User login
Lung Cancer Screening: A Need for Adjunctive Testing
- Naidch DP et al. Radiology. 1990;175(3):729-731. doi:10.1148/radiology.175.3.2343122
- Kaneko M et al. Radiology. 1996;201(3):798-802. doi:10.1148/radiology.201.3.8939234
- National Lung Screening Trial Research Team. Radiology. 2011;258(1):243-253. doi:10.1148/radiol.10091808
- National Lung Screening Trial Research Team. J Thorac Oncol. 2019;14(10):1732-1742. doi:10.1016/j.jtho.2019.05.044
- Mazzone PJ et al. Chest. 2021;160(5):e427-e494. doi:10.1016/j.chest.2021.06.063
- Tanner NT et al. Chest. 2023;S0012-3692(23)00175-7. doi:10.1016/j.chest.2023.02.003
- National Lung Screening Trial Research Team. N Engl J Med. 2011;365(5):395- 409. doi:10.1056/NEJMoa1102873
- Marmor HN et al. Curr Chall Thorac Surg. 2023;5:5. doi:10.21037/ccts-20-171
- Naidch DP et al. Radiology. 1990;175(3):729-731. doi:10.1148/radiology.175.3.2343122
- Kaneko M et al. Radiology. 1996;201(3):798-802. doi:10.1148/radiology.201.3.8939234
- National Lung Screening Trial Research Team. Radiology. 2011;258(1):243-253. doi:10.1148/radiol.10091808
- National Lung Screening Trial Research Team. J Thorac Oncol. 2019;14(10):1732-1742. doi:10.1016/j.jtho.2019.05.044
- Mazzone PJ et al. Chest. 2021;160(5):e427-e494. doi:10.1016/j.chest.2021.06.063
- Tanner NT et al. Chest. 2023;S0012-3692(23)00175-7. doi:10.1016/j.chest.2023.02.003
- National Lung Screening Trial Research Team. N Engl J Med. 2011;365(5):395- 409. doi:10.1056/NEJMoa1102873
- Marmor HN et al. Curr Chall Thorac Surg. 2023;5:5. doi:10.21037/ccts-20-171
- Naidch DP et al. Radiology. 1990;175(3):729-731. doi:10.1148/radiology.175.3.2343122
- Kaneko M et al. Radiology. 1996;201(3):798-802. doi:10.1148/radiology.201.3.8939234
- National Lung Screening Trial Research Team. Radiology. 2011;258(1):243-253. doi:10.1148/radiol.10091808
- National Lung Screening Trial Research Team. J Thorac Oncol. 2019;14(10):1732-1742. doi:10.1016/j.jtho.2019.05.044
- Mazzone PJ et al. Chest. 2021;160(5):e427-e494. doi:10.1016/j.chest.2021.06.063
- Tanner NT et al. Chest. 2023;S0012-3692(23)00175-7. doi:10.1016/j.chest.2023.02.003
- National Lung Screening Trial Research Team. N Engl J Med. 2011;365(5):395- 409. doi:10.1056/NEJMoa1102873
- Marmor HN et al. Curr Chall Thorac Surg. 2023;5:5. doi:10.21037/ccts-20-171
Advances in Lung Cancer Diagnostics and Treatment
1. Cancer facts and figures 2022. American Cancer Society. Accessed June 14, 2022. https://www.cancer.org/content/dam/ cancer-org/research/cancer-facts-and-statistics/annual-cancerfacts-and-figures/2022/2022-cancer-facts-and-figures
2. Novellis P, Maisonneuve P, Dieci E, et al. Quality of life, postoperative pain, and lymph node dissection in a robotic approach compared to VATS and OPEN for early stage lung cancer. J Clin Med. 2021;10(8):1687. doi:10.3390/jcm10081687
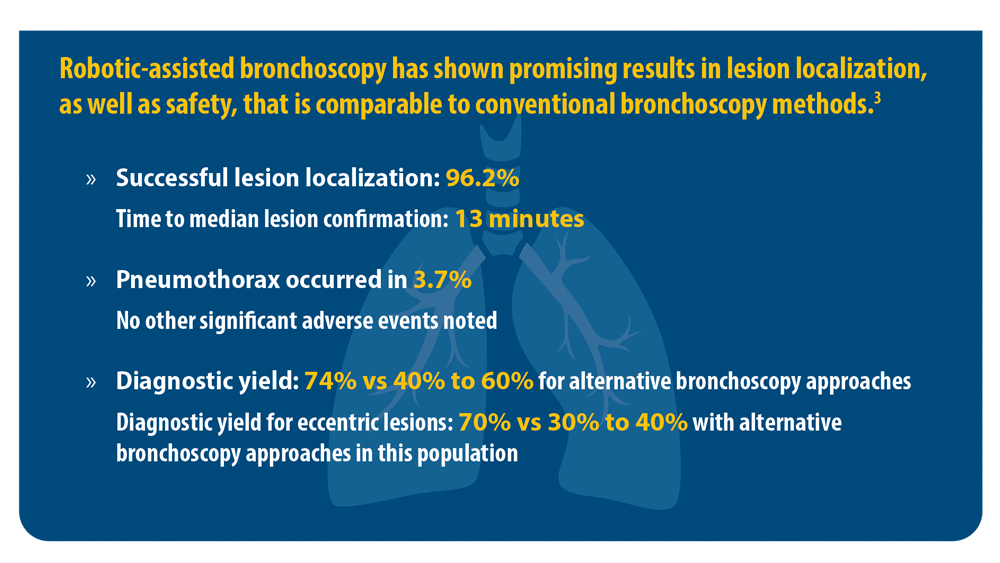
