User login
- The lidocaine patch 5% provided pain relief for mild-to-moderate carpal tunnel syndrome.
- The lidocaine patch 5% may offer patients a noninvasive treatment option with minimal risk for drug-drug interactions or systemic side effects.
- Objectives: A standard treatment option for mild-to-moderate carpal tunnel syndrome (CTS) is a local injection of anesthetic-corticosteroid, but this can be painful and may cause complications. This pilot clinical trial was designed to compare the safety and efficacy of daily applications of the lidocaine patch 5% (Lidoderm) to that of a single injection of 0.5 cc lidocaine 1% plus methylprednisolone acetate (Depo-Medrol) 40 mg.
- Methods: In this randomized, parallel-group, open-label, single-center, active-controlled, prospective pilot study, participants aged 18–75 years with clinical/electrodiagnostic evidence of CTS were randomized to receive the lidocaine patch 5% or 1 injection of 0.5 cc lidocaine 1% plus methylprednisolone acetate 40 mg. Outcome assessments included the Brief Pain Inventory (measuring pain intensity, relief, and interference with quality of life, Patient and Global Clinical Impression of Improvement, Global Assessment of Treatment Satisfaction, and safety.
- Results: Baseline characteristics of the 40 patients randomized to treatment with the lidocaine patch 5% (n=20) or injection (n=20) were similar between groups. After 4 weeks of treatment, patients in both groups reported significant changes (P<.05) in worst pain, average pain, and pain “right now.” Composite interference scores, which are measures of how much patients’ pain interfered with quality of life, also significantly improved in both treatment groups (patch, –13.9; injection, –16.7; P<.001 vs baseline for both groups). Eighty percent of patients in the lidocaine patch group and 59% of patients who received the injection reported being “satisfied” or “very satisfied,” while investigators reported improvement in 88% of patients using the lidocaine patch and in 74% of those who received the injection. Both treatments were well tolerated, with treatment-related adverse events (AEs) reported in 3 patients in each group (15%). No systemic treatment-related AEs were observed with the lidocaine patch 5%.
- Conclusions: This pilot trial demonstrated that the lidocaine patch 5% was efficacious in reducing pain associated with CTS and was well tolerated. The lidocaine patch 5% may offer patients with CTS effective, noninvasive treatment for the management of their symptoms. Further controlled trials are warranted.
Treatment options for carpal tunnel syndrome (CTS) include wrist splinting, oral corticosteroids, and local injections with anesthetics and corticosteroids for mild-to-moderate cases, and surgical release for severe cases.1
Injections work, but have drawbacks. Injecting a corticosteroid and local anesthetic into, or proximal to, the carpal tunnel gives significantly greater relief than oral steroids.2 In fact, a recent randomized, open-label trial demonstrated that local steroid injections may relieve CTS pain as well as, or better than, invasive surgery.3 However, corticosteroid injections are time consuming and costly. Moreover, inadvertent injections into the nerve can lead to chronic pain and long-term discomfort.4-6 Repeated injections carry the risk for needle injury to the median nerve, intratendinous injection and tendon rupture, adhesions, dysesthesias, and infection.1 Many clinicians limit the number of injections into the carpal tunnel to about 3 or 4 per year to minimize local complications and the possibility of systemic toxic side effects (eg, hyperglycemia or hypertension).7
The thinking behind a new approach. Because nonsurgical treatment options for CTS are suboptimal, new therapies are needed.8 The pain of peripheral nerve injury—eg, CTS-associated median nerve compression—may result from changes in voltage-gated sodium channels in the injured afferents and their uninjured neighbors.9 These changes may have a profound impact on neuronal excitability, causing abnormal sodium channel expression, spontaneous and ectopic sodium channel discharge, and neuropathic pain.10 Pharmacologically blocking these channels and the processes underlying their changes may be the most efficient way of selectively eliminating the associated pain.10 Because lidocaine is believed to stabilize the sodium channels in damaged afferent neurons,11 the lidocaine patch 5% may be an appropriate treatment option for patients with CTS.
Related evidence. The lidocaine patch 5% is indicated for treating pain associated with postherpetic neuralgia and can be used with minimal risk of drug-drug interactions.12 Recent literature suggests that the lidocaine patch 5% may relieve pain associated with multiple types of peripheral neuropathies13,14; therefore, patients with CTS may also benefit from this modality.
The formulation relieves localized pain and may be particularly appropriate for patients who are awaiting surgery or wish to limit their exposure to corticosteroids, such as those with diabetes, heart disease, or hypertension. Though there are anecdotal reports of success with topical lidocaine patches for CTS, its efficacy and safety have not been evaluated in randomized trials or documented in published literature.
Focus of our pilot study. To investigate the role of topical lidocaine in relieving pain or functional impairment caused by persistent or recurrent CTS, we conducted a randomized pilot trial comparing the safety and efficacy of daily applications of the lidocaine patch 5% (Lidoderm) with the efficacy and safety of a single injection of 0.5 cc lidocaine 1% and methylprednisolone acetate (Depo-Medrol) 40 mg in patients with mild-to-moderate CTS.
Methods
Participants and design
This trial was a 4-week, randomized, parallel-group, open-label, single-center, active-controlled, prospective pilot study conducted in the United States. The Ethical Review Committee, Inc, located in Kansas City, Kansas, reviewed and approved the study. Patients 18 to 75 years with clinical and electrodiagnostic evidence of CTS were randomly assigned to receive the lidocaine patch 5% or a single injection of 0.5 cc lidocaine 1% and methylprednisolone acetate 40 mg.
Inclusion criteria. Electrodiagnostic evidence of CTS included a median motor nerve distal latency more than 4.10 m sec or a difference of more than 1 m sec between the median and ulnar sensory latencies when recorded with the fourth finger.15 Patients also were required to have persistent or recurrent CTS as defined by the presence of pain, paresthesias, or positive Phalen’s or Tinel’s signs. We enrolled patients who met the eligibility criteria, gave consent, and attended 1 of 2 treatment centers (a family practice clinic or a physical medicine clinic) between November 2003 and May 2004. Patients were not recruited from the general population and were not given incentives to participate other than free treatment. Patients were enrolled after providing written informed consent.
Exclusion criteria. Patients were excluded from the study if they had peripheral neuropathy of any origin other than CTS, carpal tunnel injection in the study limb within the previous 8 weeks, carpal tunnel surgical release of the study limb within the previous 6 months, concomitant cervical radiculopathy, anatomic abnormalities of the wrist or hand, median nerve injury from trauma, upper motor neuron disturbance causing spastic or nonspastic paresis or plegia of the affected limb, or thenar weakness sufficient to require tendon transfer to support thumb opposition.
Other exclusion criteria were concomitant use of the lidocaine patch 5% for any other condition, participation in a clinical trial within the previous 30 days, and pregnancy. Women who were breastfeeding or were of childbearing potential who were not using a reliable form of contraception were also excluded, as were patients with thenar atrophy or significantly prolonged median motor nerve distal latencies indicative of severe CTS.
