User login
CHICAGO – Once-weekly, fixed-dose albiglutide achieved superior glycemic control compared with dose-adjusted sitagliptin in patients with type 2 diabetes who had inadequate glycemic control and varying degrees of renal impairment in a phase III trial.
Albiglutide is an investigational glucagonlike peptide–1 (GLP-1) agonist now under review at both the U.S. Food and Drug Administration and the European Medicines Agency as a potential new treatment for type 2 diabetes. It is a large molecule cleared by the reticuloendothelial system rather than by the kidney, so its metabolism is not affected by the renal dysfunction that is common in patients with type 2 diabetes.
"Albiglutide will be a useful treatment addition for patients with renal impairment and type 2 diabetes. Current treatments for such patients are limited. Several antidiabetic medications are contraindicated in this population, and many others require dose adjustment," Dr. Lawrence A. Leiter observed in presenting the trial results at the annual scientific sessions of the American Diabetes Association.
The 52-week double-blind study involved 495 patients with type 2 diabetes whose hyperglycemia wasn’t adequately controlled by diet and exercise or oral antidiabetic medications. Renal impairment was mild in slightly over half of the participants, moderate in 41%, and severe in the remainder.
Patients were randomized to self-administered subcutaneous injection of albiglutide at 30 mg once a week or the oral dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin, dose-adjusted on the basis of the degree of renal impairment as specified in the product labeling. Thirty-five percent of patients in the albiglutide group had their daily dose uptitrated from 30 mg to 50 mg owing to insufficient response to the initial dose.
The primary endpoint was the change in mean hemoglobin A1c from baseline to week 26. The HbA1c dropped by 0.8% in the albiglutide group, significantly better than the 0.5% decrease with sitagliptin (P = .0003). The advantage favoring the investigational agent was seen in all three renal impairment severity groups, reported Dr. Leiter, professor of medicine and nutritional sciences at the University of Toronto and president of the Canadian Society of Endocrinology and Metabolism.
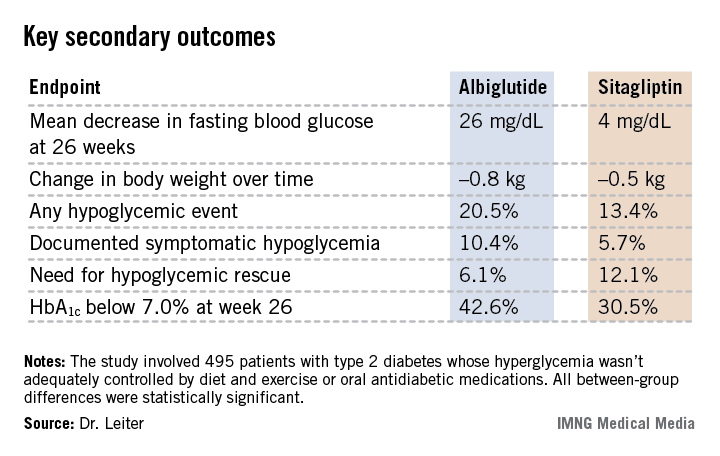
Albiglutide-treated patients also fared significantly better in terms of multiple key secondary endpoints (see chart). For example, 42.6% of them achieved a clinically meaningful HbA1c response by 26 weeks, defined as an HbA1c below 7.0%, compared with 30.5% of patients on sitagliptin.
Both drugs were well tolerated. The rate of adverse events leading to study withdrawal was 6.4% with albiglutide and 8.1% with sitagliptin. Both drugs had impressively low rates of nausea and vomiting; typically, less than 1% of patients per week reported either symptom.
Session chair Dr. Julio Rosenstock called this study noteworthy for several reasons. It is the first study of a GLP-1 agonist in patients with renal insufficiency; head-to-head comparative randomized trials in diabetes are rare. And the low-single-digit rates of nausea and vomiting seen with albiglutide in this study are "remarkable." Other GLP-1 agonists are typically associated with nausea and vomiting rates in the 15%-20% range or even higher, noted Dr. Rosenstock, director of the Dallas Diabetes and Endocrine Center at Medical City.
Dr. Leiter agreed, offering two potential explanations for the rock-bottom nausea and vomiting rates. One is that the once-weekly injection results in a very gradual increase in drug levels. Also, as a large molecule, albiglutide probably has less central action, including less activity at the central nervous system’s nausea and vomiting centers.
