscesses and meningitis are possible.46 Standard perioperative antibiotic administration helps to minimize the risk of infection. If a patient presents with signs and symptoms of an epidural abscess—back pain, fever, and variable neurologic deficits—emergent initiation of intravenous antibiotics is needed. Magnetic resonance imaging (MRI) with and without gadolinium should be obtained, as well.22
Spinal damage. Although IT catheters are placed under fluoroscopic guidance, there is a risk of direct injury to the spinal cord; this is more common if the catheter is placed above the level of the conus medullaris. Damage to the spinal cord or exiting spinal nerves will manifest as pain, sensory loss, and/or weakness over a dermatomal distribution.43
Neurologic sequelae, ranging from mild symptoms to paraplegia, can result from the formation of a granuloma at the tip of the spinal catheter. A sudden increase in pain usually occurs prior to neurologic deterioration, thereby allowing for early detection and intervention.47 Development of a granuloma appears to be related to the long-term infusion of high-concentration opioids.34 The diagnosis is confirmed by MRI, but physical exam and history are imperative in making the initial diagnosis.
In cases of mild neurologic symptoms, a transition to saline infusion through the pump may allow the granuloma to absorb; more severe cases may require neurosurgical intervention.47
Is your patient scheduled for an IT drug trial?
If a patient of yours is scheduled for an IT drug trial, ideally followed by pump implantation, microdosing—the practice of weaning the individual from oral opioids prior to the procedure so that very low doses of IT opioids will suffice—may play a role.48,49 While this approach appears promising, however, there is little in the way of definitive evidence of efficacy.
CASE › Over time, Ms. G’s maintenance IT dose of morphine had to be slowly increased from 0.5 mg to 1 mg/d. At bimonthly visits with her FP, she consistently reports pain scores of 3 on a scale of 1 to 10. The patient’s function has returned to baseline, and she has minimal adverse effects.
CORRESPONDENCE
Jessica Tsukanov, DO, Montefiore Medical Center, 3347 Steuben Avenue, Bronx, NY 10467; [email protected]
1. Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149-151.
2. Hayek SM, Hanes MC. Intrathecal therapy for chronic pain: current trends and future needs. Curr Pain Headache Rep. 2014;18:338.
3. Krames ES. Intraspinal opioid therapy for chronic nonmalignant pain: current practice and clinical guidelines. J Pain Symptom Manage. 1996;11:333-352.
4. Smith TJ, Staats PS, Deer T, et al; Implantable Drug Delivery Systems Study Group. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20:4040-4049.
5. Rauck RL, Cherry D, Boyer MF, et al. Long-term intrathecal opioid therapy with a patient-activated, implanted delivery system for the treatment of refractory cancer pain. J Pain. 2003;4:441-447.
6. Burton AW, Rajagopal A, Shah HN, et al. Epidural and intrathecal analgesia is effective in treating refractory cancer pain. Pain Med. 2004;5:239-247.
7. Hassenbusch SJ. Cost modeling for alternate routes of administration of opioids for cancer pain. Oncology. 1999;13(5 suppl 2):S63-S67.
8. Thimineur MA, Kravitz E, Vodapally MS. Intrathecal opioid treatment for chronic non-malignant pain: a 3-year prospective study. Pain. 2004;109:242-249.
9. Gerber HR. Intrathecal morphine for chronic benign pain. Best Pract Res Clin Anesthesiol. 2003;17:429-442.
10. Tuner JA, Sears JM, Loeser JD. Programmable intrathecal opioid delivery systems for chronic noncancer pain: a systematic review of effectiveness and complications. Clin J Pain. 2007;23:180-195.
11. Brown J, Klapow J, Doleys D, et al. Disease-specific and generic health outcomes: a model for the evaluation of long-term intrathecal opioid therapy in noncancer low back pain patients. Clin J Pain. 1999;15:122-131.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2006;6:432-442.
13. Ahmed SU, Martin NM, Chang Y. Patient selection and trial methods for intraspinal drug delivery for chronic pain: a national survey. Neuromodulation. 2005;8:112-120.
14. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85:2215-2222.
15. Coffey RJ, Owens ML, Broste SK, et al. Mortality associated with implantation and management of intrathecal opioid drug infusion systems to treat noncancer pain. Anesthesiology. 2009;111:881-891.
16. Sjöberg M, Nitescu P, Appelgren L, et al. Long-term intrathecal morphine and bupivacaine in patients with refractory cancer pain. Results from a morphine:bupivacaine dose regimen of 0.5:4.75 mg/ml. Anesthesiology. 1994;80:284-297.
17. Sjöberg M, Appelgen L, Einarsson S, et al. Long-term intrathecal morphine and bupivacaine in “refractory” cancer pain. I. Results from the first series of 52 patients. Acta Anaesthsiol Scand. 1991;35:30-43.