Interventions
Using a predefined randomization sequence, patients were assigned in strict consecutive order to 1 of 2 treatments: daily applications of the lidocaine patch 5% or a single injection of 0.5 cc lidocaine 1% plus Depo-Medrol 40 mg. Patients assigned to the lidocaine patch 5% were instructed to cover the volar aspect of the wrist—using up to 3 patches per day, covering a surface area of up to 420 cm2, and as much of the painful area as possible—for 24 hours a day. Patients were also instructed to change the patches each day for 4 weeks and were allowed to cut the patch to size. Just one investigator (SN), who has more than 10 years experience giving corticosteroid injections into the carpal tunnel, performed the injections on each patient in this group.
Routine concomitant use of analgesic medications for CTS was not permitted; however, patients were allowed to use analgesics as needed for acute episodes of pain. Patients were asked at each study visit about concomitant medication use, including other analgesics. Patients using splints at the time of randomization were allowed to continue using them, but patients were not permitted to begin using splints during the trial. Adherence among patients randomized to the patch was evaluated by patch counts.
Outcome assessments
Patients were evaluated at baseline, at 2 interim points (Week 1 and Week 2), and at the study’s conclusion (Week 4). The Brief Pain Inventory (BPI) was used at each evaluation to assess pain intensity, pain relief, and pain interference with various domains of quality of life (QOL).16 These domains included general activity, mood, walking ability, normal work, relationships with other people, sleep, and enjoyment of life. Global assessment of pain relief and satisfaction, using the Patient and Global Clinical Impression of Improvement (CGI-I) and the Global Assessment of Treatment Satisfaction (PGAS), was also evaluated. The Patient and Global Clinical Impression of Change are 7-point scales in which patients and clinicians rate changes in overall status since beginning study medication. The Global Assessment of Treatment Satisfaction measure assesses patient responses to the question “Overall, how satisfied are you with your treatment?” Results are rated on a 5-point scale. Safety and tolerability were assessed via adverse event monitoring.
Statistical analysis
The primary analysis included a modified intent-to-treat population consisting of all randomized subjects who had at least 1 post-randomization observation. Missing observations were replaced with the prior observation carried forward. In addition, subjects not given the randomized treatment were analyzed as treated.
For the 7-item BPI interference subscale, missing values were replaced with the average of the non-missing completed items to compute a sum score, provided only 1 response was missing. If more than 1 response was missing, the subscale was defined as incomplete. If a subject failed to complete a form after randomization, he was considered to not have any post-randomization observations for that form and was excluded from the modified intent-to-treat population for that analysis.
The null hypothesis being tested in this pilot study is that there is no difference between treatments. The level for declaring statistical significance was a 2-sided P-value (P<.05). Efficacy endpoints were analyzed using paired t-tests, while global assessments of treatment satisfaction were analyzed using nonparametric methods. Continuous variables were tested by analysis of covariance (ANCOVA) with treatment group as the between-subject factor and baseline as the covariable. For continuous variables (ordinal variables with 5 or more values), a repeated measures ANCOVA was performed with treatment as the between-subject factor and visit and its interaction with treatment as within-subject factors. Ordinal variables with less than 5 values were evaluated using the Wilcoxon rank sum test.
All patients who received study medication were included in the safety analysis. Adverse events were classified according to MedDRA and the incidence of treatment-emergent events was summarized.
Results
Forty patients (20 per group) were enrolled in the study and assigned to receive either daily applications of the lidocaine patch 5% or a single lidocaine/corticosteroid injection. Baseline characteristics of patients were similar between groups (TABLE 1). The mean age of the predominantly female (70%) population was 48 years. All patients had mild or moderate CTS at baseline as determined by Global Clinical Impression of Severity of CTS. Although some patients had previously been treated for CTS, none had undergone carpal tunnel release surgery.
Five patients randomized to the lidocaine patch 5% group did not complete the trial due to adverse events (3 patients), being out of town (1 patient), and becoming lost to follow-up (1 patient). Three patients randomized to the injection group did not complete the study because of rejection of injection (1 patient), becoming lost to follow up (1 patient), and inclement weather (1 patient). Of the 8 patients who did not complete the study, 4 patients (3 in the patch group and 1 in the injection group), had at least 1 observation after randomization and were included in the intent-to-treat population.
Patients used an average of 1 patch per day. Use of concomitant analgesics was similar between groups.
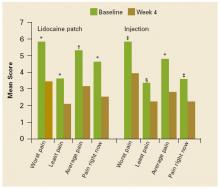
FIGURE 1 shows the mean changes in pain intensity scores, including average pain, pain right now, least pain, and worst pain, from baseline to Week 4. No statistically significant between-group differences were observed. Both groups experienced significant improvements in average pain intensity. Mean changes in average pain scores were –2.2 with the patch (P=.0009) and –2.1 with the injection (P<.0001). More than 60% of patients in both groups experienced a clinically meaningful (≥30% reduction) improvement in average daily pain intensity. Patients in both groups also reported significant changes (P<.05) in worst pain (patch, –2.4; injection, –2.2), least pain (patch, –1.6; injection, –1.1), and pain “right now” (patch, –2.1; injection, –1.3).
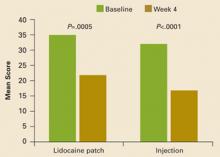
Composite interference scores, which are measures of how much patients’ pain interfered with 7 domains of QOL (general activity, mood, waking ability, normal work, relations with other people, sleep, and enjoyment of life), also significantly improved in both treatment groups (patch, –13.9; injection, –16.7; P<.001 vs baseline for both groups), as shown in FIGURE 2. Eighty percent of patients in the patch group and 59% of patients in the injection group reported being “satisfied” or “very satisfied” with treatment, while investigators reported improvement in 88% of patients on the patch and in 74% of those who received the injection (TABLE 2).
Three patients in each group (15%) reported treatment-related adverse events, all of which were mild in severity. Adverse events reported in the lidocaine patch 5% group included rash (n=1), itching (n=1), and a burning sensation (n=1). One patient in the lidocaine patch 5% group who experienced a skin rash discontinued the study due to this adverse effect. Two patients discontinued the study due to dizziness and palpitations (n=1) and nausea, diarrhea, and vomiting (n=1), adverse events considered to be unrelated to treatment. Three patients who received the injection reported hand numbness (n=1), injection site pain (n=1), and tingling in hands (n=1). No systemic treatment-related adverse events were observed with the lidocaine patch 5%.
FIGURE 1
Mean pain intensity scores
Mean pain intensity scores (worst pain, least pain, average pain, pain right now) as measured on the Brief Pain Inventory at baseline and at Week 4. *P<.0001. †P<.001. ‡P<.01. §P<.05. No significant between-group differences were observed.
FIGURE 2
Mean composite scores of pain interference with QOL
Mean composite scores of pain interference with quality of life at baseline and at Week 4.
