User login
Mitragyna speciosa, better known as kratom, is a tropical evergreen tree that is native to Southeast Asia. Botanically, it is a member of the Rubiaceae family, as is the coffee plant, and physical laborers among indigenous populations have historically chewed the leaves or brewed them as a tea to improve endurance and reduce fatigue.1 Kratom is psychoactive; small amounts (up to 5 g of plant material) possess stimulant properties, while larger doses (>5 g) produce opioid-like, sedative, euphoric, and antinociceptive effects.2
In recent years, kratom has gained popularity in Western parts of the world due to its unique properties and perceived safety as a botanical product. Individuals may use kratom to boost their energy, relieve pain, or treat a wide range of physical or mood problems. Increasingly, kratom is being used by people who abuse opioids to self-manage opioid withdrawal, or for its euphoric effects. But kratom carries several important risks, including addiction, serious adverse effects, and possibly death. In this article, we review the epidemiology and pharmacology of kratom, and provide some guidance for educating patients about this substance.
Widely used but not FDA approved
Although kratom is not regulated or approved by the FDA, 3 to 5 million Americans use it regularly.3 According to an internet survey, kratom users are mostly college-educated, employed white men, age 31 to 50, who take the substance to manage pain or to treat general anxiety and mood disorders.4 Some individuals use kratom as an opioid substitute to reduce symptoms of opioid withdrawal.4
Kratom is available from a wide range of manufacturers in various formulations, including powders, tablets, liquids, and gum. It is sometimes sold in combination with other agents as a single product. Low-cost, over-the-counter kratom products are available as “dietary supplements” in retail stores or online. Although the product packaging sometimes recommends a specific dose, the amount of active ingredients (as well as other agents) is unknown. Kratom is illegal in several states (Box5).
Box
The use and sale of kratom is illegal in several countries, including Australia, Poland, Denmark, Sweden, Malaysia, and Vietnam. In the United States, kratom was legal to grow and purchase in all 50 states until 2015, when the Drug Enforcement Administration (DEA) identified kratom as a “substance of concern.” In August 2016, the DEA submitted a notice of intent to place mitragynine and 7-hydroxymitragynine, 2 alkaloids of kratom that have opioid-like properties, into Schedule I of the Controlled Substance Act; however, due to significant public pressure, the DEA withdrew the request in October 2016.
As of February 2020, kratom was illegal to buy, sell, or use in Wisconsin, Rhode Island, Vermont, Indiana, Arkansas, Alabama, specific counties of some states, and the District of Columbia. Legislation was pending in New York, Missouri, and Louisiana.
Source: Reference 5
The 2 alkaloids of interest
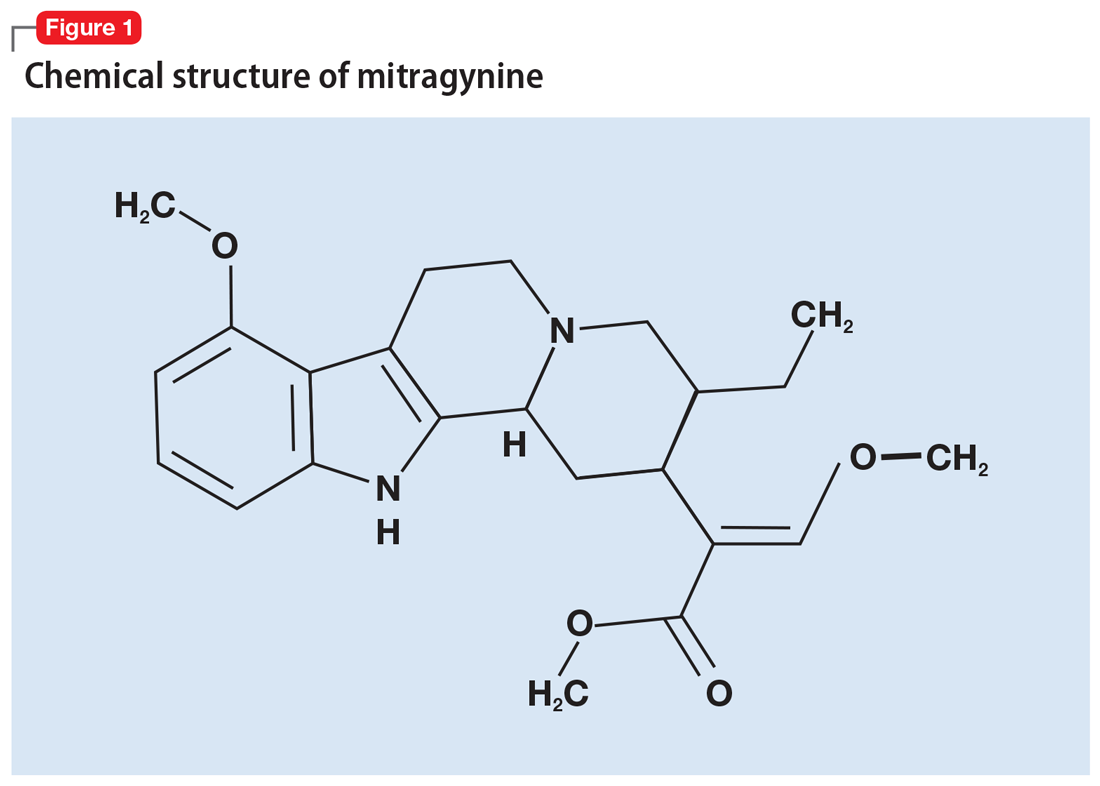
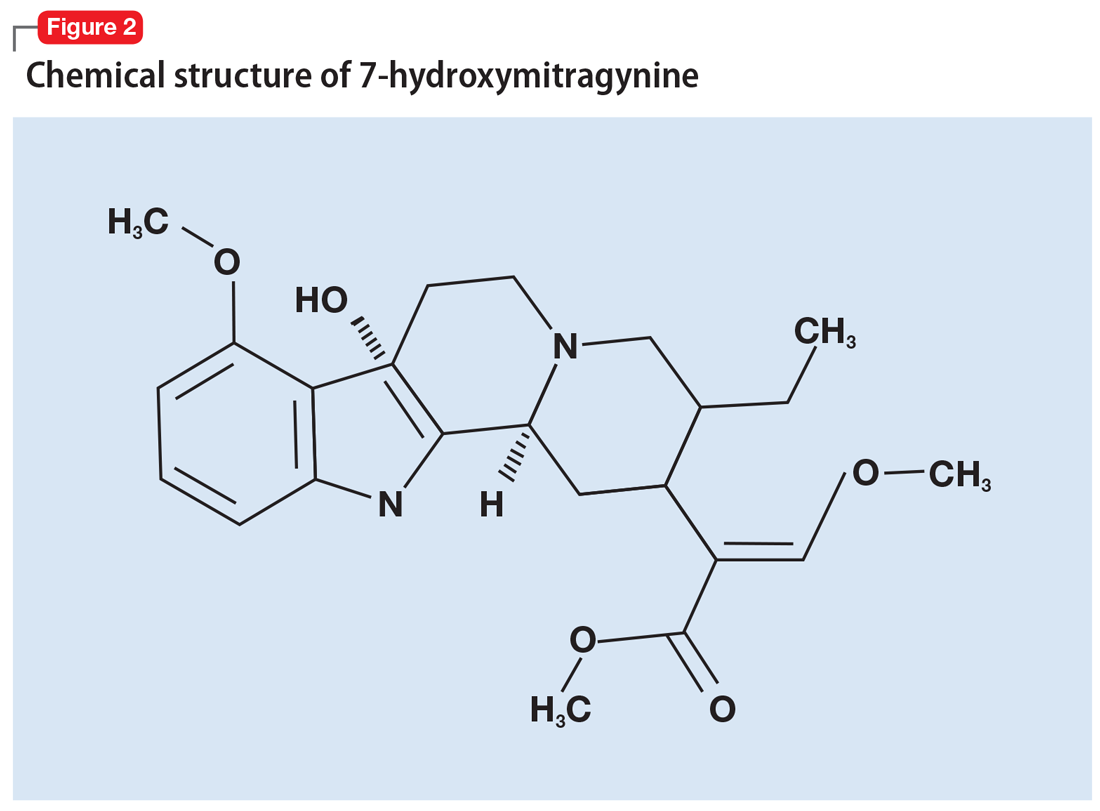
More than 40 alkaloids have been isolated from kratom leaves. The proportions of these alkaloids vary significantly depending on the environment in which the plant is grown, the breeding and harvesting techniques, and the age of the plant.6 Two alkaloids of significant interest are mitragynine (Figure 1) and 7-hydroxymitragynine (Figure 2), both of which are unique to M. speciosa and have opioid-like properties. Administering these alkaloids to morphine-dependent rats resulted in cross-tolerance and precipitated withdrawal when the rats were given naloxone.7 The potency of kratom at the mu opioid receptor has been found to exceed that of morphine.
