User login
, a new analysis suggests.
The finding comes from a small study that entered such patients with paroxysmal and especially persistent AFib who were candidates for ablation. Those shedding at least 3% of body weight in the months before the procedure while engaged in a structured risk-factor modification (RFM) program were “dramatically” more likely to be AFib-free 6 months later.
The improved ablation efficacy, compared with results in similar patients who didn’t lose as much weight, was most pronounced among those whose AFib had been the persistent form, reported investigators at the annual scientific sessions of the Heart Rhythm Society, held in New Orleans.
Of note, ablations in the study were consistently limited, as much as possible, to standard pulmonary-vein isolation (PVI).
Associations between AFib and obesity and other behavioral and lifestyle-related risk factors are well recognized, but the limited studies of their effect on AFib ablation success have been inconsistent. The current analysis, the group says, points specifically to preablation weight loss as means to improving AFib-ablation outcomes.
“Adjunctive therapy focused on weight loss should be incorporated in the treatment plan for obese patients undergoing ablation for atrial fibrillation,” Jeffrey J. Goldberger, MD, MBA, of the University of Miami, said when presenting the new results at the HRS sessions.
Such a plan is entirely consistent with recent guidelines and especially a 2020 American Heart Association (AHA) consensus statement, but is inconsistently and perhaps even seldom realized in clinical practice.
Dramatic increase in success
Even modest weight loss before ablation may help, proposed Dr. Goldberger, who directs his institution’s Center for Atrial Fibrillation. Decreases for the greater-weight-loss group actually averaged less than 6% of baseline body weight.
Yet it was apparently enough to improve ablation outcomes significantly: Eighty-eight percent were free of AFib 6 months after the procedure, compared with 61% for patients who lost less than 3% of their preablation weight.
For improving ablation success, he said, “We’re talking about a moderate amount of weight loss. These patients are not going from being obese to being thin. They’re still quite overweight.”
In an analysis limited to the four-fifths of patients with persistent AFib, “we saw the same pattern,” Dr. Goldberger said at a media presentation prior to his formal report at the HRS sessions.
Moreover, that subgroup’s benefit persisted out to 12 months, at which time 42% and 81% of patients with less and greater weight loss, respectively, were free of AFib. That represents, he said, “a really tremendous – dramatic, actually – increase in success of pulmonary vein isolation in those who lost weight.”
“We’ve known for a long time that weight loss is important for preventing atrial fibrillation or increasing the success rates of the different treatments we use,” Cynthia M. Tracy, MD, said in an interview. “Probably in some studies, weight loss has been as effective as antiarrhythmics.”
A loss of 3% body weight “is not a lot,” she said. In the current analysis, “It’s notable that it made that much difference with even a fairly modest amount of weight loss.”
Now when asked, “ ‘How much do I have to lose before you’ll consider doing my ablation?’ we have a bit more concrete data to give patients and doctors as to what amount might be beneficial,” said Dr. Tracy of George Washington University Hospital, Washington, who is not associated with the study.
Evolving view of AFib
The findings are emblematic of the profession’s evolving view of AFib and its management, Dr. Goldberger observed at the press conference. Should clinicians think of AFib as similar to “a disease like Wolff-Parkinson-White syndrome,” in which the patient usually has a successful ablation, and then “we expect that to last in perpetuity with no further interventions?”
Or, he said, “is atrial fibrillation more a disease like coronary artery disease, where even if they have an intervention, the disease process is still ongoing and requires long-term disease management? I think it’s pretty clear that we’re dealing with the latter case.”
Dr. Goldberger’s report was an interim analysis of an ongoing randomized trial called LEAF (Liraglutide Effect on Atrial Fibrillation), which is comparing patients with AFib assigned to “take” vs. “not take” the GLP-1 receptor agonist liraglutide, an antidiabetic (Victoza) and weight-loss (Saxenda) drug. The trial aims to assess the drug’s apparent ability to shrink atrial epicardial adipose tissue which, Dr. Goldberger said, is thought to contribute to AFib development and influence AFib-ablation outcomes.
It’s unknown and a limitation of the current analysis, he said, whether the observed link between improved preablation–weight ablation success “is specifically related to weight loss, liraglutide treatment, or both.”
As the invited discussant for Dr. Goldberger’s presentation, David Frankel, MD, observed that studies have been inconsistent on whether substantial weight loss may improve the results of AFib rhythm-control therapy.