TABLE 1
Patient demographics and baseline characteristics
| PARAMETER | LIDOCAINE PATCH (N=20) | INJECTION (N=20) |
|---|---|---|
| Age (mean years±SD) | 48.4±10.3 | 47.5±13.9 |
| Gender (%) | ||
| Male | 35 | 25 |
| Female | 65 | 75 |
| Average pain intensity at baseline (mean±SD) | 5.3±1.9 | 4.8±2.5 |
| Clinical Global Impression of Severity at Baseline (%) | ||
| Mild | 55 | 45 |
| Moderate | 45 | 55 |
TABLE 2
Impact of treatments on Clinicians’ Global Impression of Change and Global Assessment of Treatment Satisfaction scales
| PARAMETER | LIDOCAINE PATCH | INJECTION |
|---|---|---|
| Clinician Global Impression of Change (%) | ||
| Improved | 88 | 74 |
| No Change | 12 | 26 |
| Worse | 0 | 0 |
| Patient satisfaction | ||
| Patients satisfied or very satisfied (%) | 80 | 59 |
Discussion
The lidocaine patch 5%, a noninvasive, targeted peripheral analgesic, effectively relieved the intensity of localized pain reported by patients with CTS. The efficacy of the lidocaine patch 5% in reducing pain and improving symptoms was comparable to that of the more invasive anesthetic/corticosteroid injection. The lidocaine patch 5% significantly reduced CTS-related pain, thereby reducing the pain’s interference with QOL and resulting in a high level of treatment satisfaction. In addition, the lidocaine patch 5% was well tolerated with no reported systemic adverse effects.
These preliminary data from this small, open-label, pilot investigation suggest the possibility that the lidocaine patch 5% may be a useful option for some patients.
Study limitations. Since it was a pilot study, the number of patients included in the trial was small and the duration was short. Moreover, allocation was not concealed and the study was not blinded. Further controlled trials are needed to confirm the effects reported in this pilot study.
The lidocaine patch 5% targets localized damaged or dysfunctional nociceptors while reducing the risk of drug interactions and systemic side effects. Since this noninvasive treatment appears to relieve CTS-related pain with minimal risks, the lidocaine patch 5% may be a reasonable therapeutic option for patients with new-onset CTS who do not respond to more conservative options and who are unable or unwilling to receive more invasive therapies. Moreover, it affords clinicians a possible alternative to corticosteroid injections in patients awaiting surgery and eliminates the risk of adhesions due to injection.
CORRESPONDENCE
Srinivas Nalamachu, MD, 4601 W 109th St, Suite 302, Mid-America Physiatrists, PA, Overland Park, KS 66211. E-mail: [email protected]
1. Viera AJ. Management of carpal tunnel syndrome. Am Fam Physician 2003;68:265-272.
2. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev 2002;4:CD001554.-
3. Ly-Pen D, Andréu J-L, de Blas G, Sánchez-Olaso A, Millán I. Surgical decompression versus local steroid injection in carpal tunnel syndrome: a one-year, prospective, randomized, open, controlled, clinical trial. Arthritis Rheum 2005;52:612-619.
4. Kasten SJ, Louis DS. Carpal tunnel syndrome: a case of median nerve injection injury and a safe and effective method for injecting the carpal tunnel. J Fam Pract 1996;43:79-82.
5. Frederick HA, Carter PR, Littler JW. Injection injuries to the median and ulnar nerves at the wrist. J Hand Surg [Am] 1992;17:645-647.
6. Tavares SP, Giddins GE. Nerve injury following steroid injection for carpal tunnel syndrome. A report of two cases. J Hand Surg [Br] 1996;21:208-209.
7. Katz JN, Simmons BP. Clinical practice. Carpal tunnel syndrome. N Engl J Med 2002;346:1807-1812.
8. Goodyear-Smith F, Arroll B. What can family physicians offer patients with carpal tunnel syndrome other than surgery? A systematic review of nonsurgical management. Ann Fam Med 2004;2:267-273.
9. Gold MS. Sodium channels and pain therapy. Curr Opin Anaesthesiol 2000;13:565-572.
10. Gold MS, Weinreich D, Kim C-S, et al. Redistribution of NaV1.8 in uninjured axons enables neuropathic pain. J Neurosci 2003;23:158-166.
11. Galer BS, Sheldon E, Patel N, et al. Topical lidocaine patch 5% may target a novel underlying pain mechanism in osteoarthritis. Curr Med Res Opinion 2004;20:1455-1458.
12. Gammaitoni AR, Alvarez NA, Galer BS. Safety and tolerability of the lidocaine patch 5%, a targeted peripheral analgesic: a review of the literature. J Clin Pharmacol 2003;43:111-117.
13. Barbano RL, Herrmann DN, Hart-Gouleau S, et al. Effectiveness, tolerability, and impact on quality of life of the 5% lidocaine patch in diabetic polyneuropathy. Arch Neurol 2004;61:914-918.
14. Meier T, Wasner G, Faust M, et al. Efficacy of lidocaine patch 5% in the treatment of focal peripheral neuropathic pain syndromes: a randomized, double-blind, placebo-controlled study. Pain 2003;106:151-158.
15. American Association of Electrodiagnostic Medicine, American Academy of Neurology, and American Academy of Physical Medicine and Rehabilitation. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: summary statement. Muscle Nerve 2002;25:918-922.
16. Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain 2004;5:133-137
- The lidocaine patch 5% provided pain relief for mild-to-moderate carpal tunnel syndrome.
- The lidocaine patch 5% may offer patients a noninvasive treatment option with minimal risk for drug-drug interactions or systemic side effects.
- Objectives: A standard treatment option for mild-to-moderate carpal tunnel syndrome (CTS) is a local injection of anesthetic-corticosteroid, but this can be painful and may cause complications. This pilot clinical trial was designed to compare the safety and efficacy of daily applications of the lidocaine patch 5% (Lidoderm) to that of a single injection of 0.5 cc lidocaine 1% plus methylprednisolone acetate (Depo-Medrol) 40 mg.
- Methods: In this randomized, parallel-group, open-label, single-center, active-controlled, prospective pilot study, participants aged 18–75 years with clinical/electrodiagnostic evidence of CTS were randomized to receive the lidocaine patch 5% or 1 injection of 0.5 cc lidocaine 1% plus methylprednisolone acetate 40 mg. Outcome assessments included the Brief Pain Inventory (measuring pain intensity, relief, and interference with quality of life, Patient and Global Clinical Impression of Improvement, Global Assessment of Treatment Satisfaction, and safety.
- Results: Baseline characteristics of the 40 patients randomized to treatment with the lidocaine patch 5% (n=20) or injection (n=20) were similar between groups. After 4 weeks of treatment, patients in both groups reported significant changes (P<.05) in worst pain, average pain, and pain “right now.” Composite interference scores, which are measures of how much patients’ pain interfered with quality of life, also significantly improved in both treatment groups (patch, –13.9; injection, –16.7; P<.001 vs baseline for both groups). Eighty percent of patients in the lidocaine patch group and 59% of patients who received the injection reported being “satisfied” or “very satisfied,” while investigators reported improvement in 88% of patients using the lidocaine patch and in 74% of those who received the injection. Both treatments were well tolerated, with treatment-related adverse events (AEs) reported in 3 patients in each group (15%). No systemic treatment-related AEs were observed with the lidocaine patch 5%.
- Conclusions: This pilot trial demonstrated that the lidocaine patch 5% was efficacious in reducing pain associated with CTS and was well tolerated. The lidocaine patch 5% may offer patients with CTS effective, noninvasive treatment for the management of their symptoms. Further controlled trials are warranted.
