User login
- Arrange for echocardiography or radionuclide angiography within 72 hours of a heart failure exacerbation. An ejection fraction >50% in the presence of signs and symptoms of heart failure makes the diagnosis of diastolic heart failure probable (B).
- To treat associated hypertension, use angiotensin receptor blockers (ARBs), angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, calcium channel blockers, or diuretics to achieve a blood pressure goal of <130/80 mm Hg (C).
- When using beta-blockers to control heart rate, titrate doses more aggressively than would be done for systolic failure, to reach a goal of 60 to 70 bpm (B).
- Use ACE inhibitors/ARBs to decrease hospitalizations, decrease symptoms, and prevent left ventricular remodeling (A).
Heart failure is a growing epidemic in the US, estimated to cause at least 20% of all hospitalizations in persons over 65 years of age. It is also the leading inpatient diagnosis among Medicare recipients with this age group.1,2,3 More than 5 million people in the US have heart failure, with approximately 550,000 new cases diagnosed annually.
Growing epidemiologic evidence suggests that studies of heart failure have underrepresented a large patient population with a natural history different from that of left ventricular (LV) systolic dysfunction.4-8One third to one half of patients with signs and symptoms of heart failure have preserved left ventricular function (LVF). They are said to have diastolic heart failure (DHF).
Identifying persons with this less-understood form of heart failure can be challenging. Skillful discernment is needed to avoid mistakenly attributing symptoms to other causes. DHF is particularly common among elderly women with hypertension; every patient with signs and symptoms of heart failure should undergo echocardiography to determine LV function.
Though the evidence base for DHF treatment is less well established than it is for systolic heart failure (SHF), data from recent trials have offered a promising direction.
New categorization of heart failure
The relative scopes of DHF and SHF will be better appreciated by understanding how recently developed guidelines have restructured the historical classification of heart failure.
Heart failure is defined by the American College of Cardiology (ACC) and the American Heart Association (AHA) as a complex syndrome resulting from any structural or functional cardiac disorder that impairs the ability of the ventricles to fill with or eject blood.9 The older terms, low-vs high-output failure, are now regarded as obsolete and have been abandoned in favor of distinguishing between abnormalities of systolic and diastolic function.10-12
ACC/AHA heart failure staging system
Severity of heart failure symptoms has traditionally been gauged by the New York Heart Association (NYHA) classification system. A criticism of the NYHA scale, however, is that patients may fluctuate in and out of the varying functional classes. To correct this shortcoming of the NYHA scale, the ACC and the AHA devised a new staging system to describe the progression of heart failure.9 The premise of this new system is to provide permanence to each sequential progression through the stages of heart failure while complementing the existing NYHA scale.9,13
New model. Patients with Stage A heart failure are at high risk of developing heart failure based on comorbidities and medical history.
Patients with Stage B heart failure have some component of structural heart disease but are asymptomatic.
Patients with Stage C heart failure have underlying structural abnormalities and have symptoms, or have had symptoms of heart failure in the past.
Patients with Stage D heart failure are refractory to conventional medical therapy and have end-stage symptoms.
TABLE 1 shows how the ACC/AHA Heart Failure Staging System correlates with the NYHA Classification scheme. Family practitioners can use the new heart failure staging system to identify and recognize risk factors for the development of heart failure and then seek to aggressively prevent or reverse them.
TABLE 1
Relationship of the ACC/AHA Heart Failure
| ACC/AHA STAGES OF HEART FAILURE | NYHA FUNCTIONAL CLASSIFICATION |
|---|---|
| A- high risk for development of HF; no underlying structural cardiac disease (ie, hypertension, diabetes, hyperlipidemia, etc) | No correlation |
| B- Structural heart disease but asymptomatic (ie, LVH) | I- patients with no limitation of activities; they suffer no symptoms from ordinary physical activity |
| C- Structural heart disease with past or current symptoms of heart failure | II- patients with slight, mild limitation of activity; they are comfortable with rest or with mild exertion |
| III- patients with marked limitation of activity; they are comfortable only at rest | |
| D- Refractory heart failure | IV- patients who should be at complete rest, confined to bed or chair; any physical activity brings on discomfort and symptoms occur at rest |
| Patients with Stage A heart failure are at high risk of developing clinical HF and are not representative of any patients categorized under the NHYA functional classification system, as they are not yet symptomatic. Patients with Stage B heart failure have some form of structural heart disease without associated symptoms and correlate best with NYHA Class I patients. Patients with Stage C heart failure have the same underlying structural cardiac disorders associated with Stage B, but they have past or current symptoms of HF. Depending on the severity of their condition, patients with Stage C heart failure may fall within any of the NYHA functional classes. Patients with Stage D heart failure have symptoms refractory to optimized medical and interventional therapies and are representative of NYHA Class IV patients. | |
Who is at risk for DHF?
Risk factors for the development of DHF include advanced age, female sex, hypertension, and coronary ischemia. Approximately 50% of those older than 70 years who have heart failure have preserved LV function.14-16 In a large epidemiologic study of elderly patients with heart failure, women were twice as likely as men to have preserved LV function.17 In examining post-myocardial infarction (MI) patients with heart failure, women and those with smaller infarctions were also more likely to have preserved LV function (odds ratio=1.97; 95% confidence interval [CI], 1.27–3.07).18
Hypertension is a well known cause of left ventricular hypertrophy (LVH), which is a causal mechanism for DHF.19,20 Levy et al, in a study of 5143 subjects from the original Framingham Heart Study participants and Framingham Offspring participants, found that hypertension predated the development of heart failure in 91% of cases among patients in this cohort.21 In this sample, hypertension also carried the greatest population-attributable risk for the development of heart failure of all risk factors considered (39% in men and 59% in women). Hypertension also had the highest prevalence of all risk factors in this study (60% in men and 62% in women). Untreated hypertension leads to an increasing incidence of LVH and associated diastolic dysfunction. Increased LV mass and stiffness cause noncompliance and abnormal relaxation of the ventricular wall leading to increased diastolic pressures.4,19-21
Coronary ischemia can also cause diastolic dysfunction.20 Data from the Framingham Heart Study indicate that the prevalence of MI was 10% in hypertensive men and 3% in hypertensive women.21 MI is a well known precursor of LV systolic dysfunction; however, the relationship to diastolic dysfunction is less clear. Although the prevalence of MI was associated with a 5- to 6-fold risk for heart failure in Framingham subjects, after adjustment for age and other risk factors, fewer than half of the patients who subsequently developed heart failure had a history of MI. This finding supports the role of untreated hypertension in the pathogenesis of DHF.21
Physical examination does not help distinguish between DHF and SHF. Signs and symptoms of both disorders are relatively the same.22 Therefore, the presence of one or more of these risk factors in the setting of heart failure and preserved LV function supports the diagnosis of DHF.14-17TABLE 2 summarizes known clinical characteristics and features of SHF and DHF. All patients with systolic heart failure have some component of diastolic dysfunction as well.10,12,23,24
TABLE 2
Characteristics of patients with systolic vs diastolic heart failure
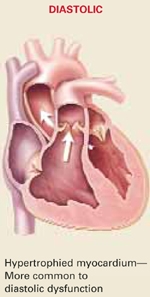
| Differentiating systolic and diastolic dysfunction | 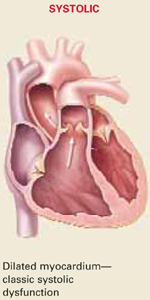
| 
|
| Etiology | Commonly associated with previous MI; exists concurrently with diastolic dysfunction | Pathogenesis is multifocal; associated more often with systemic hypertension, may exist alone without a component of systolic heart failure |
| Gender-specific differences | Both sexes affected | More common in women |
| Age-related differences | All ages affected | More common in elderly patients |
| Echocardiographic findings | Depressed LVEF <40% | Preserved LVEF >40% |
| Symptomatology | Identical—unable to differentiate with clinical examination | Identical—unable to differentiate with clinical examination |
| Long-term prognosis | 15% annual mortality rate | 5 to 8% annual mortality rate |
| MI, myocardial infarction; LVEF, left ventricular ejection fraction. | ||
Diagnosis is made clinically
No consensus exists on standardized criteria for diagnosing diastolic heart failure. However, 3 diagnostic levels—possible, probable, and definite DHF—have been proposed by Vasan and Levy.11
Possible DHF is defined as signs and symptoms of heart failure (TABLE 3) in patients with normal LV function, but lacking an assessment of ventricular function in proximity to the heart failure event.
Probable DHF is defined as (1) signs and symptoms of heart failure and (2) an ejection fraction >50% measured via echocardiography or radionuclide angio-graphy within 72 hours of the heart failure exacerbation.
Definite DHF is defined as (1) signs and symptoms of heart failure, (2) an ejection fraction >50% measured via the above methods within 72 hours of the patient’s presentation, and (3) increased left-ventricular end diastolic pressure (LVEDP) measured during cardiac catheterization.
TABLE 3
Modified Framingham criteria for diagnosing heart failure
| Need 2 major or 1 major and 2 minor fulfilled criteria for diagnosis of heart failure. |
|---|
| MAJOR CRITERIA |
| Paroxysmal nocturnal dyspnea |
| Orthopnea |
| Elevated jugular venous pressure |
| Pulmonary rales |
| Cardiomegaly on radiography |
| Acute pulmonary edema |
| S3 gallop |
| Weight loss >4.5 kg in response to treatment of heart failure |
| MINOR CRITERIA |
| Bilateral ankle/leg edema |
| Nocturnal cough |
| Dyspnea on ordinary exertion |
| Hepatomegaly |
| Pleural effusion |
| Tachycardia >120 bpm |
| MAJOR OR MINOR |
| Weight loss >4.5 kg in 5 days in response to treatment of heart failure |
| From: McKee et al, N Engl J Med 1971; 285:1441-1446.26 |
Direct assessment of diastolic function unnecessary
Evidence of diastolic dysfunction as determined by echocardiography or cardiac catheterization has been debated as a necessary third diagnostic criterion.24 The problem, though, is that there is no simple means of reliably diagnosing diastolic dysfunction with echocardiography (E:A ratios, deceleration or relaxation times), and that performing cardiac catheterization to measure LVEDP is impractical.22
Furthermore, Zile et al have shown that, though cardiac catheterization helps to confirm diastolic dysfunction, it is not necessary to establish the diagnosis. In this study, 63 patients with clinically defined diastolic heart failure based on the Framingham criteria underwent diagnostic cardiac catheterization; 58 (92%) of these patients were also found to have an abnormal LVEDP, indicative of diastolic dysfunction.25 Therefore, the diagnosis of DHF can be made in the setting of heart failure in a patient with a normal ejection fraction.
Order echocardiography within 72 hours of symptom onset
A major challenge for clinicians is to determine whether a patient’s dyspnea is a true symptom of heart failure. Signs and symptoms of heart failure must be defined using clinical indicators such as the Framingham heart failure criteria (FIGURE).26 Diagnosis of heart failure is more easily made for a patient presenting to the emergency department with acute pulmonary edema than it is for an outpatient seen repeatedly for shortness of breath over months.
For a patient presenting with acute pulmonary edema, an echocardiogram should be performed within 72 hours of symptoms to document cardiac function in proximity to the heart failure exacerbation. The ejection fraction of patients with DHF can remain within normal range, even during acute decompensation.27,28 Stroke volume and cardiac output may be decreased despite a normal ejection fraction.
