User login
Today, “normal” aging is no longer acceptable. From aesthetics to physical, mental, and sexual health, the maturing population seeks effective minimally invasive and practical methods to halt time and reverse its adverse effects. Nowhere is this more apparent than when dealing with urinary and fecal incontinence, conditions that can be not only embarrassing to patients but also debilitating, with potential crippling adverse affects on quality of life. As the US population ages, the prevalence of incontinence is increasing.
Patients commonly present with questions about their incontinence with preconceived notions on their available treatment options based on Internet searches and advertisements from magazines and television. Thus, as gynecologists, we have a pivotal role in educating women on their conditions and management options in a comprehensive, informative, and reassuring manner. By educating patients on the success rates and limitations of available treatments, patients can make informed decisions and reinforce their sense of autonomy. In this article we present the evidence on current, new, and investigative products available for the treatment of both stress urinary incontinence and overactive bladder, as well as fecal incontinence.
Case 1: Stress urinary incontinence
A 46-year-old woman (G2P2) presents with loss of urine with exercise, dancing, and sneezing that began after the birth of her last baby 5 years ago and is progressively becoming more frequent. She performs Kegel exercises occasionally and denies urinary urgency and/or urge incontinence. She reports a 20-lb weight gain in the past 3 years. Physical examination findings reveal normal pelvic examination with adequate pelvic organ support but weakened pelvic floor muscles during contraction. When you ask her to cough, you observe a small amount of urine loss from the urethral meatus. She has heard of “slings” before, but she is anxious about surgery.
Stress urinary incontinence (SUI) is the involuntary loss of urine with effort, physical exertion, sneezing, or coughing.1 It is the most common type of incontinence in younger women, with risk factors including increasing age, parity, and obesity.2,3 SUI treatment options, beginning from least to most invasive, include pelvic floor exercises, biofeedback and/or physical therapy, continence devices, off-label use of medications, urethral bulking agents, and surgical correction with slings. Midurethral tension-free slings are highly efficacious for the treatment of SUI. While a sling is a minimally invasive procedure, patients typically voice concerns regarding surgery and appropriately begin with conservative treatments.
A new FDA-approved OTC option for SUI
First-line conservative therapies offered to patients for SUI include pelvic floor muscle exercises and intravaginal continence devices. Disappointingly, such devices—including pessaries and the incontinence dish—have not been popular among patients for SUI. Authors of a randomized control trial evaluating incontinence pessaries versus behavioral therapy, including pelvic floor muscle training, found that, after 3 months, use of a pes‑ sary was not as effective as behavioral therapy in terms of patient satisfaction and improvement in bothersome urinary incontinence.4 In our experience, many patients wearing incontinence rings discontinue their use due to ineffectiveness or discomfort.
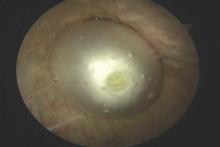
Patients now have an FDA-approved, over-the-counter option for SUI symptom management. The Poise Impressa is a disposable, nonabsorbent, flexible intravaginal device for patients with SUI (FIGURE 1). The device is comprised of a silicone core with a soft, nonwoven polypropylene fabric cover. It is inserted similar to a tampon, using an applicator, and provides nonobstructive support to the urethra to prevent stress urinary leakage. To find the proper fit, patients purchase the sizing kit, which includes 3 sizes. Patients are to insert size 1 first and monitor their comfort as well as improvement in leakage. Should size 1not sufficiently relieve leakage, the patient may try sizes 2 and 3 successively, with the goal of finding the most comfortable and effective insert. The insert is approved for up to 8 hours of wear in a 24-hour period, at which time the patient removes the device by pulling the string in a similar manner as removing a tampon.
Efficacy and quality of life data. Over 28 days, 85% of women with severe SUI confirmed on urodynamic testing achieved greater than 70% leakage reduction according to measured pad weights.5 Seventy percent of women reported 90% improvement in quality of life using validated questionnaires. In addition, 92% reported feeling dry with an improved perception of incontinence and greater confidence during strenuous activities.6 There were no serious adverse events, and the most common mild adverse events were discomfort, pain, and spotting.
As more patients become aware of the device through advertising and word of mouth, we expect patients to seek advice from their gynecologists on the safety and efficacy of the insert. In our experience, most patients report improvement in bothersome symptoms with the device and are overall satisfied. For patients who have discomfort with device placement, a water-based lubricant can be used. Patients using vaginal estrogen may apply the medication at night and wear the device during the day.
Office-based bladder control system in the pipeline
For SUI, options are limited for patients who would rather seek office-based procedures than invasive surgeries. Injections of urethral bulking agents can be performed in an office setting by injecting them transurethrally with a cystoscope slightly distal to the bladder neck. While bulking agents have a role in certain patients with SUI, especially those who are not interested in pursuing more invasive surgeries, only 43% have short-term (less than 6 months) cure and 75% report short-term improvement.7
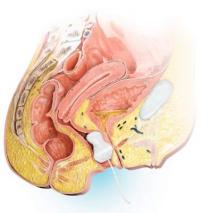
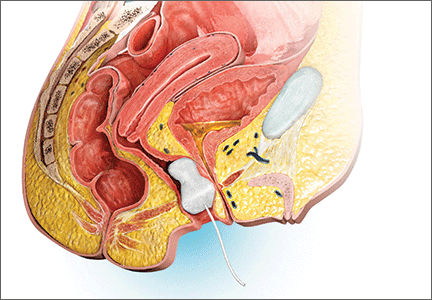
A minimally invasive office-based procedure to treat SUI symptoms is under investigation in clinical trials currently. The Vesair Balloon bladder control system (Solace Therapeutics) is performed with cystoscopic guidance and is being tested at multiple sites throughout the United States (FIGURE 2).
The Vesair Balloon acts like a “shock absorber” to reduce momentary increases in bladder pressure due to external forces or stressors. The balloon is a small device, approximately the size of a quarter, and is implanted through the urethra via a specially designed applicator under cystoscopic guidance in the office setting. Pretreatment with pain medication usually is unnecessary. The VesairBalloon may be retained in situ for up to 12 months, at which time it is removed using a device-specific grasper under direct visualization with a cystoscope in the office.
