User login
Data Trends 2025: Hepatology
Data Trends 2025: Hepatology
Click here to view more from Federal Health Care Data Trends 2025.
- Niezen S, et al. Am J Gastroenterol. Published online January 7, 2025. doi:10.14309/ajg.0000000000003312
- Beydoun HA, Tsai J. J Viral Hepat. 2024;31(10):601-613. doi:10.1111/jvh.13981
- Yeoh A, et al. J Clin Gastroenterol. 2024;58(7):718-725. doi:10.1097/MCG.0000000000001921
- Varley CD, et al. Clin Infect Dis. 2024;78(6):1571-1579. doi:10.1093/cid/ciae025
- Njei B, et al. Dig Dis Sci. 2025;70(2):802-813. doi:10.1007/s10620-024-08764-4
Click here to view more from Federal Health Care Data Trends 2025.
Click here to view more from Federal Health Care Data Trends 2025.
- Niezen S, et al. Am J Gastroenterol. Published online January 7, 2025. doi:10.14309/ajg.0000000000003312
- Beydoun HA, Tsai J. J Viral Hepat. 2024;31(10):601-613. doi:10.1111/jvh.13981
- Yeoh A, et al. J Clin Gastroenterol. 2024;58(7):718-725. doi:10.1097/MCG.0000000000001921
- Varley CD, et al. Clin Infect Dis. 2024;78(6):1571-1579. doi:10.1093/cid/ciae025
- Njei B, et al. Dig Dis Sci. 2025;70(2):802-813. doi:10.1007/s10620-024-08764-4
- Niezen S, et al. Am J Gastroenterol. Published online January 7, 2025. doi:10.14309/ajg.0000000000003312
- Beydoun HA, Tsai J. J Viral Hepat. 2024;31(10):601-613. doi:10.1111/jvh.13981
- Yeoh A, et al. J Clin Gastroenterol. 2024;58(7):718-725. doi:10.1097/MCG.0000000000001921
- Varley CD, et al. Clin Infect Dis. 2024;78(6):1571-1579. doi:10.1093/cid/ciae025
- Njei B, et al. Dig Dis Sci. 2025;70(2):802-813. doi:10.1007/s10620-024-08764-4
Data Trends 2025: Hepatology
Data Trends 2025: Hepatology
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Histology is the gold standard for cirrhosis diagnosis. However, a combination of clinical history, physical examination findings, and supportive laboratory and radiographic features is generally sufficient to make the diagnosis. Routine ultrasound and computed tomography (CT) imaging often identifies a nodular liver contour with sequelae of portal hypertension, including splenomegaly, varices, and ascites, which can suggest cirrhosis when supported by laboratory parameters and clinical features. As a result, the diagnosis is typically made clinically.1 Many patients with compensated cirrhosis go undetected. The presence of a decompensation event (ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) often leads to index diagnosis when patients were previously compensated. When a patient presents with suspected decompensated cirrhosis, it is important to consider other diagnoses with similar presentations and ensure that multiple disease processes are not contributing to the symptoms.
CASE PRESENTATION
A 64-year-old male with a history of intravenous (IV) methamphetamine use and prior incarceration presented with a 3-week history of progressively worsening generalized swelling. Prior to the onset of his symptoms, the patient injured his right lower extremity (RLE) in a bicycle accident, resulting in edema that progressed to bilateral lower extremity (BLE) edema and worsening fatigue, despite resolution of the initial injury. The patient gained weight though he could not quantify the amount. He experienced progressive hunger, thirst, and fatigue as well as increased sleep. Additionally, the patient experienced worsening dyspnea on exertion and orthopnea. He started using 2 pillows instead of 1 pillow at night.
The patient reported no fevers, chills, sputum production, chest pain, or paroxysmal nocturnal dyspnea. He had no known history of sexually transmitted infections, no significant history of alcohol use, and occasional tobacco and marijuana use. He had been incarcerated > 10 years before and last used IV methamphetamine 3 years before. He did not regularly take any medications.
The patient’s vital signs included a temperature of 98.2 °F; 78/min heart rate; 15/min respiratory rate; 159/109 mm Hg blood pressure; and 98% oxygen saturation on room air. He had gained 20 lbs in the past 4 months. He had pitting edema in both legs and arms, as well as periorbital swelling, but no jugular venous distention, abnormal heart sounds, or murmurs. Breath sounds were distant but clear to auscultation. His abdomen was distended with normal bowel sounds and no fluid wave; mild epigastric tenderness was present, but no intra-abdominal masses were palpated. He had spider angiomata on the upper chest but no other stigmata of cirrhosis, such as caput medusae or jaundice. Tattoos were noted.
Laboratory test results showed a platelet count of 178 x 103/μL (reference range, 140- 440 ~ 103μL).Creatinine was 0.80 mg/dL (reference range, < 1.28 mg/dL), with an estimated glomerular filtration rate (eGFR) of 99 mL/min/1.73 m2 using the Chronic Kidney Disease-Epidemiology equation (reference range, > 60 mL/min/1.73 m2), (reference range, > 60 mL/min/1.73 m2), and Cystatin C was 1.14 mg/L (reference range, < 1.15 mg/L). His electrolytes and complete blood count were within normal limits, including sodium, 134 mmol/L; potassium, 4.4 mmol/L; chloride, 108 mmol/L; and carbon dioxide, 22.5 mmol/L.
Additional test results included alkaline phosphatase, 126 U/L (reference range, < 94 U/L); alanine transaminase, 41 U/L (reference range, < 45 U/L); aspartate aminotransferase, 70 U/L (reference range, < 35 U/L); total bilirubin, 0.6 mg/dL (reference range, < 1 mg/dL); albumin, 1.8 g/dL (reference range, 3.2-4.8 g/dL); and total protein, 6.3 g/dL (reference range, 5.9-8.3 g/dL). The patient’s international normalized ratio was 0.96 (reference range, 0.8-1.1), and brain natriuretic peptide was normal at 56 pg/mL. No prior laboratory results were available for comparison.
Urine toxicology was positive for amphetamines. Urinalysis demonstrated large occult blood, with a red blood cell count of 26/ HPF (reference range, 0/HPF) and proteinuria (100 mg/dL; reference range, negative), without bacteria, nitrites, or leukocyte esterase. Urine white blood cell count was 10/ HPF (reference range, 0/HPF), and fine granular casts and hyaline casts were present.
A noncontrast CT of the abdomen and pelvis in the emergency department showed an irregular liver contour with diffuse nodularity, multiple portosystemic collaterals, moderate abdominal and pelvic ascites, small bilateral pleural effusions with associated atelectasis, and anasarca consistent with cirrhosis (Figure 1). The patient was admitted to the internal medicine service for workup and management of newly diagnosed cirrhosis.

Paracentesis revealed straw-colored fluid with an ascitic fluid neutrophil count of 17/μL, a protein level of < 3 g/dL and albumin level of < 1.5 g/dL. Gram stain of the ascitic fluid showed a moderate white blood cell count with no organisms. Fluid culture showed no microbial growth.
Initial workup for cirrhosis demonstrated a positive total hepatitis A antibody. The patient had a nonreactive hepatitis B surface antigen and surface antibody, but a reactive hepatitis B core antibody; a hepatitis B DNA level was not ordered. He had a reactive hepatitis C antibody with a viral load of 4,490,000 II/mL (genotype 1a). The patient’s iron level was 120 μg/dL, with a calculated total iron-binding capacity (TIBC) of 126.2 μg/dL. His transferrin saturation (TSAT) (serum iron divided by TIBC) was 95%. The patient had nonreactive antinuclear antibody and antimitochondrial antibody tests and a positive antismooth muscle antibody test with a titer of 1:40. His α-fetoprotein (AFP) level was 505 ng/mL (reference range, < 8 ng/mL).
Follow-up MRI of the abdomen and pelvis showed cirrhotic morphology with large volume ascites and portosystemic collaterals, consistent with portal hypertension. Additionally, it showed multiple scattered peripheral sub centimeter hyperenhancing foci, most likely representing benign lesions.
The patient's spot urine protein-creatinine ratio was 3.76. To better quantify proteinuria, a 24-hour urine collection was performed and revealed 12.8 g/d of urine protein (reference range, 0-0.17 g/d). His serum triglyceride level was 175 mg/dL (reference range, 40-60 mg/dL); total cholesterol was 177 mg/ dL (reference range, ≤ 200 mg/dL); low density lipoprotein cholesterol was 98 mg/ dL (reference range, ≤ 130 mg/dL); and highdensity lipoprotein cholesterol was 43.8 mg/ dL (reference range, ≥ 40 mg/dL); C3 complement level was 71 mg/dL (reference range, 82-185 mg/dL); and C4 complement level was 22 mg/dL (reference range, 15-53 mg/ dL). His rheumatoid factor was < 14 IU/mL. Tests for rapid plasma reagin and HIV antigen- antibody were nonreactive, and the phospholipase A2 receptor antibody test was negative. The patient tested positive for QuantiFERON-TB Gold and qualitative cryoglobulin, which indicated a cryocrit of 1%.
A renal biopsy was performed, revealing diffuse podocyte foot process effacement and glomerulonephritis with low-grade C3 and immunoglobulin (Ig) G deposits, consistent with early membranoproliferative glomerulonephritis (MPGN) (Figures 2 and 3).


The patient was initially diuresed with IV furosemide without significant urine output. He was then diuresed with IV 25% albumin (total, 25 g), followed by IV furosemide 40 mg twice daily, which led to significant urine output and resolution of his anasarca. Given the patient’s hypoalbuminemic state, IV albumin was necessary to deliver furosemide to the proximal tubule. He was started on lisinopril for renal protection and discharged with spironolactone and furosemide for fluid management in the context of cirrhosis.
The patient was evaluated by the Liver Nodule Clinic, which includes specialists from hepatology, medical oncology, radiation oncology, interventional radiology, and diagnostic radiology. The team considered the patient’s medical history and characteristics of the nodules on imaging. Notable aspects of the patient’s history included hepatitis C virus (HCV) infection and an elevated AFP level, although imaging showed no lesion concerning for malignancy. Given these findings, the patient was scheduled for a liver biopsy to establish a tissue diagnosis of cirrhosis. Hepatology, nephrology, and infectious disease specialists coordinated to plan the management and treatment of latent tuberculosis (TB), chronic HCV, MPGN, compensated cirrhosis, and suspicious liver lesions.
The patient chose to handle management and treatment as an outpatient. He was discharged with furosemide and spironolactone for anasarca management, and amlodipine and lisinopril for his hypertension and MPGN. Follow-up appointments were scheduled with infectious disease for management of latent TB and HCV, nephrology for MPGN, gastroenterology for cirrhosis, and interventional radiology for liver biopsy. Unfortunately, the patient was unhoused with limited access to transportation, which prevented timely follow-up. Given these social factors, immunosuppression was not started. Additionally, he did not start on HCV therapy because the viral load was still pending at time of discharge.