Cardiogenic pulmonary edema in DHF patients results from the stiffened ventricle’s inability to compensate for increased venous return due to an expansion in central blood volume or sodium retention. Subsequently, diastolic pressures elevate and impede lung compliance, which increases the work of breathing and dyspnea.20,29 A normal ejection fraction and symptom diminishment following diuresis in the setting of acute decompensation help confirm the diagnosis of DHF, especially when other disease states are complicating the clinical picture.30
Elevated BNP levels may be helpful
An elevated level of b-type natriuretic peptide (BNP) can help confirm the clinical diagnosis of heart failure, and it has been shown in small studies to be a valid marker of DHF.31,32 In a study of 294 patients referred for echocardiography to evaluate LV function, Lubien et al found that a BNP value of at least 62 pg/mL had a sensitivity of 85%, a specificity of 83%, and an accuracy of 84% for heart failure in patients with a normal ejection fraction.32 All patients with systolic dysfunction defined by an ejection fraction <50% were excluded from this study. These results, though promising, must be confirmed by further studies evaluating the diagnostic utility of BNP to detect active heart failure symptoms in patients with diastolic dysfunction.
Treatment of symptomatic diastolic dysfunction
For SHF patients, multiple large outcome trials have clearly documented the benefit of angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, and aldosterone antagonists in reducing mortality.33-36 The relative paucity of outcome data for DHF has resulted in medical therapy primarily centered on modifying physiologic factors to improve LV filling and relaxation. Specifically, treatment should focus on symptom reduction, balancing fluid status, controlling heart rate, decreasing any ischemia, and achieving blood pressure goals.19,20,22,31 Though many of the medications used to treat SHF are also used for DHF, there are several important differences in appropriate initiation and subsequent titration of these drugs in the 2 settings.20,31
While treatment of DHF is largely theoretical, a limited number of well-designed, randomized studies are available to help determine appropriate therapy.37-39TABLE 4 provides a summary of the evidence base for evaluation and treatment of systolic vs diastolic heart failure.40TABLE 5 gives a synopsis of these studies. A suggested diagnostic and treatment approach for patients with DHF is outlined in the FIGURE. After determining whether a patient has DHF— primarily through the ruling out of other conditions and confirmation with echocardiographic studies—consider the applicability of each treatment based on a patient’s medical history and present condition.
TABLE 4
Comparative evidence base for evaluation and treatment of systolic vs diastolic heart failure
| LEVEL OF EVIDENCE* | ||
|---|---|---|
| FEATURE | SYSTOLIC HEART FAILURE | DIASTOLIC HEART FAILURE |
| Prevalence and risk factors | III | III |
| Non-invasive diagnostic methodologies | I - assessment of LVEF | IV, VII† |
| I - measurement of BNP levels | ||
| Prognosis | I - II | II, III |
| Treatment with ACE inhibitor, ARB, beta-blockers, and digitalis | I‡ | II, V-VII |
| Prevention trials (treatment of asymptomatic precursor condition) | I | None |
*
| ||
| † Diagnosis is primarily by exclusion of systolic heart failure; measurement of LVEF and BNP is also useful. | ||
| ‡ Cochrane review and meta-analysis. | ||
| ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; LVEF, left ventricular ejection fraction; BNP, b-type natriuretic peptide. Adapted and reproduced with permission from the BMJ Publishing Group and Dr. Ramachandran S. Vasan. BMJ 2003; 327:1181-1182.40 | ||
TABLE 5
Diastolic heart failure outcome trials
| TRIAL | BACKGROUND AND CONTEXT | REPRESENTATIVE PATIENT POPULATION | AVG LVEF OF PARTICIPANTS | NNT | SOR* (LOE) |
|---|---|---|---|---|---|
| CHARM-Preserved | Candesartan added to standard heart failure therapy in patients with LVEF >40% | N=3023 | 54% | 36† | A (1b) |
| 60% NYHA Class II | 42‡ | ||||
| 38% NYHA Class III | |||||
| 2% NYHA Class IV | |||||
| DIG Ancillary Trial | Digoxin + ACE inhibitors and diuretics in patients with LVEF >45% | N=988 | Not reported | N/A§ | B (1b) |
| NYHA classification not specified | |||||
| Propranolol Study, Aronow et al | Propranolol added to ACE inhibitors and diuretics in post-MI patients with LVEF 40% | N=158 | 56% | 5¶ | A (1b) |
| 52% NYHA Class II | |||||
| 48% NYHA Class III | |||||
| *Based on the guidelines for evidence quality outlined by the Center for Evidence-Based Medicine, available at: www.cebm.net/levels_ of_evidence.asp. A(1b) = consistent level 1 studies; individual randomized controlled trial (with narrow confidence interval). B(1b) = consistent level 2 or 3 studies or extrapolations from level 1 studies; individual randomized controlled trial (with narrow confidence interval) | |||||
| † For the composite of cardiovascular death, hospital admission for heart failure, MI, or cerebrovascular accident over 3 years | |||||
| ‡ For recurrent admissions for heart failure exacerbations over 3 years | |||||
| § No statistical differences between groups in rates of hospitalization or mortality over 3 years | |||||
| ¶ All-cause mortality over a mean of 32 months | |||||
| NNT, number needed to treat to prevent one death or other specified endpoint; LVEF, left ventricular ejection fraction; ACE, angiotensin-converting enzyme; NYHA, New York Heart Association classification; CHARM, Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity; DIG, Digitalis Investigation Group. | |||||
Medications to control blood pressure
Hypertension is a major risk factor for DHF, and the ACC/AHA heart failure guidelines recommend a lower blood pressure goal for patients with diastolic heart failure than for those with uncomplicated hypertension (ie, <130/80 mm Hg).9 Angiotensin receptor blockers (ARBs), ACE inhibitors, beta-blockers, calcium channel blockers, and diuretics may all be employed to achieve this blood pressure goal.
Angiotensin II receptor blockers. The use of ARBs in the treatment of DHF was recently evaluated in the CHARM-Preserved Study. Candesartan, 32 mg once daily, when added to a background therapy of mostly diuretics and beta-blockers (initially excluding the use of ACE inhibitors but later permitted in appropriate patients following the release of the HOPE trial results), was found to have a modest impact in preventing recurrent admissions for heart failure exacerbations (number needed to treat [NNT]=42 over 3 years).37 Candesartan also demonstrated a more favorable impact on the composite end-point of cardiovascular death, hospitalization for heart failure, MI, and stroke (NNT=36).
ACE inhibitors. For post-MI patients with DHF, ACE inhibitors have improved treadmill duration and NYHA functional class.41 Further studies are needed to determine whether an ACE inhibitor or an ARB is preferred or whether they may be used safely together in the management of DHF.
Beta-blockers. Propranolol, when added to an ACE inhibitor and diuretic, has been shown to significantly reduce mortality in a small prospective study of 158 post-MI patients with an average LVEF of 56% and NYHA Class II or III symptoms.38 Seventy percent of the study patients were women (n=111) and the mean age was 81 years. The dose of pro-pranolol in this study was increased in 10-mg increments at 10-day intervals up to a total daily dose of 30 mg 3 times daily.
All 79 patients randomized to receive propranolol successfully reached the target dose; however, 14% (n=11) discontinued therapy due to worsening heart failure or hypotension. The absolute reduction in total mortality among patients receiving propranolol was 20%, compared with the study group receiving only standard heart failure therapy (NNT=5 for a median of 32 months of follow-up, P=.007). The positive effect of beta-blocker therapy in this small study merits another larger, complementary trial to confirm its benefits in a bigger patient population with the same characteristics.
Control of volume status
Diuretics. It has long been recognized that diuretics are a useful and necessary adjunct in the management of volume overload in patients with heart failure42; however, no large, long-term studies are available to evaluate the effects of these medications on mortality.43 Without concurrent ACE inhibitor/ARB and beta-blocker therapy, diuretics have been shown to cause rebound sympathetic activation.44,45
For patients with either systolic or diastolic dysfunction, diuretics may be dosed aggressively to achieve euvolemia. But for patients with DHF who are partly dependent on volume coupled with increased heart rate to maintain cardiac output, excessive diuresis can cause a significant reduction in preload, which can worsen symptoms.20,22,30 It is advocated that long-term diuretics should be used judiciously in the treatment of both SHF and DHF, with individualized, tailored therapy being preferred and daily weights used as a guide to determine optimum fluid status.9
Medications to control heart rate
Beta-blockers. In addition to their anti-hypertensive effects, beta-blockers may also be used as rate-lowering therapy in the treatment of DHF. Dosing and titration in this setting are handled differently than for SHF. Whereas titration of beta-blockers in SHF requires careful adjustment to avoid worsening of the patients’ symptoms and subsequent exacerbation,46-48 dosing in DHF can be more aggressive, with a resting heart rate goal of 60 to 70 bpm.20,49 Beta-blockers are used as negative chronotropes in this instance to improve left ventricular filling. Beta-blockers are also useful in the management of ischemia and angina associated with diastolic heart failure.19,20
Calcium channel blockers. For patients with contraindications to beta-blocker therapy, non-dihydropyridine calcium channel blockers (verapamil, diltiazem) may be employed as rate-lowering therapy for DHF.19 Unlike the other drugs used in DHF, non-dihydropyridine calcium channel blockers have no role in the treatment of SHF except in the presence of tachyarrhythmias.20
Dihyropyridine calcium channel blockers (ie, amlodipine, felodipine) should be reserved for heart failure patients in general with angina refractory to beta-blockers. Amlodipine and felodipine are probably the safest of the dihydropyridine calcium channel blockers to use for the treatment of angina as they have not been shown to worsen existing SHF.50,51 Verapamil has been shown in a small study to increase exercise capacity and heart failure score in patients with DHF.52
Digitalis. The use of digoxin in patients with DHF was evaluated in the Digitalis Investigation Group (DIG) ancillary trial, a parallel substudy of the overall DIG Trial that enrolled 988 patients with diastolic dysfunction.39 DHF patients receiving digoxin were found to have fewer symptoms and hospitalizations, although this finding was not statistically significant. These findings should be weighed against recent data suggesting that digoxin predisposes women with depressed left ventricular systolic dysfunction to an increased risk of death.53 The role of digoxin in DHF is unclear, and it is recommended that its use be restricted to patients with recurrent hospitalizations and refractory tachyarrhythmias despite optimized medical therapy.9,20,30,54
Prognosis
The annual mortality of patients with DHF has been reported as 5% to 8%, whereas mortality associated with SHF approximates 10% to 15%. However, in patients aged >70 years, both SHF and DHF have a 5-year mortality of 50% and both have an estimated 50% annual hospital admission rate.58
Looking forward
Greater recognition of the disorder and more enrollment of patients with DHF in outcome-based studies will hopefully improve our understanding and approach to treatment of this specific form of heart failure.40,55
Ongoing studies that may provide more evidence-based data to guide therapy for DHF include the Irbesartan in Heart Failure with Preserved Systolic Function Trial (I-PRESERVE), Perindopril for Elderly People with Chronic Heart Failure Study (PEP-CHF) and Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure (SENIORS).56-58
- Amlodipine • Norvasc
- Candesartan • Atacand
- Digoxin • Lanoxin
- Diltiazem • Cardizem, Cartia, Pilacor, Tiazac
- Enalapril • Vasotec
- Felodipine • Plendil
- Hydrazaline • Apresoline
- Propanolol • Betachron, Inderal
- Verapamil • Calan, Covem, Isoptin, Verelan
Acknowledgments
The authors wish to thank Thomas Hill and JoAnn Moates for their invaluable research assistance in preparation of this manuscript.
