User login
Case Report
A 58-year-old man presented for evaluation of a pruritic rash involving the pubic area of 30 years’ duration. Multiple primary care physicians and dermatologists had evaluated the patient during this period, but he noted a specific diagnosis had not been rendered and multiple treatments had been unsuccessful. The patient described a rash, which was absent at the time of evaluation, as a self-remitting and exacerbating irritation typically induced by sweating and physical activity. The patient also stated that the irritation was associated with a strong, distinct, musty odor that severely interrupted his sex life and decreased his quality of life. Prior treatments included various topical corticosteroids, topical and oral antibiotics, and various homeopathic treatments that were minimally efficacious or nonefficacious. He was unsure if antifungals had previously been prescribed.
The patient’s medical history was notable for pulmonary interstitial fibrosis, anxiety, posttraumatic stress disorder, and mild glucose intolerance. The patient had no pertinent surgical history and no known drug allergies. Current medications included a bronchodilating inhaler, escitalopram, trazodone, buspirone, clonazepam, prazosin, gabapentin, and azithromycin for current upper respiratory tract infection. The patient was a former smoker and a social drinker.
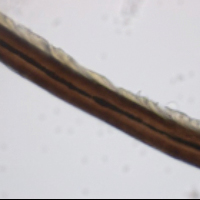
On physical evaluation the pubic area displayed slight patchy erythema without a papular component and was otherwise unremarkable to the unaided eye. Upon palpation of the skin, there were no remarkable findings. Under dermoscopic evaluation, small white-yellow concretions along the hair shaft were noticed. Evaluation with a Wood lamp is shown in Figure 1.
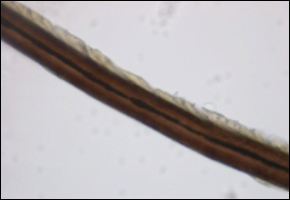
The patient was treated empirically with ketoconazole cream 2% applied to the affected area once daily until follow-up 3 weeks later. The patient also was advised to shave the pubic area to remove potentially infected hairs, as white piedra (WP) was suspected. A diagnosis of WP was confirmed on histologic evaluation of pubic hair samples approximately 1 to 2 weeks later (Figure 2).
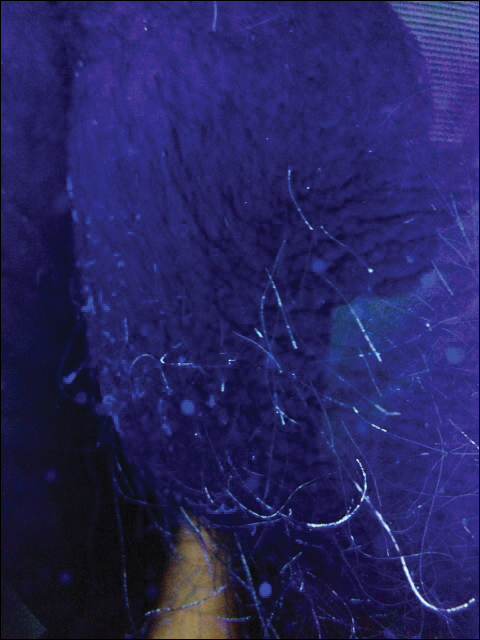
At 3-week follow-up, Wood lamp evaluation did not identify concretions along the pubic hair shafts. The patient was symptom free and extremely pleased. Of note, the patient did not shave the pubic area and was counseled on recurrence.
Comment
Piedra (meaning stone in Spanish) describes a group of fungal infections that present with gritty concretions on the hair shaft.1,2 In 1911, Horta3 classified piedra into 2 subtypes: black piedra, caused by Piedraia hortae, and WP, caused by Trichosporon species. Black piedra occurs more frequently in tropical countries and commonly affects hair shafts on the scalp.4 White piedra most commonly affects the pubic area, with rare cases in scalp and facial hair.1,5-7
Epidemiology
White piedra is seen worldwide, including Europe, South America, India, Southeast Asia, Africa, South America, and southern parts of the United States. The majority of cases occur in tropical and temperate regions.1 White piedra likely is underdiagnosed; for example, in a study of 166 young men with genital concerns in Houston, Texas, Trichosporon was isolated in 40% of cultured scrotal hairs.8
Species Identification
There are several species of WP; special techniques must be used to differentiate them, which is beyond the scope of this case. The known species include Trichosporon asahii, Trichosporon asteroides, Trichosporon cutaneum, Trichosporon inkin, Trichosporon mucoides, and Trichosporon ovoides.1Trichosporon asahii and T mucoides have been known to cause systemic infections in immunocompromised hosts known as trichosporonosis.1,9 As an example of a special technique used for species recognition, Sugita et al10 used sequence analysis of the ribosomal DNA intergenic spacer 1 regions to distinguish T asahii isolates. Identification of species may be warranted in the proper clinical scenario; however, histologic evaluation by an experienced dermatopathologist frequently is sufficient to identify the Trichosporon genus.