DISCUSSION
The diagnosis of decompensated cirrhosis was prematurely established, resulting in a diagnostic delay, a form of diagnostic error. However, on hospital day 2, the initial hypothesis of decompensated cirrhosis as the sole driver of the patient’s presentation was reconsidered due to the disconnect between the severity of hypoalbuminemia and diffuse edema (anasarca), and the absence of laboratory evidence of hepatic decompensation (normal international normalized ratio, bilirubin, and low but normal platelet count). Although image findings supported cirrhosis, laboratory markers did not indicate hepatic decompensation. The severity of hypoalbuminemia and anasarca, along with an indeterminate Serum-Ascites Albumin Gradient, prompted the patient’s care team to consider other causes, specifically, nephrotic syndrome.
The patien’s spot protein-to-creatinine ratio was 3.76 (reference range < 0.2 mg/mg creatinine), but a 24-hour urine protein collection was 12.8 g/day (reference range < 150 mg/day). While most spot urine protein- to-creatinine ratios (UPCR) correlate with a 24-hour urine collection, discrepancies can occur, as in this case. It is important to recognize that the spot UPCR assumes that patients are excreting 1000 mg of creatinine daily in their urine, which is not always the case. In addition, changes in urine osmolality can lead to different values. The gold standard for proteinuria is a 24-hour urine collection for protein and creatinine.
The patient’s nephrotic-range proteinuria and severe hypoalbuminemia are not solely explained by cirrhosis. In addition, the patient’s lower extremity edema pointed to nephrotic syndrome. The differential diagnosis for nephrotic syndrome includes both primary and secondary forms of membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, and MPGN, a histopathological diagnosis that requires distinguishing between immune complex-mediated and complement-mediated forms. Other causes of nephrotic syndrome that do not fit in any of these buckets include amyloidosis, IgA nephropathy, and diabetes mellitus (DM). Despite DM being a common cause of nephrotic range proteinuria, it rarely leads to full nephrotic syndrome.
When considering the diagnosis, we reframed the patient’s clinical syndrome as compensated cirrhosis plus nephrotic syndrome. This approach prioritized identifying a cause that could explain both cirrhosis (from any cause) leading to IgA nephropathy or injection drug use serving as a risk factor for cirrhosis and nephrotic syndrome through HCV or AA amyloidosis, respectively. This problem representation guided us to the correct diagnosis. There are multiple renal diseases associated with HCV infection, including MPGN, membranous nephropathy, focal segmental glomerulosclerosis, and IgA nephropathy.2 MPGN and mixed cryoglobulinemia are the most common. In the past, MPGN was classified as type I, II, and III.
The patient’s urine toxicology revealed recent amphetamine use, which can also lead to acute kidney injury through rhabdomyolysis or acute interstitial nephritis (AIN).3 In the cases of rhabdomyolysis, urinalysis would show positive heme without any red blood cell on microscopic analysis, which was not present in this case. AIN commonly manifests as acute kidney injury, pyuria, and proteinuria but without a decrease in complement levels.4 While the patient’s urine sediment included white blood cell (10/high-power field), the presence of microscopic hematuria, decreased complement levels, and proteinuria in the context of HCV positivity makes MPGN more likely than AIN.
Recently, there has been greater emphasis on using immunofluorescence for kidney biopsies. MPGN is now classified into 2 main categories: MPGN with mesangial immunoglobulins and C3 deposits in the capillary walls, and MPGN with C3 deposits but without Ig.5 MPGN with Ig-complement deposits is seen in autoimmune diseases and infections and is associated with dysproteinemias.
The renal biopsy in this patient was consistent with MPGN with immunofluorescence, a common finding in patients with infection. By synthesizing these data, we concluded that the patient represented a case of chronic HCV infection that led to MPGN with cryoglobulinemia. The normal C4 and negative RF do not suggest cryoglobulinemic crisis. Compensated cirrhosis was seen on imaging, pending liver biopsy.
Treatment
The management of MPGN secondary to HCV infection relies on the treatment of the underlying infection and clearance of viral load. Direct-acting antivirals have been used successfully in the treatment of HCV-associated MPGN. When cryoglobulinemia is present, immunosuppressive therapy is recommended. These regimens commonly include rituximab and steroids.5 Rituximab is also used for nephrotic syndrome associated with MPGN, as recommended in the 2018 Kidney Disease: Improving Global Outcomes guidelines.6
When initiating rituximab therapy in a patient who tests positive for hepatitis B (HBcAb positive or HBsAb positive), it is recommended to follow the established guidelines, which include treating them with entecavir for prophylaxis to prevent reactivation or a flare of hepatitis B.7 The patient in this case needed close follow-up in the nephrology and hepatology clinic. Immunosuppressive therapy was not pursued while the patient was admitted to the hospital due to instability with housing, transportation, and difficulty in ensuring close follow-up.
CONCLUSIONS
Clinicians should maintain a broad differential even in the face of confirmatory imaging and other objective findings. In the case of anasarca, nephrotic syndrome should be considered. Key causes of nephrotic syndromes include MPGN, membranous nephropathy, minimal change disease, and focal segmental glomerulosclerosis. MPGN is a histopathological diagnosis, and it is essential to identify if it is secondary to immune complexes or only complement mediated because Ig-complement deposits are seen in autoimmune disease and infection. The management of MPGN due to HCV infection relies on antiviral therapy. In the presence of cryoglobulinemia, immunosuppressive therapy is recommended.
- Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023;329(18):1589–1602. doi:10.1001/jama.2023.5997
- Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20(24):7544-7554. doi:10.3748/wjg.v20.i24.7544
- Foley RJ, Kapatkin K, Vrani R, Weinman EJ. Amphetamineinduced acute renal failure. South Med J. 1984;77(2):258- 260. doi:10.1097/00007611-198402000-00035
- Rossert J. Drug - induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817. doi:10.1046/j.1523-1755.2001.060002804.x
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31(4):341-348. doi:10.1016/j.semnephrol.2011.06.005
- Jadoul M, Berenguer MC, Doss W, et al. Executive summary of the 2018 KDIGO hepatitis C in CKD guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94(4):663-673. doi:10.1016/j.kint.2018.06.011
- Myint A, Tong MJ, Beaven SW. Reactivation of hepatitis b virus: a review of clinical guidelines. Clin Liver Dis (Hoboken). 2020;15(4):162-167. doi:10.1002/cld.883
Histology is the gold standard for cirrhosis diagnosis. However, a combination of clinical history, physical examination findings, and supportive laboratory and radiographic features is generally sufficient to make the diagnosis. Routine ultrasound and computed tomography (CT) imaging often identifies a nodular liver contour with sequelae of portal hypertension, including splenomegaly, varices, and ascites, which can suggest cirrhosis when supported by laboratory parameters and clinical features. As a result, the diagnosis is typically made clinically.1 Many patients with compensated cirrhosis go undetected. The presence of a decompensation event (ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) often leads to index diagnosis when patients were previously compensated. When a patient presents with suspected decompensated cirrhosis, it is important to consider other diagnoses with similar presentations and ensure that multiple disease processes are not contributing to the symptoms.
CASE PRESENTATION
A 64-year-old male with a history of intravenous (IV) methamphetamine use and prior incarceration presented with a 3-week history of progressively worsening generalized swelling. Prior to the onset of his symptoms, the patient injured his right lower extremity (RLE) in a bicycle accident, resulting in edema that progressed to bilateral lower extremity (BLE) edema and worsening fatigue, despite resolution of the initial injury. The patient gained weight though he could not quantify the amount. He experienced progressive hunger, thirst, and fatigue as well as increased sleep. Additionally, the patient experienced worsening dyspnea on exertion and orthopnea. He started using 2 pillows instead of 1 pillow at night.
The patient reported no fevers, chills, sputum production, chest pain, or paroxysmal nocturnal dyspnea. He had no known history of sexually transmitted infections, no significant history of alcohol use, and occasional tobacco and marijuana use. He had been incarcerated > 10 years before and last used IV methamphetamine 3 years before. He did not regularly take any medications.
The patient’s vital signs included a temperature of 98.2 °F; 78/min heart rate; 15/min respiratory rate; 159/109 mm Hg blood pressure; and 98% oxygen saturation on room air. He had gained 20 lbs in the past 4 months. He had pitting edema in both legs and arms, as well as periorbital swelling, but no jugular venous distention, abnormal heart sounds, or murmurs. Breath sounds were distant but clear to auscultation. His abdomen was distended with normal bowel sounds and no fluid wave; mild epigastric tenderness was present, but no intra-abdominal masses were palpated. He had spider angiomata on the upper chest but no other stigmata of cirrhosis, such as caput medusae or jaundice. Tattoos were noted.
Laboratory test results showed a platelet count of 178 x 103/μL (reference range, 140- 440 ~ 103μL).Creatinine was 0.80 mg/dL (reference range, < 1.28 mg/dL), with an estimated glomerular filtration rate (eGFR) of 99 mL/min/1.73 m2 using the Chronic Kidney Disease-Epidemiology equation (reference range, > 60 mL/min/1.73 m2), (reference range, > 60 mL/min/1.73 m2), and Cystatin C was 1.14 mg/L (reference range, < 1.15 mg/L). His electrolytes and complete blood count were within normal limits, including sodium, 134 mmol/L; potassium, 4.4 mmol/L; chloride, 108 mmol/L; and carbon dioxide, 22.5 mmol/L.
Additional test results included alkaline phosphatase, 126 U/L (reference range, < 94 U/L); alanine transaminase, 41 U/L (reference range, < 45 U/L); aspartate aminotransferase, 70 U/L (reference range, < 35 U/L); total bilirubin, 0.6 mg/dL (reference range, < 1 mg/dL); albumin, 1.8 g/dL (reference range, 3.2-4.8 g/dL); and total protein, 6.3 g/dL (reference range, 5.9-8.3 g/dL). The patient’s international normalized ratio was 0.96 (reference range, 0.8-1.1), and brain natriuretic peptide was normal at 56 pg/mL. No prior laboratory results were available for comparison.
Urine toxicology was positive for amphetamines. Urinalysis demonstrated large occult blood, with a red blood cell count of 26/ HPF (reference range, 0/HPF) and proteinuria (100 mg/dL; reference range, negative), without bacteria, nitrites, or leukocyte esterase. Urine white blood cell count was 10/ HPF (reference range, 0/HPF), and fine granular casts and hyaline casts were present.
A noncontrast CT of the abdomen and pelvis in the emergency department showed an irregular liver contour with diffuse nodularity, multiple portosystemic collaterals, moderate abdominal and pelvic ascites, small bilateral pleural effusions with associated atelectasis, and anasarca consistent with cirrhosis (Figure 1). The patient was admitted to the internal medicine service for workup and management of newly diagnosed cirrhosis.

Paracentesis revealed straw-colored fluid with an ascitic fluid neutrophil count of 17/μL, a protein level of < 3 g/dL and albumin level of < 1.5 g/dL. Gram stain of the ascitic fluid showed a moderate white blood cell count with no organisms. Fluid culture showed no microbial growth.
Initial workup for cirrhosis demonstrated a positive total hepatitis A antibody. The patient had a nonreactive hepatitis B surface antigen and surface antibody, but a reactive hepatitis B core antibody; a hepatitis B DNA level was not ordered. He had a reactive hepatitis C antibody with a viral load of 4,490,000 II/mL (genotype 1a). The patient’s iron level was 120 μg/dL, with a calculated total iron-binding capacity (TIBC) of 126.2 μg/dL. His transferrin saturation (TSAT) (serum iron divided by TIBC) was 95%. The patient had nonreactive antinuclear antibody and antimitochondrial antibody tests and a positive antismooth muscle antibody test with a titer of 1:40. His α-fetoprotein (AFP) level was 505 ng/mL (reference range, < 8 ng/mL).