Competitive binding studies that examined the affinity of mitragynine and 7-hydroxymitragynine at the various opioid receptor subtypes found a preference for the kappa receptors (antagonism), followed by mu (partial agonism), and lastly delta. This profile of mitragynine is very similar to that of buprenorphine.8 The affinity of 7-hydroxymitragynine for the mu receptor (agonism) is significantly greater than that of mitragynine.9 Mitragynine also interacts with noradrenergic and serotonergic pathways by stimulating postsynaptic alpha-2 adrenergic receptors and inhibiting 5-HT2A receptors.9 These properties are responsible for kratom’s ability to manage opioid withdrawal symptoms, which are generally attributed to a hyperactive noradrenergic system. There also is evidence that the hepatic metabolite 7-hydroxymitragynine is important in mediating the analgesic component of mitragynine.10
The initial effects of kratom typically begin within 10 to 20 minutes of consumption, and the full effects are experienced in 30 to 60 minutes.1 The half-life of mitragynine in humans has not yet been determined, but is believed to be relatively short.11 In rats, the half-life of mitragynine is 2 to 3 hours.12 Individuals who use kratom to prevent opioid withdrawal have reported taking it as often as every 6 to 12 hours.13
Continue to: Metabolism of mitragynine...
Metabolism of mitragynine is predominantly carried out through cytochrome P450 (CYP) 3A4, with minor contributions by 2D6 and 2C9. A total of 13 metabolites are produced, including 7-hydroxymitragynine.14 Kratom’s constituents also interact with the CYP system, inhibiting 2C9, 2D6, and 3A4 isoenzymes, and to some extent, 1A2.
Adverse effects can be fatal
An animal study revealed that when administered intravenously, mitragynine and 7-hydroxymitragynine have a similar toxicity profile to heroin.15 When these alkaloids were administered in ascending doses, increases in blood pressure and elevations in liver function tests and creatinine levels from baseline were observed.
Chronic kratom use can result in weight loss, insomnia, constipation, dehydration, skin hyperpigmentation, and extreme fatigue.16 There have also been reports of seizures, delusions, hallucinations, respiratory depression, hepatotoxicity, coma, and death.17,18 An emerging concern is the potential development of fatty liver infiltrates leading to cholestatic liver damage.19-25 One case report described a young man who developed a serum aspartate aminotransferase level of 1,300 IU/L (reference range: 5 to 45 IU/L) and a serum alanine aminotransaminase level of 3,700 IU/L (reference range: 5 to 60 IU/L) after he ingested a kratom product.26 Histologically, the pattern of liver injury mimics primary biliary cholangitis.27
In recent years, calls to poison control centers in the United States related to kratom exposure have risen. Between 2011 and 2017, the number of calls increased from 1 a month to 2 each day.28 The US National Poison Data System has also noted an increase in the number of calls in reference to kratom. It received 2,312 calls from January 2011 through July 2018, with 18 calls occurring in 2011, and 357 within the first 7 months of 2018.29
As of February 2018, the FDA had received reports of 44 deaths associated with kratom.30 There have been reports of fatal overdoses involving kratom, particularly when kratom is co-ingested or used with adulterated and/or combination agents, including one case that involved quetiapine.31-33 There have been reports of deaths believed to be attributed to the use of kratom alone; in one such case, a 35-year-old man experienced a fatal cardiac arrest due to kratom use with no other coingestants.34 Among the reports of deaths in which kratom was the only substance consumed, the mitragynine blood levels of the deceased individuals were found to be higher than the levels associated with individuals who had consumed traditional kratom teas.29
Continue to: There is a lack of quality control...
There is a lack of quality control of commercially available kratom preparations. The FDA has found kratom products that exceeded the level of safe exposure to nickel and lead.35 There have also been reports of Salmonella outbreaks associated with kratom products.36
Detecting kratom use
Mitragynine is a lipophilic alkaloid that is poorly soluble in water37 and eliminated primarily in urine.12 Based on data from treatment center admissions, kratom can be detected in urine samples for 5 to 6 days after use.24,38,39 However, kratom is not detectable by a standard urine toxicology screen; therefore, a high degree of suspicion and special confirmatory testing are necessary. The breakdown products of mitragynine can be detected through gas chromatography coupled with mass spectrometry (GC/MS), liquid chromatography with linear ion trap mass spectrometry, or electrospray tandem mass spectrometry.40-42
A familiar withdrawal syndrome
Abrupt discontinuation of high-dose, long-term kratom use can produce withdrawal symptoms.13 Symptoms of kratom withdrawal resemble those of opioid withdrawal. These include physiological symptoms (mydriasis, nausea, sweating and chills, muscle and body aches, tremors and twitches, diarrhea, rhinorrhea, and lacrimation) and psychological symptoms (insomnia, restlessness, irritability/hostility, fatigue, anxiety, mood disturbances, and hallucinations).13 Symptoms are first noted starting 12 hours after the last use of kratom, and can last up to 7 days.43 Withdrawal intensity has been positively correlated with the daily amount of kratom consumed, as well as the duration and frequency of use.13,16
In 2 case reports, the newborns of women who used kratom during pregnancy experienced neonatal abstinence syndrome.44,45 In these 2 reports, symptoms such as jitteriness, irritability, feeding intolerance, and vomiting emerged on postpartum Day 2. The newborns were admitted to a neonatal ICU and started on a standard opioid protocol with IV morphine and subsequently tapered with an oral formulation over 5 days.44,45
Helping patients who use kratom
The best approach to treating a patient who is experiencing kratom withdrawal is symptomatic management, as would be appropriate for a patient experiencing opioid withdrawal.13 However, the use of agents such as methadone or buprenorphine for patients undergoing kratom withdrawal has not been thoroughly evaluated; very few reports have been published.46,47
Continue to: Similarly, while the standard of care...
Similarly, while the standard of care for treating a patient with opioid use disorder is medication-assisted treatment in combination with counseling and behavioral therapies, there is little evidence on the efficacy of such treatments for patients who use kratom. There are no specific guidelines, and the risk of relapsing to kratom use is high.48,49 Nonetheless, some clinicians have used the same protocol for patients with opioid use disorder to treat patients using kratom, and several published case reports describe this approach.50,51 Because administering buprenorphine/naltrexone to a patient who is dependent on kratom can precipitate withdrawal, clinicians should follow a similar initiation protocol as for opioid dependence when starting a patient on these agents (ie, a washout period with a challenge test would be prudent prior to starting naltrexone).
In cases of kratom overdose, naloxone has been shown to reverse the analgesic effects of mitragynine in rats. However, in a case report of an individual who accidently overdosed on a kratom product, naloxone had a modest effect.52
Bottom Line
Kratom is a botanical substance that acts like a stimulant at low doses and an opioid at higher doses. Patients might use it to treat mood-related symptoms, relieve pain, or manage opioid withdrawal. Kratom use has been associated with the development of addiction as well as a multitude of serious adverse effects, including hepatotoxicity and overdose. Long-term management may be required for a patient who uses kratom.
Related Resources
- White CM. Pharmacologic and clinical assessment of kratom: an update. Am J Health Syst Pharm. 2019;76(23):1915-1925.
- Smith KE, Lawson T. Prevalence and motivations for kratom use in a sample of substance users enrolled in a residential treatment program. Drug Alcohol Depend. 2017;180:340-348.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naltrexone • Suboxone
Methadone • Methadose
Naltrexone • Revia
Naloxone • Narcan
Quetiapine • Seroquel
1. Henningfield JE, Fant RV, Wang DW. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology (Berl). 2018;235(2):573-589.