Treatment options for carpal tunnel syndrome (CTS) include wrist splinting, oral corticosteroids, and local injections with anesthetics and corticosteroids for mild-to-moderate cases, and surgical release for severe cases.1
Injections work, but have drawbacks. Injecting a corticosteroid and local anesthetic into, or proximal to, the carpal tunnel gives significantly greater relief than oral steroids.2 In fact, a recent randomized, open-label trial demonstrated that local steroid injections may relieve CTS pain as well as, or better than, invasive surgery.3 However, corticosteroid injections are time consuming and costly. Moreover, inadvertent injections into the nerve can lead to chronic pain and long-term discomfort.4-6 Repeated injections carry the risk for needle injury to the median nerve, intratendinous injection and tendon rupture, adhesions, dysesthesias, and infection.1 Many clinicians limit the number of injections into the carpal tunnel to about 3 or 4 per year to minimize local complications and the possibility of systemic toxic side effects (eg, hyperglycemia or hypertension).7
The thinking behind a new approach. Because nonsurgical treatment options for CTS are suboptimal, new therapies are needed.8 The pain of peripheral nerve injury—eg, CTS-associated median nerve compression—may result from changes in voltage-gated sodium channels in the injured afferents and their uninjured neighbors.9 These changes may have a profound impact on neuronal excitability, causing abnormal sodium channel expression, spontaneous and ectopic sodium channel discharge, and neuropathic pain.10 Pharmacologically blocking these channels and the processes underlying their changes may be the most efficient way of selectively eliminating the associated pain.10 Because lidocaine is believed to stabilize the sodium channels in damaged afferent neurons,11 the lidocaine patch 5% may be an appropriate treatment option for patients with CTS.
Related evidence. The lidocaine patch 5% is indicated for treating pain associated with postherpetic neuralgia and can be used with minimal risk of drug-drug interactions.12 Recent literature suggests that the lidocaine patch 5% may relieve pain associated with multiple types of peripheral neuropathies13,14; therefore, patients with CTS may also benefit from this modality.
The formulation relieves localized pain and may be particularly appropriate for patients who are awaiting surgery or wish to limit their exposure to corticosteroids, such as those with diabetes, heart disease, or hypertension. Though there are anecdotal reports of success with topical lidocaine patches for CTS, its efficacy and safety have not been evaluated in randomized trials or documented in published literature.
Focus of our pilot study. To investigate the role of topical lidocaine in relieving pain or functional impairment caused by persistent or recurrent CTS, we conducted a randomized pilot trial comparing the safety and efficacy of daily applications of the lidocaine patch 5% (Lidoderm) with the efficacy and safety of a single injection of 0.5 cc lidocaine 1% and methylprednisolone acetate (Depo-Medrol) 40 mg in patients with mild-to-moderate CTS.
Methods
Participants and design
This trial was a 4-week, randomized, parallel-group, open-label, single-center, active-controlled, prospective pilot study conducted in the United States. The Ethical Review Committee, Inc, located in Kansas City, Kansas, reviewed and approved the study. Patients 18 to 75 years with clinical and electrodiagnostic evidence of CTS were randomly assigned to receive the lidocaine patch 5% or a single injection of 0.5 cc lidocaine 1% and methylprednisolone acetate 40 mg.
Inclusion criteria. Electrodiagnostic evidence of CTS included a median motor nerve distal latency more than 4.10 m sec or a difference of more than 1 m sec between the median and ulnar sensory latencies when recorded with the fourth finger.15 Patients also were required to have persistent or recurrent CTS as defined by the presence of pain, paresthesias, or positive Phalen’s or Tinel’s signs. We enrolled patients who met the eligibility criteria, gave consent, and attended 1 of 2 treatment centers (a family practice clinic or a physical medicine clinic) between November 2003 and May 2004. Patients were not recruited from the general population and were not given incentives to participate other than free treatment. Patients were enrolled after providing written informed consent.
Exclusion criteria. Patients were excluded from the study if they had peripheral neuropathy of any origin other than CTS, carpal tunnel injection in the study limb within the previous 8 weeks, carpal tunnel surgical release of the study limb within the previous 6 months, concomitant cervical radiculopathy, anatomic abnormalities of the wrist or hand, median nerve injury from trauma, upper motor neuron disturbance causing spastic or nonspastic paresis or plegia of the affected limb, or thenar weakness sufficient to require tendon transfer to support thumb opposition.
Other exclusion criteria were concomitant use of the lidocaine patch 5% for any other condition, participation in a clinical trial within the previous 30 days, and pregnancy. Women who were breastfeeding or were of childbearing potential who were not using a reliable form of contraception were also excluded, as were patients with thenar atrophy or significantly prolonged median motor nerve distal latencies indicative of severe CTS.
Interventions
Using a predefined randomization sequence, patients were assigned in strict consecutive order to 1 of 2 treatments: daily applications of the lidocaine patch 5% or a single injection of 0.5 cc lidocaine 1% plus Depo-Medrol 40 mg. Patients assigned to the lidocaine patch 5% were instructed to cover the volar aspect of the wrist—using up to 3 patches per day, covering a surface area of up to 420 cm2, and as much of the painful area as possible—for 24 hours a day. Patients were also instructed to change the patches each day for 4 weeks and were allowed to cut the patch to size. Just one investigator (SN), who has more than 10 years experience giving corticosteroid injections into the carpal tunnel, performed the injections on each patient in this group.
Routine concomitant use of analgesic medications for CTS was not permitted; however, patients were allowed to use analgesics as needed for acute episodes of pain. Patients were asked at each study visit about concomitant medication use, including other analgesics. Patients using splints at the time of randomization were allowed to continue using them, but patients were not permitted to begin using splints during the trial. Adherence among patients randomized to the patch was evaluated by patch counts.
Outcome assessments
Patients were evaluated at baseline, at 2 interim points (Week 1 and Week 2), and at the study’s conclusion (Week 4). The Brief Pain Inventory (BPI) was used at each evaluation to assess pain intensity, pain relief, and pain interference with various domains of quality of life (QOL).16 These domains included general activity, mood, walking ability, normal work, relationships with other people, sleep, and enjoyment of life. Global assessment of pain relief and satisfaction, using the Patient and Global Clinical Impression of Improvement (CGI-I) and the Global Assessment of Treatment Satisfaction (PGAS), was also evaluated. The Patient and Global Clinical Impression of Change are 7-point scales in which patients and clinicians rate changes in overall status since beginning study medication. The Global Assessment of Treatment Satisfaction measure assesses patient responses to the question “Overall, how satisfied are you with your treatment?” Results are rated on a 5-point scale. Safety and tolerability were assessed via adverse event monitoring.
Statistical analysis
The primary analysis included a modified intent-to-treat population consisting of all randomized subjects who had at least 1 post-randomization observation. Missing observations were replaced with the prior observation carried forward. In addition, subjects not given the randomized treatment were analyzed as treated.