Those finding such an association, including LEAF and the influential LEGACY study, differed from others showing a null effect by including “a comprehensive risk factor management” program, observed Dr. Frankel, of the Hospital of the University of Pennsylvania and Penn Heart and Vascular Center, Philadelphia.
Rather than focusing solely on weight loss or sleep apnea as AFib risk factors, he said, the studies linking weight loss to AFib rhythm control also included “hypertension, diabetes, hyperlipidemia, smoking cessation, and alcohol reduction,” Dr. Frankel said. “So it seems clear that to significantly impact AF recurrence, we need to focus on all these contributors to metabolic syndrome.”
Comprehensive risk-factor management
LEAF entered patients with AFib, 79% of whom had persistent AF and the rest paroxysmal AF, who followed the RFM program and were randomly assigned also to take liraglutide or placebo. The “nurse-practitioner-led” RFM program, conducted both in-clinic and online, featured “established goals for each patient” using AHA diet and lifestyle recommendations, an exercise prescription, dietary counseling, evaluation and treatment of sleep apnea, and measures to control any diabetes, hyperlipidemia, or hypertension, Dr. Goldberger said. And patients “were counseled on alcohol reduction and smoking cessation as necessary.”
After 3 months, 29 and 30 patients – regardless of randomization assignment – had lost < 3% and at least 3% of baseline body weight, respectively.
Catheter ablation achieved PVI in all patients. A 3-month blanking period followed, after which they went off antiarrhythmic meds.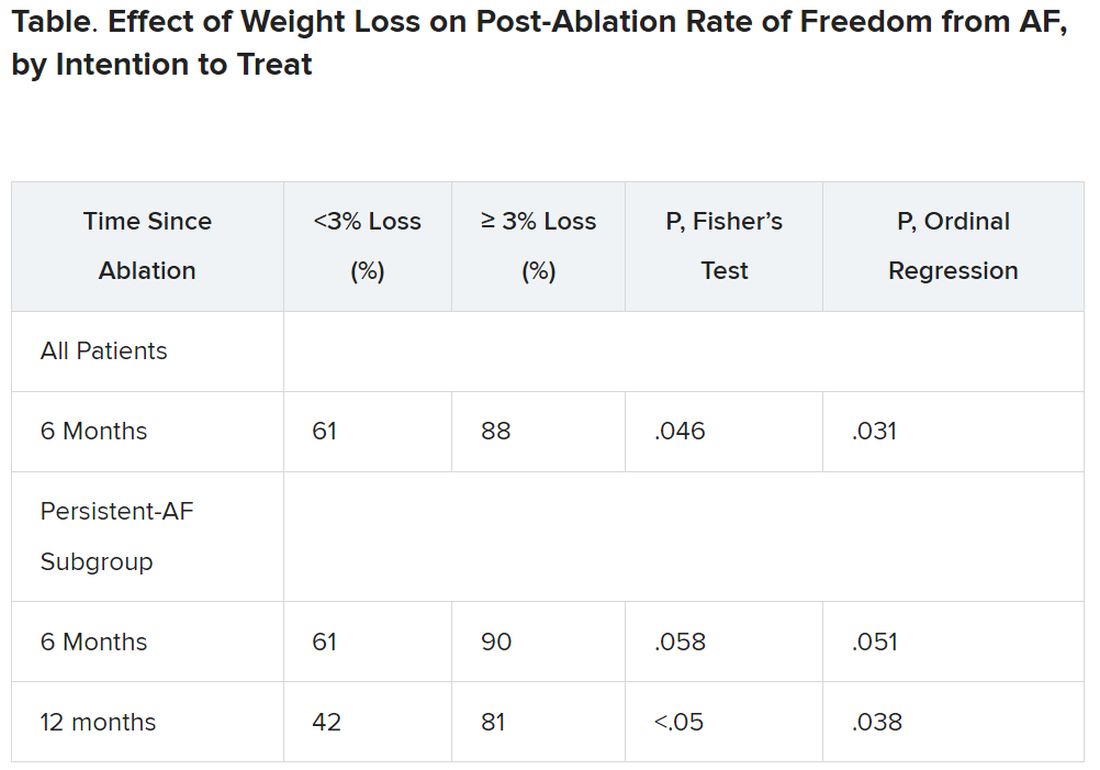
It’s very difficult for patients to lose 10% or more of body weight, “and it would not happen overnight,” Dr. Tracy observed. “These are symptomatic patients, for the most part, if they get referred to an electrophysiologist. So you don’t want to defer them indefinitely.”