18. Van Dongen RT, Crul BJ, De Bock M. Long-term intrathecal infusion of morphine and morphine/bupivacaine mixtures in the treatment of cancer pain: a retrospective analysis of 51 cases. Pain. 1993;55:119-123.
19. van Dongen RT, Crul BJ, van Egmond J. Intrathecal coadministration of bupivacaine diminishes morphine dose progression during long-term intrathecal infusion in cancer patients. Clin J Pain. 1999;15:166-172.
20. Deer TR, Caraway DL, Kim CK, et al. Clinical experience with intrathecal bupivacaine in combination with opioid for the treatment of chronic pain related to failed back surgery syndrome and metastatic cancer pain of the spine. Spine J. 2002;2:274-278.
21. Krames ES, Lanning RM. Intrathecal infusional analgesia for nonmalignant pain: analgesic efficacy of intrathecal opioid with or without bupivacaine. J Pain Symptom Manage. 1993;8:539-548.
22. Rainov NG, Heidecke V, Burkert W. Long-term intrathecal infusion of drug combinations for chronic back and leg pain. J Pain Symptom Manage. 2001;22:862-871.
23. Veizi IE, Hayek SM, Narouze S, et al. Combination of intrathecal opioids with bupivacaine attenuates opioid dose escalation in chronic noncancer pain patients. Pain Med. 2011;12:1481-1489.
24. Mironer YE, Haasis JC, Chapple I, et al. Efficacy and safety of intrathecal opioid/bupivacaine mixture in chronic nonmalignant pain: A double blind, randomized, crossover, multicenter study by the National Forum of Independent Pain Clinicians (NFIPC). Neuromodulation. 2002;5:208-213.
25. Staats PS, Yearwood T, Charapata SG, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291:63-70.
26. Rauck RL, Wallace MS, Leong MS, et al; Ziconotide 301 Study Group. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J Pain Symptom Manage. 2006;31:393-406.
27. Wallace MS, Rauck R, Fisher R, et al; Ziconotide 98-022 Study Group. Intrathecal ziconotide for severe chronic pain: safety and tolerability results of an open-label, long-term trial. Anesth Analg. 2008;106:628-637.
28. Maier C, Gockel HH, Gruhn K, et al. Increased risk of suicide under intrathecal ziconotide treatment? - a warning. Pain. 2011;152:235-237.
29. Ackerman LL, Follett KA, Rosenquist RW. Long-term outcomes during treatment of chronic pain with intrathecal clonidine or clonidine/opioid combinations. J Pain Symptom Manage. 2003;26:668-677.
30. Tarrico M, Adone R, Pagliacci C, et al. Pharmacological interventions for spasticity following spinal cord injury. Cochrane Database Syst Rev. 2000;(2):CD001131.
31. van Hilten BJ, van de Beek WT, Hoff JI, et al. Intrathecal baclofen for the treatment of dystonia in patients with reflex sympathetic dystrophy. N Engl J Med. 2000;343:625-630.
32. Herman RM, D’Luzansky SC, Ippolito R. Intrathecal baclofen suppresses central pain in patients with spinal lesions. A pilot study. Clin J Pain. 1992;8:338-345.
33. Deer T, Prager J, Levy R, et al. Polyanalgesic consensus conference 2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation. 2012;15:436-466.
34. Yaksh TL, Coffey RJ. Spinal opiate toxicity. In: Proceedings of American Society of Regional Anesthesia and Pain Medication Conference; November 18-21, 2004; Phoenix, AZ.
35. Levy MH. Pharmacologic management of cancer pain. Semin Oncol. 1994;21:718-739.
36. Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61:276-310.
37. Zeppetella G, O’Doherty CA, Collins S. Prevalence and characteristics of breakthrough pain in patients with non-malignant terminal disease admitted to a hospice. Palliat Med. 2001;15:243-246.
38. Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41:273-281.
39. Hanks GW, Forbes K. Opioid responsiveness. Acta Anaesthesiol Scan. 1997;41:154-158.
40. Paice JA, Penn RD, Ryan WG. Altered sexual function and decreased testosterone in patients receiving intraspinal opioids. J Pain Symptom Manage. 1994;9:126-131.
41. Brennan MJ. The effect of opioid therapy on endocrine function. Am J Med. 2013;126(3 suppl 1):S12-S18.
42. Beard S, Hunn A. Wight J. Treatments for spasticity and pain in multiple sclerosis: a systematic review. Health Technol Assess. 2003;7:iii,ix-x,1-111.