For the 7-item BPI interference subscale, missing values were replaced with the average of the non-missing completed items to compute a sum score, provided only 1 response was missing. If more than 1 response was missing, the subscale was defined as incomplete. If a subject failed to complete a form after randomization, he was considered to not have any post-randomization observations for that form and was excluded from the modified intent-to-treat population for that analysis.
The null hypothesis being tested in this pilot study is that there is no difference between treatments. The level for declaring statistical significance was a 2-sided P-value (P<.05). Efficacy endpoints were analyzed using paired t-tests, while global assessments of treatment satisfaction were analyzed using nonparametric methods. Continuous variables were tested by analysis of covariance (ANCOVA) with treatment group as the between-subject factor and baseline as the covariable. For continuous variables (ordinal variables with 5 or more values), a repeated measures ANCOVA was performed with treatment as the between-subject factor and visit and its interaction with treatment as within-subject factors. Ordinal variables with less than 5 values were evaluated using the Wilcoxon rank sum test.
All patients who received study medication were included in the safety analysis. Adverse events were classified according to MedDRA and the incidence of treatment-emergent events was summarized.
Results
Forty patients (20 per group) were enrolled in the study and assigned to receive either daily applications of the lidocaine patch 5% or a single lidocaine/corticosteroid injection. Baseline characteristics of patients were similar between groups (TABLE 1). The mean age of the predominantly female (70%) population was 48 years. All patients had mild or moderate CTS at baseline as determined by Global Clinical Impression of Severity of CTS. Although some patients had previously been treated for CTS, none had undergone carpal tunnel release surgery.
Five patients randomized to the lidocaine patch 5% group did not complete the trial due to adverse events (3 patients), being out of town (1 patient), and becoming lost to follow-up (1 patient). Three patients randomized to the injection group did not complete the study because of rejection of injection (1 patient), becoming lost to follow up (1 patient), and inclement weather (1 patient). Of the 8 patients who did not complete the study, 4 patients (3 in the patch group and 1 in the injection group), had at least 1 observation after randomization and were included in the intent-to-treat population.
Patients used an average of 1 patch per day. Use of concomitant analgesics was similar between groups.
FIGURE 1 shows the mean changes in pain intensity scores, including average pain, pain right now, least pain, and worst pain, from baseline to Week 4. No statistically significant between-group differences were observed. Both groups experienced significant improvements in average pain intensity. Mean changes in average pain scores were –2.2 with the patch (P=.0009) and –2.1 with the injection (P<.0001). More than 60% of patients in both groups experienced a clinically meaningful (≥30% reduction) improvement in average daily pain intensity. Patients in both groups also reported significant changes (P<.05) in worst pain (patch, –2.4; injection, –2.2), least pain (patch, –1.6; injection, –1.1), and pain “right now” (patch, –2.1; injection, –1.3).
Composite interference scores, which are measures of how much patients’ pain interfered with 7 domains of QOL (general activity, mood, waking ability, normal work, relations with other people, sleep, and enjoyment of life), also significantly improved in both treatment groups (patch, –13.9; injection, –16.7; P<.001 vs baseline for both groups), as shown in FIGURE 2. Eighty percent of patients in the patch group and 59% of patients in the injection group reported being “satisfied” or “very satisfied” with treatment, while investigators reported improvement in 88% of patients on the patch and in 74% of those who received the injection (TABLE 2).
Three patients in each group (15%) reported treatment-related adverse events, all of which were mild in severity. Adverse events reported in the lidocaine patch 5% group included rash (n=1), itching (n=1), and a burning sensation (n=1). One patient in the lidocaine patch 5% group who experienced a skin rash discontinued the study due to this adverse effect. Two patients discontinued the study due to dizziness and palpitations (n=1) and nausea, diarrhea, and vomiting (n=1), adverse events considered to be unrelated to treatment. Three patients who received the injection reported hand numbness (n=1), injection site pain (n=1), and tingling in hands (n=1). No systemic treatment-related adverse events were observed with the lidocaine patch 5%.
FIGURE 1
Mean pain intensity scores
Mean pain intensity scores (worst pain, least pain, average pain, pain right now) as measured on the Brief Pain Inventory at baseline and at Week 4. *P<.0001. †P<.001. ‡P<.01. §P<.05. No significant between-group differences were observed.
FIGURE 2
Mean composite scores of pain interference with QOL
Mean composite scores of pain interference with quality of life at baseline and at Week 4.
TABLE 1
Patient demographics and baseline characteristics
| PARAMETER | LIDOCAINE PATCH (N=20) | INJECTION (N=20) |
|---|---|---|
| Age (mean years±SD) | 48.4±10.3 | 47.5±13.9 |
| Gender (%) | ||
| Male | 35 | 25 |
| Female | 65 | 75 |
| Average pain intensity at baseline (mean±SD) | 5.3±1.9 | 4.8±2.5 |
| Clinical Global Impression of Severity at Baseline (%) | ||
| Mild | 55 | 45 |
| Moderate | 45 | 55 |
TABLE 2
Impact of treatments on Clinicians’ Global Impression of Change and Global Assessment of Treatment Satisfaction scales
| PARAMETER | LIDOCAINE PATCH | INJECTION |
|---|---|---|
| Clinician Global Impression of Change (%) | ||
| Improved | 88 | 74 |
| No Change | 12 | 26 |
| Worse | 0 | 0 |
| Patient satisfaction | ||
| Patients satisfied or very satisfied (%) | 80 | 59 |
Discussion
The lidocaine patch 5%, a noninvasive, targeted peripheral analgesic, effectively relieved the intensity of localized pain reported by patients with CTS. The efficacy of the lidocaine patch 5% in reducing pain and improving symptoms was comparable to that of the more invasive anesthetic/corticosteroid injection. The lidocaine patch 5% significantly reduced CTS-related pain, thereby reducing the pain’s interference with QOL and resulting in a high level of treatment satisfaction. In addition, the lidocaine patch 5% was well tolerated with no reported systemic adverse effects.
These preliminary data from this small, open-label, pilot investigation suggest the possibility that the lidocaine patch 5% may be a useful option for some patients.
Study limitations. Since it was a pilot study, the number of patients included in the trial was small and the duration was short. Moreover, allocation was not concealed and the study was not blinded. Further controlled trials are needed to confirm the effects reported in this pilot study.
The lidocaine patch 5% targets localized damaged or dysfunctional nociceptors while reducing the risk of drug interactions and systemic side effects. Since this noninvasive treatment appears to relieve CTS-related pain with minimal risks, the lidocaine patch 5% may be a reasonable therapeutic option for patients with new-onset CTS who do not respond to more conservative options and who are unable or unwilling to receive more invasive therapies. Moreover, it affords clinicians a possible alternative to corticosteroid injections in patients awaiting surgery and eliminates the risk of adhesions due to injection.
CORRESPONDENCE
Srinivas Nalamachu, MD, 4601 W 109th St, Suite 302, Mid-America Physiatrists, PA, Overland Park, KS 66211. E-mail: [email protected]
- The lidocaine patch 5% provided pain relief for mild-to-moderate carpal tunnel syndrome.
- The lidocaine patch 5% may offer patients a noninvasive treatment option with minimal risk for drug-drug interactions or systemic side effects.
