User login
SAN DIEGO – How to prevent sudden arrhythmic death in vulnerable but currently unprotected populations is being addressed by ongoing studies that variously evaluate a pharmacologic, an implanted device-based, or a wearable solution, according to Dr. Bruce D. Lindsay.
Dr. Lindsay, head of the cardiac electrophysiology and pacing section at the Cleveland Clinic, presented an overview of selected major ongoing clinical trials in electrophysiology at the annual meeting of the American College of Cardiology. He focused on five hot topics: prevention of sudden death, atrial fibrillation (AF) ablation, prevention of implantable device-related infections, device-based treatment of heart failure, and leadless pacing systems.
Preventing sudden death
Ongoing phase III trials are evaluating the safety, tolerability, and efficacy of an oral Gilead drug known for now as GS-6615. The drug, a selective late sodium current inhibitor, is designed to shorten the corrected QTc interval in patients with several forms of long QT syndrome.
A different approach is being studied in REFINE-ICD (Risk Estimation Following Infarction Noninvasive Evaluation – ICD Efficacy), a 1,400-subject trial recruiting patients who’ve had an acute MI within the previous year, have abnormal findings on 24-hour Holter monitoring, and have moderate left ventricular dysfunction as defined by an ejection fraction of 35%-50%. The trial will assess whether prophylactic placement of an implantable cardioverter-defibrillator (ICD) guided by noninvasive risk assessment based on heart rate turbulence and T-wave alternans analysis will reduce mortality in MI survivors.
“This is a group that’s at lower risk than current ICD recipients, but because it’s such a large group they account for a lot of sudden deaths,” the cardiologist said.
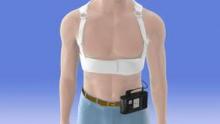
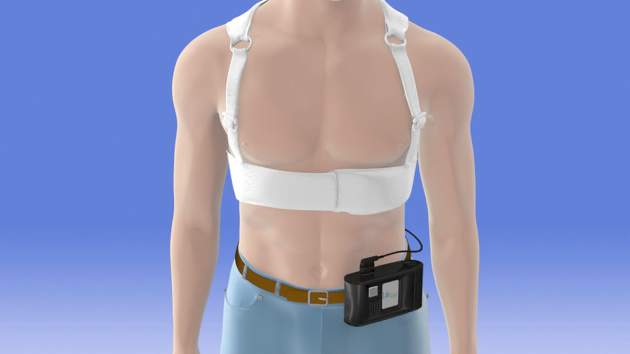
Another phase III trial currently recruiting participants is a 1,900-patient postmarketing study aimed at defining which patients benefit from using the LifeVest wearable defibrillator during the first 3 months following an acute MI resulting in ventricular dysfunction.
AF ablation
The most important ongoing study in this field, in Dr. Lindsay’s view, is CABANA (Catheter Ablation Versus Anti-Arrhythmic Drug Therapy for Atrial Fibrillation Trial). This National Institutes of Health–sponsored study is aimed at showing whether ablation is superior to rate or rhythm control drug therapy in terms of all-cause mortality, disabling stroke, serious bleeding, and/or cardiac arrest. Secondary endpoints include cost, quality of life, hospitalization rates, and the relationship of left atrial size to progression of AF and its contribution to morbidity and mortality.
A promising, innovative ablation strategy known as focal impulse and rotor ablation for paroxysmal AF is under evaluation in the German REAFFIRM (Randomized Evaluation of Atrial Fibrillation Treatment With Focal Impulse and Rotor Modulation Guided Procedures). A similar U.S. study known as FIRMAT-PAF (Focal Impulse and Rotor Modulation Ablation Trial for Treatment of Paroxysmal Atrial Fibrillation) had to be abandoned, however, because of its inability to recruit patients.
New ablation technologies are also being introduced. Three pivotal trials totaling roughly 1,500 patients are evaluating the Biosense Webster nMARQ multielectrode irrigated catheter for paroxysmal AF in the reMARQable trial; a Medtronic phased radiofrequency ablation catheter for persistent AF in the VICTORY AF study; and a CardioFocus endoscopic ablation catheter for paroxysmal AF.