43. Kamran S, Wright BD. Complications of intrathecal drug delivery systems. Neuromodulation. 2001;4:111-115.
44. Follett KA, Naumann CP. A prospective study of catheter-related complications of intrathecal drug delivery systems. J Pain Symptom Manage. 2000;19:209-215.
45. Follett KA, Burchiel K, Deer T, et al. Prevention of intrathecal drug delivery catheter-related complications. Neuromodulation. 2003;6:32-41.
46. Paice JA, Penn RD, Shott S. Intraspinal morphine for chronic pain: a retrospective, multicenter study. J Pain Symptom Manage. 1996;11:71-80.
47. Miele VJ, Price KO, Bloomfield S, et al. A review of intrathecal morphine therapy related granulomas. Eur J Pain. 2006;10:251-261.
48. Hayek SM. Intrathecal “microdosing”: reality or artifact? Pain Med. 2012;13:1664-1665.
49. Grider JS, Harned ME, Etscheidt MA. Patient selection and outcomes using a low-dose intrathecal opioid trialing method for chronic nonmalignant pain. Pain Physician. 2011;14:343-351.
1. Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149-151.
2. Hayek SM, Hanes MC. Intrathecal therapy for chronic pain: current trends and future needs. Curr Pain Headache Rep. 2014;18:338.
3. Krames ES. Intraspinal opioid therapy for chronic nonmalignant pain: current practice and clinical guidelines. J Pain Symptom Manage. 1996;11:333-352.
4. Smith TJ, Staats PS, Deer T, et al; Implantable Drug Delivery Systems Study Group. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20:4040-4049.
5. Rauck RL, Cherry D, Boyer MF, et al. Long-term intrathecal opioid therapy with a patient-activated, implanted delivery system for the treatment of refractory cancer pain. J Pain. 2003;4:441-447.
6. Burton AW, Rajagopal A, Shah HN, et al. Epidural and intrathecal analgesia is effective in treating refractory cancer pain. Pain Med. 2004;5:239-247.
7. Hassenbusch SJ. Cost modeling for alternate routes of administration of opioids for cancer pain. Oncology. 1999;13(5 suppl 2):S63-S67.
8. Thimineur MA, Kravitz E, Vodapally MS. Intrathecal opioid treatment for chronic non-malignant pain: a 3-year prospective study. Pain. 2004;109:242-249.
9. Gerber HR. Intrathecal morphine for chronic benign pain. Best Pract Res Clin Anesthesiol. 2003;17:429-442.
10. Tuner JA, Sears JM, Loeser JD. Programmable intrathecal opioid delivery systems for chronic noncancer pain: a systematic review of effectiveness and complications. Clin J Pain. 2007;23:180-195.
11. Brown J, Klapow J, Doleys D, et al. Disease-specific and generic health outcomes: a model for the evaluation of long-term intrathecal opioid therapy in noncancer low back pain patients. Clin J Pain. 1999;15:122-131.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2006;6:432-442.
13. Ahmed SU, Martin NM, Chang Y. Patient selection and trial methods for intraspinal drug delivery for chronic pain: a national survey. Neuromodulation. 2005;8:112-120.
14. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85:2215-2222.
15. Coffey RJ, Owens ML, Broste SK, et al. Mortality associated with implantation and management of intrathecal opioid drug infusion systems to treat noncancer pain. Anesthesiology. 2009;111:881-891.
16. Sjöberg M, Nitescu P, Appelgren L, et al. Long-term intrathecal morphine and bupivacaine in patients with refractory cancer pain. Results from a morphine:bupivacaine dose regimen of 0.5:4.75 mg/ml. Anesthesiology. 1994;80:284-297.
17. Sjöberg M, Appelgen L, Einarsson S, et al. Long-term intrathecal morphine and bupivacaine in “refractory” cancer pain. I. Results from the first series of 52 patients. Acta Anaesthsiol Scand. 1991;35:30-43.
18. Van Dongen RT, Crul BJ, De Bock M. Long-term intrathecal infusion of morphine and morphine/bupivacaine mixtures in the treatment of cancer pain: a retrospective analysis of 51 cases. Pain. 1993;55:119-123.
19. van Dongen RT, Crul BJ, van Egmond J. Intrathecal coadministration of bupivacaine diminishes morphine dose progression during long-term intrathecal infusion in cancer patients. Clin J Pain. 1999;15:166-172.
20. Deer TR, Caraway DL, Kim CK, et al. Clinical experience with intrathecal bupivacaine in combination with opioid for the treatment of chronic pain related to failed back surgery syndrome and metastatic cancer pain of the spine. Spine J. 2002;2:274-278.
21. Krames ES, Lanning RM. Intrathecal infusional analgesia for nonmalignant pain: analgesic efficacy of intrathecal opioid with or without bupivacaine. J Pain Symptom Manage. 1993;8:539-548.