- Objectives: A standard treatment option for mild-to-moderate carpal tunnel syndrome (CTS) is a local injection of anesthetic-corticosteroid, but this can be painful and may cause complications. This pilot clinical trial was designed to compare the safety and efficacy of daily applications of the lidocaine patch 5% (Lidoderm) to that of a single injection of 0.5 cc lidocaine 1% plus methylprednisolone acetate (Depo-Medrol) 40 mg.
- Methods: In this randomized, parallel-group, open-label, single-center, active-controlled, prospective pilot study, participants aged 18–75 years with clinical/electrodiagnostic evidence of CTS were randomized to receive the lidocaine patch 5% or 1 injection of 0.5 cc lidocaine 1% plus methylprednisolone acetate 40 mg. Outcome assessments included the Brief Pain Inventory (measuring pain intensity, relief, and interference with quality of life, Patient and Global Clinical Impression of Improvement, Global Assessment of Treatment Satisfaction, and safety.
- Results: Baseline characteristics of the 40 patients randomized to treatment with the lidocaine patch 5% (n=20) or injection (n=20) were similar between groups. After 4 weeks of treatment, patients in both groups reported significant changes (P<.05) in worst pain, average pain, and pain “right now.” Composite interference scores, which are measures of how much patients’ pain interfered with quality of life, also significantly improved in both treatment groups (patch, –13.9; injection, –16.7; P<.001 vs baseline for both groups). Eighty percent of patients in the lidocaine patch group and 59% of patients who received the injection reported being “satisfied” or “very satisfied,” while investigators reported improvement in 88% of patients using the lidocaine patch and in 74% of those who received the injection. Both treatments were well tolerated, with treatment-related adverse events (AEs) reported in 3 patients in each group (15%). No systemic treatment-related AEs were observed with the lidocaine patch 5%.
- Conclusions: This pilot trial demonstrated that the lidocaine patch 5% was efficacious in reducing pain associated with CTS and was well tolerated. The lidocaine patch 5% may offer patients with CTS effective, noninvasive treatment for the management of their symptoms. Further controlled trials are warranted.
Treatment options for carpal tunnel syndrome (CTS) include wrist splinting, oral corticosteroids, and local injections with anesthetics and corticosteroids for mild-to-moderate cases, and surgical release for severe cases.1
Injections work, but have drawbacks. Injecting a corticosteroid and local anesthetic into, or proximal to, the carpal tunnel gives significantly greater relief than oral steroids.2 In fact, a recent randomized, open-label trial demonstrated that local steroid injections may relieve CTS pain as well as, or better than, invasive surgery.3 However, corticosteroid injections are time consuming and costly. Moreover, inadvertent injections into the nerve can lead to chronic pain and long-term discomfort.4-6 Repeated injections carry the risk for needle injury to the median nerve, intratendinous injection and tendon rupture, adhesions, dysesthesias, and infection.1 Many clinicians limit the number of injections into the carpal tunnel to about 3 or 4 per year to minimize local complications and the possibility of systemic toxic side effects (eg, hyperglycemia or hypertension).7
The thinking behind a new approach. Because nonsurgical treatment options for CTS are suboptimal, new therapies are needed.8 The pain of peripheral nerve injury—eg, CTS-associated median nerve compression—may result from changes in voltage-gated sodium channels in the injured afferents and their uninjured neighbors.9 These changes may have a profound impact on neuronal excitability, causing abnormal sodium channel expression, spontaneous and ectopic sodium channel discharge, and neuropathic pain.10 Pharmacologically blocking these channels and the processes underlying their changes may be the most efficient way of selectively eliminating the associated pain.10 Because lidocaine is believed to stabilize the sodium channels in damaged afferent neurons,11 the lidocaine patch 5% may be an appropriate treatment option for patients with CTS.
Related evidence. The lidocaine patch 5% is indicated for treating pain associated with postherpetic neuralgia and can be used with minimal risk of drug-drug interactions.12 Recent literature suggests that the lidocaine patch 5% may relieve pain associated with multiple types of peripheral neuropathies13,14; therefore, patients with CTS may also benefit from this modality.
The formulation relieves localized pain and may be particularly appropriate for patients who are awaiting surgery or wish to limit their exposure to corticosteroids, such as those with diabetes, heart disease, or hypertension. Though there are anecdotal reports of success with topical lidocaine patches for CTS, its efficacy and safety have not been evaluated in randomized trials or documented in published literature.
Focus of our pilot study. To investigate the role of topical lidocaine in relieving pain or functional impairment caused by persistent or recurrent CTS, we conducted a randomized pilot trial comparing the safety and efficacy of daily applications of the lidocaine patch 5% (Lidoderm) with the efficacy and safety of a single injection of 0.5 cc lidocaine 1% and methylprednisolone acetate (Depo-Medrol) 40 mg in patients with mild-to-moderate CTS.
Methods
Participants and design
This trial was a 4-week, randomized, parallel-group, open-label, single-center, active-controlled, prospective pilot study conducted in the United States. The Ethical Review Committee, Inc, located in Kansas City, Kansas, reviewed and approved the study. Patients 18 to 75 years with clinical and electrodiagnostic evidence of CTS were randomly assigned to receive the lidocaine patch 5% or a single injection of 0.5 cc lidocaine 1% and methylprednisolone acetate 40 mg.
Inclusion criteria. Electrodiagnostic evidence of CTS included a median motor nerve distal latency more than 4.10 m sec or a difference of more than 1 m sec between the median and ulnar sensory latencies when recorded with the fourth finger.15 Patients also were required to have persistent or recurrent CTS as defined by the presence of pain, paresthesias, or positive Phalen’s or Tinel’s signs. We enrolled patients who met the eligibility criteria, gave consent, and attended 1 of 2 treatment centers (a family practice clinic or a physical medicine clinic) between November 2003 and May 2004. Patients were not recruited from the general population and were not given incentives to participate other than free treatment. Patients were enrolled after providing written informed consent.
Exclusion criteria. Patients were excluded from the study if they had peripheral neuropathy of any origin other than CTS, carpal tunnel injection in the study limb within the previous 8 weeks, carpal tunnel surgical release of the study limb within the previous 6 months, concomitant cervical radiculopathy, anatomic abnormalities of the wrist or hand, median nerve injury from trauma, upper motor neuron disturbance causing spastic or nonspastic paresis or plegia of the affected limb, or thenar weakness sufficient to require tendon transfer to support thumb opposition.
Other exclusion criteria were concomitant use of the lidocaine patch 5% for any other condition, participation in a clinical trial within the previous 30 days, and pregnancy. Women who were breastfeeding or were of childbearing potential who were not using a reliable form of contraception were also excluded, as were patients with thenar atrophy or significantly prolonged median motor nerve distal latencies indicative of severe CTS.
Interventions
Using a predefined randomization sequence, patients were assigned in strict consecutive order to 1 of 2 treatments: daily applications of the lidocaine patch 5% or a single injection of 0.5 cc lidocaine 1% plus Depo-Medrol 40 mg. Patients assigned to the lidocaine patch 5% were instructed to cover the volar aspect of the wrist—using up to 3 patches per day, covering a surface area of up to 420 cm2, and as much of the painful area as possible—for 24 hours a day. Patients were also instructed to change the patches each day for 4 weeks and were allowed to cut the patch to size. Just one investigator (SN), who has more than 10 years experience giving corticosteroid injections into the carpal tunnel, performed the injections on each patient in this group.