Transmission
The 2 most common causative organisms of WP are T inkin and T ovoides. Furthermore, T inkin causes the vast majority of WP in the pubic region.8
Diagnosis and Differential
White piedra is characterized by the presence of adherent tan to white nodules along the hair shafts. The concretions tend to be softer than black piedra and, unlike trichomycosis, normally do not fluoresce.1 They do not encircle the hair shaft as hair casts do and can be readily distinguished from Trichomycosis axillaris, black piedra, pediculosis, and trichorrhexis nodosa on microscopic examination.14 The hair shaft concretions of WP are difficult to visualize with the unaided eye. As a result, it is easily misdiagnosed.15 Upon palpation of the infected hair shafts, a grainy sensation is evident. Dermoscopy improves visualization, and fluorescence was useful in our case. Microscopic evaluation will identify the adherent organism and is readily cultured on Sabouraud agar.16 Although Trichosporon species typically do not fluoresce,1 Wood lamp examination occasionally may reveal the organism, such as in our case. A possible explanation for this finding is the synergistic relationship with Corynebacterium,17 some producing fluorescent chemicals. Growth of Trichosporon species may be enhanced by or even dependent on Corynebacterium; therefore, WP is likely a coinfection of fungus and bacteria.17,18 Studies also name a novel species of Brevibacterium in relationship with genital WP.19 This species was described as producing a foul odor, as in our patient.
Treatment
The American Academy of Dermatology’s Guidelines Committee recommends complete removal of the infected hairs.1 The recommendation traditionally is hair removal in conjunction with topical or oral medications,1 such as topical imidazoles, ciclopirox olamine, selenium sulfide 2%, chlorhexidine solution, zinc pyrithione, amphotericin B lotion, and oral itraconazole. Recurrence rates are high and spontaneous remission sometimes occurs.9,20 Triazole antifungals currently are preferred for treatment of Trichosporon infections.5 Patients should be counseled to dispose of undergarments, as the organism has been recovered from cotton fibers and are a source of reinfection.1,21
Conclusion
White piedra, though relatively uncommon, is likely underdiagnosed in the United States and should be suspected in any patient presenting with irritation and a foul odor in the genital area or multiple failed therapies for a nonspecific genital dermatitis. This clinical scenario warrants dermoscopic and Wood lamp examination of the affected skin and hair shafts in addition to microscopic examination of pubic hair shafts by a dermatopathologist. Fluorescence under Wood lamp may aid in diagnosis, and conflicting findings may be attributed to its synergistic relationship with Corynebacterium and Brevibacterium coinfection. Proper treatment includes shaving of the affected hair, oral or topical antifungal treatment, and disposal of affected clothing.
- Kiken DA, Sekaran A, Antaya RJ, et al. White piedra in children [published online September 18, 2006]. J Am Acad Dermatol. 2006;55:956-961.
- Walzam M, Leeming JG. White piedra and Trichosporon beigelii: the incidence in patients attending a clinic in genitourinary medicine. Genitourin Med. 1989;16:331-334.
- Horta P. Sobre una nova forma de piedra. Mem Inst Oswaldo Cruz. 1911;3:86-107.
- Fischman O. Black piedra in Brazil: a contribution to its study in Manaus (State of Amazonas). Mycopathol Mycol Appl. 1965;25:201-204.
- Benson PM, Lapins NA, Odom RB. White piedra. Arch Dermatol. 1983;119:602-604.
- Fischman O, Pires de Camargo Z, Meireles MCA. Genital white piedra: an emerging fungal disease? Fifth International Conference on Mycoses. PAHO Sci Publ. 1989;396:70-76.
