User login
Article Type
Changed
Display Headline
Lung Cancer Screening: A Need for Adjunctive Testing
References
- Naidch DP et al. Radiology. 1990;175(3):729-731. doi:10.1148/radiology.175.3.2343122
- Kaneko M et al. Radiology. 1996;201(3):798-802. doi:10.1148/radiology.201.3.8939234
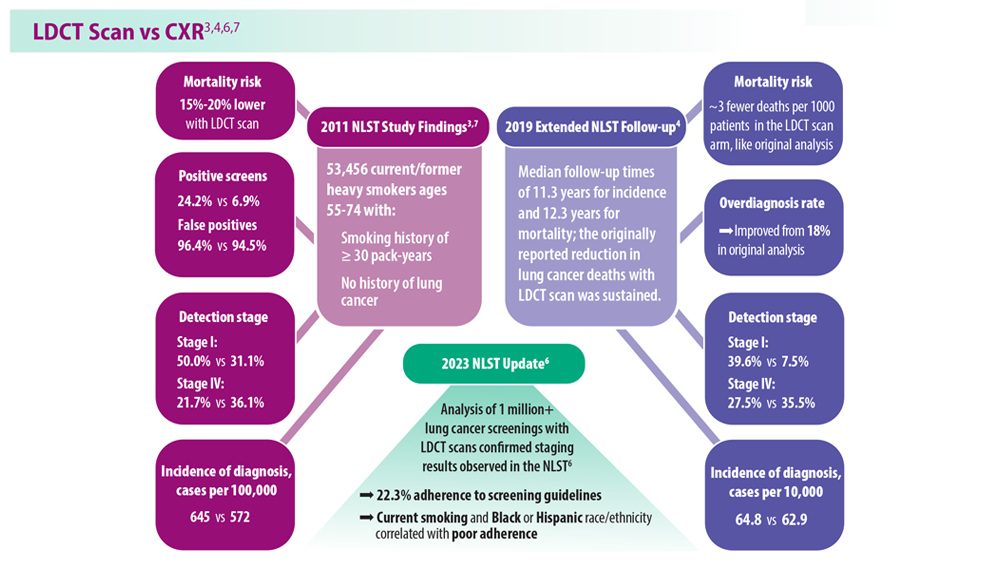
- National Lung Screening Trial Research Team. Radiology. 2011;258(1):243-253. doi:10.1148/radiol.10091808
- National Lung Screening Trial Research Team. J Thorac Oncol. 2019;14(10):1732-1742. doi:10.1016/j.jtho.2019.05.044
- Mazzone PJ et al. Chest. 2021;160(5):e427-e494. doi:10.1016/j.chest.2021.06.063
- Tanner NT et al. Chest. 2023;S0012-3692(23)00175-7. doi:10.1016/j.chest.2023.02.003
- National Lung Screening Trial Research Team. N Engl J Med. 2011;365(5):395- 409. doi:10.1056/NEJMoa1102873
- Marmor HN et al. Curr Chall Thorac Surg. 2023;5:5. doi:10.21037/ccts-20-171
Publications
Topics
References
- Naidch DP et al. Radiology. 1990;175(3):729-731. doi:10.1148/radiology.175.3.2343122
- Kaneko M et al. Radiology. 1996;201(3):798-802. doi:10.1148/radiology.201.3.8939234
- National Lung Screening Trial Research Team. Radiology. 2011;258(1):243-253. doi:10.1148/radiol.10091808
- National Lung Screening Trial Research Team. J Thorac Oncol. 2019;14(10):1732-1742. doi:10.1016/j.jtho.2019.05.044
- Mazzone PJ et al. Chest. 2021;160(5):e427-e494. doi:10.1016/j.chest.2021.06.063
- Tanner NT et al. Chest. 2023;S0012-3692(23)00175-7. doi:10.1016/j.chest.2023.02.003
- National Lung Screening Trial Research Team. N Engl J Med. 2011;365(5):395- 409. doi:10.1056/NEJMoa1102873
- Marmor HN et al. Curr Chall Thorac Surg. 2023;5:5. doi:10.21037/ccts-20-171
References
- Naidch DP et al. Radiology. 1990;175(3):729-731. doi:10.1148/radiology.175.3.2343122
- Kaneko M et al. Radiology. 1996;201(3):798-802. doi:10.1148/radiology.201.3.8939234
- National Lung Screening Trial Research Team. Radiology. 2011;258(1):243-253. doi:10.1148/radiol.10091808
- National Lung Screening Trial Research Team. J Thorac Oncol. 2019;14(10):1732-1742. doi:10.1016/j.jtho.2019.05.044
- Mazzone PJ et al. Chest. 2021;160(5):e427-e494. doi:10.1016/j.chest.2021.06.063
- Tanner NT et al. Chest. 2023;S0012-3692(23)00175-7. doi:10.1016/j.chest.2023.02.003
- National Lung Screening Trial Research Team. N Engl J Med. 2011;365(5):395- 409. doi:10.1056/NEJMoa1102873
- Marmor HN et al. Curr Chall Thorac Surg. 2023;5:5. doi:10.21037/ccts-20-171
Publications
Publications
Topics
Article Type
Display Headline
Lung Cancer Screening: A Need for Adjunctive Testing
Display Headline
Lung Cancer Screening: A Need for Adjunctive Testing
Disallow All Ads
Content Gating
Open Access (article Unlocked/Open Access)
Alternative CME
Disqus Comments
Default
Eyebrow Default
Slideshow
Consolidated Pubs: Do Not Show Source Publication Logo
Use ProPublica
Conference Recap Checkbox
Not Conference Recap
Clinical Edge
Medscape Article
Display survey writer
Reuters content
Disable Inline Native ads
WebMD Article