3. Chen AC, Pastis NJ Jr, Mahajan AK, et al. Robotic bronchoscopy for peripheral pulmonary lesions: a multicenter pilot and feasibility study (BENEFIT). Chest. 2021;159(2):845-852. doi:10.1016/j. chest.2020.08.2047
4. Current cigarette smoking among adults in the United States. Centers for Disease Control and Prevention. Updated March 17, 2022. Accessed June 15, 2022. https://www.cdc.gov/tobacco/ data_statistics/fact_sheets/adult_data/cig_smoking/index.htm
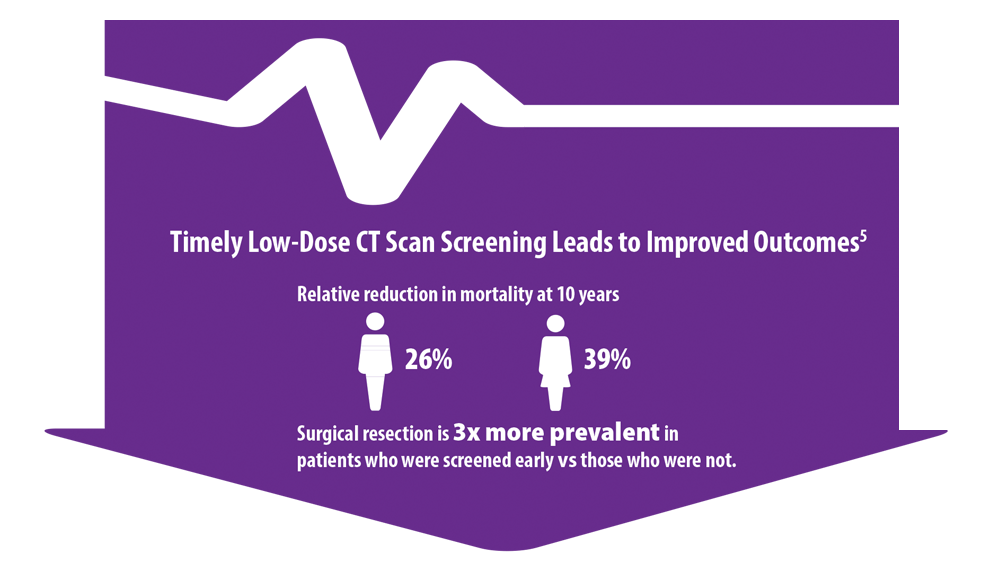
5. Haddad DN, Sandler KL, Henderson LM, Rivera MP, Aldrich MC. Disparities in lung cancer screening: a review. Ann Am Thorac Soc. 2020;17(4):399-405. doi:10.1513/AnnalsATS.201907- 556CME
6. US Preventive Services Task Force issues final recommendation statement on screening for lung cancer. USPSTF Bulletin. Published March 9, 2021. Accessed June 15, 2022. https://www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/lung-cancer-newsbulletin.pdf
7. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST guideline and expert panel report. Chest. 2021;160(5):e427-e494. doi:10.1016/j.chest.2021.06.063
8. Lung cancer screening report. National Cancer Institute Cancer Trends Progress Report. Updated April 2022. Accessed June 15, 2022. https://progressreport.cancer.gov/detection/lung_cancer
9. Huang L, Li L, Zhou Y, et al. Clinical characteristics correlate with outcomes of immunotherapy in advanced non-small cell lung cancer. J Cancer. 2020;11(24):7137-7145. doi:10.7150/ jca.49213
10. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985. doi:10.1056/NEJMoa2202170
11. Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med. 2020;383(18):1711-1723. doi:10.1056/NEJMoa2027071
1. Cancer facts and figures 2022. American Cancer Society. Accessed June 14, 2022. https://www.cancer.org/content/dam/ cancer-org/research/cancer-facts-and-statistics/annual-cancerfacts-and-figures/2022/2022-cancer-facts-and-figures
2. Novellis P, Maisonneuve P, Dieci E, et al. Quality of life, postoperative pain, and lymph node dissection in a robotic approach compared to VATS and OPEN for early stage lung cancer. J Clin Med. 2021;10(8):1687. doi:10.3390/jcm10081687
3. Chen AC, Pastis NJ Jr, Mahajan AK, et al. Robotic bronchoscopy for peripheral pulmonary lesions: a multicenter pilot and feasibility study (BENEFIT). Chest. 2021;159(2):845-852. doi:10.1016/j. chest.2020.08.2047
4. Current cigarette smoking among adults in the United States. Centers for Disease Control and Prevention. Updated March 17, 2022. Accessed June 15, 2022. https://www.cdc.gov/tobacco/ data_statistics/fact_sheets/adult_data/cig_smoking/index.htm
5. Haddad DN, Sandler KL, Henderson LM, Rivera MP, Aldrich MC. Disparities in lung cancer screening: a review. Ann Am Thorac Soc. 2020;17(4):399-405. doi:10.1513/AnnalsATS.201907- 556CME
6. US Preventive Services Task Force issues final recommendation statement on screening for lung cancer. USPSTF Bulletin. Published March 9, 2021. Accessed June 15, 2022. https://www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/lung-cancer-newsbulletin.pdf
7. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST guideline and expert panel report. Chest. 2021;160(5):e427-e494. doi:10.1016/j.chest.2021.06.063
8. Lung cancer screening report. National Cancer Institute Cancer Trends Progress Report. Updated April 2022. Accessed June 15, 2022. https://progressreport.cancer.gov/detection/lung_cancer
9. Huang L, Li L, Zhou Y, et al. Clinical characteristics correlate with outcomes of immunotherapy in advanced non-small cell lung cancer. J Cancer. 2020;11(24):7137-7145. doi:10.7150/ jca.49213
10. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985. doi:10.1056/NEJMoa2202170
11. Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med. 2020;383(18):1711-1723. doi:10.1056/NEJMoa2027071
1. Cancer facts and figures 2022. American Cancer Society. Accessed June 14, 2022. https://www.cancer.org/content/dam/ cancer-org/research/cancer-facts-and-statistics/annual-cancerfacts-and-figures/2022/2022-cancer-facts-and-figures
2. Novellis P, Maisonneuve P, Dieci E, et al. Quality of life, postoperative pain, and lymph node dissection in a robotic approach compared to VATS and OPEN for early stage lung cancer. J Clin Med. 2021;10(8):1687. doi:10.3390/jcm10081687
3. Chen AC, Pastis NJ Jr, Mahajan AK, et al. Robotic bronchoscopy for peripheral pulmonary lesions: a multicenter pilot and feasibility study (BENEFIT). Chest. 2021;159(2):845-852. doi:10.1016/j. chest.2020.08.2047
4. Current cigarette smoking among adults in the United States. Centers for Disease Control and Prevention. Updated March 17, 2022. Accessed June 15, 2022. https://www.cdc.gov/tobacco/ data_statistics/fact_sheets/adult_data/cig_smoking/index.htm
5. Haddad DN, Sandler KL, Henderson LM, Rivera MP, Aldrich MC. Disparities in lung cancer screening: a review. Ann Am Thorac Soc. 2020;17(4):399-405. doi:10.1513/AnnalsATS.201907- 556CME
6. US Preventive Services Task Force issues final recommendation statement on screening for lung cancer. USPSTF Bulletin. Published March 9, 2021. Accessed June 15, 2022. https://www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/lung-cancer-newsbulletin.pdf
7. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST guideline and expert panel report. Chest. 2021;160(5):e427-e494. doi:10.1016/j.chest.2021.06.063
8. Lung cancer screening report. National Cancer Institute Cancer Trends Progress Report. Updated April 2022. Accessed June 15, 2022. https://progressreport.cancer.gov/detection/lung_cancer
9. Huang L, Li L, Zhou Y, et al. Clinical characteristics correlate with outcomes of immunotherapy in advanced non-small cell lung cancer. J Cancer. 2020;11(24):7137-7145. doi:10.7150/ jca.49213
10. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985. doi:10.1056/NEJMoa2202170
11. Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med. 2020;383(18):1711-1723. doi:10.1056/NEJMoa2027071