Routine concomitant use of analgesic medications for CTS was not permitted; however, patients were allowed to use analgesics as needed for acute episodes of pain. Patients were asked at each study visit about concomitant medication use, including other analgesics. Patients using splints at the time of randomization were allowed to continue using them, but patients were not permitted to begin using splints during the trial. Adherence among patients randomized to the patch was evaluated by patch counts.
Outcome assessments
Patients were evaluated at baseline, at 2 interim points (Week 1 and Week 2), and at the study’s conclusion (Week 4). The Brief Pain Inventory (BPI) was used at each evaluation to assess pain intensity, pain relief, and pain interference with various domains of quality of life (QOL).16 These domains included general activity, mood, walking ability, normal work, relationships with other people, sleep, and enjoyment of life. Global assessment of pain relief and satisfaction, using the Patient and Global Clinical Impression of Improvement (CGI-I) and the Global Assessment of Treatment Satisfaction (PGAS), was also evaluated. The Patient and Global Clinical Impression of Change are 7-point scales in which patients and clinicians rate changes in overall status since beginning study medication. The Global Assessment of Treatment Satisfaction measure assesses patient responses to the question “Overall, how satisfied are you with your treatment?” Results are rated on a 5-point scale. Safety and tolerability were assessed via adverse event monitoring.
Statistical analysis
The primary analysis included a modified intent-to-treat population consisting of all randomized subjects who had at least 1 post-randomization observation. Missing observations were replaced with the prior observation carried forward. In addition, subjects not given the randomized treatment were analyzed as treated.
For the 7-item BPI interference subscale, missing values were replaced with the average of the non-missing completed items to compute a sum score, provided only 1 response was missing. If more than 1 response was missing, the subscale was defined as incomplete. If a subject failed to complete a form after randomization, he was considered to not have any post-randomization observations for that form and was excluded from the modified intent-to-treat population for that analysis.
The null hypothesis being tested in this pilot study is that there is no difference between treatments. The level for declaring statistical significance was a 2-sided P-value (P<.05). Efficacy endpoints were analyzed using paired t-tests, while global assessments of treatment satisfaction were analyzed using nonparametric methods. Continuous variables were tested by analysis of covariance (ANCOVA) with treatment group as the between-subject factor and baseline as the covariable. For continuous variables (ordinal variables with 5 or more values), a repeated measures ANCOVA was performed with treatment as the between-subject factor and visit and its interaction with treatment as within-subject factors. Ordinal variables with less than 5 values were evaluated using the Wilcoxon rank sum test.
All patients who received study medication were included in the safety analysis. Adverse events were classified according to MedDRA and the incidence of treatment-emergent events was summarized.
Results
Forty patients (20 per group) were enrolled in the study and assigned to receive either daily applications of the lidocaine patch 5% or a single lidocaine/corticosteroid injection. Baseline characteristics of patients were similar between groups (TABLE 1). The mean age of the predominantly female (70%) population was 48 years. All patients had mild or moderate CTS at baseline as determined by Global Clinical Impression of Severity of CTS. Although some patients had previously been treated for CTS, none had undergone carpal tunnel release surgery.
Five patients randomized to the lidocaine patch 5% group did not complete the trial due to adverse events (3 patients), being out of town (1 patient), and becoming lost to follow-up (1 patient). Three patients randomized to the injection group did not complete the study because of rejection of injection (1 patient), becoming lost to follow up (1 patient), and inclement weather (1 patient). Of the 8 patients who did not complete the study, 4 patients (3 in the patch group and 1 in the injection group), had at least 1 observation after randomization and were included in the intent-to-treat population.
Patients used an average of 1 patch per day. Use of concomitant analgesics was similar between groups.
FIGURE 1 shows the mean changes in pain intensity scores, including average pain, pain right now, least pain, and worst pain, from baseline to Week 4. No statistically significant between-group differences were observed. Both groups experienced significant improvements in average pain intensity. Mean changes in average pain scores were –2.2 with the patch (P=.0009) and –2.1 with the injection (P<.0001). More than 60% of patients in both groups experienced a clinically meaningful (≥30% reduction) improvement in average daily pain intensity. Patients in both groups also reported significant changes (P<.05) in worst pain (patch, –2.4; injection, –2.2), least pain (patch, –1.6; injection, –1.1), and pain “right now” (patch, –2.1; injection, –1.3).
Composite interference scores, which are measures of how much patients’ pain interfered with 7 domains of QOL (general activity, mood, waking ability, normal work, relations with other people, sleep, and enjoyment of life), also significantly improved in both treatment groups (patch, –13.9; injection, –16.7; P<.001 vs baseline for both groups), as shown in FIGURE 2. Eighty percent of patients in the patch group and 59% of patients in the injection group reported being “satisfied” or “very satisfied” with treatment, while investigators reported improvement in 88% of patients on the patch and in 74% of those who received the injection (TABLE 2).
Three patients in each group (15%) reported treatment-related adverse events, all of which were mild in severity. Adverse events reported in the lidocaine patch 5% group included rash (n=1), itching (n=1), and a burning sensation (n=1). One patient in the lidocaine patch 5% group who experienced a skin rash discontinued the study due to this adverse effect. Two patients discontinued the study due to dizziness and palpitations (n=1) and nausea, diarrhea, and vomiting (n=1), adverse events considered to be unrelated to treatment. Three patients who received the injection reported hand numbness (n=1), injection site pain (n=1), and tingling in hands (n=1). No systemic treatment-related adverse events were observed with the lidocaine patch 5%.
FIGURE 1
Mean pain intensity scores
Mean pain intensity scores (worst pain, least pain, average pain, pain right now) as measured on the Brief Pain Inventory at baseline and at Week 4. *P<.0001. †P<.001. ‡P<.01. §P<.05. No significant between-group differences were observed.
FIGURE 2
Mean composite scores of pain interference with QOL
Mean composite scores of pain interference with quality of life at baseline and at Week 4.
TABLE 1
Patient demographics and baseline characteristics
| PARAMETER | LIDOCAINE PATCH (N=20) | INJECTION (N=20) |
|---|---|---|
| Age (mean years±SD) | 48.4±10.3 | 47.5±13.9 |
| Gender (%) | ||
| Male | 35 | 25 |
| Female | 65 | 75 |
| Average pain intensity at baseline (mean±SD) | 5.3±1.9 | 4.8±2.5 |
| Clinical Global Impression of Severity at Baseline (%) | ||
| Mild | 55 | 45 |
| Moderate | 45 | 55 |
TABLE 2
Impact of treatments on Clinicians’ Global Impression of Change and Global Assessment of Treatment Satisfaction scales
| PARAMETER | LIDOCAINE PATCH | INJECTION |
|---|---|---|
| Clinician Global Impression of Change (%) | ||
| Improved | 88 | 74 |
| No Change | 12 | 26 |
| Worse | 0 | 0 |
| Patient satisfaction | ||
| Patients satisfied or very satisfied (%) | 80 | 59 |
Discussion
The lidocaine patch 5%, a noninvasive, targeted peripheral analgesic, effectively relieved the intensity of localized pain reported by patients with CTS. The efficacy of the lidocaine patch 5% in reducing pain and improving symptoms was comparable to that of the more invasive anesthetic/corticosteroid injection. The lidocaine patch 5% significantly reduced CTS-related pain, thereby reducing the pain’s interference with QOL and resulting in a high level of treatment satisfaction. In addition, the lidocaine patch 5% was well tolerated with no reported systemic adverse effects.