- Tambe SA, Dhurat SR, Kumar CA, et al. Two cases of scalp piedra caused by Trichosporon ovoides. Indian J Dermatol Venereol Leprol. 2009;75:293-295.
- Kalter DC, Tschen JA, Cernoch PL, et al. Genital white piedra: epidemiology, microbiology, and therapy. J Am Acad Dermatol. 1986;14:982-993.
- James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. United Kingdom: Saunders Elsevier; 2011.
- Sugita T, Nakajima M, Ikeda R, et al. Sequence analysis of the ribosomal DNA intergenic spacer 1 regions of Trichosporon species. J Clin Microbiol. 2002;40:1826-1830.
- Kaplan W. Piedra in lower animals: a case report of white piedra in a monkey and a review of the literature. J Am Vet Med Assoc. 1959;134:113-117.
- Carneiro JA, Assis FA, Filho JT. Piedra branca genital. An Bras Dermatol. 1971;46:265-269.
- Avram A, Buot G, Binet A, et al. Étude clinique et mycologique concernant 11 cas de trichosporie noueuse (piedra blanche) génito-pubienne. Ann Dermatol Venereol. 1987;114:819-827.
- So
bera JO, Elewski BE. Fungal diseases. In: Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. New York, NY: Mosby; 2003:1135-1138. - Gold I, Sommer B, Urson S, et al. White piedra: a frequently misdiagnosed infection of hair. Int J Dermatol. 1984;23:621-623.
- Smith JD, Murtishaw WA, McBride ME. White piedra (Trichosporosis). Arch Dermatol. 1973;107:439-442.
- Youker SR, Andreozzi RJ, Appelbaum PC, et al. White piedra: further evidence of a synergistic infection. J Am Acad Dermatol. 2003;49:746-749.
- Ellner KM, McBride ME, Kalter DC, et al. White piedra: evidence for a synergistic infection. Br J Dermatol. 1990;123:355-363.
- McBride ME, Ellner KM, Black HS, et al. A new Brevibacterium sp. isolated from infected genital hair of patients with white piedra. J Med Microbiol. 1993;39:255-261.
- Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for superficial mycotic infections of the skin: piedra. J Am Acad Dermatol. 1996;34:122-124.
- de Almeida HL Jr, Rivitti EA, Jaeger RG. White piedra: ultrastructure and a new microecological aspect. Mycoses. 1990;33:491-497.
Case Report
A 58-year-old man presented for evaluation of a pruritic rash involving the pubic area of 30 years’ duration. Multiple primary care physicians and dermatologists had evaluated the patient during this period, but he noted a specific diagnosis had not been rendered and multiple treatments had been unsuccessful. The patient described a rash, which was absent at the time of evaluation, as a self-remitting and exacerbating irritation typically induced by sweating and physical activity. The patient also stated that the irritation was associated with a strong, distinct, musty odor that severely interrupted his sex life and decreased his quality of life. Prior treatments included various topical corticosteroids, topical and oral antibiotics, and various homeopathic treatments that were minimally efficacious or nonefficacious. He was unsure if antifungals had previously been prescribed.
The patient’s medical history was notable for pulmonary interstitial fibrosis, anxiety, posttraumatic stress disorder, and mild glucose intolerance. The patient had no pertinent surgical history and no known drug allergies. Current medications included a bronchodilating inhaler, escitalopram, trazodone, buspirone, clonazepam, prazosin, gabapentin, and azithromycin for current upper respiratory tract infection. The patient was a former smoker and a social drinker.
On physical evaluation the pubic area displayed slight patchy erythema without a papular component and was otherwise unremarkable to the unaided eye. Upon palpation of the skin, there were no remarkable findings. Under dermoscopic evaluation, small white-yellow concretions along the hair shaft were noticed. Evaluation with a Wood lamp is shown in Figure 1.

The patient was treated empirically with ketoconazole cream 2% applied to the affected area once daily until follow-up 3 weeks later. The patient also was advised to shave the pubic area to remove potentially infected hairs, as white piedra (WP) was suspected. A diagnosis of WP was confirmed on histologic evaluation of pubic hair samples approximately 1 to 2 weeks later (Figure 2).

At 3-week follow-up, Wood lamp evaluation did not identify concretions along the pubic hair shafts. The patient was symptom free and extremely pleased. Of note, the patient did not shave the pubic area and was counseled on recurrence.