Follow-up MRI of the abdomen and pelvis showed cirrhotic morphology with large volume ascites and portosystemic collaterals, consistent with portal hypertension. Additionally, it showed multiple scattered peripheral sub centimeter hyperenhancing foci, most likely representing benign lesions.
The patient's spot urine protein-creatinine ratio was 3.76. To better quantify proteinuria, a 24-hour urine collection was performed and revealed 12.8 g/d of urine protein (reference range, 0-0.17 g/d). His serum triglyceride level was 175 mg/dL (reference range, 40-60 mg/dL); total cholesterol was 177 mg/ dL (reference range, ≤ 200 mg/dL); low density lipoprotein cholesterol was 98 mg/ dL (reference range, ≤ 130 mg/dL); and highdensity lipoprotein cholesterol was 43.8 mg/ dL (reference range, ≥ 40 mg/dL); C3 complement level was 71 mg/dL (reference range, 82-185 mg/dL); and C4 complement level was 22 mg/dL (reference range, 15-53 mg/ dL). His rheumatoid factor was < 14 IU/mL. Tests for rapid plasma reagin and HIV antigen- antibody were nonreactive, and the phospholipase A2 receptor antibody test was negative. The patient tested positive for QuantiFERON-TB Gold and qualitative cryoglobulin, which indicated a cryocrit of 1%.
A renal biopsy was performed, revealing diffuse podocyte foot process effacement and glomerulonephritis with low-grade C3 and immunoglobulin (Ig) G deposits, consistent with early membranoproliferative glomerulonephritis (MPGN) (Figures 2 and 3).


The patient was initially diuresed with IV furosemide without significant urine output. He was then diuresed with IV 25% albumin (total, 25 g), followed by IV furosemide 40 mg twice daily, which led to significant urine output and resolution of his anasarca. Given the patient’s hypoalbuminemic state, IV albumin was necessary to deliver furosemide to the proximal tubule. He was started on lisinopril for renal protection and discharged with spironolactone and furosemide for fluid management in the context of cirrhosis.
The patient was evaluated by the Liver Nodule Clinic, which includes specialists from hepatology, medical oncology, radiation oncology, interventional radiology, and diagnostic radiology. The team considered the patient’s medical history and characteristics of the nodules on imaging. Notable aspects of the patient’s history included hepatitis C virus (HCV) infection and an elevated AFP level, although imaging showed no lesion concerning for malignancy. Given these findings, the patient was scheduled for a liver biopsy to establish a tissue diagnosis of cirrhosis. Hepatology, nephrology, and infectious disease specialists coordinated to plan the management and treatment of latent tuberculosis (TB), chronic HCV, MPGN, compensated cirrhosis, and suspicious liver lesions.
The patient chose to handle management and treatment as an outpatient. He was discharged with furosemide and spironolactone for anasarca management, and amlodipine and lisinopril for his hypertension and MPGN. Follow-up appointments were scheduled with infectious disease for management of latent TB and HCV, nephrology for MPGN, gastroenterology for cirrhosis, and interventional radiology for liver biopsy. Unfortunately, the patient was unhoused with limited access to transportation, which prevented timely follow-up. Given these social factors, immunosuppression was not started. Additionally, he did not start on HCV therapy because the viral load was still pending at time of discharge.
DISCUSSION
The diagnosis of decompensated cirrhosis was prematurely established, resulting in a diagnostic delay, a form of diagnostic error. However, on hospital day 2, the initial hypothesis of decompensated cirrhosis as the sole driver of the patient’s presentation was reconsidered due to the disconnect between the severity of hypoalbuminemia and diffuse edema (anasarca), and the absence of laboratory evidence of hepatic decompensation (normal international normalized ratio, bilirubin, and low but normal platelet count). Although image findings supported cirrhosis, laboratory markers did not indicate hepatic decompensation. The severity of hypoalbuminemia and anasarca, along with an indeterminate Serum-Ascites Albumin Gradient, prompted the patient’s care team to consider other causes, specifically, nephrotic syndrome.
The patien’s spot protein-to-creatinine ratio was 3.76 (reference range < 0.2 mg/mg creatinine), but a 24-hour urine protein collection was 12.8 g/day (reference range < 150 mg/day). While most spot urine protein- to-creatinine ratios (UPCR) correlate with a 24-hour urine collection, discrepancies can occur, as in this case. It is important to recognize that the spot UPCR assumes that patients are excreting 1000 mg of creatinine daily in their urine, which is not always the case. In addition, changes in urine osmolality can lead to different values. The gold standard for proteinuria is a 24-hour urine collection for protein and creatinine.
The patient’s nephrotic-range proteinuria and severe hypoalbuminemia are not solely explained by cirrhosis. In addition, the patient’s lower extremity edema pointed to nephrotic syndrome. The differential diagnosis for nephrotic syndrome includes both primary and secondary forms of membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, and MPGN, a histopathological diagnosis that requires distinguishing between immune complex-mediated and complement-mediated forms. Other causes of nephrotic syndrome that do not fit in any of these buckets include amyloidosis, IgA nephropathy, and diabetes mellitus (DM). Despite DM being a common cause of nephrotic range proteinuria, it rarely leads to full nephrotic syndrome.
When considering the diagnosis, we reframed the patient’s clinical syndrome as compensated cirrhosis plus nephrotic syndrome. This approach prioritized identifying a cause that could explain both cirrhosis (from any cause) leading to IgA nephropathy or injection drug use serving as a risk factor for cirrhosis and nephrotic syndrome through HCV or AA amyloidosis, respectively. This problem representation guided us to the correct diagnosis. There are multiple renal diseases associated with HCV infection, including MPGN, membranous nephropathy, focal segmental glomerulosclerosis, and IgA nephropathy.2 MPGN and mixed cryoglobulinemia are the most common. In the past, MPGN was classified as type I, II, and III.
The patient’s urine toxicology revealed recent amphetamine use, which can also lead to acute kidney injury through rhabdomyolysis or acute interstitial nephritis (AIN).3 In the cases of rhabdomyolysis, urinalysis would show positive heme without any red blood cell on microscopic analysis, which was not present in this case. AIN commonly manifests as acute kidney injury, pyuria, and proteinuria but without a decrease in complement levels.4 While the patient’s urine sediment included white blood cell (10/high-power field), the presence of microscopic hematuria, decreased complement levels, and proteinuria in the context of HCV positivity makes MPGN more likely than AIN.
Recently, there has been greater emphasis on using immunofluorescence for kidney biopsies. MPGN is now classified into 2 main categories: MPGN with mesangial immunoglobulins and C3 deposits in the capillary walls, and MPGN with C3 deposits but without Ig.5 MPGN with Ig-complement deposits is seen in autoimmune diseases and infections and is associated with dysproteinemias.
The renal biopsy in this patient was consistent with MPGN with immunofluorescence, a common finding in patients with infection. By synthesizing these data, we concluded that the patient represented a case of chronic HCV infection that led to MPGN with cryoglobulinemia. The normal C4 and negative RF do not suggest cryoglobulinemic crisis. Compensated cirrhosis was seen on imaging, pending liver biopsy.
Treatment
The management of MPGN secondary to HCV infection relies on the treatment of the underlying infection and clearance of viral load. Direct-acting antivirals have been used successfully in the treatment of HCV-associated MPGN. When cryoglobulinemia is present, immunosuppressive therapy is recommended. These regimens commonly include rituximab and steroids.5 Rituximab is also used for nephrotic syndrome associated with MPGN, as recommended in the 2018 Kidney Disease: Improving Global Outcomes guidelines.6
When initiating rituximab therapy in a patient who tests positive for hepatitis B (HBcAb positive or HBsAb positive), it is recommended to follow the established guidelines, which include treating them with entecavir for prophylaxis to prevent reactivation or a flare of hepatitis B.7 The patient in this case needed close follow-up in the nephrology and hepatology clinic. Immunosuppressive therapy was not pursued while the patient was admitted to the hospital due to instability with housing, transportation, and difficulty in ensuring close follow-up.
CONCLUSIONS
Clinicians should maintain a broad differential even in the face of confirmatory imaging and other objective findings. In the case of anasarca, nephrotic syndrome should be considered. Key causes of nephrotic syndromes include MPGN, membranous nephropathy, minimal change disease, and focal segmental glomerulosclerosis. MPGN is a histopathological diagnosis, and it is essential to identify if it is secondary to immune complexes or only complement mediated because Ig-complement deposits are seen in autoimmune disease and infection. The management of MPGN due to HCV infection relies on antiviral therapy. In the presence of cryoglobulinemia, immunosuppressive therapy is recommended.
Histology is the gold standard for cirrhosis diagnosis. However, a combination of clinical history, physical examination findings, and supportive laboratory and radiographic features is generally sufficient to make the diagnosis. Routine ultrasound and computed tomography (CT) imaging often identifies a nodular liver contour with sequelae of portal hypertension, including splenomegaly, varices, and ascites, which can suggest cirrhosis when supported by laboratory parameters and clinical features. As a result, the diagnosis is typically made clinically.1 Many patients with compensated cirrhosis go undetected. The presence of a decompensation event (ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) often leads to index diagnosis when patients were previously compensated. When a patient presents with suspected decompensated cirrhosis, it is important to consider other diagnoses with similar presentations and ensure that multiple disease processes are not contributing to the symptoms.
CASE PRESENTATION
A 64-year-old male with a history of intravenous (IV) methamphetamine use and prior incarceration presented with a 3-week history of progressively worsening generalized swelling. Prior to the onset of his symptoms, the patient injured his right lower extremity (RLE) in a bicycle accident, resulting in edema that progressed to bilateral lower extremity (BLE) edema and worsening fatigue, despite resolution of the initial injury. The patient gained weight though he could not quantify the amount. He experienced progressive hunger, thirst, and fatigue as well as increased sleep. Additionally, the patient experienced worsening dyspnea on exertion and orthopnea. He started using 2 pillows instead of 1 pillow at night.
The patient reported no fevers, chills, sputum production, chest pain, or paroxysmal nocturnal dyspnea. He had no known history of sexually transmitted infections, no significant history of alcohol use, and occasional tobacco and marijuana use. He had been incarcerated > 10 years before and last used IV methamphetamine 3 years before. He did not regularly take any medications.
The patient’s vital signs included a temperature of 98.2 °F; 78/min heart rate; 15/min respiratory rate; 159/109 mm Hg blood pressure; and 98% oxygen saturation on room air. He had gained 20 lbs in the past 4 months. He had pitting edema in both legs and arms, as well as periorbital swelling, but no jugular venous distention, abnormal heart sounds, or murmurs. Breath sounds were distant but clear to auscultation. His abdomen was distended with normal bowel sounds and no fluid wave; mild epigastric tenderness was present, but no intra-abdominal masses were palpated. He had spider angiomata on the upper chest but no other stigmata of cirrhosis, such as caput medusae or jaundice. Tattoos were noted.