Preliminary efficacy and safety data. In a single-blinded randomized controlled trial, 63% of women in the Vesair Balloon group had significant improvement in provocative pad weights and quality-of-life questionnaire scores at 3 months, compared with 31% in the control group.8 No serious adverse events were observed. Eleven of 63 patients (17%) withdrew from the study—most commonly for bladder irritation and dysuria.
We anxiously await the results of a second single-blinded randomized control trial currently being conducted.
Best surgical options for SUI
Today, the standard surgical procedure for SUI is a midurethral sling. Midurethral slings may be placed through 3 routes: retropubic; transobturator; and single-incision, otherwise known as “mini-slings.” Subjective cure rates of retropubic versus transobturator slings are similar, with lower rates of bladder perforation, major vascular/visceral injury, and operative blood loss in the transobturator group.9 However, rates of groin pain are higher in the trans‑ obturator group.
Single-incision slings were developed in an effort to avoid the morbidity and pain with passing traditional sling trocars through the obturator space and skin of the groin. In a randomized controlled trial, the Miniarc single- incision sling (Astora Women’s Health) was found to be noninferior to the Monarc transobturator sling (Astora) at 12 and 36 months.10 There were no statistically significant differences between subjective and objective cure rates on cough stress tests. Postoperative pain and groin pain were significantly less in patients with the Miniarc sling, compared with the Monarc sling.
It is our opinion that as more data become available, single-incision slings will find their foothold in a subset of patients with SUI.
Case 2: Overactive bladder: Failed medication therapy
A healthy 63-year-old woman presents with a 9-month history of loss of urine with strong urges, urinating 4 times per night, and a feeling of urgency when she needs to urinate. She denies pain with urination, difficulty emptying her bladder fully, and pain with a full bladder. She has restricted her fluid intake to 4 glasses of water per day and has stopped drinking fluids 4 hours before bedtime.
She described her symptoms to her intern‑ ist, who prescribed oxybutynin. She took the medication for 3 months but stopped after she developed severe constipation and dry mouth. She states the medication did not help her urinary symptoms. You discuss with her trials of other medications including topical anticholinergics and mirabegron. She is frustrated with her symptoms and asks if there are any other options besides medications.
Overactive bladder (OAB) is present in up to 16% of the US population, with the percentage estimated to increase by 20% within the next 2 years.11,12 The drastic increase in prevalence, likely due to the aging population, may result in an increased counseling and management burden placed on general practitioners and gynecologists.
First-line management options for OAB are behavioral modifications and/or medications. Our patient in case 2 failed both first-line therapies. When a patient fails or is intolerant to an anticholinergic medication, we offer mirabegron, a beta-3 agonist (after excluding any contraindications to the medication). Beyond medications, the therapeutic options are rather limited.
Second-line OAB treatment options
In January 2013, the FDA expanded the approved use of onabotulinum toxin A (Botox, Allergan) for the treatment of OAB in those who are intolerant of or have failed treatment with anticholinergic medications. Using a cystoscope, 100 units of onabotulinum toxin A are injected into 20 sites within the bladder wall. Due to the risk of urinary retention in up to 6% of patients, it is recommended to administer onabotulinum toxin A to patients who are willing and capable of performing clean intermittent catheterization.13
Efficacy data. In a recent systematic review and meta-analysis, the authors concluded onabotulinum toxin A to be effective in the treatment of idiopathic OAB with a statistically significant reduction compared with baseline in the number of incontinence episodes per day (-2.77 in the treatment group vs -1.01 in the placebo group) and the number of voids per day (-1.61 in the treatment group vs -0.87 in the placebo group).14 Patients who received onabotulinum toxin A experienced a higher rate of adverse effects, such as urinary tract infections, and were more likely to require clean intermittent catheterization due to incomplete bladder emptying.13 Patients can expect symptom improvement for approximately 6 months or longer.15 Based on the manufacturers’ recommendations, patients are not to be reinjected sooner than 12 weeks from prior onabotulinum toxin A injection.
In women with refractory OAB, available second-line treatments include neuromodulation by sacral nerve or posterior tibial nerve stimulation (PTNS). The latter therapy is an office-based procedure that involves placement of a lead percutaneous to the medial aspect of the ankle near the tibial nerve. It is postulated that stimulation of the tibial nerve results in retrograde stimulation of the S3 sacral nerve plexus, resulting in OAB symptom relief in 54% to 70% of patients.16
Case 3: Fecal incontinence
A 57-year-old, otherwise healthy, multiparous woman presents with a 3-year history of fecal incontinence. She reports that it is embarrassing and distressing. She avoids certain social activities and is not currently sexually active due to the frequency of bowel leakage episodes.
In an effort to decrease her episodes of incontinence, she takes loperamide hydrochloride (Imodium) regularly with little improvement in the frequency of accidents. She has no history of gastrointestinal, rectal, or gynecologic surgery. She had 2 full-term vaginal deliveries that were uncomplicated. On review of systems, she also discloses occasional urinary incontinence.
Physical examination reveals normal vaginal anatomy with adequate pelvic organ support and no neurologic abnormalities. Rectal examination demonstrates normal tone and no evidence of rectal prolapse. Contractions of the pelvic floor muscles are weak. She is frustrated with her condition and seeks your guidance.
Fecal incontinence affects more than 20 million women in the United States, with only one-third of those with the condition disclosing their symptoms to their physician.17 Many etiologies for accidental bowel leakage exist, with some of the most common being advancing age and obstetric trauma. Up to one-third of women presenting for evaluation of urinary incontinence have fecal incontinence; therefore, one must be vigilant in screening for this potentially devastating condition.18
In case 3, the patient has tried medical therapies for fecal incontinence, including stool-bulking agents and motility regulators such as loperamide hydrochloride. Besides offering fiber supplements (or other stool-bulking agents) or physical therapy, nonsurgical options for this patient are limited.
Newly available: A vaginal insert for fecal incontinence
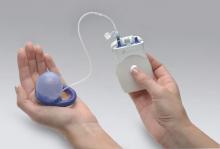
In 2015, the Eclipse System (Pelvalon) became the first FDA-approved vaginal insert for the treatment of fecal incontinence. The manufacturer recently was granted clearance for its second-generation device (FIGURE 3). The device consists of a silicone-coated stainless steel base with a posteriorly facing balloon and a pressure-regulated pump that allows the patient to control her bowel movements. After a patient is fitted with the device in the office setting, she is independently able to insert and remove it as well as deflate the balloon to allow for bowel movements and inflate the balloon to prevent accidental bowel leakage.
