User login
Case
A 56-year-old woman presented to the ED with palpitations and lightheadedness, which began upon awakening that morning. The patient had a history of atrial fibrillation (AF), and believed this was the cause of her symptoms. Over the past 18 months, the patient had twice undergone successful cardioversion for AF with a rapid ventricular response (RVR); both cardioversions were performed by her cardiologist.
The patient denied experiencing any chest pain, shortness of breath, nausea, or vomiting. Her medical history was significant only for AF. Regarding her medication history, the patient had been prescribed metoprolol, but admitted that she frequently forgot to take it. She further stated that she was not taking aspirin or anticoagulation therapy for AF. She denied past or current alcohol consumption or tobacco use.
On physical examination, the patient’s vital signs were: heart rate (HR), 186 beats/min; blood pressure, 137/82 mm Hg; respiratory rate, 20 breaths/min, and temperature, afebrile. Oxygen saturation was 96% on room air. The head, eye, ears, nose, and throat examination was normal. Auscultation of the lungs revealed clear breath sounds bilaterally. On examination of the heart, the patient had an irregularly irregular rhythm that was tachycardic; no murmurs, rubs, or gallops were appreciated. The abdomen was soft and nontender. There was no edema or redness of the lower extremities.
The emergency physician (EP) placed the patient on a cardiac monitor and administered 2 L of oxygen via nasal cannula. An electrocardiogram (ECG), portable chest X-ray (CXR), and laboratory evaluation were ordered, and an intravenous (IV) line was established. The ECG revealed AF with RVR, without evidence of ischemia. The CXR was interpreted as normal. Laboratory studies, including complete blood count, basic metabolic profile, and serum troponin levels, were likewise within normal limits.
Based on the patient’s history and evaluation, the EP decided to cardiovert the patient rather than attempt rate control with IV medications. The patient consented to the cardioversion, based on the two previous successful cardioversions performed by her cardiologist. The EP gave the patient midazolam 2 mg IV and performed synchronized cardioversion at 200 joules. The patient converted to normal sinus rhythm with an HR of 86 beats/min. She was observed in the ED for 1 hour, given metoprolol 50 mg by mouth, and discharged home with instructions to follow up with her cardiologist the following week.
The next day, the patient suffered a large ischemic stroke in the distribution of the left middle cerebral artery, resulting in a dense right hemiparesis. The neurological deficit was significant, necessitating the patient’s placement in a nursing home.
The patient and her family sued the EP for malpractice for not anticoagulating the patient prior to and following cardioversion. A $3.3 million settlement was agreed upon prior to trial.
Discussion
Patients commonly present to the ED for complaints related to AF. In some cases, the EP is the first to diagnose the patient’s AF; in other cases, the patient has a history of AF and is presenting with a complication. The focus of this discussion is solely on the management of AF with RVR.
When managing a patient in AF with RVR, the EP must consider three issues: ventricular rate control (VRC), rhythm control, and anticoagulation. Selecting the best treatment strategy will depend on the patient’s hemodynamic stability, duration of her or his symptoms, local custom and preference, and the length of time the AF has been present.
Ventricular Rate Control and Cardioversion
For many stable patients, VRC is frequently the treatment of choice, with a goal HR of less than 100 beats/min. Intravenous diltiazem, esmolol, or metoprolol can be used to achieve VRC in patients in AF. Because these drugs only control ventricular rate and do not typically cardiovert, the risk of embolization is small.
Synchronized cardioversion has the benefit of providing both rate and rhythm control, but at the expense of the increased risk of arterial embolization. Some patients, including those with rheumatic heart disease, mitral stenosis, prosthetic heart valves, severe left ventricular dysfunction, or a history of thromboembolism, are at a constant high risk of developing a thromboembolism.1
Risk-Benefit Ratio and Anticoagulation Therapy
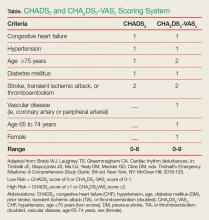
To help determine the risk-benefit ratio in patients without the risk factors mentioned above, the EP should calculate the CHADS2 (congestive heart failure [CHF], hypertension, age, diabetes mellitus [DM], prior stroke, transient ischemic attack [TIA], or thromboembolism [doubled]) score or CHA2DS2-VASC (CHF, hypertension, age 75 years or older [two scores], DM, previous stroke, TIA, or thromboembolism [doubled], vascular disease, age 65-74 years, sex [female]) score to help identify patients at risk for arterial embolic complications (Table).