CORRESPONDING AUTHOR
Spencer A. Morris, PharmD, BCPS, Georgetown Hospital System, Georgetown Memorial Hospital, 606 Black River Road, Georgetown, SC 29440. E-mail: [email protected].
1. American Heart Association. Heart Disease and Stroke Statistics—2004 Update. Dallas, Texas: American Heart Association; 2003.
2. Popvic JR, Hall MJ. 1999 National Hospital Discharge Survey. Advance data from vital and health statistics; no. 319. Hyattsville, Maryland: Nation Center for Health Statistics, 2001.
3. Krumholz HM, Parent EM, Tu N, et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med 1997;157:99-104.
4. Krum H, Gilbert RE. Demographics and concomitant disorders in heart failure. Lancet 2003;362:147-158.
5. Redfield MM, Jacobsen SJ, Burnett JC, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289:194-202.
6. Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation 1998;98:2282.-
7. Vasan RS, Larson MG, Benjamin EJ, et al. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol 1999;33:1948.-
8. Kitzman DW, Gardin JM, Arnold A, et al. Heart failure with preserved LV function in the elderly: clinical and echocardiographic correlates from the Cardiovascular Health Study. Circulation 1999;94:1433.-
9. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: full text: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). Available at: http://www.acc.org/clinical/guidelines/failure/pdfs/hf_fulltext.pdf.
10. Gaasch WH. Diagnosis and treatment of heart failure based on left ventricular systolic or diastolic dysfunction. JAMA 1994;271:1276-1280.
11. Vasan RS, Levy D. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation 2000;101:2118-2121.
12. Grossman W. Defining diastolic dysfunction. Circulation 2000;101:2020-2021.
13. Brozena SC, Jessup M. The new staging system for heart failure: what every primary care physician should know. Geriatrics 2003;58:31-36.
14. McDermott MM, Feinglas J, Sy J, et al. Hospitalized congestive heart failure patients with preserved versus abnormal left ventricular systolic function; clinical characteristics and drug therapy. Am J Med 1995;99:629-635.
15. Vasan RS, Benjamin EJ, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure; an epidemiologic perspective. J Am Coll Cardiol 1995;26:1565.-
16. Kitzman DW, Gardin JM, Gottdiener JS, et al. for the CHS Research Group. Importance of heart failure with preserved systolic function in patients >65 years of age. Am J Cardiol 2001;87:413-419.
17. Masoudi FA, Havranek EP, Smith G, et al. Gender, age and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol 2003;41:217-223.
18. Hellermann JP, Jacobsen SJ, Reeder GS, et al. Heart failure after myocardial infarction: Prevalence of preserved left ventricular systolic function in the community. Am Heart J 2003;145:742-748.
19. Bonow RO, Udelson JE. Left ventricular diastolic dysfunction as a cause of congestive heart failure: mechanisms and management. Ann Intern Med 1992;117:502-510.
20. Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatment. Circulation 2002;105:1503-1508.
21. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA 1996;275:1557-1562.
22. Vasan RS, Benjamin EJ, Levy D. Congestive heart failure with normal left ventricular systolic function: clinical approaches to the diagnosis and treatment of diastolic heart failure. Arch Intern Med 1996;156:146-157.
23. Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 2002;105:1387-1393.
24. How to diagnose diastolic heart failure. European Study Group on Diastolic Heart Failure. Eur Heart J 1998;19:990.-
25. Zile MR, Gaasch WH, Carroll JD, et al. Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation 2001;104:779-782.
26. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971;285:1441-1446.
27. Gandi SK, Powers JC, Nomeir A, et al. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med 2001;344:17-60.
28. Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated dias-tolic heart failure in comparison to systolic heart failure. JAMA 2002;288:2144-2150.
29. Zile MR, Baicu CF, Gaasch WH. Diastolic heart fail-ure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 2004;350:1953-1959.
30. Elesber AA, Redfield MM. Approach to patients with heart failure and normal ejection fraction. Mayo Clin Proc 2001;76:1047-1052.
31. Angeja BG, Grossman W. Evaluation and management of diastolic heart failure. Circulation 2003;107:659.-
32. Lubien E, DeMaria A, Krishnaswamy P, et al. Utility of b-natriuretic peptide in detecting diastolic dysfunction: comparison with Doppler velocity recordings. Circulation 2002;105:595-601.
33. Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA 1995;273:1450-1456.
34. Foody JM, Farrell MH, Krumholz HM. β-blocker therapy in heart failure: scientific review. JAMA 2002;287:883-889.
35. Pitt B, Zannad F, Remme WJ. The effect of spirono-lactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709-717.
36. Pitt B, Remme W, Zannad F. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309-1321.
37. Yusuf S, Pfeffer MA, Swedberg, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003;362:777-781.
38. Aronow WS, Ahn C, Kronzon I. Effects of propranolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction >40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol 1997;80:207-209.
39. The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525.-
40. Vasan RS. Diastolic heart failure: the condition exists and needs to be recognized, prevented, and treated. BMJ 2003;327:1181-1182.
41. Aronow WS, Kronzon I. Effect of enalapril on congestive heart failure treated with diuretics in elderly patients with prior myocardial infarction and normal left ventricular ejection fraction. Am J Cardiol 1993;71:602-604.
42. Wilson JR, Reichek N, Dunkman WB, Golberg S. Effect of diuresis on the performance of the failing left ventricle in man. Am J Med 1981;70:234-239.
43. Faris R, Flather M, Purcell H, et al. Current evidence supporting the role of diuretics in heart failure: a meta analysis of randomized controlled trials. Int J Cardiol 2002;82:149-158.
44. Cowie MR, Zaphiriou A. Management of chronic heart failure. Br Med J 2002;325:422-425.
45. Cody RJ, Kubo SH, Pickworth KK. Diuretic treatment for the sodium retention of congestive heart failure. Arch Intern Med 1994;154:1905-1914.
46. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial Lancet 1999;353:9-13.
47. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet 1999;353:2001-2007.
48. Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-1658.
49. Levine HJ. Optimum heart rate of large failing hearts. Am J Cardiol 1988;61:633-636.
50. O’Connor CM, Carson PE, Miller AB, et al. Effect of amlodipine on mode of death among patients with advanced heart failure in the PRAISE trial. Prospective Randomized Amlodipine Survival Evaluation. Am J Cardiol 1998;82:881-887.
51. Amabile CM, Spencer AP. Keeping your patient with heart failure safe: a review of potentially dangerous medications. Arch Intern Med 2004;164:709-720.
52. Setaro J, Zaret BL, Schueman Ds, et al. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic performance. Am J Cardiol 1990;66:981-986.
53. Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med 2002;347:1403-1411.
54. Yamani MH. When should digoxin be used in patients with diastolic dysfunction? Cleve Clin J Med 2001;68:481,-485.
55. Vasan RS, Benjamin EJ. Diastolic heart failure—no time to relax. N Engl J Med 2001;344:56-58.
56. Carson P, Massie B. I-PRESERVE (Irbesartan in Heart Failure with Preserved Systolic Function) Study initiation presented at the European Society of Cardiology Annual Meeting, September 3, 2002, Berlin.
57. Cleland JG, Tendera M, Adamus J, et al. Perindopril for elderly people with chronic heart failure: the PEP-CHF study.
- Arrange for echocardiography or radionuclide angiography within 72 hours of a heart failure exacerbation. An ejection fraction >50% in the presence of signs and symptoms of heart failure makes the diagnosis of diastolic heart failure probable (B).
- To treat associated hypertension, use angiotensin receptor blockers (ARBs), angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, calcium channel blockers, or diuretics to achieve a blood pressure goal of <130/80 mm Hg (C).
- When using beta-blockers to control heart rate, titrate doses more aggressively than would be done for systolic failure, to reach a goal of 60 to 70 bpm (B).
- Use ACE inhibitors/ARBs to decrease hospitalizations, decrease symptoms, and prevent left ventricular remodeling (A).
Heart failure is a growing epidemic in the US, estimated to cause at least 20% of all hospitalizations in persons over 65 years of age. It is also the leading inpatient diagnosis among Medicare recipients with this age group.1,2,3 More than 5 million people in the US have heart failure, with approximately 550,000 new cases diagnosed annually.
Growing epidemiologic evidence suggests that studies of heart failure have underrepresented a large patient population with a natural history different from that of left ventricular (LV) systolic dysfunction.4-8One third to one half of patients with signs and symptoms of heart failure have preserved left ventricular function (LVF). They are said to have diastolic heart failure (DHF).
Identifying persons with this less-understood form of heart failure can be challenging. Skillful discernment is needed to avoid mistakenly attributing symptoms to other causes. DHF is particularly common among elderly women with hypertension; every patient with signs and symptoms of heart failure should undergo echocardiography to determine LV function.
Though the evidence base for DHF treatment is less well established than it is for systolic heart failure (SHF), data from recent trials have offered a promising direction.
New categorization of heart failure
The relative scopes of DHF and SHF will be better appreciated by understanding how recently developed guidelines have restructured the historical classification of heart failure.
Heart failure is defined by the American College of Cardiology (ACC) and the American Heart Association (AHA) as a complex syndrome resulting from any structural or functional cardiac disorder that impairs the ability of the ventricles to fill with or eject blood.9 The older terms, low-vs high-output failure, are now regarded as obsolete and have been abandoned in favor of distinguishing between abnormalities of systolic and diastolic function.10-12
ACC/AHA heart failure staging system
Severity of heart failure symptoms has traditionally been gauged by the New York Heart Association (NYHA) classification system. A criticism of the NYHA scale, however, is that patients may fluctuate in and out of the varying functional classes. To correct this shortcoming of the NYHA scale, the ACC and the AHA devised a new staging system to describe the progression of heart failure.9 The premise of this new system is to provide permanence to each sequential progression through the stages of heart failure while complementing the existing NYHA scale.9,13
New model. Patients with Stage A heart failure are at high risk of developing heart failure based on comorbidities and medical history.
Patients with Stage B heart failure have some component of structural heart disease but are asymptomatic.
Patients with Stage C heart failure have underlying structural abnormalities and have symptoms, or have had symptoms of heart failure in the past.
Patients with Stage D heart failure are refractory to conventional medical therapy and have end-stage symptoms.
TABLE 1 shows how the ACC/AHA Heart Failure Staging System correlates with the NYHA Classification scheme. Family practitioners can use the new heart failure staging system to identify and recognize risk factors for the development of heart failure and then seek to aggressively prevent or reverse them.