In a multicenter trial conducted by Richter and colleagues,19 78% of women successfully fitted with the device had a 50% mean reduction of fecal incontinence episodes. Two-week mean incontinence episodes decreased from 11 to 2 after 1 month of continued use of the insert. In addition, there was significant improvement in quality-of-life questionnaire scores.
Of the 110 patients fitted with the device, 32 (29%) withdrew due to unsatisfactory device fit or were unable to remove or insert the device themselves. Common adverse effects included pelvic cramping and discomfort during device fitting. One month after insertion, pelvic pain and cramping continued in up to 10% of patients. No serious adverse events related to the device were observed during the 1-month trial.19
In the approximate 70% of women successfully fitted with the vaginal insert, the system was highly efficacious in improving subjective and objective outcomes with no unexpected serious adverse events. Currently the device is available at investigative sites across the United States, and the company plans for sales to begin later this year.
Surgical options for fecal incontinence
In patients for whom conservative and medical therapies have failed, surgical treatments may be offered. Surgical options vary from minimally invasive procedures to colostomy. One of the minimally invasive procedures available is the InterStim procedure, or sacral nerve stimulation (SNS). An electrode is inserted percutaneously through the S3 foramen and is connected to an implanted battery under the skin of the buttocks. Low-voltage stimulation is applied to the leads that lie adjacent to the S3 sacral nerve roots.
Patients with SNS experience fewer episodes of fecal incontinence, with over 80% maintaining a reduction in fecal incontinent episodes by greater than 50% up to 5 years after implantation.20,21
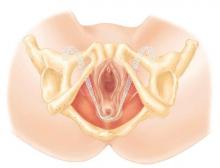
The transobturator postanal sling system (TOPAS, Astora) is a new investigational surgical device. It is inserted in a minimally invasive procedure and is currently undergoing a prospective, multicenter clinical trial (FIGURE 4). It consists of a polypropylene mesh sling placed perianally, with the mesh arms exiting through the obturator foramen bilaterally. It is intended to increase posterior pelvic support at the level of the anorectal junction. Efficacy and safety of the product have yet to be determined.
We need to stay up to date on new treatment options
As the prevalence increases for urinary and fecal incontinence, ObGyns are challenged to remain knowledgeable about the condition, the prognosis, and the success of interventions. Currently, patients have a range of options to manage their urinary and fecal incontinence symptoms, with the number of products and clinical data increasing over time. With the advent of novel products and the widespread availability of information via the Internet, physicians must remain the established source on new innovative treatments and up-to-date clinical data in order to provide competent and comprehensive care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189(2):428–434.
- Lensen EJ, Withagen MI, Kluivers KB, Milani AL, Vierhout ME. Urinary incontinence after surgery for pelvic organ prolapse. Neurourol Urodyn. 2013;32(5):455–459.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115(3):609–617.
- Ziv E, Stanton SL, Abarbanel J. Efficacy and safety of a novel disposable intravaginal device for treating stress urinary incontinence. Am J Obstet Gynecol. 2008;198(5):594.e1–e7.
- Ziv E, Stanton SL, Abarbanel J. Significant improvement in the quality of life in women treated with a novel disposable intravaginal device for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(6):651–658.
- Ghoniem GM, Miller CJ. A systematic review and meta-analysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24(1):27–36.
- Wyndaele JJ, De Wachter S, Tommaselli GA, et al. A randomized, controlled clinical trial of an intravesical pressure-attenuation balloon system for the treatment of stress urinary incontinence in females [published online ahead of print January 16, 2015]. Neurourol Urodyn. doi:10.1002/nau.22708.
- Ford AA, Rogerson L, Cody JD, Ogah J. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2015;7:CD006375.
- Lee JK, Rosamilia A, Dwyer PL, Lim YN, Muller R. Randomized trial of a single incision versus an outside-in transobturator midurethral sling in women with stress urinary incontinence: 12 month results. Am J Obstet Gynecol. 2015;213(1):35.e1–e9.
- Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–1138.
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336.
- Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013;189(6):2186−2193.
- Cui Y, Zhou X, Zong H, Yan H, Zhang Y. The efficacy and safety of onabotulinumtoxinA in treating idiopathic OAB: A systematic review and meta-analysis. Neurourol Urodyn. 2015;34(5):413–419.
- Apostolidis A, Dasgupta P, Denys P, et al; European Consensus Panel. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55(1):100–119.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- Johanson JF, Lafferty J. Epidemiology of fecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91(1):33–36.
- Jackson SL, Weber AM, Hull TL, Mitchinson AR, Walters MD. Fecal incontinence in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1997;89(3):423–427.
- Richter HE, Matthews CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125(3):540–547.
- Thaha MA, Abukar AA, Thin NN, Ramsanahie A, Knowles CH. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015;8:CD004464.
- Hull T, Giese C, Wexner SD, et al; SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56(2):234–245.
Today, “normal” aging is no longer acceptable. From aesthetics to physical, mental, and sexual health, the maturing population seeks effective minimally invasive and practical methods to halt time and reverse its adverse effects. Nowhere is this more apparent than when dealing with urinary and fecal incontinence, conditions that can be not only embarrassing to patients but also debilitating, with potential crippling adverse affects on quality of life. As the US population ages, the prevalence of incontinence is increasing.
Patients commonly present with questions about their incontinence with preconceived notions on their available treatment options based on Internet searches and advertisements from magazines and television. Thus, as gynecologists, we have a pivotal role in educating women on their conditions and management options in a comprehensive, informative, and reassuring manner. By educating patients on the success rates and limitations of available treatments, patients can make informed decisions and reinforce their sense of autonomy. In this article we present the evidence on current, new, and investigative products available for the treatment of both stress urinary incontinence and overactive bladder, as well as fecal incontinence.
Case 1: Stress urinary incontinence
A 46-year-old woman (G2P2) presents with loss of urine with exercise, dancing, and sneezing that began after the birth of her last baby 5 years ago and is progressively becoming more frequent. She performs Kegel exercises occasionally and denies urinary urgency and/or urge incontinence. She reports a 20-lb weight gain in the past 3 years. Physical examination findings reveal normal pelvic examination with adequate pelvic organ support but weakened pelvic floor muscles during contraction. When you ask her to cough, you observe a small amount of urine loss from the urethral meatus. She has heard of “slings” before, but she is anxious about surgery.