These preliminary data from this small, open-label, pilot investigation suggest the possibility that the lidocaine patch 5% may be a useful option for some patients.
Study limitations. Since it was a pilot study, the number of patients included in the trial was small and the duration was short. Moreover, allocation was not concealed and the study was not blinded. Further controlled trials are needed to confirm the effects reported in this pilot study.
The lidocaine patch 5% targets localized damaged or dysfunctional nociceptors while reducing the risk of drug interactions and systemic side effects. Since this noninvasive treatment appears to relieve CTS-related pain with minimal risks, the lidocaine patch 5% may be a reasonable therapeutic option for patients with new-onset CTS who do not respond to more conservative options and who are unable or unwilling to receive more invasive therapies. Moreover, it affords clinicians a possible alternative to corticosteroid injections in patients awaiting surgery and eliminates the risk of adhesions due to injection.
CORRESPONDENCE
Srinivas Nalamachu, MD, 4601 W 109th St, Suite 302, Mid-America Physiatrists, PA, Overland Park, KS 66211. E-mail: [email protected]
1. Viera AJ. Management of carpal tunnel syndrome. Am Fam Physician 2003;68:265-272.
2. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev 2002;4:CD001554.-
3. Ly-Pen D, Andréu J-L, de Blas G, Sánchez-Olaso A, Millán I. Surgical decompression versus local steroid injection in carpal tunnel syndrome: a one-year, prospective, randomized, open, controlled, clinical trial. Arthritis Rheum 2005;52:612-619.
4. Kasten SJ, Louis DS. Carpal tunnel syndrome: a case of median nerve injection injury and a safe and effective method for injecting the carpal tunnel. J Fam Pract 1996;43:79-82.
5. Frederick HA, Carter PR, Littler JW. Injection injuries to the median and ulnar nerves at the wrist. J Hand Surg [Am] 1992;17:645-647.
6. Tavares SP, Giddins GE. Nerve injury following steroid injection for carpal tunnel syndrome. A report of two cases. J Hand Surg [Br] 1996;21:208-209.
7. Katz JN, Simmons BP. Clinical practice. Carpal tunnel syndrome. N Engl J Med 2002;346:1807-1812.
8. Goodyear-Smith F, Arroll B. What can family physicians offer patients with carpal tunnel syndrome other than surgery? A systematic review of nonsurgical management. Ann Fam Med 2004;2:267-273.
9. Gold MS. Sodium channels and pain therapy. Curr Opin Anaesthesiol 2000;13:565-572.
10. Gold MS, Weinreich D, Kim C-S, et al. Redistribution of NaV1.8 in uninjured axons enables neuropathic pain. J Neurosci 2003;23:158-166.
11. Galer BS, Sheldon E, Patel N, et al. Topical lidocaine patch 5% may target a novel underlying pain mechanism in osteoarthritis. Curr Med Res Opinion 2004;20:1455-1458.
12. Gammaitoni AR, Alvarez NA, Galer BS. Safety and tolerability of the lidocaine patch 5%, a targeted peripheral analgesic: a review of the literature. J Clin Pharmacol 2003;43:111-117.
13. Barbano RL, Herrmann DN, Hart-Gouleau S, et al. Effectiveness, tolerability, and impact on quality of life of the 5% lidocaine patch in diabetic polyneuropathy. Arch Neurol 2004;61:914-918.
14. Meier T, Wasner G, Faust M, et al. Efficacy of lidocaine patch 5% in the treatment of focal peripheral neuropathic pain syndromes: a randomized, double-blind, placebo-controlled study. Pain 2003;106:151-158.
15. American Association of Electrodiagnostic Medicine, American Academy of Neurology, and American Academy of Physical Medicine and Rehabilitation. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: summary statement. Muscle Nerve 2002;25:918-922.
16. Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain 2004;5:133-137
1. Viera AJ. Management of carpal tunnel syndrome. Am Fam Physician 2003;68:265-272.
2. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev 2002;4:CD001554.-
3. Ly-Pen D, Andréu J-L, de Blas G, Sánchez-Olaso A, Millán I. Surgical decompression versus local steroid injection in carpal tunnel syndrome: a one-year, prospective, randomized, open, controlled, clinical trial. Arthritis Rheum 2005;52:612-619.
4. Kasten SJ, Louis DS. Carpal tunnel syndrome: a case of median nerve injection injury and a safe and effective method for injecting the carpal tunnel. J Fam Pract 1996;43:79-82.
5. Frederick HA, Carter PR, Littler JW. Injection injuries to the median and ulnar nerves at the wrist. J Hand Surg [Am] 1992;17:645-647.
6. Tavares SP, Giddins GE. Nerve injury following steroid injection for carpal tunnel syndrome. A report of two cases. J Hand Surg [Br] 1996;21:208-209.
7. Katz JN, Simmons BP. Clinical practice. Carpal tunnel syndrome. N Engl J Med 2002;346:1807-1812.
8. Goodyear-Smith F, Arroll B. What can family physicians offer patients with carpal tunnel syndrome other than surgery? A systematic review of nonsurgical management. Ann Fam Med 2004;2:267-273.
9. Gold MS. Sodium channels and pain therapy. Curr Opin Anaesthesiol 2000;13:565-572.
10. Gold MS, Weinreich D, Kim C-S, et al. Redistribution of NaV1.8 in uninjured axons enables neuropathic pain. J Neurosci 2003;23:158-166.
11. Galer BS, Sheldon E, Patel N, et al. Topical lidocaine patch 5% may target a novel underlying pain mechanism in osteoarthritis. Curr Med Res Opinion 2004;20:1455-1458.
12. Gammaitoni AR, Alvarez NA, Galer BS. Safety and tolerability of the lidocaine patch 5%, a targeted peripheral analgesic: a review of the literature. J Clin Pharmacol 2003;43:111-117.
13. Barbano RL, Herrmann DN, Hart-Gouleau S, et al. Effectiveness, tolerability, and impact on quality of life of the 5% lidocaine patch in diabetic polyneuropathy. Arch Neurol 2004;61:914-918.
14. Meier T, Wasner G, Faust M, et al. Efficacy of lidocaine patch 5% in the treatment of focal peripheral neuropathic pain syndromes: a randomized, double-blind, placebo-controlled study. Pain 2003;106:151-158.
15. American Association of Electrodiagnostic Medicine, American Academy of Neurology, and American Academy of Physical Medicine and Rehabilitation. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: summary statement. Muscle Nerve 2002;25:918-922.
16. Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain 2004;5:133-137