For patients who have been in AF for less than 48 hours and who are at a very low-embolic risk (CHA2DS2-VASC score of 0), some experts suggest cardioversion without anticoagulation. However, other experts recommend anticoagulation prior to cardioversion—even in low-risk patients. Unfortunately, there is disagreement between professional organizations, with the American Heart Association/American College of Cardiology/Heart Rhythm Society stating that cardioversion may be performed with or without procedural anticoagulation,2 while the 2016 European Society of Cardiology guidelines recommend immediate initiation of anticoagulants in all such patients scheduled for cardioversion.3
The reasoning in favor of anticoagulation prior to cardioversion is supported by an observational study by Airaksinen et al4 of 2,481 patients undergoing cardioversion for AF of less than 48 hours duration. This study demonstrated a definite thromboembolic event in 38 (0.7%) of the patients within 30 days (median of 2 days). The thromboembolic event was stroke in 31 of the 38 patients.4 Airaksinen et al4 found that age older than 60 years, female sex, heart failure (HF), and DM were the strongest predictors of embolization. The risk of stroke in patients without HF and those younger than age 60 years was only 0.2%.4
In a similar observational study by Hansen et al5 of 16,274 patients in AF undergoing cardioversion with and without anticoagulation therapy, the absence of postcardioversion anticoagulation increased the risk of thromboembolism 2-fold—regardless of CHA2DS2-VASC scores.
Summary
While the management of AF with a duration of more than 48 hours should always include some type of anticoagulation therapy (pre- or postcardioversion, or both), the role of anticoagulation in low-risk patients with AF of less than 48 hours is not as clear. As this situation is not uncommon, the emergency medicine and cardiology physicians should consider developing a mutually agreed upon protocol on how best to manage these patients at their institution. When considering cardioversion without pre- or postanticoagulation in low-risk patients with AF, EPs should always involve the patient in the decision-making process.
1. Phang R, Manning WJ. Prevention of embolization prior to and after restoration of sinus rhythm in atrial fibrillation. UptoDate Web site. http://www.uptodate.com/contents/prevention-of-embolization-prior-to-and-after-restoration-of-sinus-rhythm-in-atrial-fibrillation. Updated October 10, 2016. Accessed March 6, 2017.
2. January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-e267. Erratum in Circulation. 2014;130(23):e272-e274. doi:10.1161/CIR.0000000000000041.
3. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016:37(38):2893-2962. doi:10.1093/eurheartj/ehw210.
4. Airaksinen KE, Grönberg T, Nuotio I, et al. Thromboembolic complications after cardioversion of acute atrial fibrillation: the FinCV (Finnish CardioVersion) study. J Am Coll Cardiol. 2013;62(13):1187-1192. doi:10.1016/j.jacc.2013.04.089.
5. Hansen ML, Jepsen RM, Olesen JB, et al. Thromboembolic risk in 16 274 atrial fibrillation patients undergoing direct current cardioversion with and without oral anticoagulant therapy. Europace. 2015;17(1):18-23. doi:10.1093/europace/euu189.
Case
A 56-year-old woman presented to the ED with palpitations and lightheadedness, which began upon awakening that morning. The patient had a history of atrial fibrillation (AF), and believed this was the cause of her symptoms. Over the past 18 months, the patient had twice undergone successful cardioversion for AF with a rapid ventricular response (RVR); both cardioversions were performed by her cardiologist.
The patient denied experiencing any chest pain, shortness of breath, nausea, or vomiting. Her medical history was significant only for AF. Regarding her medication history, the patient had been prescribed metoprolol, but admitted that she frequently forgot to take it. She further stated that she was not taking aspirin or anticoagulation therapy for AF. She denied past or current alcohol consumption or tobacco use.
On physical examination, the patient’s vital signs were: heart rate (HR), 186 beats/min; blood pressure, 137/82 mm Hg; respiratory rate, 20 breaths/min, and temperature, afebrile. Oxygen saturation was 96% on room air. The head, eye, ears, nose, and throat examination was normal. Auscultation of the lungs revealed clear breath sounds bilaterally. On examination of the heart, the patient had an irregularly irregular rhythm that was tachycardic; no murmurs, rubs, or gallops were appreciated. The abdomen was soft and nontender. There was no edema or redness of the lower extremities.