Stress urinary incontinence (SUI) is the involuntary loss of urine with effort, physical exertion, sneezing, or coughing.1 It is the most common type of incontinence in younger women, with risk factors including increasing age, parity, and obesity.2,3 SUI treatment options, beginning from least to most invasive, include pelvic floor exercises, biofeedback and/or physical therapy, continence devices, off-label use of medications, urethral bulking agents, and surgical correction with slings. Midurethral tension-free slings are highly efficacious for the treatment of SUI. While a sling is a minimally invasive procedure, patients typically voice concerns regarding surgery and appropriately begin with conservative treatments.
A new FDA-approved OTC option for SUI
First-line conservative therapies offered to patients for SUI include pelvic floor muscle exercises and intravaginal continence devices. Disappointingly, such devices—including pessaries and the incontinence dish—have not been popular among patients for SUI. Authors of a randomized control trial evaluating incontinence pessaries versus behavioral therapy, including pelvic floor muscle training, found that, after 3 months, use of a pes‑ sary was not as effective as behavioral therapy in terms of patient satisfaction and improvement in bothersome urinary incontinence.4 In our experience, many patients wearing incontinence rings discontinue their use due to ineffectiveness or discomfort.
Patients now have an FDA-approved, over-the-counter option for SUI symptom management. The Poise Impressa is a disposable, nonabsorbent, flexible intravaginal device for patients with SUI (FIGURE 1). The device is comprised of a silicone core with a soft, nonwoven polypropylene fabric cover. It is inserted similar to a tampon, using an applicator, and provides nonobstructive support to the urethra to prevent stress urinary leakage. To find the proper fit, patients purchase the sizing kit, which includes 3 sizes. Patients are to insert size 1 first and monitor their comfort as well as improvement in leakage. Should size 1not sufficiently relieve leakage, the patient may try sizes 2 and 3 successively, with the goal of finding the most comfortable and effective insert. The insert is approved for up to 8 hours of wear in a 24-hour period, at which time the patient removes the device by pulling the string in a similar manner as removing a tampon.
Efficacy and quality of life data. Over 28 days, 85% of women with severe SUI confirmed on urodynamic testing achieved greater than 70% leakage reduction according to measured pad weights.5 Seventy percent of women reported 90% improvement in quality of life using validated questionnaires. In addition, 92% reported feeling dry with an improved perception of incontinence and greater confidence during strenuous activities.6 There were no serious adverse events, and the most common mild adverse events were discomfort, pain, and spotting.
As more patients become aware of the device through advertising and word of mouth, we expect patients to seek advice from their gynecologists on the safety and efficacy of the insert. In our experience, most patients report improvement in bothersome symptoms with the device and are overall satisfied. For patients who have discomfort with device placement, a water-based lubricant can be used. Patients using vaginal estrogen may apply the medication at night and wear the device during the day.
Office-based bladder control system in the pipeline
For SUI, options are limited for patients who would rather seek office-based procedures than invasive surgeries. Injections of urethral bulking agents can be performed in an office setting by injecting them transurethrally with a cystoscope slightly distal to the bladder neck. While bulking agents have a role in certain patients with SUI, especially those who are not interested in pursuing more invasive surgeries, only 43% have short-term (less than 6 months) cure and 75% report short-term improvement.7
A minimally invasive office-based procedure to treat SUI symptoms is under investigation in clinical trials currently. The Vesair Balloon bladder control system (Solace Therapeutics) is performed with cystoscopic guidance and is being tested at multiple sites throughout the United States (FIGURE 2).
The Vesair Balloon acts like a “shock absorber” to reduce momentary increases in bladder pressure due to external forces or stressors. The balloon is a small device, approximately the size of a quarter, and is implanted through the urethra via a specially designed applicator under cystoscopic guidance in the office setting. Pretreatment with pain medication usually is unnecessary. The VesairBalloon may be retained in situ for up to 12 months, at which time it is removed using a device-specific grasper under direct visualization with a cystoscope in the office.
Preliminary efficacy and safety data. In a single-blinded randomized controlled trial, 63% of women in the Vesair Balloon group had significant improvement in provocative pad weights and quality-of-life questionnaire scores at 3 months, compared with 31% in the control group.8 No serious adverse events were observed. Eleven of 63 patients (17%) withdrew from the study—most commonly for bladder irritation and dysuria.
We anxiously await the results of a second single-blinded randomized control trial currently being conducted.
Best surgical options for SUI
Today, the standard surgical procedure for SUI is a midurethral sling. Midurethral slings may be placed through 3 routes: retropubic; transobturator; and single-incision, otherwise known as “mini-slings.” Subjective cure rates of retropubic versus transobturator slings are similar, with lower rates of bladder perforation, major vascular/visceral injury, and operative blood loss in the transobturator group.9 However, rates of groin pain are higher in the trans‑ obturator group.
Single-incision slings were developed in an effort to avoid the morbidity and pain with passing traditional sling trocars through the obturator space and skin of the groin. In a randomized controlled trial, the Miniarc single- incision sling (Astora Women’s Health) was found to be noninferior to the Monarc transobturator sling (Astora) at 12 and 36 months.10 There were no statistically significant differences between subjective and objective cure rates on cough stress tests. Postoperative pain and groin pain were significantly less in patients with the Miniarc sling, compared with the Monarc sling.
It is our opinion that as more data become available, single-incision slings will find their foothold in a subset of patients with SUI.
Case 2: Overactive bladder: Failed medication therapy
A healthy 63-year-old woman presents with a 9-month history of loss of urine with strong urges, urinating 4 times per night, and a feeling of urgency when she needs to urinate. She denies pain with urination, difficulty emptying her bladder fully, and pain with a full bladder. She has restricted her fluid intake to 4 glasses of water per day and has stopped drinking fluids 4 hours before bedtime.
She described her symptoms to her intern‑ ist, who prescribed oxybutynin. She took the medication for 3 months but stopped after she developed severe constipation and dry mouth. She states the medication did not help her urinary symptoms. You discuss with her trials of other medications including topical anticholinergics and mirabegron. She is frustrated with her symptoms and asks if there are any other options besides medications.
Overactive bladder (OAB) is present in up to 16% of the US population, with the percentage estimated to increase by 20% within the next 2 years.11,12 The drastic increase in prevalence, likely due to the aging population, may result in an increased counseling and management burden placed on general practitioners and gynecologists.