2. Chang-Chien GC, Odonkor CA, Amorapanth P, et al. Is kratom the new ‘legal high’ on the block?: the case of an emerging opioid receptor agonist with substance abuse potential. Pain Physician. 2017;20(1):E195-E198.
3. Penders T, Jones WB. Kratom, a substance of increasing concern [PCSS webinar]. Providers Clinical Support System. November 28, 2018. https://pcssnow.org/event/kratom-a-substance-of-increasing-concern. Accessed January 29, 2020.
4. Grundmann O. Patterns of kratom use and health impact in the US-results from an online survey. Drug Alcohol Depend. 2017;176:63-70.
5. US Drug Enforcement Administration. Drugs of concern. https://www.dea.gov/sites/default/files/sites/getsmartaboutdrugs.com/files/publications/DoA_2017Ed_Updated_6.16.17.pdf#page=84. Updated June 16, 2017. Accessed January 29, 2020.
6. Matsumoto K, Horie S, Ishikawa H, et al. Antinociceptive effect of 7-hydroxymitragynine in mice: discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sciences. 2004;74(17):2143-2155.
7. Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull (Tokyo). 2004;52(8):916-928.
8. Suhaimi FW, Yusoff NH, Hassan R, et al. Neurobiology of kratom and its main alkaloid mitragynine. Brain Res Bull. 2016;126(pt 1):29-40.
9. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
10. Kruegel AC, Uprety R, Grinnell SG, et al. 7-hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci. 2019;5(6):992-1001.
11. Trakulsrichai S, Sathirakul K, Auparakkitanon S, et al. Pharmacokinetics of mitragynine in man. Drug Des Devel Ther. 2015:9:2421-2429.
12. Warner ML, Kaufman NC, Grundmann O, et al. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Intl J Legal Med. 2016;130(1):127-138.
13. Stanciu CN, Gnanasegaram SA, Ahmed S, et al. Kratom withdrawal: a systematic review with case series. J Psychoactive Drugs. 2019;51(1):12-18.
14. Kamble SH, Sharma A, King TI, et al. Metabolite profiling and identification of enzymes responsible for the metabolism of mitragynine, the major alkaloid of Mitragyna speciosa (kratom). Xenobiotica. 2019;49(11):1279-1288.
15. Smith LC, Lin L, Hwang CS, et al. Lateral flow assessment and unanticipated toxicity of kratom. Chem Res Toxicol. 2019;32(1):113-121.
16. Saingam D, Assanangkornchai S, Geater AF, et al. Factor analytical investigation of Krathom (Mitragyna speciosa Korth.) withdrawal syndrome in Thailand. J Psychoactive Drugs. 2016;48(2):76-85.
17. Vicknasingam B, Narayanan S, Beng GT, et al. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010;21(4):283-288.
18. Saingam D, Assanangkornchai S, Geater AF, et al. Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: a qualitative study. Int J Drug Policy. 2013;24(4):351-358.
19. Fernandes CT, Iqbal U, Tighe SP, et al. Kratom-induced cholestatic liver injury and its conservative management. J Investig Med High Impact Case Rep. 2019;7:2324709619836138. doi: 10.1177/2324709619836138.
20. Dorman C, Wong M, Khan A. Cholestatic hepatitis from prolonged kratom use: a case report. Hepatology. 2015;61(3):1086-1087.
21. Osborne CS, Overstreet AN, Rockey DC, et al. Drug-induced liver injury caused by kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J Investig Med High Impact Case Rep. 2019;7:2324709619826167. doi: 10.1177/2324709619826167.
22. Mousa MS, Sephien A, Gutierrez J, et al. N-acetylcysteine for acute hepatitis induced by kratom herbal tea. Am J Ther. 2018;25(5):e550-e551.
23. Riverso M, Chang M, Soldevila-Pico C, et al. Histologic characterization of kratom use-associated liver injury. Gastroenterology Res. 2018;11(1):79-82.
24. Kapp FG, Maurer HH, Auwärter V, et al. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). J Med Toxicol. 2011;7(3):227-231.
25. Antony A, Lee TP. Herb-induced liver injury with cholestasis and renal injury secondary to short-term use of kratom (Mitragyna speciosa). Am J Ther. 2019;26(4):e546-e547.
26. Palasamudram Shekar S, Rojas EE, D’Angelo CC, et al. Legally lethal kratom: a herbal supplement with overdose potential. J Psychoactive Drugs. 2019;51(1):28-30.
27. Aldyab M, Ells PF, Bui R, et al. Kratom-induced cholestatic liver injury mimicking anti-mitochondrial antibody-negative primary biliary cholangitis: a case report and review of literature. Gastroenterology Res. 2019;12(4):211-215.
28. Post S, Spiller HA, Chounthirath T. Kratom exposures reported to United States poison control centers: 2011-2017. Clinical Toxicol (Phila). 2019;57(10):847-854.
29. Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacotherapy. 2019;39(7):775-777.
30. US Food & Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on the agency’s scientific evidence on the presence of opioid compounds in kratom , underscoring its potential for abuse. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-scientific-evidence-presence-opioid-compounds. Published February 6, 2019. Accessed January 29, 2020.
31. Gershman K, Timm K, Frank M, et al. Deaths in Colorado attributed to kratom. N Engl J Med. 2019;380(1):97-98.
32. Kronstrand R, Roman M, Thelander G, et al. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend krypton. J Anal Toxicol. 2011;35(4):242-247.
33. Hughes RL. Fatal combination of mitragynine and quetiapine - a case report with discussion of a potential herb-drug interaction. Forensic Sci Med Pathol. 2019;15(1):110-113.
34. Abdullah HMA, Haq I, Lamfers R. Cardiac arrest in a young healthy male patient secondary to kratom ingestion: is this ‘legal high’ substance more dangerous than initially thought? BMJ Case Rep. 2019;12(7):pii: e229778. doi: 10.1136/bcr-2019-229778.
35. Laboratory analysis of kratom products for heavy metals. US FDA. https://www.fda.gov/news-events/public-health-focus/laboratory-analysis-kratom-products-heavy-metals. Updated April 3, 2019. Accessed January 29, 2020.
36. FDA investigated multistate outbreak of salmonella infections linked to products reported to contain kratom. US FDA. https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigated-multistate-outbreak-salmonella-infections-linked-products-reported-contain-kratom. Updated June 29, 2018. Accessed January 14, 2020.
37. Aggarwal G, Robertson E, McKinlay J, et a., Death from kratom toxicity and the possible role of intralipid. J Intensive Care Soc. 2018;19(1):61-63.
38. Drug Facts. Kratom. Confirm Biosciences. https://www.confirmbiosciences.com/knowledge/drug-facts/kratom/. Accessed January 14, 2020.
39. Grinspoon P. How long does kratom stay in the system? Addiction Resource. https://addictionresource.com/drugs/kratom/how-long-kratom-stay-in-your-system/. Updated December 18, 2019. Accessed January 29, 2020.
40. Kaewklum D, Kaewklum M, Pootrakronchai R, et al. Detection of mitragynine and its metaboilite in urine following ingestion of leaves of Mitragyna speciosa korth. Recent Advances in Doping Analysis (13). Proceedings of the Manfred Donike Workshop, 23rd Cologne Workshop on Dope Analysis. 2005:403-406.
41. Lu S, Tran BN, Nelsen JL, et al. Quantitative analysis of mitragynine in human urine by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(24):2499-2505.
42. Philipp AA, Wissenbach DK, Zoerntlein SW, et al. Studies on the metabolism of mitragynine, the main alkaloid of the herbal drug kratom, in rat and human urine using liquid chromatography-linear ion trap mass spectrometry. J Mass Spectrom. 2009;44(8):1249-1261.
43. Manda VK, Bharathi A, Ali Z, et al. Evaluation of in vitro absorption, distribution, metabolism, and excretion (ADME) properties of mitragynine, 7-hydroxymitragynine, and mitraphylline. Planta Med. 2014;80(7):568-576.
44. Davidson L, Rawat M, Stojanovski S, et al. Natural drugs, not so natural effects: neonatal abstinence syndrome secondary to ‘kratom‘. J Neonatal Perinatal Med. 2019;12(1):109-112.