Other large, completed phase III trials have shown benefits for albiglutide in additional, common clinical scenarios, including yearlong studies of albiglutide as add-on therapy in patients with type 2 diabetes not controlled on pioglitazone and metformin, albiglutide monotherapy in drug-naive type 2 diabetic patients, a comparison of the novel GLP-1 agonist to insulin glargine in patients with type 2 diabetes, and albiglutide versus pioglitazone as add-on therapy in patients on background metformin and glimepiride.
Albiglutide is being developed by GlaxoSmithKline. Dr. Leiter has received research grants from and served as a consultant to GSK and other companies.
CHICAGO – Once-weekly, fixed-dose albiglutide achieved superior glycemic control compared with dose-adjusted sitagliptin in patients with type 2 diabetes who had inadequate glycemic control and varying degrees of renal impairment in a phase III trial.
Albiglutide is an investigational glucagonlike peptide–1 (GLP-1) agonist now under review at both the U.S. Food and Drug Administration and the European Medicines Agency as a potential new treatment for type 2 diabetes. It is a large molecule cleared by the reticuloendothelial system rather than by the kidney, so its metabolism is not affected by the renal dysfunction that is common in patients with type 2 diabetes.

"Albiglutide will be a useful treatment addition for patients with renal impairment and type 2 diabetes. Current treatments for such patients are limited. Several antidiabetic medications are contraindicated in this population, and many others require dose adjustment," Dr. Lawrence A. Leiter observed in presenting the trial results at the annual scientific sessions of the American Diabetes Association.
The 52-week double-blind study involved 495 patients with type 2 diabetes whose hyperglycemia wasn’t adequately controlled by diet and exercise or oral antidiabetic medications. Renal impairment was mild in slightly over half of the participants, moderate in 41%, and severe in the remainder.
Patients were randomized to self-administered subcutaneous injection of albiglutide at 30 mg once a week or the oral dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin, dose-adjusted on the basis of the degree of renal impairment as specified in the product labeling. Thirty-five percent of patients in the albiglutide group had their daily dose uptitrated from 30 mg to 50 mg owing to insufficient response to the initial dose.
The primary endpoint was the change in mean hemoglobin A1c from baseline to week 26. The HbA1c dropped by 0.8% in the albiglutide group, significantly better than the 0.5% decrease with sitagliptin (P = .0003). The advantage favoring the investigational agent was seen in all three renal impairment severity groups, reported Dr. Leiter, professor of medicine and nutritional sciences at the University of Toronto and president of the Canadian Society of Endocrinology and Metabolism.
Albiglutide-treated patients also fared significantly better in terms of multiple key secondary endpoints (see chart). For example, 42.6% of them achieved a clinically meaningful HbA1c response by 26 weeks, defined as an HbA1c below 7.0%, compared with 30.5% of patients on sitagliptin.
Both drugs were well tolerated. The rate of adverse events leading to study withdrawal was 6.4% with albiglutide and 8.1% with sitagliptin. Both drugs had impressively low rates of nausea and vomiting; typically, less than 1% of patients per week reported either symptom.
Session chair Dr. Julio Rosenstock called this study noteworthy for several reasons. It is the first study of a GLP-1 agonist in patients with renal insufficiency; head-to-head comparative randomized trials in diabetes are rare. And the low-single-digit rates of nausea and vomiting seen with albiglutide in this study are "remarkable." Other GLP-1 agonists are typically associated with nausea and vomiting rates in the 15%-20% range or even higher, noted Dr. Rosenstock, director of the Dallas Diabetes and Endocrine Center at Medical City.
Dr. Leiter agreed, offering two potential explanations for the rock-bottom nausea and vomiting rates. One is that the once-weekly injection results in a very gradual increase in drug levels. Also, as a large molecule, albiglutide probably has less central action, including less activity at the central nervous system’s nausea and vomiting centers.
Other large, completed phase III trials have shown benefits for albiglutide in additional, common clinical scenarios, including yearlong studies of albiglutide as add-on therapy in patients with type 2 diabetes not controlled on pioglitazone and metformin, albiglutide monotherapy in drug-naive type 2 diabetic patients, a comparison of the novel GLP-1 agonist to insulin glargine in patients with type 2 diabetes, and albiglutide versus pioglitazone as add-on therapy in patients on background metformin and glimepiride.
Albiglutide is being developed by GlaxoSmithKline. Dr. Leiter has received research grants from and served as a consultant to GSK and other companies.