First-line management options for OAB are behavioral modifications and/or medications. Our patient in case 2 failed both first-line therapies. When a patient fails or is intolerant to an anticholinergic medication, we offer mirabegron, a beta-3 agonist (after excluding any contraindications to the medication). Beyond medications, the therapeutic options are rather limited.
Second-line OAB treatment options
In January 2013, the FDA expanded the approved use of onabotulinum toxin A (Botox, Allergan) for the treatment of OAB in those who are intolerant of or have failed treatment with anticholinergic medications. Using a cystoscope, 100 units of onabotulinum toxin A are injected into 20 sites within the bladder wall. Due to the risk of urinary retention in up to 6% of patients, it is recommended to administer onabotulinum toxin A to patients who are willing and capable of performing clean intermittent catheterization.13
Efficacy data. In a recent systematic review and meta-analysis, the authors concluded onabotulinum toxin A to be effective in the treatment of idiopathic OAB with a statistically significant reduction compared with baseline in the number of incontinence episodes per day (-2.77 in the treatment group vs -1.01 in the placebo group) and the number of voids per day (-1.61 in the treatment group vs -0.87 in the placebo group).14 Patients who received onabotulinum toxin A experienced a higher rate of adverse effects, such as urinary tract infections, and were more likely to require clean intermittent catheterization due to incomplete bladder emptying.13 Patients can expect symptom improvement for approximately 6 months or longer.15 Based on the manufacturers’ recommendations, patients are not to be reinjected sooner than 12 weeks from prior onabotulinum toxin A injection.
In women with refractory OAB, available second-line treatments include neuromodulation by sacral nerve or posterior tibial nerve stimulation (PTNS). The latter therapy is an office-based procedure that involves placement of a lead percutaneous to the medial aspect of the ankle near the tibial nerve. It is postulated that stimulation of the tibial nerve results in retrograde stimulation of the S3 sacral nerve plexus, resulting in OAB symptom relief in 54% to 70% of patients.16
Case 3: Fecal incontinence
A 57-year-old, otherwise healthy, multiparous woman presents with a 3-year history of fecal incontinence. She reports that it is embarrassing and distressing. She avoids certain social activities and is not currently sexually active due to the frequency of bowel leakage episodes.
In an effort to decrease her episodes of incontinence, she takes loperamide hydrochloride (Imodium) regularly with little improvement in the frequency of accidents. She has no history of gastrointestinal, rectal, or gynecologic surgery. She had 2 full-term vaginal deliveries that were uncomplicated. On review of systems, she also discloses occasional urinary incontinence.
Physical examination reveals normal vaginal anatomy with adequate pelvic organ support and no neurologic abnormalities. Rectal examination demonstrates normal tone and no evidence of rectal prolapse. Contractions of the pelvic floor muscles are weak. She is frustrated with her condition and seeks your guidance.
Fecal incontinence affects more than 20 million women in the United States, with only one-third of those with the condition disclosing their symptoms to their physician.17 Many etiologies for accidental bowel leakage exist, with some of the most common being advancing age and obstetric trauma. Up to one-third of women presenting for evaluation of urinary incontinence have fecal incontinence; therefore, one must be vigilant in screening for this potentially devastating condition.18
In case 3, the patient has tried medical therapies for fecal incontinence, including stool-bulking agents and motility regulators such as loperamide hydrochloride. Besides offering fiber supplements (or other stool-bulking agents) or physical therapy, nonsurgical options for this patient are limited.
Newly available: A vaginal insert for fecal incontinence
In 2015, the Eclipse System (Pelvalon) became the first FDA-approved vaginal insert for the treatment of fecal incontinence. The manufacturer recently was granted clearance for its second-generation device (FIGURE 3). The device consists of a silicone-coated stainless steel base with a posteriorly facing balloon and a pressure-regulated pump that allows the patient to control her bowel movements. After a patient is fitted with the device in the office setting, she is independently able to insert and remove it as well as deflate the balloon to allow for bowel movements and inflate the balloon to prevent accidental bowel leakage.
In a multicenter trial conducted by Richter and colleagues,19 78% of women successfully fitted with the device had a 50% mean reduction of fecal incontinence episodes. Two-week mean incontinence episodes decreased from 11 to 2 after 1 month of continued use of the insert. In addition, there was significant improvement in quality-of-life questionnaire scores.
Of the 110 patients fitted with the device, 32 (29%) withdrew due to unsatisfactory device fit or were unable to remove or insert the device themselves. Common adverse effects included pelvic cramping and discomfort during device fitting. One month after insertion, pelvic pain and cramping continued in up to 10% of patients. No serious adverse events related to the device were observed during the 1-month trial.19
In the approximate 70% of women successfully fitted with the vaginal insert, the system was highly efficacious in improving subjective and objective outcomes with no unexpected serious adverse events. Currently the device is available at investigative sites across the United States, and the company plans for sales to begin later this year.
Surgical options for fecal incontinence
In patients for whom conservative and medical therapies have failed, surgical treatments may be offered. Surgical options vary from minimally invasive procedures to colostomy. One of the minimally invasive procedures available is the InterStim procedure, or sacral nerve stimulation (SNS). An electrode is inserted percutaneously through the S3 foramen and is connected to an implanted battery under the skin of the buttocks. Low-voltage stimulation is applied to the leads that lie adjacent to the S3 sacral nerve roots.
Patients with SNS experience fewer episodes of fecal incontinence, with over 80% maintaining a reduction in fecal incontinent episodes by greater than 50% up to 5 years after implantation.20,21
The transobturator postanal sling system (TOPAS, Astora) is a new investigational surgical device. It is inserted in a minimally invasive procedure and is currently undergoing a prospective, multicenter clinical trial (FIGURE 4). It consists of a polypropylene mesh sling placed perianally, with the mesh arms exiting through the obturator foramen bilaterally. It is intended to increase posterior pelvic support at the level of the anorectal junction. Efficacy and safety of the product have yet to be determined.