TABLE 1
Relationship of the ACC/AHA Heart Failure
| ACC/AHA STAGES OF HEART FAILURE | NYHA FUNCTIONAL CLASSIFICATION |
|---|---|
| A- high risk for development of HF; no underlying structural cardiac disease (ie, hypertension, diabetes, hyperlipidemia, etc) | No correlation |
| B- Structural heart disease but asymptomatic (ie, LVH) | I- patients with no limitation of activities; they suffer no symptoms from ordinary physical activity |
| C- Structural heart disease with past or current symptoms of heart failure | II- patients with slight, mild limitation of activity; they are comfortable with rest or with mild exertion |
| III- patients with marked limitation of activity; they are comfortable only at rest | |
| D- Refractory heart failure | IV- patients who should be at complete rest, confined to bed or chair; any physical activity brings on discomfort and symptoms occur at rest |
| Patients with Stage A heart failure are at high risk of developing clinical HF and are not representative of any patients categorized under the NHYA functional classification system, as they are not yet symptomatic. Patients with Stage B heart failure have some form of structural heart disease without associated symptoms and correlate best with NYHA Class I patients. Patients with Stage C heart failure have the same underlying structural cardiac disorders associated with Stage B, but they have past or current symptoms of HF. Depending on the severity of their condition, patients with Stage C heart failure may fall within any of the NYHA functional classes. Patients with Stage D heart failure have symptoms refractory to optimized medical and interventional therapies and are representative of NYHA Class IV patients. | |
Who is at risk for DHF?
Risk factors for the development of DHF include advanced age, female sex, hypertension, and coronary ischemia. Approximately 50% of those older than 70 years who have heart failure have preserved LV function.14-16 In a large epidemiologic study of elderly patients with heart failure, women were twice as likely as men to have preserved LV function.17 In examining post-myocardial infarction (MI) patients with heart failure, women and those with smaller infarctions were also more likely to have preserved LV function (odds ratio=1.97; 95% confidence interval [CI], 1.27–3.07).18
Hypertension is a well known cause of left ventricular hypertrophy (LVH), which is a causal mechanism for DHF.19,20 Levy et al, in a study of 5143 subjects from the original Framingham Heart Study participants and Framingham Offspring participants, found that hypertension predated the development of heart failure in 91% of cases among patients in this cohort.21 In this sample, hypertension also carried the greatest population-attributable risk for the development of heart failure of all risk factors considered (39% in men and 59% in women). Hypertension also had the highest prevalence of all risk factors in this study (60% in men and 62% in women). Untreated hypertension leads to an increasing incidence of LVH and associated diastolic dysfunction. Increased LV mass and stiffness cause noncompliance and abnormal relaxation of the ventricular wall leading to increased diastolic pressures.4,19-21
Coronary ischemia can also cause diastolic dysfunction.20 Data from the Framingham Heart Study indicate that the prevalence of MI was 10% in hypertensive men and 3% in hypertensive women.21 MI is a well known precursor of LV systolic dysfunction; however, the relationship to diastolic dysfunction is less clear. Although the prevalence of MI was associated with a 5- to 6-fold risk for heart failure in Framingham subjects, after adjustment for age and other risk factors, fewer than half of the patients who subsequently developed heart failure had a history of MI. This finding supports the role of untreated hypertension in the pathogenesis of DHF.21
Physical examination does not help distinguish between DHF and SHF. Signs and symptoms of both disorders are relatively the same.22 Therefore, the presence of one or more of these risk factors in the setting of heart failure and preserved LV function supports the diagnosis of DHF.14-17TABLE 2 summarizes known clinical characteristics and features of SHF and DHF. All patients with systolic heart failure have some component of diastolic dysfunction as well.10,12,23,24
TABLE 2
Characteristics of patients with systolic vs diastolic heart failure
| Differentiating systolic and diastolic dysfunction | 
| 
|
| Etiology | Commonly associated with previous MI; exists concurrently with diastolic dysfunction | Pathogenesis is multifocal; associated more often with systemic hypertension, may exist alone without a component of systolic heart failure |
| Gender-specific differences | Both sexes affected | More common in women |
| Age-related differences | All ages affected | More common in elderly patients |
| Echocardiographic findings | Depressed LVEF <40% | Preserved LVEF >40% |
| Symptomatology | Identical—unable to differentiate with clinical examination | Identical—unable to differentiate with clinical examination |
| Long-term prognosis | 15% annual mortality rate | 5 to 8% annual mortality rate |
| MI, myocardial infarction; LVEF, left ventricular ejection fraction. | ||
Diagnosis is made clinically
No consensus exists on standardized criteria for diagnosing diastolic heart failure. However, 3 diagnostic levels—possible, probable, and definite DHF—have been proposed by Vasan and Levy.11
Possible DHF is defined as signs and symptoms of heart failure (TABLE 3) in patients with normal LV function, but lacking an assessment of ventricular function in proximity to the heart failure event.
Probable DHF is defined as (1) signs and symptoms of heart failure and (2) an ejection fraction >50% measured via echocardiography or radionuclide angio-graphy within 72 hours of the heart failure exacerbation.
Definite DHF is defined as (1) signs and symptoms of heart failure, (2) an ejection fraction >50% measured via the above methods within 72 hours of the patient’s presentation, and (3) increased left-ventricular end diastolic pressure (LVEDP) measured during cardiac catheterization.
TABLE 3
Modified Framingham criteria for diagnosing heart failure
| Need 2 major or 1 major and 2 minor fulfilled criteria for diagnosis of heart failure. |
|---|
| MAJOR CRITERIA |
| Paroxysmal nocturnal dyspnea |
| Orthopnea |
| Elevated jugular venous pressure |
| Pulmonary rales |
| Cardiomegaly on radiography |
| Acute pulmonary edema |
| S3 gallop |
| Weight loss >4.5 kg in response to treatment of heart failure |
| MINOR CRITERIA |
| Bilateral ankle/leg edema |
| Nocturnal cough |
| Dyspnea on ordinary exertion |
| Hepatomegaly |
| Pleural effusion |
| Tachycardia >120 bpm |
| MAJOR OR MINOR |
| Weight loss >4.5 kg in 5 days in response to treatment of heart failure |
| From: McKee et al, N Engl J Med 1971; 285:1441-1446.26 |
Direct assessment of diastolic function unnecessary
Evidence of diastolic dysfunction as determined by echocardiography or cardiac catheterization has been debated as a necessary third diagnostic criterion.24 The problem, though, is that there is no simple means of reliably diagnosing diastolic dysfunction with echocardiography (E:A ratios, deceleration or relaxation times), and that performing cardiac catheterization to measure LVEDP is impractical.22
Furthermore, Zile et al have shown that, though cardiac catheterization helps to confirm diastolic dysfunction, it is not necessary to establish the diagnosis. In this study, 63 patients with clinically defined diastolic heart failure based on the Framingham criteria underwent diagnostic cardiac catheterization; 58 (92%) of these patients were also found to have an abnormal LVEDP, indicative of diastolic dysfunction.25 Therefore, the diagnosis of DHF can be made in the setting of heart failure in a patient with a normal ejection fraction.
Order echocardiography within 72 hours of symptom onset
A major challenge for clinicians is to determine whether a patient’s dyspnea is a true symptom of heart failure. Signs and symptoms of heart failure must be defined using clinical indicators such as the Framingham heart failure criteria (FIGURE).26 Diagnosis of heart failure is more easily made for a patient presenting to the emergency department with acute pulmonary edema than it is for an outpatient seen repeatedly for shortness of breath over months.
For a patient presenting with acute pulmonary edema, an echocardiogram should be performed within 72 hours of symptoms to document cardiac function in proximity to the heart failure exacerbation. The ejection fraction of patients with DHF can remain within normal range, even during acute decompensation.27,28 Stroke volume and cardiac output may be decreased despite a normal ejection fraction.
Cardiogenic pulmonary edema in DHF patients results from the stiffened ventricle’s inability to compensate for increased venous return due to an expansion in central blood volume or sodium retention. Subsequently, diastolic pressures elevate and impede lung compliance, which increases the work of breathing and dyspnea.20,29 A normal ejection fraction and symptom diminishment following diuresis in the setting of acute decompensation help confirm the diagnosis of DHF, especially when other disease states are complicating the clinical picture.30
Elevated BNP levels may be helpful
An elevated level of b-type natriuretic peptide (BNP) can help confirm the clinical diagnosis of heart failure, and it has been shown in small studies to be a valid marker of DHF.31,32 In a study of 294 patients referred for echocardiography to evaluate LV function, Lubien et al found that a BNP value of at least 62 pg/mL had a sensitivity of 85%, a specificity of 83%, and an accuracy of 84% for heart failure in patients with a normal ejection fraction.32 All patients with systolic dysfunction defined by an ejection fraction <50% were excluded from this study. These results, though promising, must be confirmed by further studies evaluating the diagnostic utility of BNP to detect active heart failure symptoms in patients with diastolic dysfunction.
Treatment of symptomatic diastolic dysfunction
For SHF patients, multiple large outcome trials have clearly documented the benefit of angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, and aldosterone antagonists in reducing mortality.33-36 The relative paucity of outcome data for DHF has resulted in medical therapy primarily centered on modifying physiologic factors to improve LV filling and relaxation. Specifically, treatment should focus on symptom reduction, balancing fluid status, controlling heart rate, decreasing any ischemia, and achieving blood pressure goals.19,20,22,31 Though many of the medications used to treat SHF are also used for DHF, there are several important differences in appropriate initiation and subsequent titration of these drugs in the 2 settings.20,31
While treatment of DHF is largely theoretical, a limited number of well-designed, randomized studies are available to help determine appropriate therapy.37-39TABLE 4 provides a summary of the evidence base for evaluation and treatment of systolic vs diastolic heart failure.40TABLE 5 gives a synopsis of these studies. A suggested diagnostic and treatment approach for patients with DHF is outlined in the FIGURE. After determining whether a patient has DHF— primarily through the ruling out of other conditions and confirmation with echocardiographic studies—consider the applicability of each treatment based on a patient’s medical history and present condition.