CHICAGO – Once-weekly, fixed-dose albiglutide achieved superior glycemic control compared with dose-adjusted sitagliptin in patients with type 2 diabetes who had inadequate glycemic control and varying degrees of renal impairment in a phase III trial.
Albiglutide is an investigational glucagonlike peptide–1 (GLP-1) agonist now under review at both the U.S. Food and Drug Administration and the European Medicines Agency as a potential new treatment for type 2 diabetes. It is a large molecule cleared by the reticuloendothelial system rather than by the kidney, so its metabolism is not affected by the renal dysfunction that is common in patients with type 2 diabetes.

"Albiglutide will be a useful treatment addition for patients with renal impairment and type 2 diabetes. Current treatments for such patients are limited. Several antidiabetic medications are contraindicated in this population, and many others require dose adjustment," Dr. Lawrence A. Leiter observed in presenting the trial results at the annual scientific sessions of the American Diabetes Association.
The 52-week double-blind study involved 495 patients with type 2 diabetes whose hyperglycemia wasn’t adequately controlled by diet and exercise or oral antidiabetic medications. Renal impairment was mild in slightly over half of the participants, moderate in 41%, and severe in the remainder.
Patients were randomized to self-administered subcutaneous injection of albiglutide at 30 mg once a week or the oral dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin, dose-adjusted on the basis of the degree of renal impairment as specified in the product labeling. Thirty-five percent of patients in the albiglutide group had their daily dose uptitrated from 30 mg to 50 mg owing to insufficient response to the initial dose.
The primary endpoint was the change in mean hemoglobin A1c from baseline to week 26. The HbA1c dropped by 0.8% in the albiglutide group, significantly better than the 0.5% decrease with sitagliptin (P = .0003). The advantage favoring the investigational agent was seen in all three renal impairment severity groups, reported Dr. Leiter, professor of medicine and nutritional sciences at the University of Toronto and president of the Canadian Society of Endocrinology and Metabolism.
Albiglutide-treated patients also fared significantly better in terms of multiple key secondary endpoints (see chart). For example, 42.6% of them achieved a clinically meaningful HbA1c response by 26 weeks, defined as an HbA1c below 7.0%, compared with 30.5% of patients on sitagliptin.
Both drugs were well tolerated. The rate of adverse events leading to study withdrawal was 6.4% with albiglutide and 8.1% with sitagliptin. Both drugs had impressively low rates of nausea and vomiting; typically, less than 1% of patients per week reported either symptom.
Session chair Dr. Julio Rosenstock called this study noteworthy for several reasons. It is the first study of a GLP-1 agonist in patients with renal insufficiency; head-to-head comparative randomized trials in diabetes are rare. And the low-single-digit rates of nausea and vomiting seen with albiglutide in this study are "remarkable." Other GLP-1 agonists are typically associated with nausea and vomiting rates in the 15%-20% range or even higher, noted Dr. Rosenstock, director of the Dallas Diabetes and Endocrine Center at Medical City.
Dr. Leiter agreed, offering two potential explanations for the rock-bottom nausea and vomiting rates. One is that the once-weekly injection results in a very gradual increase in drug levels. Also, as a large molecule, albiglutide probably has less central action, including less activity at the central nervous system’s nausea and vomiting centers.
Other large, completed phase III trials have shown benefits for albiglutide in additional, common clinical scenarios, including yearlong studies of albiglutide as add-on therapy in patients with type 2 diabetes not controlled on pioglitazone and metformin, albiglutide monotherapy in drug-naive type 2 diabetic patients, a comparison of the novel GLP-1 agonist to insulin glargine in patients with type 2 diabetes, and albiglutide versus pioglitazone as add-on therapy in patients on background metformin and glimepiride.
Albiglutide is being developed by GlaxoSmithKline. Dr. Leiter has received research grants from and served as a consultant to GSK and other companies.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Mean HbA1c levels dropped by 0.8% after 26 weeks on albiglutide in renally impaired type 2 diabetic patients, a significantly better result than the mean 0.5% decrease with sitagliptin. Patients on albiglutide also fared significantly better in terms of numerous secondary endpoints.
Data source: A phase III, randomized, double-blind trial in 495 patients with type 2 diabetes and varying degrees of renal insufficiency and baseline inadequate glycemic control assigned to either the novel once-weekly GLP-1 agonist albiglutide or sitagliptin.
Disclosures: Albiglutide is being developed by GlaxoSmithKline. Dr. Leiter has received research grants from and served as a consultant to GSK and other companies.