Laboratory test results showed a platelet count of 178 x 103/μL (reference range, 140- 440 ~ 103μL).Creatinine was 0.80 mg/dL (reference range, < 1.28 mg/dL), with an estimated glomerular filtration rate (eGFR) of 99 mL/min/1.73 m2 using the Chronic Kidney Disease-Epidemiology equation (reference range, > 60 mL/min/1.73 m2), (reference range, > 60 mL/min/1.73 m2), and Cystatin C was 1.14 mg/L (reference range, < 1.15 mg/L). His electrolytes and complete blood count were within normal limits, including sodium, 134 mmol/L; potassium, 4.4 mmol/L; chloride, 108 mmol/L; and carbon dioxide, 22.5 mmol/L.
Additional test results included alkaline phosphatase, 126 U/L (reference range, < 94 U/L); alanine transaminase, 41 U/L (reference range, < 45 U/L); aspartate aminotransferase, 70 U/L (reference range, < 35 U/L); total bilirubin, 0.6 mg/dL (reference range, < 1 mg/dL); albumin, 1.8 g/dL (reference range, 3.2-4.8 g/dL); and total protein, 6.3 g/dL (reference range, 5.9-8.3 g/dL). The patient’s international normalized ratio was 0.96 (reference range, 0.8-1.1), and brain natriuretic peptide was normal at 56 pg/mL. No prior laboratory results were available for comparison.
Urine toxicology was positive for amphetamines. Urinalysis demonstrated large occult blood, with a red blood cell count of 26/ HPF (reference range, 0/HPF) and proteinuria (100 mg/dL; reference range, negative), without bacteria, nitrites, or leukocyte esterase. Urine white blood cell count was 10/ HPF (reference range, 0/HPF), and fine granular casts and hyaline casts were present.
A noncontrast CT of the abdomen and pelvis in the emergency department showed an irregular liver contour with diffuse nodularity, multiple portosystemic collaterals, moderate abdominal and pelvic ascites, small bilateral pleural effusions with associated atelectasis, and anasarca consistent with cirrhosis (Figure 1). The patient was admitted to the internal medicine service for workup and management of newly diagnosed cirrhosis.

Paracentesis revealed straw-colored fluid with an ascitic fluid neutrophil count of 17/μL, a protein level of < 3 g/dL and albumin level of < 1.5 g/dL. Gram stain of the ascitic fluid showed a moderate white blood cell count with no organisms. Fluid culture showed no microbial growth.
Initial workup for cirrhosis demonstrated a positive total hepatitis A antibody. The patient had a nonreactive hepatitis B surface antigen and surface antibody, but a reactive hepatitis B core antibody; a hepatitis B DNA level was not ordered. He had a reactive hepatitis C antibody with a viral load of 4,490,000 II/mL (genotype 1a). The patient’s iron level was 120 μg/dL, with a calculated total iron-binding capacity (TIBC) of 126.2 μg/dL. His transferrin saturation (TSAT) (serum iron divided by TIBC) was 95%. The patient had nonreactive antinuclear antibody and antimitochondrial antibody tests and a positive antismooth muscle antibody test with a titer of 1:40. His α-fetoprotein (AFP) level was 505 ng/mL (reference range, < 8 ng/mL).
Follow-up MRI of the abdomen and pelvis showed cirrhotic morphology with large volume ascites and portosystemic collaterals, consistent with portal hypertension. Additionally, it showed multiple scattered peripheral sub centimeter hyperenhancing foci, most likely representing benign lesions.
The patient's spot urine protein-creatinine ratio was 3.76. To better quantify proteinuria, a 24-hour urine collection was performed and revealed 12.8 g/d of urine protein (reference range, 0-0.17 g/d). His serum triglyceride level was 175 mg/dL (reference range, 40-60 mg/dL); total cholesterol was 177 mg/ dL (reference range, ≤ 200 mg/dL); low density lipoprotein cholesterol was 98 mg/ dL (reference range, ≤ 130 mg/dL); and highdensity lipoprotein cholesterol was 43.8 mg/ dL (reference range, ≥ 40 mg/dL); C3 complement level was 71 mg/dL (reference range, 82-185 mg/dL); and C4 complement level was 22 mg/dL (reference range, 15-53 mg/ dL). His rheumatoid factor was < 14 IU/mL. Tests for rapid plasma reagin and HIV antigen- antibody were nonreactive, and the phospholipase A2 receptor antibody test was negative. The patient tested positive for QuantiFERON-TB Gold and qualitative cryoglobulin, which indicated a cryocrit of 1%.
A renal biopsy was performed, revealing diffuse podocyte foot process effacement and glomerulonephritis with low-grade C3 and immunoglobulin (Ig) G deposits, consistent with early membranoproliferative glomerulonephritis (MPGN) (Figures 2 and 3).


The patient was initially diuresed with IV furosemide without significant urine output. He was then diuresed with IV 25% albumin (total, 25 g), followed by IV furosemide 40 mg twice daily, which led to significant urine output and resolution of his anasarca. Given the patient’s hypoalbuminemic state, IV albumin was necessary to deliver furosemide to the proximal tubule. He was started on lisinopril for renal protection and discharged with spironolactone and furosemide for fluid management in the context of cirrhosis.
The patient was evaluated by the Liver Nodule Clinic, which includes specialists from hepatology, medical oncology, radiation oncology, interventional radiology, and diagnostic radiology. The team considered the patient’s medical history and characteristics of the nodules on imaging. Notable aspects of the patient’s history included hepatitis C virus (HCV) infection and an elevated AFP level, although imaging showed no lesion concerning for malignancy. Given these findings, the patient was scheduled for a liver biopsy to establish a tissue diagnosis of cirrhosis. Hepatology, nephrology, and infectious disease specialists coordinated to plan the management and treatment of latent tuberculosis (TB), chronic HCV, MPGN, compensated cirrhosis, and suspicious liver lesions.
The patient chose to handle management and treatment as an outpatient. He was discharged with furosemide and spironolactone for anasarca management, and amlodipine and lisinopril for his hypertension and MPGN. Follow-up appointments were scheduled with infectious disease for management of latent TB and HCV, nephrology for MPGN, gastroenterology for cirrhosis, and interventional radiology for liver biopsy. Unfortunately, the patient was unhoused with limited access to transportation, which prevented timely follow-up. Given these social factors, immunosuppression was not started. Additionally, he did not start on HCV therapy because the viral load was still pending at time of discharge.
DISCUSSION
The diagnosis of decompensated cirrhosis was prematurely established, resulting in a diagnostic delay, a form of diagnostic error. However, on hospital day 2, the initial hypothesis of decompensated cirrhosis as the sole driver of the patient’s presentation was reconsidered due to the disconnect between the severity of hypoalbuminemia and diffuse edema (anasarca), and the absence of laboratory evidence of hepatic decompensation (normal international normalized ratio, bilirubin, and low but normal platelet count). Although image findings supported cirrhosis, laboratory markers did not indicate hepatic decompensation. The severity of hypoalbuminemia and anasarca, along with an indeterminate Serum-Ascites Albumin Gradient, prompted the patient’s care team to consider other causes, specifically, nephrotic syndrome.
The patien’s spot protein-to-creatinine ratio was 3.76 (reference range < 0.2 mg/mg creatinine), but a 24-hour urine protein collection was 12.8 g/day (reference range < 150 mg/day). While most spot urine protein- to-creatinine ratios (UPCR) correlate with a 24-hour urine collection, discrepancies can occur, as in this case. It is important to recognize that the spot UPCR assumes that patients are excreting 1000 mg of creatinine daily in their urine, which is not always the case. In addition, changes in urine osmolality can lead to different values. The gold standard for proteinuria is a 24-hour urine collection for protein and creatinine.
The patient’s nephrotic-range proteinuria and severe hypoalbuminemia are not solely explained by cirrhosis. In addition, the patient’s lower extremity edema pointed to nephrotic syndrome. The differential diagnosis for nephrotic syndrome includes both primary and secondary forms of membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, and MPGN, a histopathological diagnosis that requires distinguishing between immune complex-mediated and complement-mediated forms. Other causes of nephrotic syndrome that do not fit in any of these buckets include amyloidosis, IgA nephropathy, and diabetes mellitus (DM). Despite DM being a common cause of nephrotic range proteinuria, it rarely leads to full nephrotic syndrome.
When considering the diagnosis, we reframed the patient’s clinical syndrome as compensated cirrhosis plus nephrotic syndrome. This approach prioritized identifying a cause that could explain both cirrhosis (from any cause) leading to IgA nephropathy or injection drug use serving as a risk factor for cirrhosis and nephrotic syndrome through HCV or AA amyloidosis, respectively. This problem representation guided us to the correct diagnosis. There are multiple renal diseases associated with HCV infection, including MPGN, membranous nephropathy, focal segmental glomerulosclerosis, and IgA nephropathy.2 MPGN and mixed cryoglobulinemia are the most common. In the past, MPGN was classified as type I, II, and III.
The patient’s urine toxicology revealed recent amphetamine use, which can also lead to acute kidney injury through rhabdomyolysis or acute interstitial nephritis (AIN).3 In the cases of rhabdomyolysis, urinalysis would show positive heme without any red blood cell on microscopic analysis, which was not present in this case. AIN commonly manifests as acute kidney injury, pyuria, and proteinuria but without a decrease in complement levels.4 While the patient’s urine sediment included white blood cell (10/high-power field), the presence of microscopic hematuria, decreased complement levels, and proteinuria in the context of HCV positivity makes MPGN more likely than AIN.
Recently, there has been greater emphasis on using immunofluorescence for kidney biopsies. MPGN is now classified into 2 main categories: MPGN with mesangial immunoglobulins and C3 deposits in the capillary walls, and MPGN with C3 deposits but without Ig.5 MPGN with Ig-complement deposits is seen in autoimmune diseases and infections and is associated with dysproteinemias.
The renal biopsy in this patient was consistent with MPGN with immunofluorescence, a common finding in patients with infection. By synthesizing these data, we concluded that the patient represented a case of chronic HCV infection that led to MPGN with cryoglobulinemia. The normal C4 and negative RF do not suggest cryoglobulinemic crisis. Compensated cirrhosis was seen on imaging, pending liver biopsy.
Treatment
The management of MPGN secondary to HCV infection relies on the treatment of the underlying infection and clearance of viral load. Direct-acting antivirals have been used successfully in the treatment of HCV-associated MPGN. When cryoglobulinemia is present, immunosuppressive therapy is recommended. These regimens commonly include rituximab and steroids.5 Rituximab is also used for nephrotic syndrome associated with MPGN, as recommended in the 2018 Kidney Disease: Improving Global Outcomes guidelines.6
When initiating rituximab therapy in a patient who tests positive for hepatitis B (HBcAb positive or HBsAb positive), it is recommended to follow the established guidelines, which include treating them with entecavir for prophylaxis to prevent reactivation or a flare of hepatitis B.7 The patient in this case needed close follow-up in the nephrology and hepatology clinic. Immunosuppressive therapy was not pursued while the patient was admitted to the hospital due to instability with housing, transportation, and difficulty in ensuring close follow-up.