The current findings, she said, point to “a more realistic target,” suggesting that weight loss of at least 3% should improve AFib ablation’s chances for success.
Dr. Goldberger disclosed ties to Medtronic. Dr. Frankel disclosed ties to Medtronic, Stryker, Biosense Webster, and Boston Scientific. Dr. Tracy reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new analysis suggests.
The finding comes from a small study that entered such patients with paroxysmal and especially persistent AFib who were candidates for ablation. Those shedding at least 3% of body weight in the months before the procedure while engaged in a structured risk-factor modification (RFM) program were “dramatically” more likely to be AFib-free 6 months later.
The improved ablation efficacy, compared with results in similar patients who didn’t lose as much weight, was most pronounced among those whose AFib had been the persistent form, reported investigators at the annual scientific sessions of the Heart Rhythm Society, held in New Orleans.
Of note, ablations in the study were consistently limited, as much as possible, to standard pulmonary-vein isolation (PVI).
Associations between AFib and obesity and other behavioral and lifestyle-related risk factors are well recognized, but the limited studies of their effect on AFib ablation success have been inconsistent. The current analysis, the group says, points specifically to preablation weight loss as means to improving AFib-ablation outcomes.
“Adjunctive therapy focused on weight loss should be incorporated in the treatment plan for obese patients undergoing ablation for atrial fibrillation,” Jeffrey J. Goldberger, MD, MBA, of the University of Miami, said when presenting the new results at the HRS sessions.
Such a plan is entirely consistent with recent guidelines and especially a 2020 American Heart Association (AHA) consensus statement, but is inconsistently and perhaps even seldom realized in clinical practice.
Dramatic increase in success
Even modest weight loss before ablation may help, proposed Dr. Goldberger, who directs his institution’s Center for Atrial Fibrillation. Decreases for the greater-weight-loss group actually averaged less than 6% of baseline body weight.
Yet it was apparently enough to improve ablation outcomes significantly: Eighty-eight percent were free of AFib 6 months after the procedure, compared with 61% for patients who lost less than 3% of their preablation weight.
For improving ablation success, he said, “We’re talking about a moderate amount of weight loss. These patients are not going from being obese to being thin. They’re still quite overweight.”
In an analysis limited to the four-fifths of patients with persistent AFib, “we saw the same pattern,” Dr. Goldberger said at a media presentation prior to his formal report at the HRS sessions.
Moreover, that subgroup’s benefit persisted out to 12 months, at which time 42% and 81% of patients with less and greater weight loss, respectively, were free of AFib. That represents, he said, “a really tremendous – dramatic, actually – increase in success of pulmonary vein isolation in those who lost weight.”
“We’ve known for a long time that weight loss is important for preventing atrial fibrillation or increasing the success rates of the different treatments we use,” Cynthia M. Tracy, MD, said in an interview. “Probably in some studies, weight loss has been as effective as antiarrhythmics.”
A loss of 3% body weight “is not a lot,” she said. In the current analysis, “It’s notable that it made that much difference with even a fairly modest amount of weight loss.”
Now when asked, “ ‘How much do I have to lose before you’ll consider doing my ablation?’ we have a bit more concrete data to give patients and doctors as to what amount might be beneficial,” said Dr. Tracy of George Washington University Hospital, Washington, who is not associated with the study.
Evolving view of AFib
The findings are emblematic of the profession’s evolving view of AFib and its management, Dr. Goldberger observed at the press conference. Should clinicians think of AFib as similar to “a disease like Wolff-Parkinson-White syndrome,” in which the patient usually has a successful ablation, and then “we expect that to last in perpetuity with no further interventions?”
Or, he said, “is atrial fibrillation more a disease like coronary artery disease, where even if they have an intervention, the disease process is still ongoing and requires long-term disease management? I think it’s pretty clear that we’re dealing with the latter case.”
Dr. Goldberger’s report was an interim analysis of an ongoing randomized trial called LEAF (Liraglutide Effect on Atrial Fibrillation), which is comparing patients with AFib assigned to “take” vs. “not take” the GLP-1 receptor agonist liraglutide, an antidiabetic (Victoza) and weight-loss (Saxenda) drug. The trial aims to assess the drug’s apparent ability to shrink atrial epicardial adipose tissue which, Dr. Goldberger said, is thought to contribute to AFib development and influence AFib-ablation outcomes.