We need to stay up to date on new treatment options
As the prevalence increases for urinary and fecal incontinence, ObGyns are challenged to remain knowledgeable about the condition, the prognosis, and the success of interventions. Currently, patients have a range of options to manage their urinary and fecal incontinence symptoms, with the number of products and clinical data increasing over time. With the advent of novel products and the widespread availability of information via the Internet, physicians must remain the established source on new innovative treatments and up-to-date clinical data in order to provide competent and comprehensive care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Today, “normal” aging is no longer acceptable. From aesthetics to physical, mental, and sexual health, the maturing population seeks effective minimally invasive and practical methods to halt time and reverse its adverse effects. Nowhere is this more apparent than when dealing with urinary and fecal incontinence, conditions that can be not only embarrassing to patients but also debilitating, with potential crippling adverse affects on quality of life. As the US population ages, the prevalence of incontinence is increasing.
Patients commonly present with questions about their incontinence with preconceived notions on their available treatment options based on Internet searches and advertisements from magazines and television. Thus, as gynecologists, we have a pivotal role in educating women on their conditions and management options in a comprehensive, informative, and reassuring manner. By educating patients on the success rates and limitations of available treatments, patients can make informed decisions and reinforce their sense of autonomy. In this article we present the evidence on current, new, and investigative products available for the treatment of both stress urinary incontinence and overactive bladder, as well as fecal incontinence.
Case 1: Stress urinary incontinence
A 46-year-old woman (G2P2) presents with loss of urine with exercise, dancing, and sneezing that began after the birth of her last baby 5 years ago and is progressively becoming more frequent. She performs Kegel exercises occasionally and denies urinary urgency and/or urge incontinence. She reports a 20-lb weight gain in the past 3 years. Physical examination findings reveal normal pelvic examination with adequate pelvic organ support but weakened pelvic floor muscles during contraction. When you ask her to cough, you observe a small amount of urine loss from the urethral meatus. She has heard of “slings” before, but she is anxious about surgery.
Stress urinary incontinence (SUI) is the involuntary loss of urine with effort, physical exertion, sneezing, or coughing.1 It is the most common type of incontinence in younger women, with risk factors including increasing age, parity, and obesity.2,3 SUI treatment options, beginning from least to most invasive, include pelvic floor exercises, biofeedback and/or physical therapy, continence devices, off-label use of medications, urethral bulking agents, and surgical correction with slings. Midurethral tension-free slings are highly efficacious for the treatment of SUI. While a sling is a minimally invasive procedure, patients typically voice concerns regarding surgery and appropriately begin with conservative treatments.
A new FDA-approved OTC option for SUI
First-line conservative therapies offered to patients for SUI include pelvic floor muscle exercises and intravaginal continence devices. Disappointingly, such devices—including pessaries and the incontinence dish—have not been popular among patients for SUI. Authors of a randomized control trial evaluating incontinence pessaries versus behavioral therapy, including pelvic floor muscle training, found that, after 3 months, use of a pes‑ sary was not as effective as behavioral therapy in terms of patient satisfaction and improvement in bothersome urinary incontinence.4 In our experience, many patients wearing incontinence rings discontinue their use due to ineffectiveness or discomfort.
Patients now have an FDA-approved, over-the-counter option for SUI symptom management. The Poise Impressa is a disposable, nonabsorbent, flexible intravaginal device for patients with SUI (FIGURE 1). The device is comprised of a silicone core with a soft, nonwoven polypropylene fabric cover. It is inserted similar to a tampon, using an applicator, and provides nonobstructive support to the urethra to prevent stress urinary leakage. To find the proper fit, patients purchase the sizing kit, which includes 3 sizes. Patients are to insert size 1 first and monitor their comfort as well as improvement in leakage. Should size 1not sufficiently relieve leakage, the patient may try sizes 2 and 3 successively, with the goal of finding the most comfortable and effective insert. The insert is approved for up to 8 hours of wear in a 24-hour period, at which time the patient removes the device by pulling the string in a similar manner as removing a tampon.
Efficacy and quality of life data. Over 28 days, 85% of women with severe SUI confirmed on urodynamic testing achieved greater than 70% leakage reduction according to measured pad weights.5 Seventy percent of women reported 90% improvement in quality of life using validated questionnaires. In addition, 92% reported feeling dry with an improved perception of incontinence and greater confidence during strenuous activities.6 There were no serious adverse events, and the most common mild adverse events were discomfort, pain, and spotting.
As more patients become aware of the device through advertising and word of mouth, we expect patients to seek advice from their gynecologists on the safety and efficacy of the insert. In our experience, most patients report improvement in bothersome symptoms with the device and are overall satisfied. For patients who have discomfort with device placement, a water-based lubricant can be used. Patients using vaginal estrogen may apply the medication at night and wear the device during the day.
Office-based bladder control system in the pipeline
For SUI, options are limited for patients who would rather seek office-based procedures than invasive surgeries. Injections of urethral bulking agents can be performed in an office setting by injecting them transurethrally with a cystoscope slightly distal to the bladder neck. While bulking agents have a role in certain patients with SUI, especially those who are not interested in pursuing more invasive surgeries, only 43% have short-term (less than 6 months) cure and 75% report short-term improvement.7
A minimally invasive office-based procedure to treat SUI symptoms is under investigation in clinical trials currently. The Vesair Balloon bladder control system (Solace Therapeutics) is performed with cystoscopic guidance and is being tested at multiple sites throughout the United States (FIGURE 2).
The Vesair Balloon acts like a “shock absorber” to reduce momentary increases in bladder pressure due to external forces or stressors. The balloon is a small device, approximately the size of a quarter, and is implanted through the urethra via a specially designed applicator under cystoscopic guidance in the office setting. Pretreatment with pain medication usually is unnecessary. The VesairBalloon may be retained in situ for up to 12 months, at which time it is removed using a device-specific grasper under direct visualization with a cystoscope in the office.
Preliminary efficacy and safety data. In a single-blinded randomized controlled trial, 63% of women in the Vesair Balloon group had significant improvement in provocative pad weights and quality-of-life questionnaire scores at 3 months, compared with 31% in the control group.8 No serious adverse events were observed. Eleven of 63 patients (17%) withdrew from the study—most commonly for bladder irritation and dysuria.
We anxiously await the results of a second single-blinded randomized control trial currently being conducted.
Best surgical options for SUI
Today, the standard surgical procedure for SUI is a midurethral sling. Midurethral slings may be placed through 3 routes: retropubic; transobturator; and single-incision, otherwise known as “mini-slings.” Subjective cure rates of retropubic versus transobturator slings are similar, with lower rates of bladder perforation, major vascular/visceral injury, and operative blood loss in the transobturator group.9 However, rates of groin pain are higher in the trans‑ obturator group.