45. Mackay L, Abrahams R. Novel case of maternal and neonatal kratom dependence and withdrawal. Can Fam Physician. 2018;64(2):121-122.
46. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
47. Galbis-Reig David. A case report of kratom addiction and withdrawal. WMJ. 2016;115(1):49-52; quiz 53.
48. Singh D, Müller CP, Vicknasingam BK. Kratom (Mitragyna speciose) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132-137.
49. Singh D, Müller CP, Vicknasingam, et al. Social functioning of kratom (Mitragyna speciosa) users in Malaysia. J Psychoactive Drugs. 2015;47(2):125-131.
50. Khazaeli A, Jerry JM, Vazirian M. Treatment of kratom withdrawal and addiction with buprenorphine. J Addict Med. 2018;12(6):493-495.
51. Buresh M. Treatment of kratom dependence with buprenorphine-naloxone maintenance. J Addict Med. 2018;12(6):481-483.
52. Overbeek DL, Abraham J, Munzer BW. Kratom (mitragynine) ingestion requiring naloxone reversal. Clin Pract Cases Emerg Med. 2019;3(1):24-26.
Mitragyna speciosa, better known as kratom, is a tropical evergreen tree that is native to Southeast Asia. Botanically, it is a member of the Rubiaceae family, as is the coffee plant, and physical laborers among indigenous populations have historically chewed the leaves or brewed them as a tea to improve endurance and reduce fatigue.1 Kratom is psychoactive; small amounts (up to 5 g of plant material) possess stimulant properties, while larger doses (>5 g) produce opioid-like, sedative, euphoric, and antinociceptive effects.2
In recent years, kratom has gained popularity in Western parts of the world due to its unique properties and perceived safety as a botanical product. Individuals may use kratom to boost their energy, relieve pain, or treat a wide range of physical or mood problems. Increasingly, kratom is being used by people who abuse opioids to self-manage opioid withdrawal, or for its euphoric effects. But kratom carries several important risks, including addiction, serious adverse effects, and possibly death. In this article, we review the epidemiology and pharmacology of kratom, and provide some guidance for educating patients about this substance.
Widely used but not FDA approved
Although kratom is not regulated or approved by the FDA, 3 to 5 million Americans use it regularly.3 According to an internet survey, kratom users are mostly college-educated, employed white men, age 31 to 50, who take the substance to manage pain or to treat general anxiety and mood disorders.4 Some individuals use kratom as an opioid substitute to reduce symptoms of opioid withdrawal.4
Kratom is available from a wide range of manufacturers in various formulations, including powders, tablets, liquids, and gum. It is sometimes sold in combination with other agents as a single product. Low-cost, over-the-counter kratom products are available as “dietary supplements” in retail stores or online. Although the product packaging sometimes recommends a specific dose, the amount of active ingredients (as well as other agents) is unknown. Kratom is illegal in several states (Box5).
Box
The use and sale of kratom is illegal in several countries, including Australia, Poland, Denmark, Sweden, Malaysia, and Vietnam. In the United States, kratom was legal to grow and purchase in all 50 states until 2015, when the Drug Enforcement Administration (DEA) identified kratom as a “substance of concern.” In August 2016, the DEA submitted a notice of intent to place mitragynine and 7-hydroxymitragynine, 2 alkaloids of kratom that have opioid-like properties, into Schedule I of the Controlled Substance Act; however, due to significant public pressure, the DEA withdrew the request in October 2016.
As of February 2020, kratom was illegal to buy, sell, or use in Wisconsin, Rhode Island, Vermont, Indiana, Arkansas, Alabama, specific counties of some states, and the District of Columbia. Legislation was pending in New York, Missouri, and Louisiana.
Source: Reference 5
The 2 alkaloids of interest
More than 40 alkaloids have been isolated from kratom leaves. The proportions of these alkaloids vary significantly depending on the environment in which the plant is grown, the breeding and harvesting techniques, and the age of the plant.6 Two alkaloids of significant interest are mitragynine (Figure 1) and 7-hydroxymitragynine (Figure 2), both of which are unique to M. speciosa and have opioid-like properties. Administering these alkaloids to morphine-dependent rats resulted in cross-tolerance and precipitated withdrawal when the rats were given naloxone.7 The potency of kratom at the mu opioid receptor has been found to exceed that of morphine.

Competitive binding studies that examined the affinity of mitragynine and 7-hydroxymitragynine at the various opioid receptor subtypes found a preference for the kappa receptors (antagonism), followed by mu (partial agonism), and lastly delta. This profile of mitragynine is very similar to that of buprenorphine.8 The affinity of 7-hydroxymitragynine for the mu receptor (agonism) is significantly greater than that of mitragynine.9 Mitragynine also interacts with noradrenergic and serotonergic pathways by stimulating postsynaptic alpha-2 adrenergic receptors and inhibiting 5-HT2A receptors.9 These properties are responsible for kratom’s ability to manage opioid withdrawal symptoms, which are generally attributed to a hyperactive noradrenergic system. There also is evidence that the hepatic metabolite 7-hydroxymitragynine is important in mediating the analgesic component of mitragynine.10

The initial effects of kratom typically begin within 10 to 20 minutes of consumption, and the full effects are experienced in 30 to 60 minutes.1 The half-life of mitragynine in humans has not yet been determined, but is believed to be relatively short.11 In rats, the half-life of mitragynine is 2 to 3 hours.12 Individuals who use kratom to prevent opioid withdrawal have reported taking it as often as every 6 to 12 hours.13
Continue to: Metabolism of mitragynine...
Metabolism of mitragynine is predominantly carried out through cytochrome P450 (CYP) 3A4, with minor contributions by 2D6 and 2C9. A total of 13 metabolites are produced, including 7-hydroxymitragynine.14 Kratom’s constituents also interact with the CYP system, inhibiting 2C9, 2D6, and 3A4 isoenzymes, and to some extent, 1A2.
Adverse effects can be fatal
An animal study revealed that when administered intravenously, mitragynine and 7-hydroxymitragynine have a similar toxicity profile to heroin.15 When these alkaloids were administered in ascending doses, increases in blood pressure and elevations in liver function tests and creatinine levels from baseline were observed.
Chronic kratom use can result in weight loss, insomnia, constipation, dehydration, skin hyperpigmentation, and extreme fatigue.16 There have also been reports of seizures, delusions, hallucinations, respiratory depression, hepatotoxicity, coma, and death.17,18 An emerging concern is the potential development of fatty liver infiltrates leading to cholestatic liver damage.19-25 One case report described a young man who developed a serum aspartate aminotransferase level of 1,300 IU/L (reference range: 5 to 45 IU/L) and a serum alanine aminotransaminase level of 3,700 IU/L (reference range: 5 to 60 IU/L) after he ingested a kratom product.26 Histologically, the pattern of liver injury mimics primary biliary cholangitis.27
In recent years, calls to poison control centers in the United States related to kratom exposure have risen. Between 2011 and 2017, the number of calls increased from 1 a month to 2 each day.28 The US National Poison Data System has also noted an increase in the number of calls in reference to kratom. It received 2,312 calls from January 2011 through July 2018, with 18 calls occurring in 2011, and 357 within the first 7 months of 2018.29
As of February 2018, the FDA had received reports of 44 deaths associated with kratom.30 There have been reports of fatal overdoses involving kratom, particularly when kratom is co-ingested or used with adulterated and/or combination agents, including one case that involved quetiapine.31-33 There have been reports of deaths believed to be attributed to the use of kratom alone; in one such case, a 35-year-old man experienced a fatal cardiac arrest due to kratom use with no other coingestants.34 Among the reports of deaths in which kratom was the only substance consumed, the mitragynine blood levels of the deceased individuals were found to be higher than the levels associated with individuals who had consumed traditional kratom teas.29
Continue to: There is a lack of quality control...