The emergency physician (EP) placed the patient on a cardiac monitor and administered 2 L of oxygen via nasal cannula. An electrocardiogram (ECG), portable chest X-ray (CXR), and laboratory evaluation were ordered, and an intravenous (IV) line was established. The ECG revealed AF with RVR, without evidence of ischemia. The CXR was interpreted as normal. Laboratory studies, including complete blood count, basic metabolic profile, and serum troponin levels, were likewise within normal limits.
Based on the patient’s history and evaluation, the EP decided to cardiovert the patient rather than attempt rate control with IV medications. The patient consented to the cardioversion, based on the two previous successful cardioversions performed by her cardiologist. The EP gave the patient midazolam 2 mg IV and performed synchronized cardioversion at 200 joules. The patient converted to normal sinus rhythm with an HR of 86 beats/min. She was observed in the ED for 1 hour, given metoprolol 50 mg by mouth, and discharged home with instructions to follow up with her cardiologist the following week.
The next day, the patient suffered a large ischemic stroke in the distribution of the left middle cerebral artery, resulting in a dense right hemiparesis. The neurological deficit was significant, necessitating the patient’s placement in a nursing home.
The patient and her family sued the EP for malpractice for not anticoagulating the patient prior to and following cardioversion. A $3.3 million settlement was agreed upon prior to trial.
Discussion
Patients commonly present to the ED for complaints related to AF. In some cases, the EP is the first to diagnose the patient’s AF; in other cases, the patient has a history of AF and is presenting with a complication. The focus of this discussion is solely on the management of AF with RVR.
When managing a patient in AF with RVR, the EP must consider three issues: ventricular rate control (VRC), rhythm control, and anticoagulation. Selecting the best treatment strategy will depend on the patient’s hemodynamic stability, duration of her or his symptoms, local custom and preference, and the length of time the AF has been present.
Ventricular Rate Control and Cardioversion
For many stable patients, VRC is frequently the treatment of choice, with a goal HR of less than 100 beats/min. Intravenous diltiazem, esmolol, or metoprolol can be used to achieve VRC in patients in AF. Because these drugs only control ventricular rate and do not typically cardiovert, the risk of embolization is small.
Synchronized cardioversion has the benefit of providing both rate and rhythm control, but at the expense of the increased risk of arterial embolization. Some patients, including those with rheumatic heart disease, mitral stenosis, prosthetic heart valves, severe left ventricular dysfunction, or a history of thromboembolism, are at a constant high risk of developing a thromboembolism.1
Risk-Benefit Ratio and Anticoagulation Therapy
To help determine the risk-benefit ratio in patients without the risk factors mentioned above, the EP should calculate the CHADS2 (congestive heart failure [CHF], hypertension, age, diabetes mellitus [DM], prior stroke, transient ischemic attack [TIA], or thromboembolism [doubled]) score or CHA2DS2-VASC (CHF, hypertension, age 75 years or older [two scores], DM, previous stroke, TIA, or thromboembolism [doubled], vascular disease, age 65-74 years, sex [female]) score to help identify patients at risk for arterial embolic complications (Table).
For patients who have been in AF for less than 48 hours and who are at a very low-embolic risk (CHA2DS2-VASC score of 0), some experts suggest cardioversion without anticoagulation. However, other experts recommend anticoagulation prior to cardioversion—even in low-risk patients. Unfortunately, there is disagreement between professional organizations, with the American Heart Association/American College of Cardiology/Heart Rhythm Society stating that cardioversion may be performed with or without procedural anticoagulation,2 while the 2016 European Society of Cardiology guidelines recommend immediate initiation of anticoagulants in all such patients scheduled for cardioversion.3
The reasoning in favor of anticoagulation prior to cardioversion is supported by an observational study by Airaksinen et al4 of 2,481 patients undergoing cardioversion for AF of less than 48 hours duration. This study demonstrated a definite thromboembolic event in 38 (0.7%) of the patients within 30 days (median of 2 days). The thromboembolic event was stroke in 31 of the 38 patients.4 Airaksinen et al4 found that age older than 60 years, female sex, heart failure (HF), and DM were the strongest predictors of embolization. The risk of stroke in patients without HF and those younger than age 60 years was only 0.2%.4
In a similar observational study by Hansen et al5 of 16,274 patients in AF undergoing cardioversion with and without anticoagulation therapy, the absence of postcardioversion anticoagulation increased the risk of thromboembolism 2-fold—regardless of CHA2DS2-VASC scores.