TABLE 4
Comparative evidence base for evaluation and treatment of systolic vs diastolic heart failure
| LEVEL OF EVIDENCE* | ||
|---|---|---|
| FEATURE | SYSTOLIC HEART FAILURE | DIASTOLIC HEART FAILURE |
| Prevalence and risk factors | III | III |
| Non-invasive diagnostic methodologies | I - assessment of LVEF | IV, VII† |
| I - measurement of BNP levels | ||
| Prognosis | I - II | II, III |
| Treatment with ACE inhibitor, ARB, beta-blockers, and digitalis | I‡ | II, V-VII |
| Prevention trials (treatment of asymptomatic precursor condition) | I | None |
*
| ||
| † Diagnosis is primarily by exclusion of systolic heart failure; measurement of LVEF and BNP is also useful. | ||
| ‡ Cochrane review and meta-analysis. | ||
| ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; LVEF, left ventricular ejection fraction; BNP, b-type natriuretic peptide. Adapted and reproduced with permission from the BMJ Publishing Group and Dr. Ramachandran S. Vasan. BMJ 2003; 327:1181-1182.40 | ||
TABLE 5
Diastolic heart failure outcome trials
| TRIAL | BACKGROUND AND CONTEXT | REPRESENTATIVE PATIENT POPULATION | AVG LVEF OF PARTICIPANTS | NNT | SOR* (LOE) |
|---|---|---|---|---|---|
| CHARM-Preserved | Candesartan added to standard heart failure therapy in patients with LVEF >40% | N=3023 | 54% | 36† | A (1b) |
| 60% NYHA Class II | 42‡ | ||||
| 38% NYHA Class III | |||||
| 2% NYHA Class IV | |||||
| DIG Ancillary Trial | Digoxin + ACE inhibitors and diuretics in patients with LVEF >45% | N=988 | Not reported | N/A§ | B (1b) |
| NYHA classification not specified | |||||
| Propranolol Study, Aronow et al | Propranolol added to ACE inhibitors and diuretics in post-MI patients with LVEF 40% | N=158 | 56% | 5¶ | A (1b) |
| 52% NYHA Class II | |||||
| 48% NYHA Class III | |||||
| *Based on the guidelines for evidence quality outlined by the Center for Evidence-Based Medicine, available at: www.cebm.net/levels_ of_evidence.asp. A(1b) = consistent level 1 studies; individual randomized controlled trial (with narrow confidence interval). B(1b) = consistent level 2 or 3 studies or extrapolations from level 1 studies; individual randomized controlled trial (with narrow confidence interval) | |||||
| † For the composite of cardiovascular death, hospital admission for heart failure, MI, or cerebrovascular accident over 3 years | |||||
| ‡ For recurrent admissions for heart failure exacerbations over 3 years | |||||
| § No statistical differences between groups in rates of hospitalization or mortality over 3 years | |||||
| ¶ All-cause mortality over a mean of 32 months | |||||
| NNT, number needed to treat to prevent one death or other specified endpoint; LVEF, left ventricular ejection fraction; ACE, angiotensin-converting enzyme; NYHA, New York Heart Association classification; CHARM, Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity; DIG, Digitalis Investigation Group. | |||||
Medications to control blood pressure
Hypertension is a major risk factor for DHF, and the ACC/AHA heart failure guidelines recommend a lower blood pressure goal for patients with diastolic heart failure than for those with uncomplicated hypertension (ie, <130/80 mm Hg).9 Angiotensin receptor blockers (ARBs), ACE inhibitors, beta-blockers, calcium channel blockers, and diuretics may all be employed to achieve this blood pressure goal.
Angiotensin II receptor blockers. The use of ARBs in the treatment of DHF was recently evaluated in the CHARM-Preserved Study. Candesartan, 32 mg once daily, when added to a background therapy of mostly diuretics and beta-blockers (initially excluding the use of ACE inhibitors but later permitted in appropriate patients following the release of the HOPE trial results), was found to have a modest impact in preventing recurrent admissions for heart failure exacerbations (number needed to treat [NNT]=42 over 3 years).37 Candesartan also demonstrated a more favorable impact on the composite end-point of cardiovascular death, hospitalization for heart failure, MI, and stroke (NNT=36).
ACE inhibitors. For post-MI patients with DHF, ACE inhibitors have improved treadmill duration and NYHA functional class.41 Further studies are needed to determine whether an ACE inhibitor or an ARB is preferred or whether they may be used safely together in the management of DHF.
Beta-blockers. Propranolol, when added to an ACE inhibitor and diuretic, has been shown to significantly reduce mortality in a small prospective study of 158 post-MI patients with an average LVEF of 56% and NYHA Class II or III symptoms.38 Seventy percent of the study patients were women (n=111) and the mean age was 81 years. The dose of pro-pranolol in this study was increased in 10-mg increments at 10-day intervals up to a total daily dose of 30 mg 3 times daily.
All 79 patients randomized to receive propranolol successfully reached the target dose; however, 14% (n=11) discontinued therapy due to worsening heart failure or hypotension. The absolute reduction in total mortality among patients receiving propranolol was 20%, compared with the study group receiving only standard heart failure therapy (NNT=5 for a median of 32 months of follow-up, P=.007). The positive effect of beta-blocker therapy in this small study merits another larger, complementary trial to confirm its benefits in a bigger patient population with the same characteristics.
Control of volume status
Diuretics. It has long been recognized that diuretics are a useful and necessary adjunct in the management of volume overload in patients with heart failure42; however, no large, long-term studies are available to evaluate the effects of these medications on mortality.43 Without concurrent ACE inhibitor/ARB and beta-blocker therapy, diuretics have been shown to cause rebound sympathetic activation.44,45
For patients with either systolic or diastolic dysfunction, diuretics may be dosed aggressively to achieve euvolemia. But for patients with DHF who are partly dependent on volume coupled with increased heart rate to maintain cardiac output, excessive diuresis can cause a significant reduction in preload, which can worsen symptoms.20,22,30 It is advocated that long-term diuretics should be used judiciously in the treatment of both SHF and DHF, with individualized, tailored therapy being preferred and daily weights used as a guide to determine optimum fluid status.9
Medications to control heart rate
Beta-blockers. In addition to their anti-hypertensive effects, beta-blockers may also be used as rate-lowering therapy in the treatment of DHF. Dosing and titration in this setting are handled differently than for SHF. Whereas titration of beta-blockers in SHF requires careful adjustment to avoid worsening of the patients’ symptoms and subsequent exacerbation,46-48 dosing in DHF can be more aggressive, with a resting heart rate goal of 60 to 70 bpm.20,49 Beta-blockers are used as negative chronotropes in this instance to improve left ventricular filling. Beta-blockers are also useful in the management of ischemia and angina associated with diastolic heart failure.19,20
Calcium channel blockers. For patients with contraindications to beta-blocker therapy, non-dihydropyridine calcium channel blockers (verapamil, diltiazem) may be employed as rate-lowering therapy for DHF.19 Unlike the other drugs used in DHF, non-dihydropyridine calcium channel blockers have no role in the treatment of SHF except in the presence of tachyarrhythmias.20
Dihyropyridine calcium channel blockers (ie, amlodipine, felodipine) should be reserved for heart failure patients in general with angina refractory to beta-blockers. Amlodipine and felodipine are probably the safest of the dihydropyridine calcium channel blockers to use for the treatment of angina as they have not been shown to worsen existing SHF.50,51 Verapamil has been shown in a small study to increase exercise capacity and heart failure score in patients with DHF.52
Digitalis. The use of digoxin in patients with DHF was evaluated in the Digitalis Investigation Group (DIG) ancillary trial, a parallel substudy of the overall DIG Trial that enrolled 988 patients with diastolic dysfunction.39 DHF patients receiving digoxin were found to have fewer symptoms and hospitalizations, although this finding was not statistically significant. These findings should be weighed against recent data suggesting that digoxin predisposes women with depressed left ventricular systolic dysfunction to an increased risk of death.53 The role of digoxin in DHF is unclear, and it is recommended that its use be restricted to patients with recurrent hospitalizations and refractory tachyarrhythmias despite optimized medical therapy.9,20,30,54
Prognosis
The annual mortality of patients with DHF has been reported as 5% to 8%, whereas mortality associated with SHF approximates 10% to 15%. However, in patients aged >70 years, both SHF and DHF have a 5-year mortality of 50% and both have an estimated 50% annual hospital admission rate.58
Looking forward
Greater recognition of the disorder and more enrollment of patients with DHF in outcome-based studies will hopefully improve our understanding and approach to treatment of this specific form of heart failure.40,55
Ongoing studies that may provide more evidence-based data to guide therapy for DHF include the Irbesartan in Heart Failure with Preserved Systolic Function Trial (I-PRESERVE), Perindopril for Elderly People with Chronic Heart Failure Study (PEP-CHF) and Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure (SENIORS).56-58
- Amlodipine • Norvasc
- Candesartan • Atacand
- Digoxin • Lanoxin
- Diltiazem • Cardizem, Cartia, Pilacor, Tiazac
- Enalapril • Vasotec
- Felodipine • Plendil
- Hydrazaline • Apresoline
- Propanolol • Betachron, Inderal
- Verapamil • Calan, Covem, Isoptin, Verelan
Acknowledgments
The authors wish to thank Thomas Hill and JoAnn Moates for their invaluable research assistance in preparation of this manuscript.
CORRESPONDING AUTHOR
Spencer A. Morris, PharmD, BCPS, Georgetown Hospital System, Georgetown Memorial Hospital, 606 Black River Road, Georgetown, SC 29440. E-mail: [email protected].
- Arrange for echocardiography or radionuclide angiography within 72 hours of a heart failure exacerbation. An ejection fraction >50% in the presence of signs and symptoms of heart failure makes the diagnosis of diastolic heart failure probable (B).
- To treat associated hypertension, use angiotensin receptor blockers (ARBs), angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, calcium channel blockers, or diuretics to achieve a blood pressure goal of <130/80 mm Hg (C).
- When using beta-blockers to control heart rate, titrate doses more aggressively than would be done for systolic failure, to reach a goal of 60 to 70 bpm (B).
- Use ACE inhibitors/ARBs to decrease hospitalizations, decrease symptoms, and prevent left ventricular remodeling (A).
Heart failure is a growing epidemic in the US, estimated to cause at least 20% of all hospitalizations in persons over 65 years of age. It is also the leading inpatient diagnosis among Medicare recipients with this age group.1,2,3 More than 5 million people in the US have heart failure, with approximately 550,000 new cases diagnosed annually.
Growing epidemiologic evidence suggests that studies of heart failure have underrepresented a large patient population with a natural history different from that of left ventricular (LV) systolic dysfunction.4-8One third to one half of patients with signs and symptoms of heart failure have preserved left ventricular function (LVF). They are said to have diastolic heart failure (DHF).
Identifying persons with this less-understood form of heart failure can be challenging. Skillful discernment is needed to avoid mistakenly attributing symptoms to other causes. DHF is particularly common among elderly women with hypertension; every patient with signs and symptoms of heart failure should undergo echocardiography to determine LV function.
Though the evidence base for DHF treatment is less well established than it is for systolic heart failure (SHF), data from recent trials have offered a promising direction.
New categorization of heart failure
The relative scopes of DHF and SHF will be better appreciated by understanding how recently developed guidelines have restructured the historical classification of heart failure.
Heart failure is defined by the American College of Cardiology (ACC) and the American Heart Association (AHA) as a complex syndrome resulting from any structural or functional cardiac disorder that impairs the ability of the ventricles to fill with or eject blood.9 The older terms, low-vs high-output failure, are now regarded as obsolete and have been abandoned in favor of distinguishing between abnormalities of systolic and diastolic function.10-12
ACC/AHA heart failure staging system
Severity of heart failure symptoms has traditionally been gauged by the New York Heart Association (NYHA) classification system. A criticism of the NYHA scale, however, is that patients may fluctuate in and out of the varying functional classes. To correct this shortcoming of the NYHA scale, the ACC and the AHA devised a new staging system to describe the progression of heart failure.9 The premise of this new system is to provide permanence to each sequential progression through the stages of heart failure while complementing the existing NYHA scale.9,13
New model. Patients with Stage A heart failure are at high risk of developing heart failure based on comorbidities and medical history.
Patients with Stage B heart failure have some component of structural heart disease but are asymptomatic.
Patients with Stage C heart failure have underlying structural abnormalities and have symptoms, or have had symptoms of heart failure in the past.
Patients with Stage D heart failure are refractory to conventional medical therapy and have end-stage symptoms.