It’s unknown and a limitation of the current analysis, he said, whether the observed link between improved preablation–weight ablation success “is specifically related to weight loss, liraglutide treatment, or both.”
As the invited discussant for Dr. Goldberger’s presentation, David Frankel, MD, observed that studies have been inconsistent on whether substantial weight loss may improve the results of AFib rhythm-control therapy.
Those finding such an association, including LEAF and the influential LEGACY study, differed from others showing a null effect by including “a comprehensive risk factor management” program, observed Dr. Frankel, of the Hospital of the University of Pennsylvania and Penn Heart and Vascular Center, Philadelphia.
Rather than focusing solely on weight loss or sleep apnea as AFib risk factors, he said, the studies linking weight loss to AFib rhythm control also included “hypertension, diabetes, hyperlipidemia, smoking cessation, and alcohol reduction,” Dr. Frankel said. “So it seems clear that to significantly impact AF recurrence, we need to focus on all these contributors to metabolic syndrome.”
Comprehensive risk-factor management
LEAF entered patients with AFib, 79% of whom had persistent AF and the rest paroxysmal AF, who followed the RFM program and were randomly assigned also to take liraglutide or placebo. The “nurse-practitioner-led” RFM program, conducted both in-clinic and online, featured “established goals for each patient” using AHA diet and lifestyle recommendations, an exercise prescription, dietary counseling, evaluation and treatment of sleep apnea, and measures to control any diabetes, hyperlipidemia, or hypertension, Dr. Goldberger said. And patients “were counseled on alcohol reduction and smoking cessation as necessary.”
After 3 months, 29 and 30 patients – regardless of randomization assignment – had lost < 3% and at least 3% of baseline body weight, respectively.
Catheter ablation achieved PVI in all patients. A 3-month blanking period followed, after which they went off antiarrhythmic meds.
It’s very difficult for patients to lose 10% or more of body weight, “and it would not happen overnight,” Dr. Tracy observed. “These are symptomatic patients, for the most part, if they get referred to an electrophysiologist. So you don’t want to defer them indefinitely.”
The current findings, she said, point to “a more realistic target,” suggesting that weight loss of at least 3% should improve AFib ablation’s chances for success.
Dr. Goldberger disclosed ties to Medtronic. Dr. Frankel disclosed ties to Medtronic, Stryker, Biosense Webster, and Boston Scientific. Dr. Tracy reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new analysis suggests.
The finding comes from a small study that entered such patients with paroxysmal and especially persistent AFib who were candidates for ablation. Those shedding at least 3% of body weight in the months before the procedure while engaged in a structured risk-factor modification (RFM) program were “dramatically” more likely to be AFib-free 6 months later.
The improved ablation efficacy, compared with results in similar patients who didn’t lose as much weight, was most pronounced among those whose AFib had been the persistent form, reported investigators at the annual scientific sessions of the Heart Rhythm Society, held in New Orleans.
Of note, ablations in the study were consistently limited, as much as possible, to standard pulmonary-vein isolation (PVI).
Associations between AFib and obesity and other behavioral and lifestyle-related risk factors are well recognized, but the limited studies of their effect on AFib ablation success have been inconsistent. The current analysis, the group says, points specifically to preablation weight loss as means to improving AFib-ablation outcomes.
“Adjunctive therapy focused on weight loss should be incorporated in the treatment plan for obese patients undergoing ablation for atrial fibrillation,” Jeffrey J. Goldberger, MD, MBA, of the University of Miami, said when presenting the new results at the HRS sessions.
Such a plan is entirely consistent with recent guidelines and especially a 2020 American Heart Association (AHA) consensus statement, but is inconsistently and perhaps even seldom realized in clinical practice.
Dramatic increase in success
Even modest weight loss before ablation may help, proposed Dr. Goldberger, who directs his institution’s Center for Atrial Fibrillation. Decreases for the greater-weight-loss group actually averaged less than 6% of baseline body weight.
Yet it was apparently enough to improve ablation outcomes significantly: Eighty-eight percent were free of AFib 6 months after the procedure, compared with 61% for patients who lost less than 3% of their preablation weight.
For improving ablation success, he said, “We’re talking about a moderate amount of weight loss. These patients are not going from being obese to being thin. They’re still quite overweight.”
In an analysis limited to the four-fifths of patients with persistent AFib, “we saw the same pattern,” Dr. Goldberger said at a media presentation prior to his formal report at the HRS sessions.