Preventing cardiac implantable device infections
WRAP-IT (the World-Wide Randomized Antibiotic Envelope Infection Trial) is currently enrolling 7,000 patients at 225 sites. This is a Merck-sponsored randomized, prospective, single-blind postmarketing study examining the ability of a proprietary mesh envelope to reduce major infections and costs in the 12 months following device generator replacement, upgrade, or revision, or new implantation of a cardiac resynchronization device. The Tyrx mesh envelope releases minocycline and rifampin for at least 7 days, then eventually becomes fully absorbed.
“Device infection is a huge problem in our field,” Dr. Lindsay noted. “This envelope may have important implications at large, or it may prove to be especially useful in people at high risk for infection.”
Heart failure
Vagal nerve stimulation via an implantable system is one of the hottest areas in the field of heart failure, the electrophysiologist said. Two major trials are ongoing: the Sorin-sponsored VANGUARD (Vagal Nerve Stimulation: Safeguarding Heart Failure Patients) trial, and INNOVATE HF (Increase of Vagal Tone in CHF), sponsored by Biocontrol Medical.
Leadless pacing
St. Jude’s Nanostim and Medtronic’s Micra are very small leadless devices implanted in the right ventricular apex via minimally invasive techniques. Both devices are investigational in the United States, although the Nanostim is approved in Europe. Clinical interest is enormous because lead-related problems have always been the Achilles’ heel of pacemaker therapy. While the Nanostim and Micra can be utilized only for single right ventricular pacing in VVI or VVIR mode, Dr. Lindsay said further advances, including leadless dual-chamber sensing and pacing and biventricular pacing, are likely.
He reported serving as a consultant to Biosense Webster, Boston Scientific, and Medtronic.
SAN DIEGO – How to prevent sudden arrhythmic death in vulnerable but currently unprotected populations is being addressed by ongoing studies that variously evaluate a pharmacologic, an implanted device-based, or a wearable solution, according to Dr. Bruce D. Lindsay.
Dr. Lindsay, head of the cardiac electrophysiology and pacing section at the Cleveland Clinic, presented an overview of selected major ongoing clinical trials in electrophysiology at the annual meeting of the American College of Cardiology. He focused on five hot topics: prevention of sudden death, atrial fibrillation (AF) ablation, prevention of implantable device-related infections, device-based treatment of heart failure, and leadless pacing systems.
Preventing sudden death
Ongoing phase III trials are evaluating the safety, tolerability, and efficacy of an oral Gilead drug known for now as GS-6615. The drug, a selective late sodium current inhibitor, is designed to shorten the corrected QTc interval in patients with several forms of long QT syndrome.
A different approach is being studied in REFINE-ICD (Risk Estimation Following Infarction Noninvasive Evaluation – ICD Efficacy), a 1,400-subject trial recruiting patients who’ve had an acute MI within the previous year, have abnormal findings on 24-hour Holter monitoring, and have moderate left ventricular dysfunction as defined by an ejection fraction of 35%-50%. The trial will assess whether prophylactic placement of an implantable cardioverter-defibrillator (ICD) guided by noninvasive risk assessment based on heart rate turbulence and T-wave alternans analysis will reduce mortality in MI survivors.
“This is a group that’s at lower risk than current ICD recipients, but because it’s such a large group they account for a lot of sudden deaths,” the cardiologist said.
Another phase III trial currently recruiting participants is a 1,900-patient postmarketing study aimed at defining which patients benefit from using the LifeVest wearable defibrillator during the first 3 months following an acute MI resulting in ventricular dysfunction.