Single-incision slings were developed in an effort to avoid the morbidity and pain with passing traditional sling trocars through the obturator space and skin of the groin. In a randomized controlled trial, the Miniarc single- incision sling (Astora Women’s Health) was found to be noninferior to the Monarc transobturator sling (Astora) at 12 and 36 months.10 There were no statistically significant differences between subjective and objective cure rates on cough stress tests. Postoperative pain and groin pain were significantly less in patients with the Miniarc sling, compared with the Monarc sling.
It is our opinion that as more data become available, single-incision slings will find their foothold in a subset of patients with SUI.
Case 2: Overactive bladder: Failed medication therapy
A healthy 63-year-old woman presents with a 9-month history of loss of urine with strong urges, urinating 4 times per night, and a feeling of urgency when she needs to urinate. She denies pain with urination, difficulty emptying her bladder fully, and pain with a full bladder. She has restricted her fluid intake to 4 glasses of water per day and has stopped drinking fluids 4 hours before bedtime.
She described her symptoms to her intern‑ ist, who prescribed oxybutynin. She took the medication for 3 months but stopped after she developed severe constipation and dry mouth. She states the medication did not help her urinary symptoms. You discuss with her trials of other medications including topical anticholinergics and mirabegron. She is frustrated with her symptoms and asks if there are any other options besides medications.
Overactive bladder (OAB) is present in up to 16% of the US population, with the percentage estimated to increase by 20% within the next 2 years.11,12 The drastic increase in prevalence, likely due to the aging population, may result in an increased counseling and management burden placed on general practitioners and gynecologists.
First-line management options for OAB are behavioral modifications and/or medications. Our patient in case 2 failed both first-line therapies. When a patient fails or is intolerant to an anticholinergic medication, we offer mirabegron, a beta-3 agonist (after excluding any contraindications to the medication). Beyond medications, the therapeutic options are rather limited.
Second-line OAB treatment options
In January 2013, the FDA expanded the approved use of onabotulinum toxin A (Botox, Allergan) for the treatment of OAB in those who are intolerant of or have failed treatment with anticholinergic medications. Using a cystoscope, 100 units of onabotulinum toxin A are injected into 20 sites within the bladder wall. Due to the risk of urinary retention in up to 6% of patients, it is recommended to administer onabotulinum toxin A to patients who are willing and capable of performing clean intermittent catheterization.13
Efficacy data. In a recent systematic review and meta-analysis, the authors concluded onabotulinum toxin A to be effective in the treatment of idiopathic OAB with a statistically significant reduction compared with baseline in the number of incontinence episodes per day (-2.77 in the treatment group vs -1.01 in the placebo group) and the number of voids per day (-1.61 in the treatment group vs -0.87 in the placebo group).14 Patients who received onabotulinum toxin A experienced a higher rate of adverse effects, such as urinary tract infections, and were more likely to require clean intermittent catheterization due to incomplete bladder emptying.13 Patients can expect symptom improvement for approximately 6 months or longer.15 Based on the manufacturers’ recommendations, patients are not to be reinjected sooner than 12 weeks from prior onabotulinum toxin A injection.
In women with refractory OAB, available second-line treatments include neuromodulation by sacral nerve or posterior tibial nerve stimulation (PTNS). The latter therapy is an office-based procedure that involves placement of a lead percutaneous to the medial aspect of the ankle near the tibial nerve. It is postulated that stimulation of the tibial nerve results in retrograde stimulation of the S3 sacral nerve plexus, resulting in OAB symptom relief in 54% to 70% of patients.16
Case 3: Fecal incontinence
A 57-year-old, otherwise healthy, multiparous woman presents with a 3-year history of fecal incontinence. She reports that it is embarrassing and distressing. She avoids certain social activities and is not currently sexually active due to the frequency of bowel leakage episodes.
In an effort to decrease her episodes of incontinence, she takes loperamide hydrochloride (Imodium) regularly with little improvement in the frequency of accidents. She has no history of gastrointestinal, rectal, or gynecologic surgery. She had 2 full-term vaginal deliveries that were uncomplicated. On review of systems, she also discloses occasional urinary incontinence.
Physical examination reveals normal vaginal anatomy with adequate pelvic organ support and no neurologic abnormalities. Rectal examination demonstrates normal tone and no evidence of rectal prolapse. Contractions of the pelvic floor muscles are weak. She is frustrated with her condition and seeks your guidance.
Fecal incontinence affects more than 20 million women in the United States, with only one-third of those with the condition disclosing their symptoms to their physician.17 Many etiologies for accidental bowel leakage exist, with some of the most common being advancing age and obstetric trauma. Up to one-third of women presenting for evaluation of urinary incontinence have fecal incontinence; therefore, one must be vigilant in screening for this potentially devastating condition.18
In case 3, the patient has tried medical therapies for fecal incontinence, including stool-bulking agents and motility regulators such as loperamide hydrochloride. Besides offering fiber supplements (or other stool-bulking agents) or physical therapy, nonsurgical options for this patient are limited.
Newly available: A vaginal insert for fecal incontinence
In 2015, the Eclipse System (Pelvalon) became the first FDA-approved vaginal insert for the treatment of fecal incontinence. The manufacturer recently was granted clearance for its second-generation device (FIGURE 3). The device consists of a silicone-coated stainless steel base with a posteriorly facing balloon and a pressure-regulated pump that allows the patient to control her bowel movements. After a patient is fitted with the device in the office setting, she is independently able to insert and remove it as well as deflate the balloon to allow for bowel movements and inflate the balloon to prevent accidental bowel leakage.
In a multicenter trial conducted by Richter and colleagues,19 78% of women successfully fitted with the device had a 50% mean reduction of fecal incontinence episodes. Two-week mean incontinence episodes decreased from 11 to 2 after 1 month of continued use of the insert. In addition, there was significant improvement in quality-of-life questionnaire scores.
Of the 110 patients fitted with the device, 32 (29%) withdrew due to unsatisfactory device fit or were unable to remove or insert the device themselves. Common adverse effects included pelvic cramping and discomfort during device fitting. One month after insertion, pelvic pain and cramping continued in up to 10% of patients. No serious adverse events related to the device were observed during the 1-month trial.19
In the approximate 70% of women successfully fitted with the vaginal insert, the system was highly efficacious in improving subjective and objective outcomes with no unexpected serious adverse events. Currently the device is available at investigative sites across the United States, and the company plans for sales to begin later this year.