Moreover, that subgroup’s benefit persisted out to 12 months, at which time 42% and 81% of patients with less and greater weight loss, respectively, were free of AFib. That represents, he said, “a really tremendous – dramatic, actually – increase in success of pulmonary vein isolation in those who lost weight.”
“We’ve known for a long time that weight loss is important for preventing atrial fibrillation or increasing the success rates of the different treatments we use,” Cynthia M. Tracy, MD, said in an interview. “Probably in some studies, weight loss has been as effective as antiarrhythmics.”
A loss of 3% body weight “is not a lot,” she said. In the current analysis, “It’s notable that it made that much difference with even a fairly modest amount of weight loss.”
Now when asked, “ ‘How much do I have to lose before you’ll consider doing my ablation?’ we have a bit more concrete data to give patients and doctors as to what amount might be beneficial,” said Dr. Tracy of George Washington University Hospital, Washington, who is not associated with the study.
Evolving view of AFib
The findings are emblematic of the profession’s evolving view of AFib and its management, Dr. Goldberger observed at the press conference. Should clinicians think of AFib as similar to “a disease like Wolff-Parkinson-White syndrome,” in which the patient usually has a successful ablation, and then “we expect that to last in perpetuity with no further interventions?”
Or, he said, “is atrial fibrillation more a disease like coronary artery disease, where even if they have an intervention, the disease process is still ongoing and requires long-term disease management? I think it’s pretty clear that we’re dealing with the latter case.”
Dr. Goldberger’s report was an interim analysis of an ongoing randomized trial called LEAF (Liraglutide Effect on Atrial Fibrillation), which is comparing patients with AFib assigned to “take” vs. “not take” the GLP-1 receptor agonist liraglutide, an antidiabetic (Victoza) and weight-loss (Saxenda) drug. The trial aims to assess the drug’s apparent ability to shrink atrial epicardial adipose tissue which, Dr. Goldberger said, is thought to contribute to AFib development and influence AFib-ablation outcomes.
It’s unknown and a limitation of the current analysis, he said, whether the observed link between improved preablation–weight ablation success “is specifically related to weight loss, liraglutide treatment, or both.”
As the invited discussant for Dr. Goldberger’s presentation, David Frankel, MD, observed that studies have been inconsistent on whether substantial weight loss may improve the results of AFib rhythm-control therapy.
Those finding such an association, including LEAF and the influential LEGACY study, differed from others showing a null effect by including “a comprehensive risk factor management” program, observed Dr. Frankel, of the Hospital of the University of Pennsylvania and Penn Heart and Vascular Center, Philadelphia.
Rather than focusing solely on weight loss or sleep apnea as AFib risk factors, he said, the studies linking weight loss to AFib rhythm control also included “hypertension, diabetes, hyperlipidemia, smoking cessation, and alcohol reduction,” Dr. Frankel said. “So it seems clear that to significantly impact AF recurrence, we need to focus on all these contributors to metabolic syndrome.”
Comprehensive risk-factor management
LEAF entered patients with AFib, 79% of whom had persistent AF and the rest paroxysmal AF, who followed the RFM program and were randomly assigned also to take liraglutide or placebo. The “nurse-practitioner-led” RFM program, conducted both in-clinic and online, featured “established goals for each patient” using AHA diet and lifestyle recommendations, an exercise prescription, dietary counseling, evaluation and treatment of sleep apnea, and measures to control any diabetes, hyperlipidemia, or hypertension, Dr. Goldberger said. And patients “were counseled on alcohol reduction and smoking cessation as necessary.”
After 3 months, 29 and 30 patients – regardless of randomization assignment – had lost < 3% and at least 3% of baseline body weight, respectively.
Catheter ablation achieved PVI in all patients. A 3-month blanking period followed, after which they went off antiarrhythmic meds.
It’s very difficult for patients to lose 10% or more of body weight, “and it would not happen overnight,” Dr. Tracy observed. “These are symptomatic patients, for the most part, if they get referred to an electrophysiologist. So you don’t want to defer them indefinitely.”
The current findings, she said, point to “a more realistic target,” suggesting that weight loss of at least 3% should improve AFib ablation’s chances for success.
Dr. Goldberger disclosed ties to Medtronic. Dr. Frankel disclosed ties to Medtronic, Stryker, Biosense Webster, and Boston Scientific. Dr. Tracy reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HEART RHYTHM 2023