There is a lack of quality control of commercially available kratom preparations. The FDA has found kratom products that exceeded the level of safe exposure to nickel and lead.35 There have also been reports of Salmonella outbreaks associated with kratom products.36
Detecting kratom use
Mitragynine is a lipophilic alkaloid that is poorly soluble in water37 and eliminated primarily in urine.12 Based on data from treatment center admissions, kratom can be detected in urine samples for 5 to 6 days after use.24,38,39 However, kratom is not detectable by a standard urine toxicology screen; therefore, a high degree of suspicion and special confirmatory testing are necessary. The breakdown products of mitragynine can be detected through gas chromatography coupled with mass spectrometry (GC/MS), liquid chromatography with linear ion trap mass spectrometry, or electrospray tandem mass spectrometry.40-42
A familiar withdrawal syndrome
Abrupt discontinuation of high-dose, long-term kratom use can produce withdrawal symptoms.13 Symptoms of kratom withdrawal resemble those of opioid withdrawal. These include physiological symptoms (mydriasis, nausea, sweating and chills, muscle and body aches, tremors and twitches, diarrhea, rhinorrhea, and lacrimation) and psychological symptoms (insomnia, restlessness, irritability/hostility, fatigue, anxiety, mood disturbances, and hallucinations).13 Symptoms are first noted starting 12 hours after the last use of kratom, and can last up to 7 days.43 Withdrawal intensity has been positively correlated with the daily amount of kratom consumed, as well as the duration and frequency of use.13,16
In 2 case reports, the newborns of women who used kratom during pregnancy experienced neonatal abstinence syndrome.44,45 In these 2 reports, symptoms such as jitteriness, irritability, feeding intolerance, and vomiting emerged on postpartum Day 2. The newborns were admitted to a neonatal ICU and started on a standard opioid protocol with IV morphine and subsequently tapered with an oral formulation over 5 days.44,45
Helping patients who use kratom
The best approach to treating a patient who is experiencing kratom withdrawal is symptomatic management, as would be appropriate for a patient experiencing opioid withdrawal.13 However, the use of agents such as methadone or buprenorphine for patients undergoing kratom withdrawal has not been thoroughly evaluated; very few reports have been published.46,47
Continue to: Similarly, while the standard of care...
Similarly, while the standard of care for treating a patient with opioid use disorder is medication-assisted treatment in combination with counseling and behavioral therapies, there is little evidence on the efficacy of such treatments for patients who use kratom. There are no specific guidelines, and the risk of relapsing to kratom use is high.48,49 Nonetheless, some clinicians have used the same protocol for patients with opioid use disorder to treat patients using kratom, and several published case reports describe this approach.50,51 Because administering buprenorphine/naltrexone to a patient who is dependent on kratom can precipitate withdrawal, clinicians should follow a similar initiation protocol as for opioid dependence when starting a patient on these agents (ie, a washout period with a challenge test would be prudent prior to starting naltrexone).
In cases of kratom overdose, naloxone has been shown to reverse the analgesic effects of mitragynine in rats. However, in a case report of an individual who accidently overdosed on a kratom product, naloxone had a modest effect.52
Bottom Line
Kratom is a botanical substance that acts like a stimulant at low doses and an opioid at higher doses. Patients might use it to treat mood-related symptoms, relieve pain, or manage opioid withdrawal. Kratom use has been associated with the development of addiction as well as a multitude of serious adverse effects, including hepatotoxicity and overdose. Long-term management may be required for a patient who uses kratom.
Related Resources
- White CM. Pharmacologic and clinical assessment of kratom: an update. Am J Health Syst Pharm. 2019;76(23):1915-1925.
- Smith KE, Lawson T. Prevalence and motivations for kratom use in a sample of substance users enrolled in a residential treatment program. Drug Alcohol Depend. 2017;180:340-348.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naltrexone • Suboxone
Methadone • Methadose
Naltrexone • Revia
Naloxone • Narcan
Quetiapine • Seroquel
Mitragyna speciosa, better known as kratom, is a tropical evergreen tree that is native to Southeast Asia. Botanically, it is a member of the Rubiaceae family, as is the coffee plant, and physical laborers among indigenous populations have historically chewed the leaves or brewed them as a tea to improve endurance and reduce fatigue.1 Kratom is psychoactive; small amounts (up to 5 g of plant material) possess stimulant properties, while larger doses (>5 g) produce opioid-like, sedative, euphoric, and antinociceptive effects.2
In recent years, kratom has gained popularity in Western parts of the world due to its unique properties and perceived safety as a botanical product. Individuals may use kratom to boost their energy, relieve pain, or treat a wide range of physical or mood problems. Increasingly, kratom is being used by people who abuse opioids to self-manage opioid withdrawal, or for its euphoric effects. But kratom carries several important risks, including addiction, serious adverse effects, and possibly death. In this article, we review the epidemiology and pharmacology of kratom, and provide some guidance for educating patients about this substance.
Widely used but not FDA approved
Although kratom is not regulated or approved by the FDA, 3 to 5 million Americans use it regularly.3 According to an internet survey, kratom users are mostly college-educated, employed white men, age 31 to 50, who take the substance to manage pain or to treat general anxiety and mood disorders.4 Some individuals use kratom as an opioid substitute to reduce symptoms of opioid withdrawal.4
Kratom is available from a wide range of manufacturers in various formulations, including powders, tablets, liquids, and gum. It is sometimes sold in combination with other agents as a single product. Low-cost, over-the-counter kratom products are available as “dietary supplements” in retail stores or online. Although the product packaging sometimes recommends a specific dose, the amount of active ingredients (as well as other agents) is unknown. Kratom is illegal in several states (Box5).
Box
The use and sale of kratom is illegal in several countries, including Australia, Poland, Denmark, Sweden, Malaysia, and Vietnam. In the United States, kratom was legal to grow and purchase in all 50 states until 2015, when the Drug Enforcement Administration (DEA) identified kratom as a “substance of concern.” In August 2016, the DEA submitted a notice of intent to place mitragynine and 7-hydroxymitragynine, 2 alkaloids of kratom that have opioid-like properties, into Schedule I of the Controlled Substance Act; however, due to significant public pressure, the DEA withdrew the request in October 2016.
As of February 2020, kratom was illegal to buy, sell, or use in Wisconsin, Rhode Island, Vermont, Indiana, Arkansas, Alabama, specific counties of some states, and the District of Columbia. Legislation was pending in New York, Missouri, and Louisiana.
Source: Reference 5
The 2 alkaloids of interest
More than 40 alkaloids have been isolated from kratom leaves. The proportions of these alkaloids vary significantly depending on the environment in which the plant is grown, the breeding and harvesting techniques, and the age of the plant.6 Two alkaloids of significant interest are mitragynine (Figure 1) and 7-hydroxymitragynine (Figure 2), both of which are unique to M. speciosa and have opioid-like properties. Administering these alkaloids to morphine-dependent rats resulted in cross-tolerance and precipitated withdrawal when the rats were given naloxone.7 The potency of kratom at the mu opioid receptor has been found to exceed that of morphine.

Competitive binding studies that examined the affinity of mitragynine and 7-hydroxymitragynine at the various opioid receptor subtypes found a preference for the kappa receptors (antagonism), followed by mu (partial agonism), and lastly delta. This profile of mitragynine is very similar to that of buprenorphine.8 The affinity of 7-hydroxymitragynine for the mu receptor (agonism) is significantly greater than that of mitragynine.9 Mitragynine also interacts with noradrenergic and serotonergic pathways by stimulating postsynaptic alpha-2 adrenergic receptors and inhibiting 5-HT2A receptors.9 These properties are responsible for kratom’s ability to manage opioid withdrawal symptoms, which are generally attributed to a hyperactive noradrenergic system. There also is evidence that the hepatic metabolite 7-hydroxymitragynine is important in mediating the analgesic component of mitragynine.10

The initial effects of kratom typically begin within 10 to 20 minutes of consumption, and the full effects are experienced in 30 to 60 minutes.1 The half-life of mitragynine in humans has not yet been determined, but is believed to be relatively short.11 In rats, the half-life of mitragynine is 2 to 3 hours.12 Individuals who use kratom to prevent opioid withdrawal have reported taking it as often as every 6 to 12 hours.13
Continue to: Metabolism of mitragynine...
Metabolism of mitragynine is predominantly carried out through cytochrome P450 (CYP) 3A4, with minor contributions by 2D6 and 2C9. A total of 13 metabolites are produced, including 7-hydroxymitragynine.14 Kratom’s constituents also interact with the CYP system, inhibiting 2C9, 2D6, and 3A4 isoenzymes, and to some extent, 1A2.