TABLE 1 shows how the ACC/AHA Heart Failure Staging System correlates with the NYHA Classification scheme. Family practitioners can use the new heart failure staging system to identify and recognize risk factors for the development of heart failure and then seek to aggressively prevent or reverse them.
TABLE 1
Relationship of the ACC/AHA Heart Failure
| ACC/AHA STAGES OF HEART FAILURE | NYHA FUNCTIONAL CLASSIFICATION |
|---|---|
| A- high risk for development of HF; no underlying structural cardiac disease (ie, hypertension, diabetes, hyperlipidemia, etc) | No correlation |
| B- Structural heart disease but asymptomatic (ie, LVH) | I- patients with no limitation of activities; they suffer no symptoms from ordinary physical activity |
| C- Structural heart disease with past or current symptoms of heart failure | II- patients with slight, mild limitation of activity; they are comfortable with rest or with mild exertion |
| III- patients with marked limitation of activity; they are comfortable only at rest | |
| D- Refractory heart failure | IV- patients who should be at complete rest, confined to bed or chair; any physical activity brings on discomfort and symptoms occur at rest |
| Patients with Stage A heart failure are at high risk of developing clinical HF and are not representative of any patients categorized under the NHYA functional classification system, as they are not yet symptomatic. Patients with Stage B heart failure have some form of structural heart disease without associated symptoms and correlate best with NYHA Class I patients. Patients with Stage C heart failure have the same underlying structural cardiac disorders associated with Stage B, but they have past or current symptoms of HF. Depending on the severity of their condition, patients with Stage C heart failure may fall within any of the NYHA functional classes. Patients with Stage D heart failure have symptoms refractory to optimized medical and interventional therapies and are representative of NYHA Class IV patients. | |
Who is at risk for DHF?
Risk factors for the development of DHF include advanced age, female sex, hypertension, and coronary ischemia. Approximately 50% of those older than 70 years who have heart failure have preserved LV function.14-16 In a large epidemiologic study of elderly patients with heart failure, women were twice as likely as men to have preserved LV function.17 In examining post-myocardial infarction (MI) patients with heart failure, women and those with smaller infarctions were also more likely to have preserved LV function (odds ratio=1.97; 95% confidence interval [CI], 1.27–3.07).18
Hypertension is a well known cause of left ventricular hypertrophy (LVH), which is a causal mechanism for DHF.19,20 Levy et al, in a study of 5143 subjects from the original Framingham Heart Study participants and Framingham Offspring participants, found that hypertension predated the development of heart failure in 91% of cases among patients in this cohort.21 In this sample, hypertension also carried the greatest population-attributable risk for the development of heart failure of all risk factors considered (39% in men and 59% in women). Hypertension also had the highest prevalence of all risk factors in this study (60% in men and 62% in women). Untreated hypertension leads to an increasing incidence of LVH and associated diastolic dysfunction. Increased LV mass and stiffness cause noncompliance and abnormal relaxation of the ventricular wall leading to increased diastolic pressures.4,19-21
Coronary ischemia can also cause diastolic dysfunction.20 Data from the Framingham Heart Study indicate that the prevalence of MI was 10% in hypertensive men and 3% in hypertensive women.21 MI is a well known precursor of LV systolic dysfunction; however, the relationship to diastolic dysfunction is less clear. Although the prevalence of MI was associated with a 5- to 6-fold risk for heart failure in Framingham subjects, after adjustment for age and other risk factors, fewer than half of the patients who subsequently developed heart failure had a history of MI. This finding supports the role of untreated hypertension in the pathogenesis of DHF.21
Physical examination does not help distinguish between DHF and SHF. Signs and symptoms of both disorders are relatively the same.22 Therefore, the presence of one or more of these risk factors in the setting of heart failure and preserved LV function supports the diagnosis of DHF.14-17TABLE 2 summarizes known clinical characteristics and features of SHF and DHF. All patients with systolic heart failure have some component of diastolic dysfunction as well.10,12,23,24
TABLE 2
Characteristics of patients with systolic vs diastolic heart failure
| Differentiating systolic and diastolic dysfunction | 
| 
|
| Etiology | Commonly associated with previous MI; exists concurrently with diastolic dysfunction | Pathogenesis is multifocal; associated more often with systemic hypertension, may exist alone without a component of systolic heart failure |
| Gender-specific differences | Both sexes affected | More common in women |
| Age-related differences | All ages affected | More common in elderly patients |
| Echocardiographic findings | Depressed LVEF <40% | Preserved LVEF >40% |
| Symptomatology | Identical—unable to differentiate with clinical examination | Identical—unable to differentiate with clinical examination |
| Long-term prognosis | 15% annual mortality rate | 5 to 8% annual mortality rate |
| MI, myocardial infarction; LVEF, left ventricular ejection fraction. | ||
Diagnosis is made clinically
No consensus exists on standardized criteria for diagnosing diastolic heart failure. However, 3 diagnostic levels—possible, probable, and definite DHF—have been proposed by Vasan and Levy.11
Possible DHF is defined as signs and symptoms of heart failure (TABLE 3) in patients with normal LV function, but lacking an assessment of ventricular function in proximity to the heart failure event.
Probable DHF is defined as (1) signs and symptoms of heart failure and (2) an ejection fraction >50% measured via echocardiography or radionuclide angio-graphy within 72 hours of the heart failure exacerbation.
Definite DHF is defined as (1) signs and symptoms of heart failure, (2) an ejection fraction >50% measured via the above methods within 72 hours of the patient’s presentation, and (3) increased left-ventricular end diastolic pressure (LVEDP) measured during cardiac catheterization.
TABLE 3
Modified Framingham criteria for diagnosing heart failure
| Need 2 major or 1 major and 2 minor fulfilled criteria for diagnosis of heart failure. |
|---|
| MAJOR CRITERIA |
| Paroxysmal nocturnal dyspnea |
| Orthopnea |
| Elevated jugular venous pressure |
| Pulmonary rales |
| Cardiomegaly on radiography |
| Acute pulmonary edema |
| S3 gallop |
| Weight loss >4.5 kg in response to treatment of heart failure |
| MINOR CRITERIA |
| Bilateral ankle/leg edema |
| Nocturnal cough |
| Dyspnea on ordinary exertion |
| Hepatomegaly |
| Pleural effusion |
| Tachycardia >120 bpm |
| MAJOR OR MINOR |
| Weight loss >4.5 kg in 5 days in response to treatment of heart failure |
| From: McKee et al, N Engl J Med 1971; 285:1441-1446.26 |
Direct assessment of diastolic function unnecessary
Evidence of diastolic dysfunction as determined by echocardiography or cardiac catheterization has been debated as a necessary third diagnostic criterion.24 The problem, though, is that there is no simple means of reliably diagnosing diastolic dysfunction with echocardiography (E:A ratios, deceleration or relaxation times), and that performing cardiac catheterization to measure LVEDP is impractical.22
Furthermore, Zile et al have shown that, though cardiac catheterization helps to confirm diastolic dysfunction, it is not necessary to establish the diagnosis. In this study, 63 patients with clinically defined diastolic heart failure based on the Framingham criteria underwent diagnostic cardiac catheterization; 58 (92%) of these patients were also found to have an abnormal LVEDP, indicative of diastolic dysfunction.25 Therefore, the diagnosis of DHF can be made in the setting of heart failure in a patient with a normal ejection fraction.
Order echocardiography within 72 hours of symptom onset
A major challenge for clinicians is to determine whether a patient’s dyspnea is a true symptom of heart failure. Signs and symptoms of heart failure must be defined using clinical indicators such as the Framingham heart failure criteria (FIGURE).26 Diagnosis of heart failure is more easily made for a patient presenting to the emergency department with acute pulmonary edema than it is for an outpatient seen repeatedly for shortness of breath over months.
For a patient presenting with acute pulmonary edema, an echocardiogram should be performed within 72 hours of symptoms to document cardiac function in proximity to the heart failure exacerbation. The ejection fraction of patients with DHF can remain within normal range, even during acute decompensation.27,28 Stroke volume and cardiac output may be decreased despite a normal ejection fraction.
Cardiogenic pulmonary edema in DHF patients results from the stiffened ventricle’s inability to compensate for increased venous return due to an expansion in central blood volume or sodium retention. Subsequently, diastolic pressures elevate and impede lung compliance, which increases the work of breathing and dyspnea.20,29 A normal ejection fraction and symptom diminishment following diuresis in the setting of acute decompensation help confirm the diagnosis of DHF, especially when other disease states are complicating the clinical picture.30
Elevated BNP levels may be helpful
An elevated level of b-type natriuretic peptide (BNP) can help confirm the clinical diagnosis of heart failure, and it has been shown in small studies to be a valid marker of DHF.31,32 In a study of 294 patients referred for echocardiography to evaluate LV function, Lubien et al found that a BNP value of at least 62 pg/mL had a sensitivity of 85%, a specificity of 83%, and an accuracy of 84% for heart failure in patients with a normal ejection fraction.32 All patients with systolic dysfunction defined by an ejection fraction <50% were excluded from this study. These results, though promising, must be confirmed by further studies evaluating the diagnostic utility of BNP to detect active heart failure symptoms in patients with diastolic dysfunction.
Treatment of symptomatic diastolic dysfunction
For SHF patients, multiple large outcome trials have clearly documented the benefit of angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, and aldosterone antagonists in reducing mortality.33-36 The relative paucity of outcome data for DHF has resulted in medical therapy primarily centered on modifying physiologic factors to improve LV filling and relaxation. Specifically, treatment should focus on symptom reduction, balancing fluid status, controlling heart rate, decreasing any ischemia, and achieving blood pressure goals.19,20,22,31 Though many of the medications used to treat SHF are also used for DHF, there are several important differences in appropriate initiation and subsequent titration of these drugs in the 2 settings.20,31
While treatment of DHF is largely theoretical, a limited number of well-designed, randomized studies are available to help determine appropriate therapy.37-39TABLE 4 provides a summary of the evidence base for evaluation and treatment of systolic vs diastolic heart failure.40TABLE 5 gives a synopsis of these studies. A suggested diagnostic and treatment approach for patients with DHF is outlined in the FIGURE. After determining whether a patient has DHF— primarily through the ruling out of other conditions and confirmation with echocardiographic studies—consider the applicability of each treatment based on a patient’s medical history and present condition.