Comment
Piedra (meaning stone in Spanish) describes a group of fungal infections that present with gritty concretions on the hair shaft.1,2 In 1911, Horta3 classified piedra into 2 subtypes: black piedra, caused by Piedraia hortae, and WP, caused by Trichosporon species. Black piedra occurs more frequently in tropical countries and commonly affects hair shafts on the scalp.4 White piedra most commonly affects the pubic area, with rare cases in scalp and facial hair.1,5-7
Epidemiology
White piedra is seen worldwide, including Europe, South America, India, Southeast Asia, Africa, South America, and southern parts of the United States. The majority of cases occur in tropical and temperate regions.1 White piedra likely is underdiagnosed; for example, in a study of 166 young men with genital concerns in Houston, Texas, Trichosporon was isolated in 40% of cultured scrotal hairs.8
Species Identification
There are several species of WP; special techniques must be used to differentiate them, which is beyond the scope of this case. The known species include Trichosporon asahii, Trichosporon asteroides, Trichosporon cutaneum, Trichosporon inkin, Trichosporon mucoides, and Trichosporon ovoides.1Trichosporon asahii and T mucoides have been known to cause systemic infections in immunocompromised hosts known as trichosporonosis.1,9 As an example of a special technique used for species recognition, Sugita et al10 used sequence analysis of the ribosomal DNA intergenic spacer 1 regions to distinguish T asahii isolates. Identification of species may be warranted in the proper clinical scenario; however, histologic evaluation by an experienced dermatopathologist frequently is sufficient to identify the Trichosporon genus.
Transmission
The 2 most common causative organisms of WP are T inkin and T ovoides. Furthermore, T inkin causes the vast majority of WP in the pubic region.8
Diagnosis and Differential
White piedra is characterized by the presence of adherent tan to white nodules along the hair shafts. The concretions tend to be softer than black piedra and, unlike trichomycosis, normally do not fluoresce.1 They do not encircle the hair shaft as hair casts do and can be readily distinguished from Trichomycosis axillaris, black piedra, pediculosis, and trichorrhexis nodosa on microscopic examination.14 The hair shaft concretions of WP are difficult to visualize with the unaided eye. As a result, it is easily misdiagnosed.15 Upon palpation of the infected hair shafts, a grainy sensation is evident. Dermoscopy improves visualization, and fluorescence was useful in our case. Microscopic evaluation will identify the adherent organism and is readily cultured on Sabouraud agar.16 Although Trichosporon species typically do not fluoresce,1 Wood lamp examination occasionally may reveal the organism, such as in our case. A possible explanation for this finding is the synergistic relationship with Corynebacterium,17 some producing fluorescent chemicals. Growth of Trichosporon species may be enhanced by or even dependent on Corynebacterium; therefore, WP is likely a coinfection of fungus and bacteria.17,18 Studies also name a novel species of Brevibacterium in relationship with genital WP.19 This species was described as producing a foul odor, as in our patient.
Treatment
The American Academy of Dermatology’s Guidelines Committee recommends complete removal of the infected hairs.1 The recommendation traditionally is hair removal in conjunction with topical or oral medications,1 such as topical imidazoles, ciclopirox olamine, selenium sulfide 2%, chlorhexidine solution, zinc pyrithione, amphotericin B lotion, and oral itraconazole. Recurrence rates are high and spontaneous remission sometimes occurs.9,20 Triazole antifungals currently are preferred for treatment of Trichosporon infections.5 Patients should be counseled to dispose of undergarments, as the organism has been recovered from cotton fibers and are a source of reinfection.1,21
Conclusion
White piedra, though relatively uncommon, is likely underdiagnosed in the United States and should be suspected in any patient presenting with irritation and a foul odor in the genital area or multiple failed therapies for a nonspecific genital dermatitis. This clinical scenario warrants dermoscopic and Wood lamp examination of the affected skin and hair shafts in addition to microscopic examination of pubic hair shafts by a dermatopathologist. Fluorescence under Wood lamp may aid in diagnosis, and conflicting findings may be attributed to its synergistic relationship with Corynebacterium and Brevibacterium coinfection. Proper treatment includes shaving of the affected hair, oral or topical antifungal treatment, and disposal of affected clothing.