CONCLUSIONS
Clinicians should maintain a broad differential even in the face of confirmatory imaging and other objective findings. In the case of anasarca, nephrotic syndrome should be considered. Key causes of nephrotic syndromes include MPGN, membranous nephropathy, minimal change disease, and focal segmental glomerulosclerosis. MPGN is a histopathological diagnosis, and it is essential to identify if it is secondary to immune complexes or only complement mediated because Ig-complement deposits are seen in autoimmune disease and infection. The management of MPGN due to HCV infection relies on antiviral therapy. In the presence of cryoglobulinemia, immunosuppressive therapy is recommended.
- Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023;329(18):1589–1602. doi:10.1001/jama.2023.5997
- Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20(24):7544-7554. doi:10.3748/wjg.v20.i24.7544
- Foley RJ, Kapatkin K, Vrani R, Weinman EJ. Amphetamineinduced acute renal failure. South Med J. 1984;77(2):258- 260. doi:10.1097/00007611-198402000-00035
- Rossert J. Drug - induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817. doi:10.1046/j.1523-1755.2001.060002804.x
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31(4):341-348. doi:10.1016/j.semnephrol.2011.06.005
- Jadoul M, Berenguer MC, Doss W, et al. Executive summary of the 2018 KDIGO hepatitis C in CKD guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94(4):663-673. doi:10.1016/j.kint.2018.06.011
- Myint A, Tong MJ, Beaven SW. Reactivation of hepatitis b virus: a review of clinical guidelines. Clin Liver Dis (Hoboken). 2020;15(4):162-167. doi:10.1002/cld.883
- Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023;329(18):1589–1602. doi:10.1001/jama.2023.5997
- Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20(24):7544-7554. doi:10.3748/wjg.v20.i24.7544
- Foley RJ, Kapatkin K, Vrani R, Weinman EJ. Amphetamineinduced acute renal failure. South Med J. 1984;77(2):258- 260. doi:10.1097/00007611-198402000-00035
- Rossert J. Drug - induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817. doi:10.1046/j.1523-1755.2001.060002804.x
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31(4):341-348. doi:10.1016/j.semnephrol.2011.06.005
- Jadoul M, Berenguer MC, Doss W, et al. Executive summary of the 2018 KDIGO hepatitis C in CKD guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94(4):663-673. doi:10.1016/j.kint.2018.06.011
- Myint A, Tong MJ, Beaven SW. Reactivation of hepatitis b virus: a review of clinical guidelines. Clin Liver Dis (Hoboken). 2020;15(4):162-167. doi:10.1002/cld.883
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
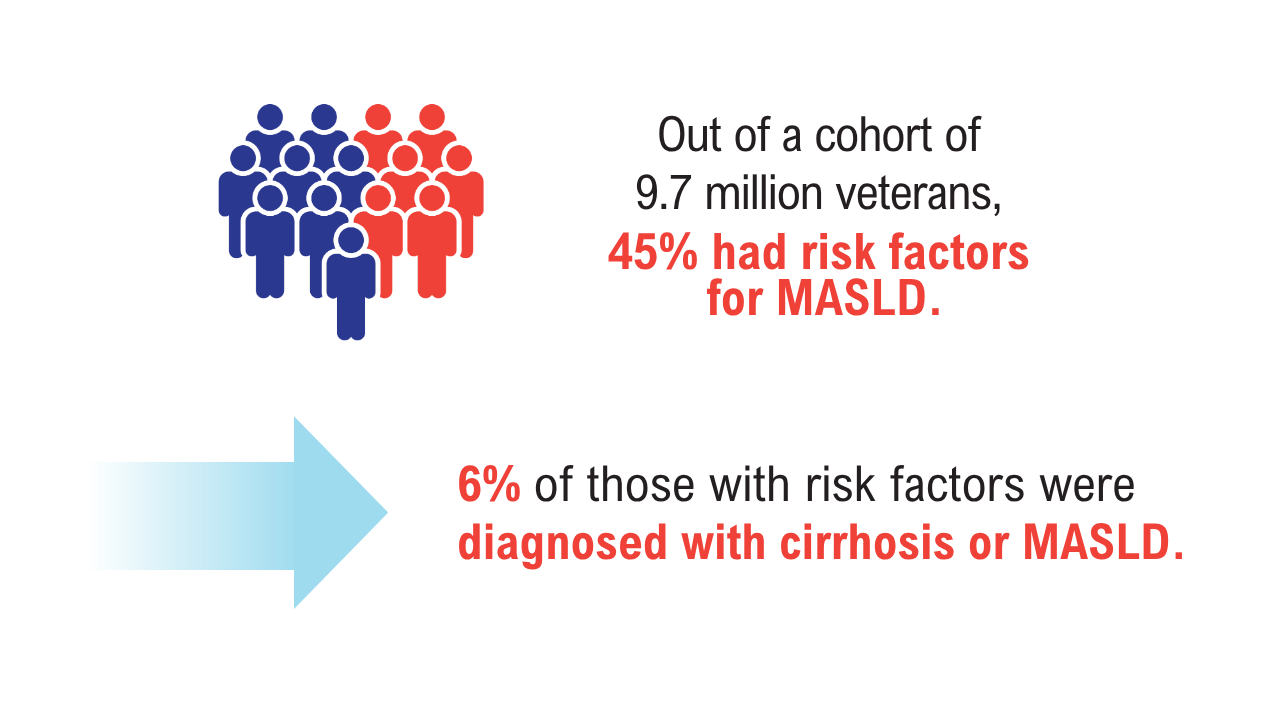
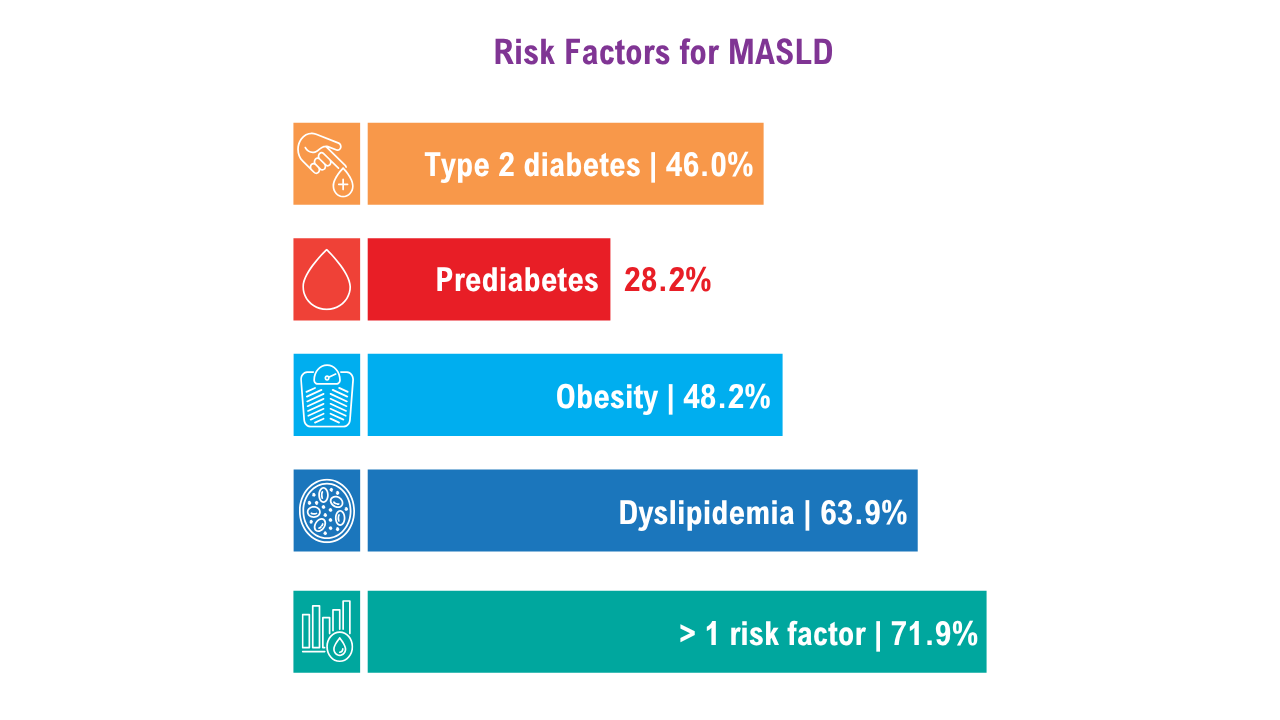
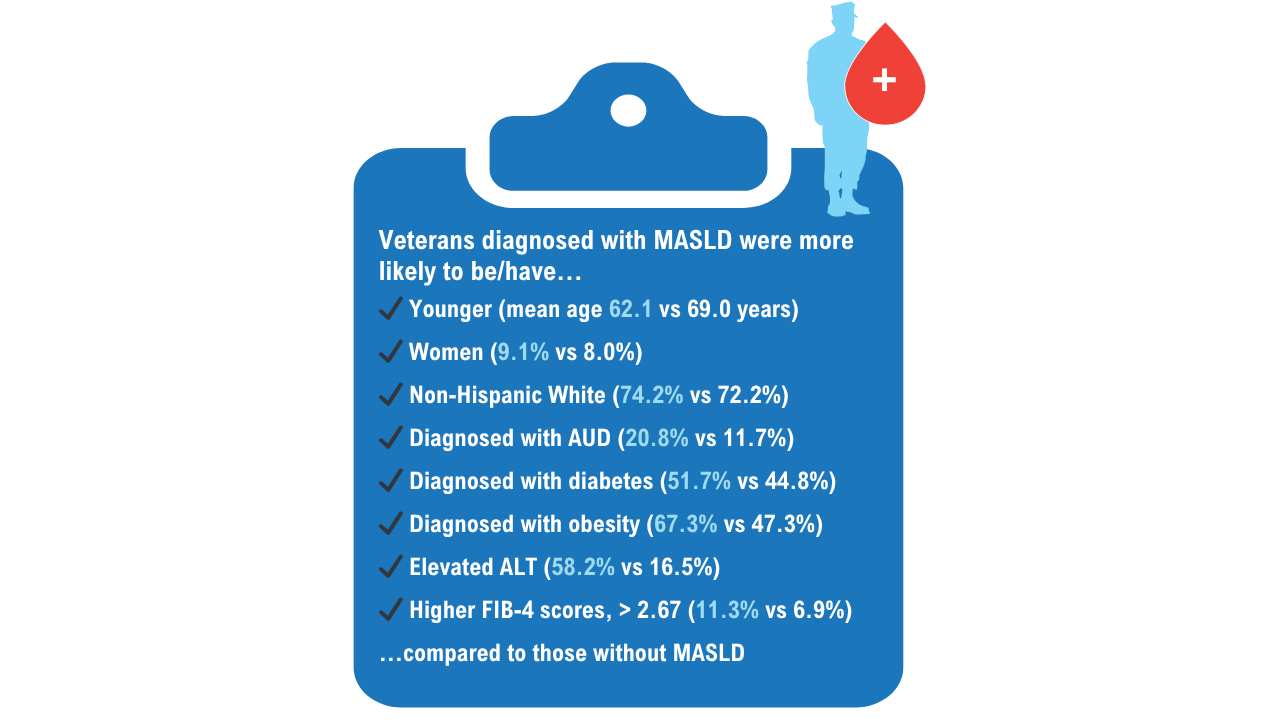
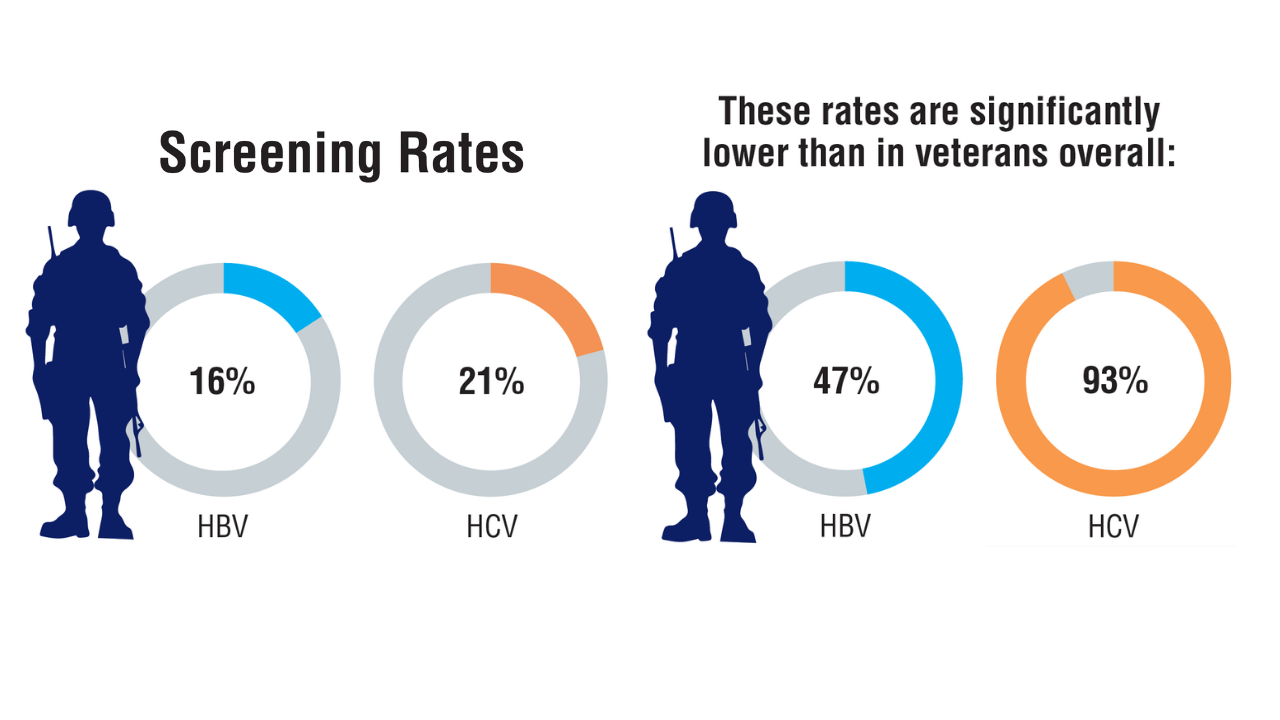
AGA Data Trends 2025: MASLD
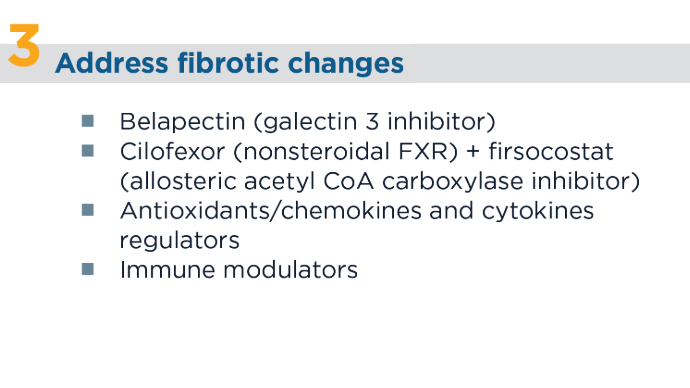
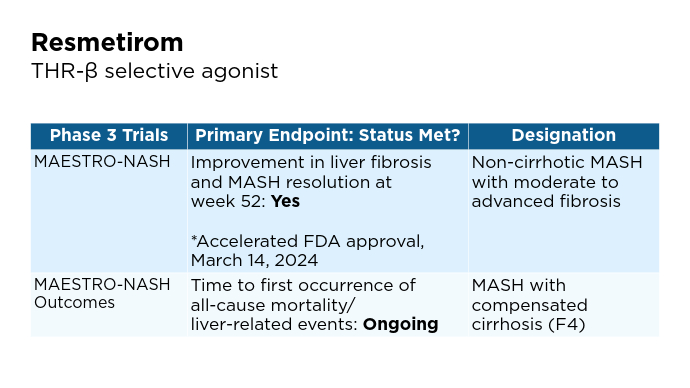
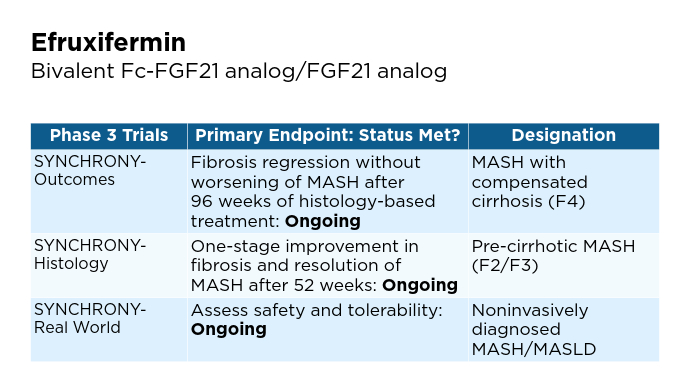
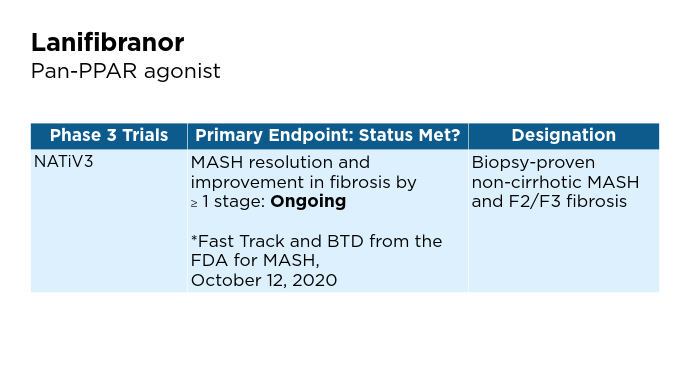
New and Emerging Treatments for MASLD/MASH
- Hu Y, Sun C, Chen Y, Liu Y-D, Fan J-G. Pipeline of New Drug Treatment for Non-alcoholic Fatty Liver Disease/Metabolic Dysfunction-associated Steatotic Liver Disease. J Clin Transl Hepatol. 2024;12(9):802-814. doi:10.14218/JCTH.2024.00123
- Petta S, Targher G, Romeo S, et al. The first MASH drug therapy on the horizon: Current perspectives of resmetirom. Liver Int. 2024;44(7):1526-1536. doi:10.14218/JCTH.2024.00123doi:10.1111/liv.15930
- Ciardullo S, Muraca E, Vergani M, Invernizzi P, Perseghin G. Advancements in pharmacological treatment of NAFLD/MASLD: a focus on metabolic and liver-targeted interventions. Gastroenterol Rep (Oxf). 2024;12:goae029. doi:10.1093/gastro/goae029
- Chen VL, Morgan TR, Rotman Y, et al. Resmetirom therapy for metabolic dysfunction-associated steatotic liver disease: October 2024 updates to AASLD Practice Guidance. Hepatology. 2025;81(1):312-320. doi:10.1097/HEP.0000000000001112
- Economist Impact 2024. MASLD/MASH in the US: A liver disease country profile. Published 2024. Accessed January 22, 2025. https://impact.economist.com/perspectives/sites/default/files/download/liver-disease-country-profile_united_states_final.pdf
- Tincopa MA, Anstee QM, Loomba R. New and emerging treatments for metabolic dysfunction-associated steatohepatitis. Cell Metab. 2024;36(5):912-926. doi:10.1016/j.cmet.2024.03.011
- Carpi S, Daniele S, de Almeida JFM, Gabbia D. Recent Advances in miRNA-Based Therapy for MASLD/MASH and MASH-Associated HCC. Int J Mol Sci. 2024;25(22):12229. https://www.mdpi.com/1422-0067/25/22/1222
- Wong RJ. Epidemiology of metabolic dysfunction-associated steatotic liver disease (MASLD) and alcohol-related liver disease (ALD). Metab Target Organ Damage. 2024;4:35. http://dx.doi.org/10.20517/mtod.2024.57
- Younossi ZM, Kalligeros M, Henry L. Epidemiology of Metabolic Dysfunction-Associated Steatotic Liver Disease. Clin Mol Hepatol. 2024. doi:10.3350/cmh.2024.0431
- Jozst L. Estimating the True Prevalence of MASH and MASLD in the US. AJMC. Published October 17, 2024. Accessed January 22, 2025. https://www.ajmc.com/view/estimating-the-true-prevalence-of-mash-and-masld-in-the-us
- Mayo Clinic website. Pediatric metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as nonalcoholic fatty liver disease (NAFLD). Published October 4, 2023. Accessed January 22, 2025. https://www.mayoclinic.org/medical-professionals/pediatrics/news/pediatric-metabolic-dysfunction-associated-steatotic-liver-disease-masld-formerly-known-as-nonalcoholic-fatty-liver-disease-nafld/mac-20555493
- Younossi ZM. Economic burden of MASLD/MASH. Conference report for NATAP. EASL 2024. Published June 5-8, 2024. Accessed January 22, 2025. https://www.natap.org/2024/EASL/EASL_41.htm
- Loomba R, Noureddin M, Kowdley KV, et al. Combination Therapies Including Cilofexor and Firsocostat for Bridging Fibrosis and Cirrhosis Attributable to NASH. Hepatol. 202;73(2):625-643. doi:10.1002/hep.31622
- Nicastro E. D’Antiga L. Nutritional Interventions, Probiotics, Synbiotics and Fecal Microbiota Transplantation in Steatoic Liver Disease. Advances in experimental medicine and Biology. Published online January 1. 202:113-133. doi:https://doi.org.10.1007/978-3-031-58572-2_7
- Shera S, Katzka W, Yang JC, et al. Bariatric-induced microbiome changes alter MASLD development in association with changes in the innate immune system. Front Microbiol. 2024;15:1407555. doi:10.3389/fmicb.2024.1407555
- Globe Newswire website. Akero Therapeutics Reports Second Quarter 2024 Financial Results and Provides Business Update [press release]. Published August 9, 2024. Accessed January 22, 2025. https://www.globenewswire.com/en/news-release/2024/08/09/2927685/0/en/Akero-Therapeutics-Reports-Second-Quarter-2024-Financial-Results-and-Provides-Business-Update.html