TABLE 4
Comparative evidence base for evaluation and treatment of systolic vs diastolic heart failure
| LEVEL OF EVIDENCE* | ||
|---|---|---|
| FEATURE | SYSTOLIC HEART FAILURE | DIASTOLIC HEART FAILURE |
| Prevalence and risk factors | III | III |
| Non-invasive diagnostic methodologies | I - assessment of LVEF | IV, VII† |
| I - measurement of BNP levels | ||
| Prognosis | I - II | II, III |
| Treatment with ACE inhibitor, ARB, beta-blockers, and digitalis | I‡ | II, V-VII |
| Prevention trials (treatment of asymptomatic precursor condition) | I | None |
*
| ||
| † Diagnosis is primarily by exclusion of systolic heart failure; measurement of LVEF and BNP is also useful. | ||
| ‡ Cochrane review and meta-analysis. | ||
| ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; LVEF, left ventricular ejection fraction; BNP, b-type natriuretic peptide. Adapted and reproduced with permission from the BMJ Publishing Group and Dr. Ramachandran S. Vasan. BMJ 2003; 327:1181-1182.40 | ||
TABLE 5
Diastolic heart failure outcome trials
| TRIAL | BACKGROUND AND CONTEXT | REPRESENTATIVE PATIENT POPULATION | AVG LVEF OF PARTICIPANTS | NNT | SOR* (LOE) |
|---|---|---|---|---|---|
| CHARM-Preserved | Candesartan added to standard heart failure therapy in patients with LVEF >40% | N=3023 | 54% | 36† | A (1b) |
| 60% NYHA Class II | 42‡ | ||||
| 38% NYHA Class III | |||||
| 2% NYHA Class IV | |||||
| DIG Ancillary Trial | Digoxin + ACE inhibitors and diuretics in patients with LVEF >45% | N=988 | Not reported | N/A§ | B (1b) |
| NYHA classification not specified | |||||
| Propranolol Study, Aronow et al | Propranolol added to ACE inhibitors and diuretics in post-MI patients with LVEF 40% | N=158 | 56% | 5¶ | A (1b) |
| 52% NYHA Class II | |||||
| 48% NYHA Class III | |||||
| *Based on the guidelines for evidence quality outlined by the Center for Evidence-Based Medicine, available at: www.cebm.net/levels_ of_evidence.asp. A(1b) = consistent level 1 studies; individual randomized controlled trial (with narrow confidence interval). B(1b) = consistent level 2 or 3 studies or extrapolations from level 1 studies; individual randomized controlled trial (with narrow confidence interval) | |||||
| † For the composite of cardiovascular death, hospital admission for heart failure, MI, or cerebrovascular accident over 3 years | |||||
| ‡ For recurrent admissions for heart failure exacerbations over 3 years | |||||
| § No statistical differences between groups in rates of hospitalization or mortality over 3 years | |||||
| ¶ All-cause mortality over a mean of 32 months | |||||
| NNT, number needed to treat to prevent one death or other specified endpoint; LVEF, left ventricular ejection fraction; ACE, angiotensin-converting enzyme; NYHA, New York Heart Association classification; CHARM, Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity; DIG, Digitalis Investigation Group. | |||||
Medications to control blood pressure
Hypertension is a major risk factor for DHF, and the ACC/AHA heart failure guidelines recommend a lower blood pressure goal for patients with diastolic heart failure than for those with uncomplicated hypertension (ie, <130/80 mm Hg).9 Angiotensin receptor blockers (ARBs), ACE inhibitors, beta-blockers, calcium channel blockers, and diuretics may all be employed to achieve this blood pressure goal.
Angiotensin II receptor blockers. The use of ARBs in the treatment of DHF was recently evaluated in the CHARM-Preserved Study. Candesartan, 32 mg once daily, when added to a background therapy of mostly diuretics and beta-blockers (initially excluding the use of ACE inhibitors but later permitted in appropriate patients following the release of the HOPE trial results), was found to have a modest impact in preventing recurrent admissions for heart failure exacerbations (number needed to treat [NNT]=42 over 3 years).37 Candesartan also demonstrated a more favorable impact on the composite end-point of cardiovascular death, hospitalization for heart failure, MI, and stroke (NNT=36).
ACE inhibitors. For post-MI patients with DHF, ACE inhibitors have improved treadmill duration and NYHA functional class.41 Further studies are needed to determine whether an ACE inhibitor or an ARB is preferred or whether they may be used safely together in the management of DHF.
Beta-blockers. Propranolol, when added to an ACE inhibitor and diuretic, has been shown to significantly reduce mortality in a small prospective study of 158 post-MI patients with an average LVEF of 56% and NYHA Class II or III symptoms.38 Seventy percent of the study patients were women (n=111) and the mean age was 81 years. The dose of pro-pranolol in this study was increased in 10-mg increments at 10-day intervals up to a total daily dose of 30 mg 3 times daily.
All 79 patients randomized to receive propranolol successfully reached the target dose; however, 14% (n=11) discontinued therapy due to worsening heart failure or hypotension. The absolute reduction in total mortality among patients receiving propranolol was 20%, compared with the study group receiving only standard heart failure therapy (NNT=5 for a median of 32 months of follow-up, P=.007). The positive effect of beta-blocker therapy in this small study merits another larger, complementary trial to confirm its benefits in a bigger patient population with the same characteristics.
Control of volume status
Diuretics. It has long been recognized that diuretics are a useful and necessary adjunct in the management of volume overload in patients with heart failure42; however, no large, long-term studies are available to evaluate the effects of these medications on mortality.43 Without concurrent ACE inhibitor/ARB and beta-blocker therapy, diuretics have been shown to cause rebound sympathetic activation.44,45
For patients with either systolic or diastolic dysfunction, diuretics may be dosed aggressively to achieve euvolemia. But for patients with DHF who are partly dependent on volume coupled with increased heart rate to maintain cardiac output, excessive diuresis can cause a significant reduction in preload, which can worsen symptoms.20,22,30 It is advocated that long-term diuretics should be used judiciously in the treatment of both SHF and DHF, with individualized, tailored therapy being preferred and daily weights used as a guide to determine optimum fluid status.9
Medications to control heart rate
Beta-blockers. In addition to their anti-hypertensive effects, beta-blockers may also be used as rate-lowering therapy in the treatment of DHF. Dosing and titration in this setting are handled differently than for SHF. Whereas titration of beta-blockers in SHF requires careful adjustment to avoid worsening of the patients’ symptoms and subsequent exacerbation,46-48 dosing in DHF can be more aggressive, with a resting heart rate goal of 60 to 70 bpm.20,49 Beta-blockers are used as negative chronotropes in this instance to improve left ventricular filling. Beta-blockers are also useful in the management of ischemia and angina associated with diastolic heart failure.19,20
Calcium channel blockers. For patients with contraindications to beta-blocker therapy, non-dihydropyridine calcium channel blockers (verapamil, diltiazem) may be employed as rate-lowering therapy for DHF.19 Unlike the other drugs used in DHF, non-dihydropyridine calcium channel blockers have no role in the treatment of SHF except in the presence of tachyarrhythmias.20
Dihyropyridine calcium channel blockers (ie, amlodipine, felodipine) should be reserved for heart failure patients in general with angina refractory to beta-blockers. Amlodipine and felodipine are probably the safest of the dihydropyridine calcium channel blockers to use for the treatment of angina as they have not been shown to worsen existing SHF.50,51 Verapamil has been shown in a small study to increase exercise capacity and heart failure score in patients with DHF.52
Digitalis. The use of digoxin in patients with DHF was evaluated in the Digitalis Investigation Group (DIG) ancillary trial, a parallel substudy of the overall DIG Trial that enrolled 988 patients with diastolic dysfunction.39 DHF patients receiving digoxin were found to have fewer symptoms and hospitalizations, although this finding was not statistically significant. These findings should be weighed against recent data suggesting that digoxin predisposes women with depressed left ventricular systolic dysfunction to an increased risk of death.53 The role of digoxin in DHF is unclear, and it is recommended that its use be restricted to patients with recurrent hospitalizations and refractory tachyarrhythmias despite optimized medical therapy.9,20,30,54
Prognosis
The annual mortality of patients with DHF has been reported as 5% to 8%, whereas mortality associated with SHF approximates 10% to 15%. However, in patients aged >70 years, both SHF and DHF have a 5-year mortality of 50% and both have an estimated 50% annual hospital admission rate.58
Looking forward
Greater recognition of the disorder and more enrollment of patients with DHF in outcome-based studies will hopefully improve our understanding and approach to treatment of this specific form of heart failure.40,55
Ongoing studies that may provide more evidence-based data to guide therapy for DHF include the Irbesartan in Heart Failure with Preserved Systolic Function Trial (I-PRESERVE), Perindopril for Elderly People with Chronic Heart Failure Study (PEP-CHF) and Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure (SENIORS).56-58
- Amlodipine • Norvasc
- Candesartan • Atacand
- Digoxin • Lanoxin
- Diltiazem • Cardizem, Cartia, Pilacor, Tiazac
- Enalapril • Vasotec
- Felodipine • Plendil
- Hydrazaline • Apresoline
- Propanolol • Betachron, Inderal
- Verapamil • Calan, Covem, Isoptin, Verelan
Acknowledgments
The authors wish to thank Thomas Hill and JoAnn Moates for their invaluable research assistance in preparation of this manuscript.
CORRESPONDING AUTHOR
Spencer A. Morris, PharmD, BCPS, Georgetown Hospital System, Georgetown Memorial Hospital, 606 Black River Road, Georgetown, SC 29440. E-mail: [email protected].
1. American Heart Association. Heart Disease and Stroke Statistics—2004 Update. Dallas, Texas: American Heart Association; 2003.
2. Popvic JR, Hall MJ. 1999 National Hospital Discharge Survey. Advance data from vital and health statistics; no. 319. Hyattsville, Maryland: Nation Center for Health Statistics, 2001.
3. Krumholz HM, Parent EM, Tu N, et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med 1997;157:99-104.
4. Krum H, Gilbert RE. Demographics and concomitant disorders in heart failure. Lancet 2003;362:147-158.
5. Redfield MM, Jacobsen SJ, Burnett JC, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289:194-202.
6. Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation 1998;98:2282.-
7. Vasan RS, Larson MG, Benjamin EJ, et al. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol 1999;33:1948.-
8. Kitzman DW, Gardin JM, Arnold A, et al. Heart failure with preserved LV function in the elderly: clinical and echocardiographic correlates from the Cardiovascular Health Study. Circulation 1999;94:1433.-
9. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: full text: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). Available at: http://www.acc.org/clinical/guidelines/failure/pdfs/hf_fulltext.pdf.
10. Gaasch WH. Diagnosis and treatment of heart failure based on left ventricular systolic or diastolic dysfunction. JAMA 1994;271:1276-1280.
11. Vasan RS, Levy D. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation 2000;101:2118-2121.
12. Grossman W. Defining diastolic dysfunction. Circulation 2000;101:2020-2021.
13. Brozena SC, Jessup M. The new staging system for heart failure: what every primary care physician should know. Geriatrics 2003;58:31-36.
14. McDermott MM, Feinglas J, Sy J, et al. Hospitalized congestive heart failure patients with preserved versus abnormal left ventricular systolic function; clinical characteristics and drug therapy. Am J Med 1995;99:629-635.
15. Vasan RS, Benjamin EJ, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure; an epidemiologic perspective. J Am Coll Cardiol 1995;26:1565.-
16. Kitzman DW, Gardin JM, Gottdiener JS, et al. for the CHS Research Group. Importance of heart failure with preserved systolic function in patients >65 years of age. Am J Cardiol 2001;87:413-419.
17. Masoudi FA, Havranek EP, Smith G, et al. Gender, age and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol 2003;41:217-223.
18. Hellermann JP, Jacobsen SJ, Reeder GS, et al. Heart failure after myocardial infarction: Prevalence of preserved left ventricular systolic function in the community. Am Heart J 2003;145:742-748.
19. Bonow RO, Udelson JE. Left ventricular diastolic dysfunction as a cause of congestive heart failure: mechanisms and management. Ann Intern Med 1992;117:502-510.
20. Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatment. Circulation 2002;105:1503-1508.
21. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA 1996;275:1557-1562.