Case Report
A 58-year-old man presented for evaluation of a pruritic rash involving the pubic area of 30 years’ duration. Multiple primary care physicians and dermatologists had evaluated the patient during this period, but he noted a specific diagnosis had not been rendered and multiple treatments had been unsuccessful. The patient described a rash, which was absent at the time of evaluation, as a self-remitting and exacerbating irritation typically induced by sweating and physical activity. The patient also stated that the irritation was associated with a strong, distinct, musty odor that severely interrupted his sex life and decreased his quality of life. Prior treatments included various topical corticosteroids, topical and oral antibiotics, and various homeopathic treatments that were minimally efficacious or nonefficacious. He was unsure if antifungals had previously been prescribed.
The patient’s medical history was notable for pulmonary interstitial fibrosis, anxiety, posttraumatic stress disorder, and mild glucose intolerance. The patient had no pertinent surgical history and no known drug allergies. Current medications included a bronchodilating inhaler, escitalopram, trazodone, buspirone, clonazepam, prazosin, gabapentin, and azithromycin for current upper respiratory tract infection. The patient was a former smoker and a social drinker.
On physical evaluation the pubic area displayed slight patchy erythema without a papular component and was otherwise unremarkable to the unaided eye. Upon palpation of the skin, there were no remarkable findings. Under dermoscopic evaluation, small white-yellow concretions along the hair shaft were noticed. Evaluation with a Wood lamp is shown in Figure 1.

The patient was treated empirically with ketoconazole cream 2% applied to the affected area once daily until follow-up 3 weeks later. The patient also was advised to shave the pubic area to remove potentially infected hairs, as white piedra (WP) was suspected. A diagnosis of WP was confirmed on histologic evaluation of pubic hair samples approximately 1 to 2 weeks later (Figure 2).

At 3-week follow-up, Wood lamp evaluation did not identify concretions along the pubic hair shafts. The patient was symptom free and extremely pleased. Of note, the patient did not shave the pubic area and was counseled on recurrence.
Comment
Piedra (meaning stone in Spanish) describes a group of fungal infections that present with gritty concretions on the hair shaft.1,2 In 1911, Horta3 classified piedra into 2 subtypes: black piedra, caused by Piedraia hortae, and WP, caused by Trichosporon species. Black piedra occurs more frequently in tropical countries and commonly affects hair shafts on the scalp.4 White piedra most commonly affects the pubic area, with rare cases in scalp and facial hair.1,5-7
Epidemiology
White piedra is seen worldwide, including Europe, South America, India, Southeast Asia, Africa, South America, and southern parts of the United States. The majority of cases occur in tropical and temperate regions.1 White piedra likely is underdiagnosed; for example, in a study of 166 young men with genital concerns in Houston, Texas, Trichosporon was isolated in 40% of cultured scrotal hairs.8
Species Identification
There are several species of WP; special techniques must be used to differentiate them, which is beyond the scope of this case. The known species include Trichosporon asahii, Trichosporon asteroides, Trichosporon cutaneum, Trichosporon inkin, Trichosporon mucoides, and Trichosporon ovoides.1Trichosporon asahii and T mucoides have been known to cause systemic infections in immunocompromised hosts known as trichosporonosis.1,9 As an example of a special technique used for species recognition, Sugita et al10 used sequence analysis of the ribosomal DNA intergenic spacer 1 regions to distinguish T asahii isolates. Identification of species may be warranted in the proper clinical scenario; however, histologic evaluation by an experienced dermatopathologist frequently is sufficient to identify the Trichosporon genus.
Transmission
The 2 most common causative organisms of WP are T inkin and T ovoides. Furthermore, T inkin causes the vast majority of WP in the pubic region.8
Diagnosis and Differential
White piedra is characterized by the presence of adherent tan to white nodules along the hair shafts. The concretions tend to be softer than black piedra and, unlike trichomycosis, normally do not fluoresce.1 They do not encircle the hair shaft as hair casts do and can be readily distinguished from Trichomycosis axillaris, black piedra, pediculosis, and trichorrhexis nodosa on microscopic examination.14 The hair shaft concretions of WP are difficult to visualize with the unaided eye. As a result, it is easily misdiagnosed.15 Upon palpation of the infected hair shafts, a grainy sensation is evident. Dermoscopy improves visualization, and fluorescence was useful in our case. Microscopic evaluation will identify the adherent organism and is readily cultured on Sabouraud agar.16 Although Trichosporon species typically do not fluoresce,1 Wood lamp examination occasionally may reveal the organism, such as in our case. A possible explanation for this finding is the synergistic relationship with Corynebacterium,17 some producing fluorescent chemicals. Growth of Trichosporon species may be enhanced by or even dependent on Corynebacterium; therefore, WP is likely a coinfection of fungus and bacteria.17,18 Studies also name a novel species of Brevibacterium in relationship with genital WP.19 This species was described as producing a foul odor, as in our patient.