- Akero website. Clinical Trials Overview. We are currently enrolling three clinical trials as part of a Phase 3 SYNCHRONY program evaluating EFX for the treatment of pre-cirrhotic MASH (F2-F3) and compensated cirrhosis (F4) due to MASH [press release]. Published 2024. Accessed January 22, 2025. https://akerotx.com/clinical-trials/
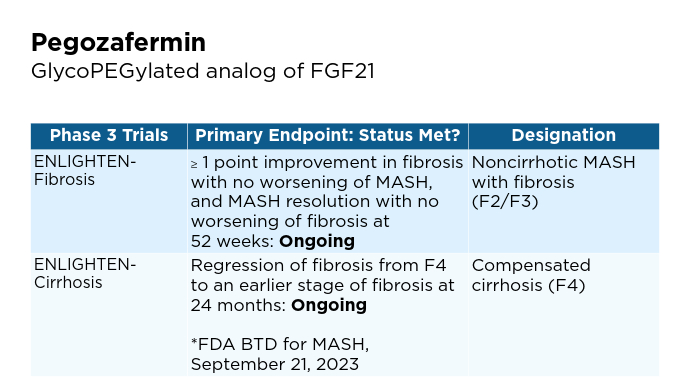
- 89bio website. 89bio Initiates Phase 3 ENLIGHTEN-Fibrosis Trial of Pegozafermin in Non-Cirrhotic Metabolic Dysfunction-Associated Steatohepatitis (MASH) Patients with Fibrosis [press release]. Published March 12, 2024. Accessed January 22, 2025. https://www.89bio.com/news/89bio-initiates-phase-3-enlighten-fibrosis-trial-of-pegozafermin-in-non-cirrhotic-metabolic-dysfunction-associated-steatohepatitis-mash-patients-with-fibrosis/
- 89bio website. 89bio Reaches Alignment with the FDA and EMA on Phase 3 Program for Pegozafermin in Nonalcoholic Steatohepatitis (NASH); Program Initiation Planned in the First Half of 2024 [press release]. Published December 4, 2023. Accessed January 22, 2025. https://www.89bio.com/news/89bio-reaches-alignment-with-the-fda-and-ema-on-phase-3-program-for-pegozafermin-in-nonalcoholic-steatohepatitis-nash-program-initiation-planned-in-the-first-half-of-2024/
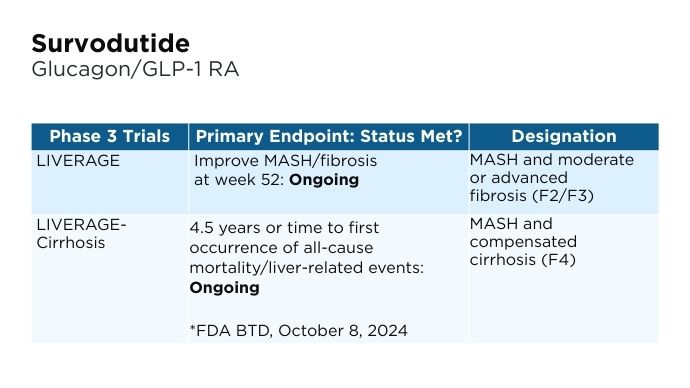
- Boehringer Ingelheim website. Boehringer receives U.S. FDA Breakthrough Therapy designation and initiates two phase III trials in MASH for survodutide [press release]. Published October 8, 2024. Accessed January 22, 2025. https://www.boehringer-ingelheim.com/human-health/metabolic-diseases/survodutide-us-fda-breakthrough-therapy-phase-3-trials-mash
- Hu Y, Sun C, Chen Y, Liu Y-D, Fan J-G. Pipeline of New Drug Treatment for Non-alcoholic Fatty Liver Disease/Metabolic Dysfunction-associated Steatotic Liver Disease. J Clin Transl Hepatol. 2024;12(9):802-814. doi:10.14218/JCTH.2024.00123
- Petta S, Targher G, Romeo S, et al. The first MASH drug therapy on the horizon: Current perspectives of resmetirom. Liver Int. 2024;44(7):1526-1536. doi:10.14218/JCTH.2024.00123doi:10.1111/liv.15930
- Ciardullo S, Muraca E, Vergani M, Invernizzi P, Perseghin G. Advancements in pharmacological treatment of NAFLD/MASLD: a focus on metabolic and liver-targeted interventions. Gastroenterol Rep (Oxf). 2024;12:goae029. doi:10.1093/gastro/goae029
- Chen VL, Morgan TR, Rotman Y, et al. Resmetirom therapy for metabolic dysfunction-associated steatotic liver disease: October 2024 updates to AASLD Practice Guidance. Hepatology. 2025;81(1):312-320. doi:10.1097/HEP.0000000000001112
- Economist Impact 2024. MASLD/MASH in the US: A liver disease country profile. Published 2024. Accessed January 22, 2025. https://impact.economist.com/perspectives/sites/default/files/download/liver-disease-country-profile_united_states_final.pdf
- Tincopa MA, Anstee QM, Loomba R. New and emerging treatments for metabolic dysfunction-associated steatohepatitis. Cell Metab. 2024;36(5):912-926. doi:10.1016/j.cmet.2024.03.011
- Carpi S, Daniele S, de Almeida JFM, Gabbia D. Recent Advances in miRNA-Based Therapy for MASLD/MASH and MASH-Associated HCC. Int J Mol Sci. 2024;25(22):12229. https://www.mdpi.com/1422-0067/25/22/1222
- Wong RJ. Epidemiology of metabolic dysfunction-associated steatotic liver disease (MASLD) and alcohol-related liver disease (ALD). Metab Target Organ Damage. 2024;4:35. http://dx.doi.org/10.20517/mtod.2024.57
- Younossi ZM, Kalligeros M, Henry L. Epidemiology of Metabolic Dysfunction-Associated Steatotic Liver Disease. Clin Mol Hepatol. 2024. doi:10.3350/cmh.2024.0431
- Jozst L. Estimating the True Prevalence of MASH and MASLD in the US. AJMC. Published October 17, 2024. Accessed January 22, 2025. https://www.ajmc.com/view/estimating-the-true-prevalence-of-mash-and-masld-in-the-us
- Mayo Clinic website. Pediatric metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as nonalcoholic fatty liver disease (NAFLD). Published October 4, 2023. Accessed January 22, 2025. https://www.mayoclinic.org/medical-professionals/pediatrics/news/pediatric-metabolic-dysfunction-associated-steatotic-liver-disease-masld-formerly-known-as-nonalcoholic-fatty-liver-disease-nafld/mac-20555493
- Younossi ZM. Economic burden of MASLD/MASH. Conference report for NATAP. EASL 2024. Published June 5-8, 2024. Accessed January 22, 2025. https://www.natap.org/2024/EASL/EASL_41.htm
- Loomba R, Noureddin M, Kowdley KV, et al. Combination Therapies Including Cilofexor and Firsocostat for Bridging Fibrosis and Cirrhosis Attributable to NASH. Hepatol. 202;73(2):625-643. doi:10.1002/hep.31622
- Nicastro E. D’Antiga L. Nutritional Interventions, Probiotics, Synbiotics and Fecal Microbiota Transplantation in Steatoic Liver Disease. Advances in experimental medicine and Biology. Published online January 1. 202:113-133. doi:https://doi.org.10.1007/978-3-031-58572-2_7
- Shera S, Katzka W, Yang JC, et al. Bariatric-induced microbiome changes alter MASLD development in association with changes in the innate immune system. Front Microbiol. 2024;15:1407555. doi:10.3389/fmicb.2024.1407555
- Globe Newswire website. Akero Therapeutics Reports Second Quarter 2024 Financial Results and Provides Business Update [press release]. Published August 9, 2024. Accessed January 22, 2025. https://www.globenewswire.com/en/news-release/2024/08/09/2927685/0/en/Akero-Therapeutics-Reports-Second-Quarter-2024-Financial-Results-and-Provides-Business-Update.html
- Akero website. Clinical Trials Overview. We are currently enrolling three clinical trials as part of a Phase 3 SYNCHRONY program evaluating EFX for the treatment of pre-cirrhotic MASH (F2-F3) and compensated cirrhosis (F4) due to MASH [press release]. Published 2024. Accessed January 22, 2025. https://akerotx.com/clinical-trials/
- 89bio website. 89bio Initiates Phase 3 ENLIGHTEN-Fibrosis Trial of Pegozafermin in Non-Cirrhotic Metabolic Dysfunction-Associated Steatohepatitis (MASH) Patients with Fibrosis [press release]. Published March 12, 2024. Accessed January 22, 2025. https://www.89bio.com/news/89bio-initiates-phase-3-enlighten-fibrosis-trial-of-pegozafermin-in-non-cirrhotic-metabolic-dysfunction-associated-steatohepatitis-mash-patients-with-fibrosis/
- 89bio website. 89bio Reaches Alignment with the FDA and EMA on Phase 3 Program for Pegozafermin in Nonalcoholic Steatohepatitis (NASH); Program Initiation Planned in the First Half of 2024 [press release]. Published December 4, 2023. Accessed January 22, 2025. https://www.89bio.com/news/89bio-reaches-alignment-with-the-fda-and-ema-on-phase-3-program-for-pegozafermin-in-nonalcoholic-steatohepatitis-nash-program-initiation-planned-in-the-first-half-of-2024/
