User login
ORLANDO – Infliximab and tacrolimus each demonstrate efficacy for treatment of moderate to severe active ulcerative colitis in published studies. However, it remains unknown if one agent offers greater efficacy than the other in this patient population, so researchers in Japan conducted a meta-analysis to find out more.
Lead investigator Shinichi Kawano, MD, at Kyushu University, Fukuoka, Japan, and his colleagues searched PubMed for relevant studies up until August 2016. They conducted a systematic review and identified 79 potential studies of tumor necrosis factor blocker infliximab (Remicade, Janssen) and the calcineurin inhibitor tacrolimus (various brands) in this patient population. They ruled out the vast majority, 75 studies, for not directly comparing therapeutic efficacy. They also excluded one additional study for insufficient data on their five outcomes of interest: rates of clinical remission, clinical response, freedom from colectomy, adverse events, and serious adverse events. They focused on three 2016 retrospective studies with a total of 244 patients, and added 13 of their own patients, 5 taking infliximab and 8 taking tacrolimus, to their dataset.
Similarly, the short-term clinical response rate favored infliximab (overall RR, 1.55), but again the difference was not statistically significant.
The investigators found the rate of colectomy was comparable between patients taking infliximab and tacrolimus (overall RR, 1.01). They reported their findings in a poster presentation at the Advances in Inflammatory Bowel Diseases meeting, sponsored by the Crohn’s & Colitis Foundation of America.
The adverse event rate favored tacrolimus over infliximab, but it was not significant (overall RR, 0.23). The serious adverse event rate slightly favored tacrolimus as well (overall RR, 0.88). The incidences of adverse events and serious adverse events were comparable between the two groups, the study authors wrote.
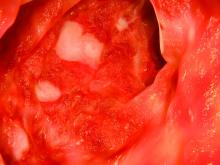
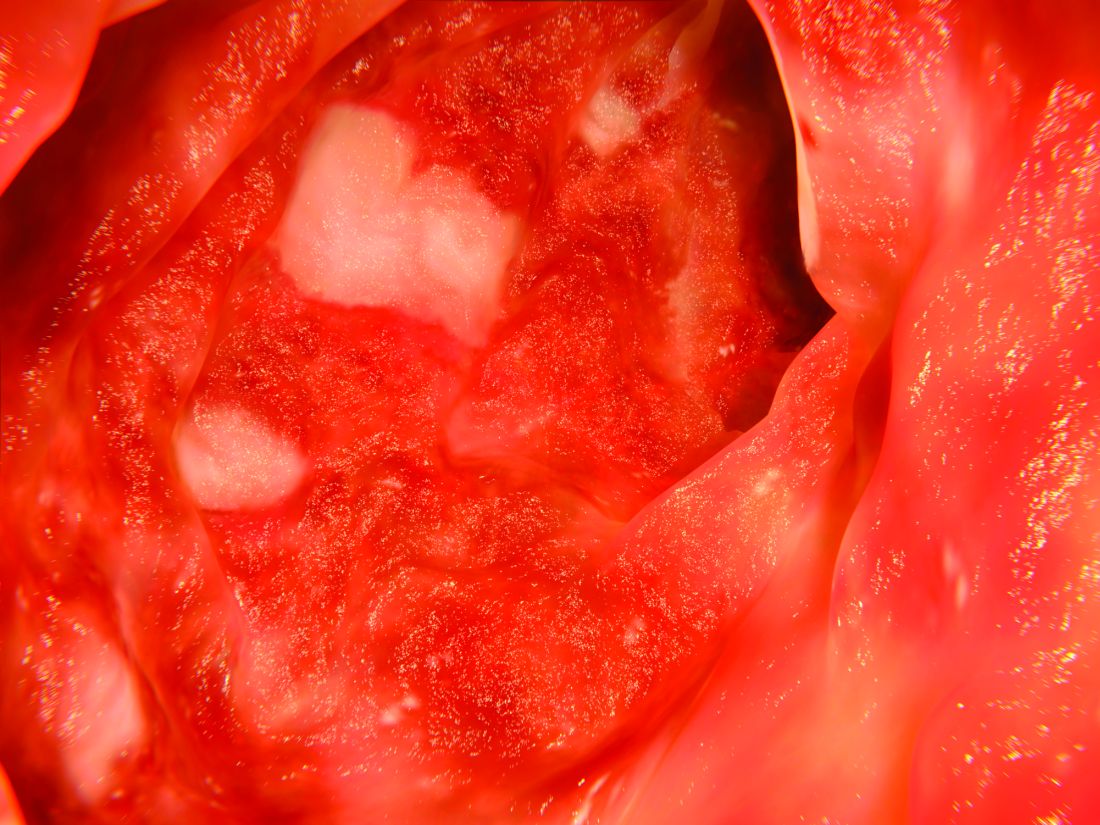
The trials included in the meta-analysis looked at either adults only or adults and pediatric patients with active ulcerative colitis. In one study, there were 40 patients taking infliximab and 50 taking tacrolimus (Aliment Pharmacol Ther. 2016;43:705-16); in another 48 patients took infliximab and 47 took tacrolimus (Gastroenterol Res Pract. 2016;2016:3162595); and in the third study 30 were treated with infliximab and 29 with tacrolimus (Scand J Gastroenterol. 2016;51:700-5).
“This meta-analysis demonstrates equivalent therapeutic efficacy and safety between infliximab and tacrolimus,” the authors continued. Dr. Kawano said he was not surprised by the findings. “Because there are only three retrospective studies, it is reasonable that there is no significant difference in efficacy between infliximab and tacrolimus.”
However, he added, “We think that further prospective, comparative trials are needed.”