22. Rainov NG, Heidecke V, Burkert W. Long-term intrathecal infusion of drug combinations for chronic back and leg pain. J Pain Symptom Manage. 2001;22:862-871.
23. Veizi IE, Hayek SM, Narouze S, et al. Combination of intrathecal opioids with bupivacaine attenuates opioid dose escalation in chronic noncancer pain patients. Pain Med. 2011;12:1481-1489.
24. Mironer YE, Haasis JC, Chapple I, et al. Efficacy and safety of intrathecal opioid/bupivacaine mixture in chronic nonmalignant pain: A double blind, randomized, crossover, multicenter study by the National Forum of Independent Pain Clinicians (NFIPC). Neuromodulation. 2002;5:208-213.
25. Staats PS, Yearwood T, Charapata SG, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291:63-70.
26. Rauck RL, Wallace MS, Leong MS, et al; Ziconotide 301 Study Group. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J Pain Symptom Manage. 2006;31:393-406.
27. Wallace MS, Rauck R, Fisher R, et al; Ziconotide 98-022 Study Group. Intrathecal ziconotide for severe chronic pain: safety and tolerability results of an open-label, long-term trial. Anesth Analg. 2008;106:628-637.
28. Maier C, Gockel HH, Gruhn K, et al. Increased risk of suicide under intrathecal ziconotide treatment? - a warning. Pain. 2011;152:235-237.
29. Ackerman LL, Follett KA, Rosenquist RW. Long-term outcomes during treatment of chronic pain with intrathecal clonidine or clonidine/opioid combinations. J Pain Symptom Manage. 2003;26:668-677.
30. Tarrico M, Adone R, Pagliacci C, et al. Pharmacological interventions for spasticity following spinal cord injury. Cochrane Database Syst Rev. 2000;(2):CD001131.
31. van Hilten BJ, van de Beek WT, Hoff JI, et al. Intrathecal baclofen for the treatment of dystonia in patients with reflex sympathetic dystrophy. N Engl J Med. 2000;343:625-630.
32. Herman RM, D’Luzansky SC, Ippolito R. Intrathecal baclofen suppresses central pain in patients with spinal lesions. A pilot study. Clin J Pain. 1992;8:338-345.
33. Deer T, Prager J, Levy R, et al. Polyanalgesic consensus conference 2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation. 2012;15:436-466.
34. Yaksh TL, Coffey RJ. Spinal opiate toxicity. In: Proceedings of American Society of Regional Anesthesia and Pain Medication Conference; November 18-21, 2004; Phoenix, AZ.
35. Levy MH. Pharmacologic management of cancer pain. Semin Oncol. 1994;21:718-739.
36. Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61:276-310.
37. Zeppetella G, O’Doherty CA, Collins S. Prevalence and characteristics of breakthrough pain in patients with non-malignant terminal disease admitted to a hospice. Palliat Med. 2001;15:243-246.
38. Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41:273-281.
39. Hanks GW, Forbes K. Opioid responsiveness. Acta Anaesthesiol Scan. 1997;41:154-158.
40. Paice JA, Penn RD, Ryan WG. Altered sexual function and decreased testosterone in patients receiving intraspinal opioids. J Pain Symptom Manage. 1994;9:126-131.
41. Brennan MJ. The effect of opioid therapy on endocrine function. Am J Med. 2013;126(3 suppl 1):S12-S18.
42. Beard S, Hunn A. Wight J. Treatments for spasticity and pain in multiple sclerosis: a systematic review. Health Technol Assess. 2003;7:iii,ix-x,1-111.
43. Kamran S, Wright BD. Complications of intrathecal drug delivery systems. Neuromodulation. 2001;4:111-115.
44. Follett KA, Naumann CP. A prospective study of catheter-related complications of intrathecal drug delivery systems. J Pain Symptom Manage. 2000;19:209-215.
45. Follett KA, Burchiel K, Deer T, et al. Prevention of intrathecal drug delivery catheter-related complications. Neuromodulation. 2003;6:32-41.
46. Paice JA, Penn RD, Shott S. Intraspinal morphine for chronic pain: a retrospective, multicenter study. J Pain Symptom Manage. 1996;11:71-80.
47. Miele VJ, Price KO, Bloomfield S, et al. A review of intrathecal morphine therapy related granulomas. Eur J Pain. 2006;10:251-261.
48. Hayek SM. Intrathecal “microdosing”: reality or artifact? Pain Med. 2012;13:1664-1665.
49. Grider JS, Harned ME, Etscheidt MA. Patient selection and outcomes using a low-dose intrathecal opioid trialing method for chronic nonmalignant pain. Pain Physician. 2011;14:343-351.