Adverse effects can be fatal
An animal study revealed that when administered intravenously, mitragynine and 7-hydroxymitragynine have a similar toxicity profile to heroin.15 When these alkaloids were administered in ascending doses, increases in blood pressure and elevations in liver function tests and creatinine levels from baseline were observed.
Chronic kratom use can result in weight loss, insomnia, constipation, dehydration, skin hyperpigmentation, and extreme fatigue.16 There have also been reports of seizures, delusions, hallucinations, respiratory depression, hepatotoxicity, coma, and death.17,18 An emerging concern is the potential development of fatty liver infiltrates leading to cholestatic liver damage.19-25 One case report described a young man who developed a serum aspartate aminotransferase level of 1,300 IU/L (reference range: 5 to 45 IU/L) and a serum alanine aminotransaminase level of 3,700 IU/L (reference range: 5 to 60 IU/L) after he ingested a kratom product.26 Histologically, the pattern of liver injury mimics primary biliary cholangitis.27
In recent years, calls to poison control centers in the United States related to kratom exposure have risen. Between 2011 and 2017, the number of calls increased from 1 a month to 2 each day.28 The US National Poison Data System has also noted an increase in the number of calls in reference to kratom. It received 2,312 calls from January 2011 through July 2018, with 18 calls occurring in 2011, and 357 within the first 7 months of 2018.29
As of February 2018, the FDA had received reports of 44 deaths associated with kratom.30 There have been reports of fatal overdoses involving kratom, particularly when kratom is co-ingested or used with adulterated and/or combination agents, including one case that involved quetiapine.31-33 There have been reports of deaths believed to be attributed to the use of kratom alone; in one such case, a 35-year-old man experienced a fatal cardiac arrest due to kratom use with no other coingestants.34 Among the reports of deaths in which kratom was the only substance consumed, the mitragynine blood levels of the deceased individuals were found to be higher than the levels associated with individuals who had consumed traditional kratom teas.29
Continue to: There is a lack of quality control...
There is a lack of quality control of commercially available kratom preparations. The FDA has found kratom products that exceeded the level of safe exposure to nickel and lead.35 There have also been reports of Salmonella outbreaks associated with kratom products.36
Detecting kratom use
Mitragynine is a lipophilic alkaloid that is poorly soluble in water37 and eliminated primarily in urine.12 Based on data from treatment center admissions, kratom can be detected in urine samples for 5 to 6 days after use.24,38,39 However, kratom is not detectable by a standard urine toxicology screen; therefore, a high degree of suspicion and special confirmatory testing are necessary. The breakdown products of mitragynine can be detected through gas chromatography coupled with mass spectrometry (GC/MS), liquid chromatography with linear ion trap mass spectrometry, or electrospray tandem mass spectrometry.40-42
A familiar withdrawal syndrome
Abrupt discontinuation of high-dose, long-term kratom use can produce withdrawal symptoms.13 Symptoms of kratom withdrawal resemble those of opioid withdrawal. These include physiological symptoms (mydriasis, nausea, sweating and chills, muscle and body aches, tremors and twitches, diarrhea, rhinorrhea, and lacrimation) and psychological symptoms (insomnia, restlessness, irritability/hostility, fatigue, anxiety, mood disturbances, and hallucinations).13 Symptoms are first noted starting 12 hours after the last use of kratom, and can last up to 7 days.43 Withdrawal intensity has been positively correlated with the daily amount of kratom consumed, as well as the duration and frequency of use.13,16
In 2 case reports, the newborns of women who used kratom during pregnancy experienced neonatal abstinence syndrome.44,45 In these 2 reports, symptoms such as jitteriness, irritability, feeding intolerance, and vomiting emerged on postpartum Day 2. The newborns were admitted to a neonatal ICU and started on a standard opioid protocol with IV morphine and subsequently tapered with an oral formulation over 5 days.44,45
Helping patients who use kratom
The best approach to treating a patient who is experiencing kratom withdrawal is symptomatic management, as would be appropriate for a patient experiencing opioid withdrawal.13 However, the use of agents such as methadone or buprenorphine for patients undergoing kratom withdrawal has not been thoroughly evaluated; very few reports have been published.46,47
Continue to: Similarly, while the standard of care...
Similarly, while the standard of care for treating a patient with opioid use disorder is medication-assisted treatment in combination with counseling and behavioral therapies, there is little evidence on the efficacy of such treatments for patients who use kratom. There are no specific guidelines, and the risk of relapsing to kratom use is high.48,49 Nonetheless, some clinicians have used the same protocol for patients with opioid use disorder to treat patients using kratom, and several published case reports describe this approach.50,51 Because administering buprenorphine/naltrexone to a patient who is dependent on kratom can precipitate withdrawal, clinicians should follow a similar initiation protocol as for opioid dependence when starting a patient on these agents (ie, a washout period with a challenge test would be prudent prior to starting naltrexone).
In cases of kratom overdose, naloxone has been shown to reverse the analgesic effects of mitragynine in rats. However, in a case report of an individual who accidently overdosed on a kratom product, naloxone had a modest effect.52
Bottom Line
Kratom is a botanical substance that acts like a stimulant at low doses and an opioid at higher doses. Patients might use it to treat mood-related symptoms, relieve pain, or manage opioid withdrawal. Kratom use has been associated with the development of addiction as well as a multitude of serious adverse effects, including hepatotoxicity and overdose. Long-term management may be required for a patient who uses kratom.
Related Resources
- White CM. Pharmacologic and clinical assessment of kratom: an update. Am J Health Syst Pharm. 2019;76(23):1915-1925.
- Smith KE, Lawson T. Prevalence and motivations for kratom use in a sample of substance users enrolled in a residential treatment program. Drug Alcohol Depend. 2017;180:340-348.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naltrexone • Suboxone
Methadone • Methadose
Naltrexone • Revia
Naloxone • Narcan
Quetiapine • Seroquel
1. Henningfield JE, Fant RV, Wang DW. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology (Berl). 2018;235(2):573-589.
2. Chang-Chien GC, Odonkor CA, Amorapanth P, et al. Is kratom the new ‘legal high’ on the block?: the case of an emerging opioid receptor agonist with substance abuse potential. Pain Physician. 2017;20(1):E195-E198.
3. Penders T, Jones WB. Kratom, a substance of increasing concern [PCSS webinar]. Providers Clinical Support System. November 28, 2018. https://pcssnow.org/event/kratom-a-substance-of-increasing-concern. Accessed January 29, 2020.
4. Grundmann O. Patterns of kratom use and health impact in the US-results from an online survey. Drug Alcohol Depend. 2017;176:63-70.
5. US Drug Enforcement Administration. Drugs of concern. https://www.dea.gov/sites/default/files/sites/getsmartaboutdrugs.com/files/publications/DoA_2017Ed_Updated_6.16.17.pdf#page=84. Updated June 16, 2017. Accessed January 29, 2020.
6. Matsumoto K, Horie S, Ishikawa H, et al. Antinociceptive effect of 7-hydroxymitragynine in mice: discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sciences. 2004;74(17):2143-2155.
7. Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull (Tokyo). 2004;52(8):916-928.
8. Suhaimi FW, Yusoff NH, Hassan R, et al. Neurobiology of kratom and its main alkaloid mitragynine. Brain Res Bull. 2016;126(pt 1):29-40.
9. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
10. Kruegel AC, Uprety R, Grinnell SG, et al. 7-hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci. 2019;5(6):992-1001.
11. Trakulsrichai S, Sathirakul K, Auparakkitanon S, et al. Pharmacokinetics of mitragynine in man. Drug Des Devel Ther. 2015:9:2421-2429.
12. Warner ML, Kaufman NC, Grundmann O, et al. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Intl J Legal Med. 2016;130(1):127-138.
13. Stanciu CN, Gnanasegaram SA, Ahmed S, et al. Kratom withdrawal: a systematic review with case series. J Psychoactive Drugs. 2019;51(1):12-18.
14. Kamble SH, Sharma A, King TI, et al. Metabolite profiling and identification of enzymes responsible for the metabolism of mitragynine, the major alkaloid of Mitragyna speciosa (kratom). Xenobiotica. 2019;49(11):1279-1288.