Dr. Kawano had no relevant financial disclosures.
ORLANDO – Infliximab and tacrolimus each demonstrate efficacy for treatment of moderate to severe active ulcerative colitis in published studies. However, it remains unknown if one agent offers greater efficacy than the other in this patient population, so researchers in Japan conducted a meta-analysis to find out more.
Lead investigator Shinichi Kawano, MD, at Kyushu University, Fukuoka, Japan, and his colleagues searched PubMed for relevant studies up until August 2016. They conducted a systematic review and identified 79 potential studies of tumor necrosis factor blocker infliximab (Remicade, Janssen) and the calcineurin inhibitor tacrolimus (various brands) in this patient population. They ruled out the vast majority, 75 studies, for not directly comparing therapeutic efficacy. They also excluded one additional study for insufficient data on their five outcomes of interest: rates of clinical remission, clinical response, freedom from colectomy, adverse events, and serious adverse events. They focused on three 2016 retrospective studies with a total of 244 patients, and added 13 of their own patients, 5 taking infliximab and 8 taking tacrolimus, to their dataset.
Similarly, the short-term clinical response rate favored infliximab (overall RR, 1.55), but again the difference was not statistically significant.
The investigators found the rate of colectomy was comparable between patients taking infliximab and tacrolimus (overall RR, 1.01). They reported their findings in a poster presentation at the Advances in Inflammatory Bowel Diseases meeting, sponsored by the Crohn’s & Colitis Foundation of America.
The adverse event rate favored tacrolimus over infliximab, but it was not significant (overall RR, 0.23). The serious adverse event rate slightly favored tacrolimus as well (overall RR, 0.88). The incidences of adverse events and serious adverse events were comparable between the two groups, the study authors wrote.
The trials included in the meta-analysis looked at either adults only or adults and pediatric patients with active ulcerative colitis. In one study, there were 40 patients taking infliximab and 50 taking tacrolimus (Aliment Pharmacol Ther. 2016;43:705-16); in another 48 patients took infliximab and 47 took tacrolimus (Gastroenterol Res Pract. 2016;2016:3162595); and in the third study 30 were treated with infliximab and 29 with tacrolimus (Scand J Gastroenterol. 2016;51:700-5).
“This meta-analysis demonstrates equivalent therapeutic efficacy and safety between infliximab and tacrolimus,” the authors continued. Dr. Kawano said he was not surprised by the findings. “Because there are only three retrospective studies, it is reasonable that there is no significant difference in efficacy between infliximab and tacrolimus.”
However, he added, “We think that further prospective, comparative trials are needed.”
Dr. Kawano had no relevant financial disclosures.
ORLANDO – Infliximab and tacrolimus each demonstrate efficacy for treatment of moderate to severe active ulcerative colitis in published studies. However, it remains unknown if one agent offers greater efficacy than the other in this patient population, so researchers in Japan conducted a meta-analysis to find out more.
Lead investigator Shinichi Kawano, MD, at Kyushu University, Fukuoka, Japan, and his colleagues searched PubMed for relevant studies up until August 2016. They conducted a systematic review and identified 79 potential studies of tumor necrosis factor blocker infliximab (Remicade, Janssen) and the calcineurin inhibitor tacrolimus (various brands) in this patient population. They ruled out the vast majority, 75 studies, for not directly comparing therapeutic efficacy. They also excluded one additional study for insufficient data on their five outcomes of interest: rates of clinical remission, clinical response, freedom from colectomy, adverse events, and serious adverse events. They focused on three 2016 retrospective studies with a total of 244 patients, and added 13 of their own patients, 5 taking infliximab and 8 taking tacrolimus, to their dataset.
Similarly, the short-term clinical response rate favored infliximab (overall RR, 1.55), but again the difference was not statistically significant.
The investigators found the rate of colectomy was comparable between patients taking infliximab and tacrolimus (overall RR, 1.01). They reported their findings in a poster presentation at the Advances in Inflammatory Bowel Diseases meeting, sponsored by the Crohn’s & Colitis Foundation of America.
The adverse event rate favored tacrolimus over infliximab, but it was not significant (overall RR, 0.23). The serious adverse event rate slightly favored tacrolimus as well (overall RR, 0.88). The incidences of adverse events and serious adverse events were comparable between the two groups, the study authors wrote.
The trials included in the meta-analysis looked at either adults only or adults and pediatric patients with active ulcerative colitis. In one study, there were 40 patients taking infliximab and 50 taking tacrolimus (Aliment Pharmacol Ther. 2016;43:705-16); in another 48 patients took infliximab and 47 took tacrolimus (Gastroenterol Res Pract. 2016;2016:3162595); and in the third study 30 were treated with infliximab and 29 with tacrolimus (Scand J Gastroenterol. 2016;51:700-5).
“This meta-analysis demonstrates equivalent therapeutic efficacy and safety between infliximab and tacrolimus,” the authors continued. Dr. Kawano said he was not surprised by the findings. “Because there are only three retrospective studies, it is reasonable that there is no significant difference in efficacy between infliximab and tacrolimus.”
However, he added, “We think that further prospective, comparative trials are needed.”
Dr. Kawano had no relevant financial disclosures.
AT AIBD 2016
Key clinical point: Infliximab and tacrolimus both demonstrate efficacy for active ulcerative colitis in published studies, but few direct comparisons exist.
Major finding: The reported rate of clinical remission with infliximab was higher than with tacrolimus (risk ratio, 1.17), but the difference was not statistically significant.
Data source: Meta-analysis of relevant articles identified in a PubMed search through August 2016.
Disclosures: Dr. Kawano had no relevant financial disclosures.