Summary
While the management of AF with a duration of more than 48 hours should always include some type of anticoagulation therapy (pre- or postcardioversion, or both), the role of anticoagulation in low-risk patients with AF of less than 48 hours is not as clear. As this situation is not uncommon, the emergency medicine and cardiology physicians should consider developing a mutually agreed upon protocol on how best to manage these patients at their institution. When considering cardioversion without pre- or postanticoagulation in low-risk patients with AF, EPs should always involve the patient in the decision-making process.
Case
A 56-year-old woman presented to the ED with palpitations and lightheadedness, which began upon awakening that morning. The patient had a history of atrial fibrillation (AF), and believed this was the cause of her symptoms. Over the past 18 months, the patient had twice undergone successful cardioversion for AF with a rapid ventricular response (RVR); both cardioversions were performed by her cardiologist.
The patient denied experiencing any chest pain, shortness of breath, nausea, or vomiting. Her medical history was significant only for AF. Regarding her medication history, the patient had been prescribed metoprolol, but admitted that she frequently forgot to take it. She further stated that she was not taking aspirin or anticoagulation therapy for AF. She denied past or current alcohol consumption or tobacco use.
On physical examination, the patient’s vital signs were: heart rate (HR), 186 beats/min; blood pressure, 137/82 mm Hg; respiratory rate, 20 breaths/min, and temperature, afebrile. Oxygen saturation was 96% on room air. The head, eye, ears, nose, and throat examination was normal. Auscultation of the lungs revealed clear breath sounds bilaterally. On examination of the heart, the patient had an irregularly irregular rhythm that was tachycardic; no murmurs, rubs, or gallops were appreciated. The abdomen was soft and nontender. There was no edema or redness of the lower extremities.
The emergency physician (EP) placed the patient on a cardiac monitor and administered 2 L of oxygen via nasal cannula. An electrocardiogram (ECG), portable chest X-ray (CXR), and laboratory evaluation were ordered, and an intravenous (IV) line was established. The ECG revealed AF with RVR, without evidence of ischemia. The CXR was interpreted as normal. Laboratory studies, including complete blood count, basic metabolic profile, and serum troponin levels, were likewise within normal limits.
Based on the patient’s history and evaluation, the EP decided to cardiovert the patient rather than attempt rate control with IV medications. The patient consented to the cardioversion, based on the two previous successful cardioversions performed by her cardiologist. The EP gave the patient midazolam 2 mg IV and performed synchronized cardioversion at 200 joules. The patient converted to normal sinus rhythm with an HR of 86 beats/min. She was observed in the ED for 1 hour, given metoprolol 50 mg by mouth, and discharged home with instructions to follow up with her cardiologist the following week.
The next day, the patient suffered a large ischemic stroke in the distribution of the left middle cerebral artery, resulting in a dense right hemiparesis. The neurological deficit was significant, necessitating the patient’s placement in a nursing home.
The patient and her family sued the EP for malpractice for not anticoagulating the patient prior to and following cardioversion. A $3.3 million settlement was agreed upon prior to trial.
Discussion
Patients commonly present to the ED for complaints related to AF. In some cases, the EP is the first to diagnose the patient’s AF; in other cases, the patient has a history of AF and is presenting with a complication. The focus of this discussion is solely on the management of AF with RVR.
When managing a patient in AF with RVR, the EP must consider three issues: ventricular rate control (VRC), rhythm control, and anticoagulation. Selecting the best treatment strategy will depend on the patient’s hemodynamic stability, duration of her or his symptoms, local custom and preference, and the length of time the AF has been present.
Ventricular Rate Control and Cardioversion
For many stable patients, VRC is frequently the treatment of choice, with a goal HR of less than 100 beats/min. Intravenous diltiazem, esmolol, or metoprolol can be used to achieve VRC in patients in AF. Because these drugs only control ventricular rate and do not typically cardiovert, the risk of embolization is small.
Synchronized cardioversion has the benefit of providing both rate and rhythm control, but at the expense of the increased risk of arterial embolization. Some patients, including those with rheumatic heart disease, mitral stenosis, prosthetic heart valves, severe left ventricular dysfunction, or a history of thromboembolism, are at a constant high risk of developing a thromboembolism.1
Risk-Benefit Ratio and Anticoagulation Therapy
To help determine the risk-benefit ratio in patients without the risk factors mentioned above, the EP should calculate the CHADS2 (congestive heart failure [CHF], hypertension, age, diabetes mellitus [DM], prior stroke, transient ischemic attack [TIA], or thromboembolism [doubled]) score or CHA2DS2-VASC (CHF, hypertension, age 75 years or older [two scores], DM, previous stroke, TIA, or thromboembolism [doubled], vascular disease, age 65-74 years, sex [female]) score to help identify patients at risk for arterial embolic complications (Table).