Treatment
The American Academy of Dermatology’s Guidelines Committee recommends complete removal of the infected hairs.1 The recommendation traditionally is hair removal in conjunction with topical or oral medications,1 such as topical imidazoles, ciclopirox olamine, selenium sulfide 2%, chlorhexidine solution, zinc pyrithione, amphotericin B lotion, and oral itraconazole. Recurrence rates are high and spontaneous remission sometimes occurs.9,20 Triazole antifungals currently are preferred for treatment of Trichosporon infections.5 Patients should be counseled to dispose of undergarments, as the organism has been recovered from cotton fibers and are a source of reinfection.1,21
Conclusion
White piedra, though relatively uncommon, is likely underdiagnosed in the United States and should be suspected in any patient presenting with irritation and a foul odor in the genital area or multiple failed therapies for a nonspecific genital dermatitis. This clinical scenario warrants dermoscopic and Wood lamp examination of the affected skin and hair shafts in addition to microscopic examination of pubic hair shafts by a dermatopathologist. Fluorescence under Wood lamp may aid in diagnosis, and conflicting findings may be attributed to its synergistic relationship with Corynebacterium and Brevibacterium coinfection. Proper treatment includes shaving of the affected hair, oral or topical antifungal treatment, and disposal of affected clothing.
- Kiken DA, Sekaran A, Antaya RJ, et al. White piedra in children [published online September 18, 2006]. J Am Acad Dermatol. 2006;55:956-961.
- Walzam M, Leeming JG. White piedra and Trichosporon beigelii: the incidence in patients attending a clinic in genitourinary medicine. Genitourin Med. 1989;16:331-334.
- Horta P. Sobre una nova forma de piedra. Mem Inst Oswaldo Cruz. 1911;3:86-107.
- Fischman O. Black piedra in Brazil: a contribution to its study in Manaus (State of Amazonas). Mycopathol Mycol Appl. 1965;25:201-204.
- Benson PM, Lapins NA, Odom RB. White piedra. Arch Dermatol. 1983;119:602-604.
- Fischman O, Pires de Camargo Z, Meireles MCA. Genital white piedra: an emerging fungal disease? Fifth International Conference on Mycoses. PAHO Sci Publ. 1989;396:70-76.
- Tambe SA, Dhurat SR, Kumar CA, et al. Two cases of scalp piedra caused by Trichosporon ovoides. Indian J Dermatol Venereol Leprol. 2009;75:293-295.
- Kalter DC, Tschen JA, Cernoch PL, et al. Genital white piedra: epidemiology, microbiology, and therapy. J Am Acad Dermatol. 1986;14:982-993.
- James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. United Kingdom: Saunders Elsevier; 2011.
- Sugita T, Nakajima M, Ikeda R, et al. Sequence analysis of the ribosomal DNA intergenic spacer 1 regions of Trichosporon species. J Clin Microbiol. 2002;40:1826-1830.
- Kaplan W. Piedra in lower animals: a case report of white piedra in a monkey and a review of the literature. J Am Vet Med Assoc. 1959;134:113-117.
- Carneiro JA, Assis FA, Filho JT. Piedra branca genital. An Bras Dermatol. 1971;46:265-269.
- Avram A, Buot G, Binet A, et al. Étude clinique et mycologique concernant 11 cas de trichosporie noueuse (piedra blanche) génito-pubienne. Ann Dermatol Venereol. 1987;114:819-827.
- So
bera JO, Elewski BE. Fungal diseases. In: Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. New York, NY: Mosby; 2003:1135-1138. - Gold I, Sommer B, Urson S, et al. White piedra: a frequently misdiagnosed infection of hair. Int J Dermatol. 1984;23:621-623.
- Smith JD, Murtishaw WA, McBride ME. White piedra (Trichosporosis). Arch Dermatol. 1973;107:439-442.
- Youker SR, Andreozzi RJ, Appelbaum PC, et al. White piedra: further evidence of a synergistic infection. J Am Acad Dermatol. 2003;49:746-749.