AF ablation
The most important ongoing study in this field, in Dr. Lindsay’s view, is CABANA (Catheter Ablation Versus Anti-Arrhythmic Drug Therapy for Atrial Fibrillation Trial). This National Institutes of Health–sponsored study is aimed at showing whether ablation is superior to rate or rhythm control drug therapy in terms of all-cause mortality, disabling stroke, serious bleeding, and/or cardiac arrest. Secondary endpoints include cost, quality of life, hospitalization rates, and the relationship of left atrial size to progression of AF and its contribution to morbidity and mortality.
A promising, innovative ablation strategy known as focal impulse and rotor ablation for paroxysmal AF is under evaluation in the German REAFFIRM (Randomized Evaluation of Atrial Fibrillation Treatment With Focal Impulse and Rotor Modulation Guided Procedures). A similar U.S. study known as FIRMAT-PAF (Focal Impulse and Rotor Modulation Ablation Trial for Treatment of Paroxysmal Atrial Fibrillation) had to be abandoned, however, because of its inability to recruit patients.
New ablation technologies are also being introduced. Three pivotal trials totaling roughly 1,500 patients are evaluating the Biosense Webster nMARQ multielectrode irrigated catheter for paroxysmal AF in the reMARQable trial; a Medtronic phased radiofrequency ablation catheter for persistent AF in the VICTORY AF study; and a CardioFocus endoscopic ablation catheter for paroxysmal AF.
Preventing cardiac implantable device infections
WRAP-IT (the World-Wide Randomized Antibiotic Envelope Infection Trial) is currently enrolling 7,000 patients at 225 sites. This is a Merck-sponsored randomized, prospective, single-blind postmarketing study examining the ability of a proprietary mesh envelope to reduce major infections and costs in the 12 months following device generator replacement, upgrade, or revision, or new implantation of a cardiac resynchronization device. The Tyrx mesh envelope releases minocycline and rifampin for at least 7 days, then eventually becomes fully absorbed.
“Device infection is a huge problem in our field,” Dr. Lindsay noted. “This envelope may have important implications at large, or it may prove to be especially useful in people at high risk for infection.”
Heart failure
Vagal nerve stimulation via an implantable system is one of the hottest areas in the field of heart failure, the electrophysiologist said. Two major trials are ongoing: the Sorin-sponsored VANGUARD (Vagal Nerve Stimulation: Safeguarding Heart Failure Patients) trial, and INNOVATE HF (Increase of Vagal Tone in CHF), sponsored by Biocontrol Medical.
Leadless pacing
St. Jude’s Nanostim and Medtronic’s Micra are very small leadless devices implanted in the right ventricular apex via minimally invasive techniques. Both devices are investigational in the United States, although the Nanostim is approved in Europe. Clinical interest is enormous because lead-related problems have always been the Achilles’ heel of pacemaker therapy. While the Nanostim and Micra can be utilized only for single right ventricular pacing in VVI or VVIR mode, Dr. Lindsay said further advances, including leadless dual-chamber sensing and pacing and biventricular pacing, are likely.
He reported serving as a consultant to Biosense Webster, Boston Scientific, and Medtronic.
SAN DIEGO – How to prevent sudden arrhythmic death in vulnerable but currently unprotected populations is being addressed by ongoing studies that variously evaluate a pharmacologic, an implanted device-based, or a wearable solution, according to Dr. Bruce D. Lindsay.
Dr. Lindsay, head of the cardiac electrophysiology and pacing section at the Cleveland Clinic, presented an overview of selected major ongoing clinical trials in electrophysiology at the annual meeting of the American College of Cardiology. He focused on five hot topics: prevention of sudden death, atrial fibrillation (AF) ablation, prevention of implantable device-related infections, device-based treatment of heart failure, and leadless pacing systems.
Preventing sudden death
Ongoing phase III trials are evaluating the safety, tolerability, and efficacy of an oral Gilead drug known for now as GS-6615. The drug, a selective late sodium current inhibitor, is designed to shorten the corrected QTc interval in patients with several forms of long QT syndrome.