15. Smith LC, Lin L, Hwang CS, et al. Lateral flow assessment and unanticipated toxicity of kratom. Chem Res Toxicol. 2019;32(1):113-121.
16. Saingam D, Assanangkornchai S, Geater AF, et al. Factor analytical investigation of Krathom (Mitragyna speciosa Korth.) withdrawal syndrome in Thailand. J Psychoactive Drugs. 2016;48(2):76-85.
17. Vicknasingam B, Narayanan S, Beng GT, et al. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010;21(4):283-288.
18. Saingam D, Assanangkornchai S, Geater AF, et al. Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: a qualitative study. Int J Drug Policy. 2013;24(4):351-358.
19. Fernandes CT, Iqbal U, Tighe SP, et al. Kratom-induced cholestatic liver injury and its conservative management. J Investig Med High Impact Case Rep. 2019;7:2324709619836138. doi: 10.1177/2324709619836138.
20. Dorman C, Wong M, Khan A. Cholestatic hepatitis from prolonged kratom use: a case report. Hepatology. 2015;61(3):1086-1087.
21. Osborne CS, Overstreet AN, Rockey DC, et al. Drug-induced liver injury caused by kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J Investig Med High Impact Case Rep. 2019;7:2324709619826167. doi: 10.1177/2324709619826167.
22. Mousa MS, Sephien A, Gutierrez J, et al. N-acetylcysteine for acute hepatitis induced by kratom herbal tea. Am J Ther. 2018;25(5):e550-e551.
23. Riverso M, Chang M, Soldevila-Pico C, et al. Histologic characterization of kratom use-associated liver injury. Gastroenterology Res. 2018;11(1):79-82.
24. Kapp FG, Maurer HH, Auwärter V, et al. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). J Med Toxicol. 2011;7(3):227-231.
25. Antony A, Lee TP. Herb-induced liver injury with cholestasis and renal injury secondary to short-term use of kratom (Mitragyna speciosa). Am J Ther. 2019;26(4):e546-e547.
26. Palasamudram Shekar S, Rojas EE, D’Angelo CC, et al. Legally lethal kratom: a herbal supplement with overdose potential. J Psychoactive Drugs. 2019;51(1):28-30.
27. Aldyab M, Ells PF, Bui R, et al. Kratom-induced cholestatic liver injury mimicking anti-mitochondrial antibody-negative primary biliary cholangitis: a case report and review of literature. Gastroenterology Res. 2019;12(4):211-215.
28. Post S, Spiller HA, Chounthirath T. Kratom exposures reported to United States poison control centers: 2011-2017. Clinical Toxicol (Phila). 2019;57(10):847-854.
29. Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacotherapy. 2019;39(7):775-777.
30. US Food & Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on the agency’s scientific evidence on the presence of opioid compounds in kratom , underscoring its potential for abuse. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-scientific-evidence-presence-opioid-compounds. Published February 6, 2019. Accessed January 29, 2020.
31. Gershman K, Timm K, Frank M, et al. Deaths in Colorado attributed to kratom. N Engl J Med. 2019;380(1):97-98.
32. Kronstrand R, Roman M, Thelander G, et al. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend krypton. J Anal Toxicol. 2011;35(4):242-247.
33. Hughes RL. Fatal combination of mitragynine and quetiapine - a case report with discussion of a potential herb-drug interaction. Forensic Sci Med Pathol. 2019;15(1):110-113.
34. Abdullah HMA, Haq I, Lamfers R. Cardiac arrest in a young healthy male patient secondary to kratom ingestion: is this ‘legal high’ substance more dangerous than initially thought? BMJ Case Rep. 2019;12(7):pii: e229778. doi: 10.1136/bcr-2019-229778.
35. Laboratory analysis of kratom products for heavy metals. US FDA. https://www.fda.gov/news-events/public-health-focus/laboratory-analysis-kratom-products-heavy-metals. Updated April 3, 2019. Accessed January 29, 2020.
36. FDA investigated multistate outbreak of salmonella infections linked to products reported to contain kratom. US FDA. https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigated-multistate-outbreak-salmonella-infections-linked-products-reported-contain-kratom. Updated June 29, 2018. Accessed January 14, 2020.
37. Aggarwal G, Robertson E, McKinlay J, et a., Death from kratom toxicity and the possible role of intralipid. J Intensive Care Soc. 2018;19(1):61-63.
38. Drug Facts. Kratom. Confirm Biosciences. https://www.confirmbiosciences.com/knowledge/drug-facts/kratom/. Accessed January 14, 2020.
39. Grinspoon P. How long does kratom stay in the system? Addiction Resource. https://addictionresource.com/drugs/kratom/how-long-kratom-stay-in-your-system/. Updated December 18, 2019. Accessed January 29, 2020.
40. Kaewklum D, Kaewklum M, Pootrakronchai R, et al. Detection of mitragynine and its metaboilite in urine following ingestion of leaves of Mitragyna speciosa korth. Recent Advances in Doping Analysis (13). Proceedings of the Manfred Donike Workshop, 23rd Cologne Workshop on Dope Analysis. 2005:403-406.
41. Lu S, Tran BN, Nelsen JL, et al. Quantitative analysis of mitragynine in human urine by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(24):2499-2505.
42. Philipp AA, Wissenbach DK, Zoerntlein SW, et al. Studies on the metabolism of mitragynine, the main alkaloid of the herbal drug kratom, in rat and human urine using liquid chromatography-linear ion trap mass spectrometry. J Mass Spectrom. 2009;44(8):1249-1261.
43. Manda VK, Bharathi A, Ali Z, et al. Evaluation of in vitro absorption, distribution, metabolism, and excretion (ADME) properties of mitragynine, 7-hydroxymitragynine, and mitraphylline. Planta Med. 2014;80(7):568-576.
44. Davidson L, Rawat M, Stojanovski S, et al. Natural drugs, not so natural effects: neonatal abstinence syndrome secondary to ‘kratom‘. J Neonatal Perinatal Med. 2019;12(1):109-112.
45. Mackay L, Abrahams R. Novel case of maternal and neonatal kratom dependence and withdrawal. Can Fam Physician. 2018;64(2):121-122.
46. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
47. Galbis-Reig David. A case report of kratom addiction and withdrawal. WMJ. 2016;115(1):49-52; quiz 53.
48. Singh D, Müller CP, Vicknasingam BK. Kratom (Mitragyna speciose) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132-137.
49. Singh D, Müller CP, Vicknasingam, et al. Social functioning of kratom (Mitragyna speciosa) users in Malaysia. J Psychoactive Drugs. 2015;47(2):125-131.
50. Khazaeli A, Jerry JM, Vazirian M. Treatment of kratom withdrawal and addiction with buprenorphine. J Addict Med. 2018;12(6):493-495.
51. Buresh M. Treatment of kratom dependence with buprenorphine-naloxone maintenance. J Addict Med. 2018;12(6):481-483.
52. Overbeek DL, Abraham J, Munzer BW. Kratom (mitragynine) ingestion requiring naloxone reversal. Clin Pract Cases Emerg Med. 2019;3(1):24-26.
1. Henningfield JE, Fant RV, Wang DW. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology (Berl). 2018;235(2):573-589.
2. Chang-Chien GC, Odonkor CA, Amorapanth P, et al. Is kratom the new ‘legal high’ on the block?: the case of an emerging opioid receptor agonist with substance abuse potential. Pain Physician. 2017;20(1):E195-E198.
3. Penders T, Jones WB. Kratom, a substance of increasing concern [PCSS webinar]. Providers Clinical Support System. November 28, 2018. https://pcssnow.org/event/kratom-a-substance-of-increasing-concern. Accessed January 29, 2020.
4. Grundmann O. Patterns of kratom use and health impact in the US-results from an online survey. Drug Alcohol Depend. 2017;176:63-70.
5. US Drug Enforcement Administration. Drugs of concern. https://www.dea.gov/sites/default/files/sites/getsmartaboutdrugs.com/files/publications/DoA_2017Ed_Updated_6.16.17.pdf#page=84. Updated June 16, 2017. Accessed January 29, 2020.
6. Matsumoto K, Horie S, Ishikawa H, et al. Antinociceptive effect of 7-hydroxymitragynine in mice: discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sciences. 2004;74(17):2143-2155.
7. Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull (Tokyo). 2004;52(8):916-928.
8. Suhaimi FW, Yusoff NH, Hassan R, et al. Neurobiology of kratom and its main alkaloid mitragynine. Brain Res Bull. 2016;126(pt 1):29-40.
9. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
10. Kruegel AC, Uprety R, Grinnell SG, et al. 7-hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci. 2019;5(6):992-1001.
11. Trakulsrichai S, Sathirakul K, Auparakkitanon S, et al. Pharmacokinetics of mitragynine in man. Drug Des Devel Ther. 2015:9:2421-2429.
12. Warner ML, Kaufman NC, Grundmann O, et al. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Intl J Legal Med. 2016;130(1):127-138.
13. Stanciu CN, Gnanasegaram SA, Ahmed S, et al. Kratom withdrawal: a systematic review with case series. J Psychoactive Drugs. 2019;51(1):12-18.
14. Kamble SH, Sharma A, King TI, et al. Metabolite profiling and identification of enzymes responsible for the metabolism of mitragynine, the major alkaloid of Mitragyna speciosa (kratom). Xenobiotica. 2019;49(11):1279-1288.
15. Smith LC, Lin L, Hwang CS, et al. Lateral flow assessment and unanticipated toxicity of kratom. Chem Res Toxicol. 2019;32(1):113-121.
16. Saingam D, Assanangkornchai S, Geater AF, et al. Factor analytical investigation of Krathom (Mitragyna speciosa Korth.) withdrawal syndrome in Thailand. J Psychoactive Drugs. 2016;48(2):76-85.
17. Vicknasingam B, Narayanan S, Beng GT, et al. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010;21(4):283-288.
18. Saingam D, Assanangkornchai S, Geater AF, et al. Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: a qualitative study. Int J Drug Policy. 2013;24(4):351-358.
19. Fernandes CT, Iqbal U, Tighe SP, et al. Kratom-induced cholestatic liver injury and its conservative management. J Investig Med High Impact Case Rep. 2019;7:2324709619836138. doi: 10.1177/2324709619836138.
20. Dorman C, Wong M, Khan A. Cholestatic hepatitis from prolonged kratom use: a case report. Hepatology. 2015;61(3):1086-1087.
21. Osborne CS, Overstreet AN, Rockey DC, et al. Drug-induced liver injury caused by kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J Investig Med High Impact Case Rep. 2019;7:2324709619826167. doi: 10.1177/2324709619826167.
22. Mousa MS, Sephien A, Gutierrez J, et al. N-acetylcysteine for acute hepatitis induced by kratom herbal tea. Am J Ther. 2018;25(5):e550-e551.
23. Riverso M, Chang M, Soldevila-Pico C, et al. Histologic characterization of kratom use-associated liver injury. Gastroenterology Res. 2018;11(1):79-82.
24. Kapp FG, Maurer HH, Auwärter V, et al. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). J Med Toxicol. 2011;7(3):227-231.
25. Antony A, Lee TP. Herb-induced liver injury with cholestasis and renal injury secondary to short-term use of kratom (Mitragyna speciosa). Am J Ther. 2019;26(4):e546-e547.
26. Palasamudram Shekar S, Rojas EE, D’Angelo CC, et al. Legally lethal kratom: a herbal supplement with overdose potential. J Psychoactive Drugs. 2019;51(1):28-30.
27. Aldyab M, Ells PF, Bui R, et al. Kratom-induced cholestatic liver injury mimicking anti-mitochondrial antibody-negative primary biliary cholangitis: a case report and review of literature. Gastroenterology Res. 2019;12(4):211-215.
28. Post S, Spiller HA, Chounthirath T. Kratom exposures reported to United States poison control centers: 2011-2017. Clinical Toxicol (Phila). 2019;57(10):847-854.
29. Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacotherapy. 2019;39(7):775-777.
30. US Food & Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on the agency’s scientific evidence on the presence of opioid compounds in kratom , underscoring its potential for abuse. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-scientific-evidence-presence-opioid-compounds. Published February 6, 2019. Accessed January 29, 2020.
31. Gershman K, Timm K, Frank M, et al. Deaths in Colorado attributed to kratom. N Engl J Med. 2019;380(1):97-98.
32. Kronstrand R, Roman M, Thelander G, et al. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend krypton. J Anal Toxicol. 2011;35(4):242-247.
33. Hughes RL. Fatal combination of mitragynine and quetiapine - a case report with discussion of a potential herb-drug interaction. Forensic Sci Med Pathol. 2019;15(1):110-113.
34. Abdullah HMA, Haq I, Lamfers R. Cardiac arrest in a young healthy male patient secondary to kratom ingestion: is this ‘legal high’ substance more dangerous than initially thought? BMJ Case Rep. 2019;12(7):pii: e229778. doi: 10.1136/bcr-2019-229778.
35. Laboratory analysis of kratom products for heavy metals. US FDA. https://www.fda.gov/news-events/public-health-focus/laboratory-analysis-kratom-products-heavy-metals. Updated April 3, 2019. Accessed January 29, 2020.
36. FDA investigated multistate outbreak of salmonella infections linked to products reported to contain kratom. US FDA. https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigated-multistate-outbreak-salmonella-infections-linked-products-reported-contain-kratom. Updated June 29, 2018. Accessed January 14, 2020.
37. Aggarwal G, Robertson E, McKinlay J, et a., Death from kratom toxicity and the possible role of intralipid. J Intensive Care Soc. 2018;19(1):61-63.
38. Drug Facts. Kratom. Confirm Biosciences. https://www.confirmbiosciences.com/knowledge/drug-facts/kratom/. Accessed January 14, 2020.
39. Grinspoon P. How long does kratom stay in the system? Addiction Resource. https://addictionresource.com/drugs/kratom/how-long-kratom-stay-in-your-system/. Updated December 18, 2019. Accessed January 29, 2020.
40. Kaewklum D, Kaewklum M, Pootrakronchai R, et al. Detection of mitragynine and its metaboilite in urine following ingestion of leaves of Mitragyna speciosa korth. Recent Advances in Doping Analysis (13). Proceedings of the Manfred Donike Workshop, 23rd Cologne Workshop on Dope Analysis. 2005:403-406.
41. Lu S, Tran BN, Nelsen JL, et al. Quantitative analysis of mitragynine in human urine by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(24):2499-2505.
42. Philipp AA, Wissenbach DK, Zoerntlein SW, et al. Studies on the metabolism of mitragynine, the main alkaloid of the herbal drug kratom, in rat and human urine using liquid chromatography-linear ion trap mass spectrometry. J Mass Spectrom. 2009;44(8):1249-1261.
43. Manda VK, Bharathi A, Ali Z, et al. Evaluation of in vitro absorption, distribution, metabolism, and excretion (ADME) properties of mitragynine, 7-hydroxymitragynine, and mitraphylline. Planta Med. 2014;80(7):568-576.
44. Davidson L, Rawat M, Stojanovski S, et al. Natural drugs, not so natural effects: neonatal abstinence syndrome secondary to ‘kratom‘. J Neonatal Perinatal Med. 2019;12(1):109-112.
45. Mackay L, Abrahams R. Novel case of maternal and neonatal kratom dependence and withdrawal. Can Fam Physician. 2018;64(2):121-122.
46. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
47. Galbis-Reig David. A case report of kratom addiction and withdrawal. WMJ. 2016;115(1):49-52; quiz 53.
48. Singh D, Müller CP, Vicknasingam BK. Kratom (Mitragyna speciose) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132-137.
49. Singh D, Müller CP, Vicknasingam, et al. Social functioning of kratom (Mitragyna speciosa) users in Malaysia. J Psychoactive Drugs. 2015;47(2):125-131.
50. Khazaeli A, Jerry JM, Vazirian M. Treatment of kratom withdrawal and addiction with buprenorphine. J Addict Med. 2018;12(6):493-495.
51. Buresh M. Treatment of kratom dependence with buprenorphine-naloxone maintenance. J Addict Med. 2018;12(6):481-483.
52. Overbeek DL, Abraham J, Munzer BW. Kratom (mitragynine) ingestion requiring naloxone reversal. Clin Pract Cases Emerg Med. 2019;3(1):24-26.