- Ellner KM, McBride ME, Kalter DC, et al. White piedra: evidence for a synergistic infection. Br J Dermatol. 1990;123:355-363.
- McBride ME, Ellner KM, Black HS, et al. A new Brevibacterium sp. isolated from infected genital hair of patients with white piedra. J Med Microbiol. 1993;39:255-261.
- Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for superficial mycotic infections of the skin: piedra. J Am Acad Dermatol. 1996;34:122-124.
- de Almeida HL Jr, Rivitti EA, Jaeger RG. White piedra: ultrastructure and a new microecological aspect. Mycoses. 1990;33:491-497.
- Kiken DA, Sekaran A, Antaya RJ, et al. White piedra in children [published online September 18, 2006]. J Am Acad Dermatol. 2006;55:956-961.
- Walzam M, Leeming JG. White piedra and Trichosporon beigelii: the incidence in patients attending a clinic in genitourinary medicine. Genitourin Med. 1989;16:331-334.
- Horta P. Sobre una nova forma de piedra. Mem Inst Oswaldo Cruz. 1911;3:86-107.
- Fischman O. Black piedra in Brazil: a contribution to its study in Manaus (State of Amazonas). Mycopathol Mycol Appl. 1965;25:201-204.
- Benson PM, Lapins NA, Odom RB. White piedra. Arch Dermatol. 1983;119:602-604.
- Fischman O, Pires de Camargo Z, Meireles MCA. Genital white piedra: an emerging fungal disease? Fifth International Conference on Mycoses. PAHO Sci Publ. 1989;396:70-76.
- Tambe SA, Dhurat SR, Kumar CA, et al. Two cases of scalp piedra caused by Trichosporon ovoides. Indian J Dermatol Venereol Leprol. 2009;75:293-295.
- Kalter DC, Tschen JA, Cernoch PL, et al. Genital white piedra: epidemiology, microbiology, and therapy. J Am Acad Dermatol. 1986;14:982-993.
- James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. United Kingdom: Saunders Elsevier; 2011.
- Sugita T, Nakajima M, Ikeda R, et al. Sequence analysis of the ribosomal DNA intergenic spacer 1 regions of Trichosporon species. J Clin Microbiol. 2002;40:1826-1830.
- Kaplan W. Piedra in lower animals: a case report of white piedra in a monkey and a review of the literature. J Am Vet Med Assoc. 1959;134:113-117.
- Carneiro JA, Assis FA, Filho JT. Piedra branca genital. An Bras Dermatol. 1971;46:265-269.
- Avram A, Buot G, Binet A, et al. Étude clinique et mycologique concernant 11 cas de trichosporie noueuse (piedra blanche) génito-pubienne. Ann Dermatol Venereol. 1987;114:819-827.
- So
bera JO, Elewski BE. Fungal diseases. In: Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. New York, NY: Mosby; 2003:1135-1138. - Gold I, Sommer B, Urson S, et al. White piedra: a frequently misdiagnosed infection of hair. Int J Dermatol. 1984;23:621-623.
- Smith JD, Murtishaw WA, McBride ME. White piedra (Trichosporosis). Arch Dermatol. 1973;107:439-442.
- Youker SR, Andreozzi RJ, Appelbaum PC, et al. White piedra: further evidence of a synergistic infection. J Am Acad Dermatol. 2003;49:746-749.
- Ellner KM, McBride ME, Kalter DC, et al. White piedra: evidence for a synergistic infection. Br J Dermatol. 1990;123:355-363.
- McBride ME, Ellner KM, Black HS, et al. A new Brevibacterium sp. isolated from infected genital hair of patients with white piedra. J Med Microbiol. 1993;39:255-261.
- Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for superficial mycotic infections of the skin: piedra. J Am Acad Dermatol. 1996;34:122-124.
- de Almeida HL Jr, Rivitti EA, Jaeger RG. White piedra: ultrastructure and a new microecological aspect. Mycoses. 1990;33:491-497.
- Although relatively uncommon, white piedra should be suspected in any patient presenting with irritation and foul odor in the genital area or multiple failed therapies for a nonspecific genital dermatitis.
- Wood lamp and dermoscopy should be used to evaluate for parasitic infections of the pubic hair shafts when nonspecific dermatitis presents in this area.