Surgical options for fecal incontinence
In patients for whom conservative and medical therapies have failed, surgical treatments may be offered. Surgical options vary from minimally invasive procedures to colostomy. One of the minimally invasive procedures available is the InterStim procedure, or sacral nerve stimulation (SNS). An electrode is inserted percutaneously through the S3 foramen and is connected to an implanted battery under the skin of the buttocks. Low-voltage stimulation is applied to the leads that lie adjacent to the S3 sacral nerve roots.
Patients with SNS experience fewer episodes of fecal incontinence, with over 80% maintaining a reduction in fecal incontinent episodes by greater than 50% up to 5 years after implantation.20,21
The transobturator postanal sling system (TOPAS, Astora) is a new investigational surgical device. It is inserted in a minimally invasive procedure and is currently undergoing a prospective, multicenter clinical trial (FIGURE 4). It consists of a polypropylene mesh sling placed perianally, with the mesh arms exiting through the obturator foramen bilaterally. It is intended to increase posterior pelvic support at the level of the anorectal junction. Efficacy and safety of the product have yet to be determined.
We need to stay up to date on new treatment options
As the prevalence increases for urinary and fecal incontinence, ObGyns are challenged to remain knowledgeable about the condition, the prognosis, and the success of interventions. Currently, patients have a range of options to manage their urinary and fecal incontinence symptoms, with the number of products and clinical data increasing over time. With the advent of novel products and the widespread availability of information via the Internet, physicians must remain the established source on new innovative treatments and up-to-date clinical data in order to provide competent and comprehensive care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189(2):428–434.
- Lensen EJ, Withagen MI, Kluivers KB, Milani AL, Vierhout ME. Urinary incontinence after surgery for pelvic organ prolapse. Neurourol Urodyn. 2013;32(5):455–459.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115(3):609–617.
- Ziv E, Stanton SL, Abarbanel J. Efficacy and safety of a novel disposable intravaginal device for treating stress urinary incontinence. Am J Obstet Gynecol. 2008;198(5):594.e1–e7.
- Ziv E, Stanton SL, Abarbanel J. Significant improvement in the quality of life in women treated with a novel disposable intravaginal device for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(6):651–658.
- Ghoniem GM, Miller CJ. A systematic review and meta-analysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24(1):27–36.
- Wyndaele JJ, De Wachter S, Tommaselli GA, et al. A randomized, controlled clinical trial of an intravesical pressure-attenuation balloon system for the treatment of stress urinary incontinence in females [published online ahead of print January 16, 2015]. Neurourol Urodyn. doi:10.1002/nau.22708.
- Ford AA, Rogerson L, Cody JD, Ogah J. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2015;7:CD006375.
- Lee JK, Rosamilia A, Dwyer PL, Lim YN, Muller R. Randomized trial of a single incision versus an outside-in transobturator midurethral sling in women with stress urinary incontinence: 12 month results. Am J Obstet Gynecol. 2015;213(1):35.e1–e9.
- Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–1138.
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336.
- Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013;189(6):2186−2193.
- Cui Y, Zhou X, Zong H, Yan H, Zhang Y. The efficacy and safety of onabotulinumtoxinA in treating idiopathic OAB: A systematic review and meta-analysis. Neurourol Urodyn. 2015;34(5):413–419.
- Apostolidis A, Dasgupta P, Denys P, et al; European Consensus Panel. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55(1):100–119.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- Johanson JF, Lafferty J. Epidemiology of fecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91(1):33–36.
- Jackson SL, Weber AM, Hull TL, Mitchinson AR, Walters MD. Fecal incontinence in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1997;89(3):423–427.
- Richter HE, Matthews CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125(3):540–547.
- Thaha MA, Abukar AA, Thin NN, Ramsanahie A, Knowles CH. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015;8:CD004464.
- Hull T, Giese C, Wexner SD, et al; SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56(2):234–245.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189(2):428–434.
- Lensen EJ, Withagen MI, Kluivers KB, Milani AL, Vierhout ME. Urinary incontinence after surgery for pelvic organ prolapse. Neurourol Urodyn. 2013;32(5):455–459.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115(3):609–617.
- Ziv E, Stanton SL, Abarbanel J. Efficacy and safety of a novel disposable intravaginal device for treating stress urinary incontinence. Am J Obstet Gynecol. 2008;198(5):594.e1–e7.
- Ziv E, Stanton SL, Abarbanel J. Significant improvement in the quality of life in women treated with a novel disposable intravaginal device for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(6):651–658.
- Ghoniem GM, Miller CJ. A systematic review and meta-analysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24(1):27–36.
- Wyndaele JJ, De Wachter S, Tommaselli GA, et al. A randomized, controlled clinical trial of an intravesical pressure-attenuation balloon system for the treatment of stress urinary incontinence in females [published online ahead of print January 16, 2015]. Neurourol Urodyn. doi:10.1002/nau.22708.
- Ford AA, Rogerson L, Cody JD, Ogah J. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2015;7:CD006375.
- Lee JK, Rosamilia A, Dwyer PL, Lim YN, Muller R. Randomized trial of a single incision versus an outside-in transobturator midurethral sling in women with stress urinary incontinence: 12 month results. Am J Obstet Gynecol. 2015;213(1):35.e1–e9.
- Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–1138.
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336.
- Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013;189(6):2186−2193.
- Cui Y, Zhou X, Zong H, Yan H, Zhang Y. The efficacy and safety of onabotulinumtoxinA in treating idiopathic OAB: A systematic review and meta-analysis. Neurourol Urodyn. 2015;34(5):413–419.
- Apostolidis A, Dasgupta P, Denys P, et al; European Consensus Panel. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55(1):100–119.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- Johanson JF, Lafferty J. Epidemiology of fecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91(1):33–36.
- Jackson SL, Weber AM, Hull TL, Mitchinson AR, Walters MD. Fecal incontinence in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1997;89(3):423–427.
- Richter HE, Matthews CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125(3):540–547.
- Thaha MA, Abukar AA, Thin NN, Ramsanahie A, Knowles CH. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015;8:CD004464.
- Hull T, Giese C, Wexner SD, et al; SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56(2):234–245.
In this Article
- New OTC option for SUI
- Second-line OAB treatments
- Promising vaginal insert for fecal incontinence