A different approach is being studied in REFINE-ICD (Risk Estimation Following Infarction Noninvasive Evaluation – ICD Efficacy), a 1,400-subject trial recruiting patients who’ve had an acute MI within the previous year, have abnormal findings on 24-hour Holter monitoring, and have moderate left ventricular dysfunction as defined by an ejection fraction of 35%-50%. The trial will assess whether prophylactic placement of an implantable cardioverter-defibrillator (ICD) guided by noninvasive risk assessment based on heart rate turbulence and T-wave alternans analysis will reduce mortality in MI survivors.
“This is a group that’s at lower risk than current ICD recipients, but because it’s such a large group they account for a lot of sudden deaths,” the cardiologist said.
Another phase III trial currently recruiting participants is a 1,900-patient postmarketing study aimed at defining which patients benefit from using the LifeVest wearable defibrillator during the first 3 months following an acute MI resulting in ventricular dysfunction.
AF ablation
The most important ongoing study in this field, in Dr. Lindsay’s view, is CABANA (Catheter Ablation Versus Anti-Arrhythmic Drug Therapy for Atrial Fibrillation Trial). This National Institutes of Health–sponsored study is aimed at showing whether ablation is superior to rate or rhythm control drug therapy in terms of all-cause mortality, disabling stroke, serious bleeding, and/or cardiac arrest. Secondary endpoints include cost, quality of life, hospitalization rates, and the relationship of left atrial size to progression of AF and its contribution to morbidity and mortality.
A promising, innovative ablation strategy known as focal impulse and rotor ablation for paroxysmal AF is under evaluation in the German REAFFIRM (Randomized Evaluation of Atrial Fibrillation Treatment With Focal Impulse and Rotor Modulation Guided Procedures). A similar U.S. study known as FIRMAT-PAF (Focal Impulse and Rotor Modulation Ablation Trial for Treatment of Paroxysmal Atrial Fibrillation) had to be abandoned, however, because of its inability to recruit patients.
New ablation technologies are also being introduced. Three pivotal trials totaling roughly 1,500 patients are evaluating the Biosense Webster nMARQ multielectrode irrigated catheter for paroxysmal AF in the reMARQable trial; a Medtronic phased radiofrequency ablation catheter for persistent AF in the VICTORY AF study; and a CardioFocus endoscopic ablation catheter for paroxysmal AF.
Preventing cardiac implantable device infections
WRAP-IT (the World-Wide Randomized Antibiotic Envelope Infection Trial) is currently enrolling 7,000 patients at 225 sites. This is a Merck-sponsored randomized, prospective, single-blind postmarketing study examining the ability of a proprietary mesh envelope to reduce major infections and costs in the 12 months following device generator replacement, upgrade, or revision, or new implantation of a cardiac resynchronization device. The Tyrx mesh envelope releases minocycline and rifampin for at least 7 days, then eventually becomes fully absorbed.
“Device infection is a huge problem in our field,” Dr. Lindsay noted. “This envelope may have important implications at large, or it may prove to be especially useful in people at high risk for infection.”
Heart failure
Vagal nerve stimulation via an implantable system is one of the hottest areas in the field of heart failure, the electrophysiologist said. Two major trials are ongoing: the Sorin-sponsored VANGUARD (Vagal Nerve Stimulation: Safeguarding Heart Failure Patients) trial, and INNOVATE HF (Increase of Vagal Tone in CHF), sponsored by Biocontrol Medical.
Leadless pacing
St. Jude’s Nanostim and Medtronic’s Micra are very small leadless devices implanted in the right ventricular apex via minimally invasive techniques. Both devices are investigational in the United States, although the Nanostim is approved in Europe. Clinical interest is enormous because lead-related problems have always been the Achilles’ heel of pacemaker therapy. While the Nanostim and Micra can be utilized only for single right ventricular pacing in VVI or VVIR mode, Dr. Lindsay said further advances, including leadless dual-chamber sensing and pacing and biventricular pacing, are likely.
He reported serving as a consultant to Biosense Webster, Boston Scientific, and Medtronic.
EXPERT ANALYSIS FROM ACC 15