For patients who have been in AF for less than 48 hours and who are at a very low-embolic risk (CHA2DS2-VASC score of 0), some experts suggest cardioversion without anticoagulation. However, other experts recommend anticoagulation prior to cardioversion—even in low-risk patients. Unfortunately, there is disagreement between professional organizations, with the American Heart Association/American College of Cardiology/Heart Rhythm Society stating that cardioversion may be performed with or without procedural anticoagulation,2 while the 2016 European Society of Cardiology guidelines recommend immediate initiation of anticoagulants in all such patients scheduled for cardioversion.3
The reasoning in favor of anticoagulation prior to cardioversion is supported by an observational study by Airaksinen et al4 of 2,481 patients undergoing cardioversion for AF of less than 48 hours duration. This study demonstrated a definite thromboembolic event in 38 (0.7%) of the patients within 30 days (median of 2 days). The thromboembolic event was stroke in 31 of the 38 patients.4 Airaksinen et al4 found that age older than 60 years, female sex, heart failure (HF), and DM were the strongest predictors of embolization. The risk of stroke in patients without HF and those younger than age 60 years was only 0.2%.4
In a similar observational study by Hansen et al5 of 16,274 patients in AF undergoing cardioversion with and without anticoagulation therapy, the absence of postcardioversion anticoagulation increased the risk of thromboembolism 2-fold—regardless of CHA2DS2-VASC scores.
Summary
While the management of AF with a duration of more than 48 hours should always include some type of anticoagulation therapy (pre- or postcardioversion, or both), the role of anticoagulation in low-risk patients with AF of less than 48 hours is not as clear. As this situation is not uncommon, the emergency medicine and cardiology physicians should consider developing a mutually agreed upon protocol on how best to manage these patients at their institution. When considering cardioversion without pre- or postanticoagulation in low-risk patients with AF, EPs should always involve the patient in the decision-making process.
1. Phang R, Manning WJ. Prevention of embolization prior to and after restoration of sinus rhythm in atrial fibrillation. UptoDate Web site. http://www.uptodate.com/contents/prevention-of-embolization-prior-to-and-after-restoration-of-sinus-rhythm-in-atrial-fibrillation. Updated October 10, 2016. Accessed March 6, 2017.
2. January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-e267. Erratum in Circulation. 2014;130(23):e272-e274. doi:10.1161/CIR.0000000000000041.
3. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016:37(38):2893-2962. doi:10.1093/eurheartj/ehw210.
4. Airaksinen KE, Grönberg T, Nuotio I, et al. Thromboembolic complications after cardioversion of acute atrial fibrillation: the FinCV (Finnish CardioVersion) study. J Am Coll Cardiol. 2013;62(13):1187-1192. doi:10.1016/j.jacc.2013.04.089.
5. Hansen ML, Jepsen RM, Olesen JB, et al. Thromboembolic risk in 16 274 atrial fibrillation patients undergoing direct current cardioversion with and without oral anticoagulant therapy. Europace. 2015;17(1):18-23. doi:10.1093/europace/euu189.
1. Phang R, Manning WJ. Prevention of embolization prior to and after restoration of sinus rhythm in atrial fibrillation. UptoDate Web site. http://www.uptodate.com/contents/prevention-of-embolization-prior-to-and-after-restoration-of-sinus-rhythm-in-atrial-fibrillation. Updated October 10, 2016. Accessed March 6, 2017.
2. January CT, Wann LS, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-e267. Erratum in Circulation. 2014;130(23):e272-e274. doi:10.1161/CIR.0000000000000041.
3. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016:37(38):2893-2962. doi:10.1093/eurheartj/ehw210.
4. Airaksinen KE, Grönberg T, Nuotio I, et al. Thromboembolic complications after cardioversion of acute atrial fibrillation: the FinCV (Finnish CardioVersion) study. J Am Coll Cardiol. 2013;62(13):1187-1192. doi:10.1016/j.jacc.2013.04.089.
5. Hansen ML, Jepsen RM, Olesen JB, et al. Thromboembolic risk in 16 274 atrial fibrillation patients undergoing direct current cardioversion with and without oral anticoagulant therapy. Europace. 2015;17(1):18-23. doi:10.1093/europace/euu189.