22. Vasan RS, Benjamin EJ, Levy D. Congestive heart failure with normal left ventricular systolic function: clinical approaches to the diagnosis and treatment of diastolic heart failure. Arch Intern Med 1996;156:146-157.
23. Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 2002;105:1387-1393.
24. How to diagnose diastolic heart failure. European Study Group on Diastolic Heart Failure. Eur Heart J 1998;19:990.-
25. Zile MR, Gaasch WH, Carroll JD, et al. Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation 2001;104:779-782.
26. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971;285:1441-1446.
27. Gandi SK, Powers JC, Nomeir A, et al. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med 2001;344:17-60.
28. Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated dias-tolic heart failure in comparison to systolic heart failure. JAMA 2002;288:2144-2150.
29. Zile MR, Baicu CF, Gaasch WH. Diastolic heart fail-ure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 2004;350:1953-1959.
30. Elesber AA, Redfield MM. Approach to patients with heart failure and normal ejection fraction. Mayo Clin Proc 2001;76:1047-1052.
31. Angeja BG, Grossman W. Evaluation and management of diastolic heart failure. Circulation 2003;107:659.-
32. Lubien E, DeMaria A, Krishnaswamy P, et al. Utility of b-natriuretic peptide in detecting diastolic dysfunction: comparison with Doppler velocity recordings. Circulation 2002;105:595-601.
33. Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA 1995;273:1450-1456.
34. Foody JM, Farrell MH, Krumholz HM. β-blocker therapy in heart failure: scientific review. JAMA 2002;287:883-889.
35. Pitt B, Zannad F, Remme WJ. The effect of spirono-lactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709-717.
36. Pitt B, Remme W, Zannad F. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309-1321.
37. Yusuf S, Pfeffer MA, Swedberg, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003;362:777-781.
38. Aronow WS, Ahn C, Kronzon I. Effects of propranolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction >40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol 1997;80:207-209.
39. The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525.-
40. Vasan RS. Diastolic heart failure: the condition exists and needs to be recognized, prevented, and treated. BMJ 2003;327:1181-1182.
41. Aronow WS, Kronzon I. Effect of enalapril on congestive heart failure treated with diuretics in elderly patients with prior myocardial infarction and normal left ventricular ejection fraction. Am J Cardiol 1993;71:602-604.
42. Wilson JR, Reichek N, Dunkman WB, Golberg S. Effect of diuresis on the performance of the failing left ventricle in man. Am J Med 1981;70:234-239.
43. Faris R, Flather M, Purcell H, et al. Current evidence supporting the role of diuretics in heart failure: a meta analysis of randomized controlled trials. Int J Cardiol 2002;82:149-158.
44. Cowie MR, Zaphiriou A. Management of chronic heart failure. Br Med J 2002;325:422-425.
45. Cody RJ, Kubo SH, Pickworth KK. Diuretic treatment for the sodium retention of congestive heart failure. Arch Intern Med 1994;154:1905-1914.
46. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial Lancet 1999;353:9-13.
47. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet 1999;353:2001-2007.
48. Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-1658.
49. Levine HJ. Optimum heart rate of large failing hearts. Am J Cardiol 1988;61:633-636.
50. O’Connor CM, Carson PE, Miller AB, et al. Effect of amlodipine on mode of death among patients with advanced heart failure in the PRAISE trial. Prospective Randomized Amlodipine Survival Evaluation. Am J Cardiol 1998;82:881-887.
51. Amabile CM, Spencer AP. Keeping your patient with heart failure safe: a review of potentially dangerous medications. Arch Intern Med 2004;164:709-720.
52. Setaro J, Zaret BL, Schueman Ds, et al. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic performance. Am J Cardiol 1990;66:981-986.
53. Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med 2002;347:1403-1411.
54. Yamani MH. When should digoxin be used in patients with diastolic dysfunction? Cleve Clin J Med 2001;68:481,-485.
55. Vasan RS, Benjamin EJ. Diastolic heart failure—no time to relax. N Engl J Med 2001;344:56-58.
56. Carson P, Massie B. I-PRESERVE (Irbesartan in Heart Failure with Preserved Systolic Function) Study initiation presented at the European Society of Cardiology Annual Meeting, September 3, 2002, Berlin.
57. Cleland JG, Tendera M, Adamus J, et al. Perindopril for elderly people with chronic heart failure: the PEP-CHF study.
1. American Heart Association. Heart Disease and Stroke Statistics—2004 Update. Dallas, Texas: American Heart Association; 2003.
2. Popvic JR, Hall MJ. 1999 National Hospital Discharge Survey. Advance data from vital and health statistics; no. 319. Hyattsville, Maryland: Nation Center for Health Statistics, 2001.
3. Krumholz HM, Parent EM, Tu N, et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med 1997;157:99-104.
4. Krum H, Gilbert RE. Demographics and concomitant disorders in heart failure. Lancet 2003;362:147-158.
5. Redfield MM, Jacobsen SJ, Burnett JC, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289:194-202.
6. Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation 1998;98:2282.-
7. Vasan RS, Larson MG, Benjamin EJ, et al. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol 1999;33:1948.-
8. Kitzman DW, Gardin JM, Arnold A, et al. Heart failure with preserved LV function in the elderly: clinical and echocardiographic correlates from the Cardiovascular Health Study. Circulation 1999;94:1433.-
9. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: full text: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). Available at: http://www.acc.org/clinical/guidelines/failure/pdfs/hf_fulltext.pdf.
10. Gaasch WH. Diagnosis and treatment of heart failure based on left ventricular systolic or diastolic dysfunction. JAMA 1994;271:1276-1280.
11. Vasan RS, Levy D. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation 2000;101:2118-2121.
12. Grossman W. Defining diastolic dysfunction. Circulation 2000;101:2020-2021.
13. Brozena SC, Jessup M. The new staging system for heart failure: what every primary care physician should know. Geriatrics 2003;58:31-36.
14. McDermott MM, Feinglas J, Sy J, et al. Hospitalized congestive heart failure patients with preserved versus abnormal left ventricular systolic function; clinical characteristics and drug therapy. Am J Med 1995;99:629-635.
15. Vasan RS, Benjamin EJ, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure; an epidemiologic perspective. J Am Coll Cardiol 1995;26:1565.-
16. Kitzman DW, Gardin JM, Gottdiener JS, et al. for the CHS Research Group. Importance of heart failure with preserved systolic function in patients >65 years of age. Am J Cardiol 2001;87:413-419.
17. Masoudi FA, Havranek EP, Smith G, et al. Gender, age and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol 2003;41:217-223.
18. Hellermann JP, Jacobsen SJ, Reeder GS, et al. Heart failure after myocardial infarction: Prevalence of preserved left ventricular systolic function in the community. Am Heart J 2003;145:742-748.
19. Bonow RO, Udelson JE. Left ventricular diastolic dysfunction as a cause of congestive heart failure: mechanisms and management. Ann Intern Med 1992;117:502-510.
20. Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatment. Circulation 2002;105:1503-1508.
21. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA 1996;275:1557-1562.
22. Vasan RS, Benjamin EJ, Levy D. Congestive heart failure with normal left ventricular systolic function: clinical approaches to the diagnosis and treatment of diastolic heart failure. Arch Intern Med 1996;156:146-157.
23. Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 2002;105:1387-1393.
24. How to diagnose diastolic heart failure. European Study Group on Diastolic Heart Failure. Eur Heart J 1998;19:990.-
25. Zile MR, Gaasch WH, Carroll JD, et al. Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation 2001;104:779-782.
26. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971;285:1441-1446.
27. Gandi SK, Powers JC, Nomeir A, et al. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med 2001;344:17-60.
28. Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated dias-tolic heart failure in comparison to systolic heart failure. JAMA 2002;288:2144-2150.
29. Zile MR, Baicu CF, Gaasch WH. Diastolic heart fail-ure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 2004;350:1953-1959.
30. Elesber AA, Redfield MM. Approach to patients with heart failure and normal ejection fraction. Mayo Clin Proc 2001;76:1047-1052.
31. Angeja BG, Grossman W. Evaluation and management of diastolic heart failure. Circulation 2003;107:659.-
32. Lubien E, DeMaria A, Krishnaswamy P, et al. Utility of b-natriuretic peptide in detecting diastolic dysfunction: comparison with Doppler velocity recordings. Circulation 2002;105:595-601.
33. Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA 1995;273:1450-1456.
34. Foody JM, Farrell MH, Krumholz HM. β-blocker therapy in heart failure: scientific review. JAMA 2002;287:883-889.
35. Pitt B, Zannad F, Remme WJ. The effect of spirono-lactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709-717.
36. Pitt B, Remme W, Zannad F. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309-1321.
37. Yusuf S, Pfeffer MA, Swedberg, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003;362:777-781.
38. Aronow WS, Ahn C, Kronzon I. Effects of propranolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction >40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol 1997;80:207-209.
39. The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525.-
40. Vasan RS. Diastolic heart failure: the condition exists and needs to be recognized, prevented, and treated. BMJ 2003;327:1181-1182.
41. Aronow WS, Kronzon I. Effect of enalapril on congestive heart failure treated with diuretics in elderly patients with prior myocardial infarction and normal left ventricular ejection fraction. Am J Cardiol 1993;71:602-604.
42. Wilson JR, Reichek N, Dunkman WB, Golberg S. Effect of diuresis on the performance of the failing left ventricle in man. Am J Med 1981;70:234-239.
43. Faris R, Flather M, Purcell H, et al. Current evidence supporting the role of diuretics in heart failure: a meta analysis of randomized controlled trials. Int J Cardiol 2002;82:149-158.
44. Cowie MR, Zaphiriou A. Management of chronic heart failure. Br Med J 2002;325:422-425.
45. Cody RJ, Kubo SH, Pickworth KK. Diuretic treatment for the sodium retention of congestive heart failure. Arch Intern Med 1994;154:1905-1914.
46. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial Lancet 1999;353:9-13.
47. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet 1999;353:2001-2007.
48. Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-1658.
49. Levine HJ. Optimum heart rate of large failing hearts. Am J Cardiol 1988;61:633-636.
50. O’Connor CM, Carson PE, Miller AB, et al. Effect of amlodipine on mode of death among patients with advanced heart failure in the PRAISE trial. Prospective Randomized Amlodipine Survival Evaluation. Am J Cardiol 1998;82:881-887.
51. Amabile CM, Spencer AP. Keeping your patient with heart failure safe: a review of potentially dangerous medications. Arch Intern Med 2004;164:709-720.
52. Setaro J, Zaret BL, Schueman Ds, et al. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic performance. Am J Cardiol 1990;66:981-986.
53. Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med 2002;347:1403-1411.
54. Yamani MH. When should digoxin be used in patients with diastolic dysfunction? Cleve Clin J Med 2001;68:481,-485.
55. Vasan RS, Benjamin EJ. Diastolic heart failure—no time to relax. N Engl J Med 2001;344:56-58.
56. Carson P, Massie B. I-PRESERVE (Irbesartan in Heart Failure with Preserved Systolic Function) Study initiation presented at the European Society of Cardiology Annual Meeting, September 3, 2002, Berlin.
57. Cleland JG, Tendera M, Adamus J, et al. Perindopril for elderly people with chronic heart failure: the PEP-CHF study.