- Boehringer Ingelheim website. Boehringer receives U.S. FDA Breakthrough Therapy designation and initiates two phase III trials in MASH for survodutide [press release]. Published October 8, 2024. Accessed January 22, 2025. https://www.boehringer-ingelheim.com/human-health/metabolic-diseases/survodutide-us-fda-breakthrough-therapy-phase-3-trials-mash
- Hu Y, Sun C, Chen Y, Liu Y-D, Fan J-G. Pipeline of New Drug Treatment for Non-alcoholic Fatty Liver Disease/Metabolic Dysfunction-associated Steatotic Liver Disease. J Clin Transl Hepatol. 2024;12(9):802-814. doi:10.14218/JCTH.2024.00123
- Petta S, Targher G, Romeo S, et al. The first MASH drug therapy on the horizon: Current perspectives of resmetirom. Liver Int. 2024;44(7):1526-1536. doi:10.14218/JCTH.2024.00123doi:10.1111/liv.15930
- Ciardullo S, Muraca E, Vergani M, Invernizzi P, Perseghin G. Advancements in pharmacological treatment of NAFLD/MASLD: a focus on metabolic and liver-targeted interventions. Gastroenterol Rep (Oxf). 2024;12:goae029. doi:10.1093/gastro/goae029
- Chen VL, Morgan TR, Rotman Y, et al. Resmetirom therapy for metabolic dysfunction-associated steatotic liver disease: October 2024 updates to AASLD Practice Guidance. Hepatology. 2025;81(1):312-320. doi:10.1097/HEP.0000000000001112
- Economist Impact 2024. MASLD/MASH in the US: A liver disease country profile. Published 2024. Accessed January 22, 2025. https://impact.economist.com/perspectives/sites/default/files/download/liver-disease-country-profile_united_states_final.pdf
- Tincopa MA, Anstee QM, Loomba R. New and emerging treatments for metabolic dysfunction-associated steatohepatitis. Cell Metab. 2024;36(5):912-926. doi:10.1016/j.cmet.2024.03.011
- Carpi S, Daniele S, de Almeida JFM, Gabbia D. Recent Advances in miRNA-Based Therapy for MASLD/MASH and MASH-Associated HCC. Int J Mol Sci. 2024;25(22):12229. https://www.mdpi.com/1422-0067/25/22/1222
- Wong RJ. Epidemiology of metabolic dysfunction-associated steatotic liver disease (MASLD) and alcohol-related liver disease (ALD). Metab Target Organ Damage. 2024;4:35. http://dx.doi.org/10.20517/mtod.2024.57
- Younossi ZM, Kalligeros M, Henry L. Epidemiology of Metabolic Dysfunction-Associated Steatotic Liver Disease. Clin Mol Hepatol. 2024. doi:10.3350/cmh.2024.0431
- Jozst L. Estimating the True Prevalence of MASH and MASLD in the US. AJMC. Published October 17, 2024. Accessed January 22, 2025. https://www.ajmc.com/view/estimating-the-true-prevalence-of-mash-and-masld-in-the-us
- Mayo Clinic website. Pediatric metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as nonalcoholic fatty liver disease (NAFLD). Published October 4, 2023. Accessed January 22, 2025. https://www.mayoclinic.org/medical-professionals/pediatrics/news/pediatric-metabolic-dysfunction-associated-steatotic-liver-disease-masld-formerly-known-as-nonalcoholic-fatty-liver-disease-nafld/mac-20555493
- Younossi ZM. Economic burden of MASLD/MASH. Conference report for NATAP. EASL 2024. Published June 5-8, 2024. Accessed January 22, 2025. https://www.natap.org/2024/EASL/EASL_41.htm
- Loomba R, Noureddin M, Kowdley KV, et al. Combination Therapies Including Cilofexor and Firsocostat for Bridging Fibrosis and Cirrhosis Attributable to NASH. Hepatol. 202;73(2):625-643. doi:10.1002/hep.31622
- Nicastro E. D’Antiga L. Nutritional Interventions, Probiotics, Synbiotics and Fecal Microbiota Transplantation in Steatoic Liver Disease. Advances in experimental medicine and Biology. Published online January 1. 202:113-133. doi:https://doi.org.10.1007/978-3-031-58572-2_7
- Shera S, Katzka W, Yang JC, et al. Bariatric-induced microbiome changes alter MASLD development in association with changes in the innate immune system. Front Microbiol. 2024;15:1407555. doi:10.3389/fmicb.2024.1407555
- Globe Newswire website. Akero Therapeutics Reports Second Quarter 2024 Financial Results and Provides Business Update [press release]. Published August 9, 2024. Accessed January 22, 2025. https://www.globenewswire.com/en/news-release/2024/08/09/2927685/0/en/Akero-Therapeutics-Reports-Second-Quarter-2024-Financial-Results-and-Provides-Business-Update.html
- Akero website. Clinical Trials Overview. We are currently enrolling three clinical trials as part of a Phase 3 SYNCHRONY program evaluating EFX for the treatment of pre-cirrhotic MASH (F2-F3) and compensated cirrhosis (F4) due to MASH [press release]. Published 2024. Accessed January 22, 2025. https://akerotx.com/clinical-trials/
- 89bio website. 89bio Initiates Phase 3 ENLIGHTEN-Fibrosis Trial of Pegozafermin in Non-Cirrhotic Metabolic Dysfunction-Associated Steatohepatitis (MASH) Patients with Fibrosis [press release]. Published March 12, 2024. Accessed January 22, 2025. https://www.89bio.com/news/89bio-initiates-phase-3-enlighten-fibrosis-trial-of-pegozafermin-in-non-cirrhotic-metabolic-dysfunction-associated-steatohepatitis-mash-patients-with-fibrosis/
- 89bio website. 89bio Reaches Alignment with the FDA and EMA on Phase 3 Program for Pegozafermin in Nonalcoholic Steatohepatitis (NASH); Program Initiation Planned in the First Half of 2024 [press release]. Published December 4, 2023. Accessed January 22, 2025. https://www.89bio.com/news/89bio-reaches-alignment-with-the-fda-and-ema-on-phase-3-program-for-pegozafermin-in-nonalcoholic-steatohepatitis-nash-program-initiation-planned-in-the-first-half-of-2024/
- Boehringer Ingelheim website. Boehringer receives U.S. FDA Breakthrough Therapy designation and initiates two phase III trials in MASH for survodutide [press release]. Published October 8, 2024. Accessed January 22, 2025. https://www.boehringer-ingelheim.com/human-health/metabolic-diseases/survodutide-us-fda-breakthrough-therapy-phase-3-trials-mash
New and Emerging Treatments for MASLD/MASH
New and Emerging Treatments for MASLD/MASH
New Model Estimates Hepatocellular Carcinoma Risk in Patients With Chronic Hepatitis B
The model, called Revised REACH-B or reREACH-B, stems from cohort studies in Hong Kong, South Korea, and Taiwan, and looks at the nonlinear parabolic association between serum hepatitis B virus (HBV) DNA levels and HCC risk.
“Current clinical practice guidelines don’t advocate antiviral treatment for patients with CHB who don’t show elevated alanine aminotransferase (ALT) levels, even in those with high HBV viral loads,” said coauthor Young-Suk Lim, MD, PhD, professor of gastroenterology at the University of Ulsan College of Medicine and Asan Medical Center in Seoul, South Korea.
“This stance is rooted in the notion that patients in the immune-tolerant phase are at very low risk for developing HCC,” Lim said. “However, the immune-tolerant phase includes patients with HBV DNA levels who face the highest risk for HCC, and many patients with moderate HBV viremia fall into an undefined gray zone.”
The study was published in Annals of Internal Medicine.
Validating reREACH-B
During a course of CHB, HBV viral loads and HCC risks evolve over time because of viral replication and host immune responses, Lim explained. Most patients typically move to seroclearance and an “inactive hepatitis” phase, but about 10%-20% can progress to a “reactivation” phase, where HBV DNA levels and ALT levels increase, which can increase HCC risk as well.
In a previous cohort study in Taiwan, a prognostic model called Risk Estimation for HCC in CHB — or REACH-B — found the risk for HCC increases tenfold with increasing levels of HBV DNA up to 5 log10IU/mL in noncirrhotic patients with CHB, regardless of ALT levels. Another cohort study in South Korea found a nonlinear parabolic association between HCC risk and HBV DNA levels up to 9 log10 IU/mL, with the highest risks found for moderate HBV DNA levels around 6 log10 IU/mL.
In this study, Lim and colleagues developed a prognostic model to integrate the nonlinear relationship and validated it externally, as well as compared it with the previous REACH-B model. The Revised REACH-B model incorporates six variables: age, sex, platelet count, HBV DNA level, ALT, and hepatitis B e-antigen (HBeAg).
The study included 14,378 treatment-naive, noncirrhotic adults with CHB and serum ALT levels < two times the upper limit of normal for at least 1 year and serum hepatitis B surface antigen for at least 6 months. The internal validation cohort included 6,949 patients from Asan Medical Center, and the external validation cohort included 7,429 patients from previous studies in Hong Kong, South Korea, and Taiwan.
Among the Asan cohort, the mean age was 45 years, 29.9% were HBeAg positive, median HBV DNA levels were 3.1 log10 IU/mL, and the median ALT level was 25 U/L. In the external cohort, the mean age was 46 years, 21% were HBeAg positive, median HBV DNA levels were 3.4 log10 IU/mL, and the median ALT level was 20 U/L.
In the Asan cohort, 435 patients (6.3%) developed HCC during a median follow-up of 10 years. The annual HCC incidence rate was 0.63 per 100 person-years, and the estimated cumulative probability of developing HCC at 10 years was 6.4%.
In the external cohort, 467 patients (6.3%) developed HCC during a median follow-up of 12 years. The annual HCC incidence rate was 0.42 per 100 person-years, and the estimated cumulative probability of developing HCC at 10 years was 3.1%.
Overall, the association between HBV viral load and HCC risk was linear in the HBeAg-negative groups and inverse in the HBeAg-positive groups, with the association between HBV viral load and HCC risk showing a nonlinear parabolic pattern.
Across both cohorts, patients with HBV DNA levels between 5 and 6 log10 IU/mL had the highest risk for HCC in both the HBeAg-negative and HBeAg-positive groups, which was more than eight times higher than those HBV DNA levels ≤ 3 log10 IU/mL.
For internal validation, the Revised REACH-B model had a c-statistic of 0.844 and 5-year area under the curve of 0.864. For external validation across the three external cohorts, the reREACH-B had c-statistics of 0.804, 0.808, and 0.813, and 5-year area under the curve of 0.839, 0.860, and 0.865.
In addition, the revised model yielded a greater positive net benefit than the REACH-B model in the threshold probability range between 0% and 18%.
“These analyses indicate the reREACH-B model can be a valuable tool in clinical practice, aiding in timely management decisions,” Lim said.
Considering Prognostic Models
This study highlights the importance of recognizing that the association between HBV DNA viral load and HCC risk isn’t linear, said Norah Terrault, MD, chief of Gastroenterology and Hepatology at the Keck School of Medicine at the University of Southern California, Los Angeles.
“In contrast to most chronic liver diseases where liver cancer develops only among those with advanced fibrosis/cirrhosis, people with chronic hepatitis B are at risk prior to the development of cirrhosis,” she said. “Risk prediction scores for HCC can be a useful means of identifying those without cirrhosis who should be enrolled in HCC surveillance programs.”
For instance, patients with HBV DNA levels < 3 log10 IU/mL or > 8 log10 IU/mL don’t have an increased risk, Terrault noted. However, the highest risk group appears to be around 5-6 log10 IU/mL.
“Future risk prediction models should acknowledge that relationship in modeling HCC risk,” she said. “The re-REACH-B provides modest improvement over the REACH-B, but further validation of this score in more diverse cohorts is essential.”
The study received financial support from the Korean government and grants from the Patient-Centered Clinical Research Coordinating Center of the National Evidence-based Healthcare Collaborating Agency and the National R&D Program for Cancer Control through the National Cancer Center, which is funded by Korea’s Ministry of Health and Welfare. Lim and Terrault reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The model, called Revised REACH-B or reREACH-B, stems from cohort studies in Hong Kong, South Korea, and Taiwan, and looks at the nonlinear parabolic association between serum hepatitis B virus (HBV) DNA levels and HCC risk.
“Current clinical practice guidelines don’t advocate antiviral treatment for patients with CHB who don’t show elevated alanine aminotransferase (ALT) levels, even in those with high HBV viral loads,” said coauthor Young-Suk Lim, MD, PhD, professor of gastroenterology at the University of Ulsan College of Medicine and Asan Medical Center in Seoul, South Korea.
“This stance is rooted in the notion that patients in the immune-tolerant phase are at very low risk for developing HCC,” Lim said. “However, the immune-tolerant phase includes patients with HBV DNA levels who face the highest risk for HCC, and many patients with moderate HBV viremia fall into an undefined gray zone.”
The study was published in Annals of Internal Medicine.
Validating reREACH-B
During a course of CHB, HBV viral loads and HCC risks evolve over time because of viral replication and host immune responses, Lim explained. Most patients typically move to seroclearance and an “inactive hepatitis” phase, but about 10%-20% can progress to a “reactivation” phase, where HBV DNA levels and ALT levels increase, which can increase HCC risk as well.
In a previous cohort study in Taiwan, a prognostic model called Risk Estimation for HCC in CHB — or REACH-B — found the risk for HCC increases tenfold with increasing levels of HBV DNA up to 5 log10IU/mL in noncirrhotic patients with CHB, regardless of ALT levels. Another cohort study in South Korea found a nonlinear parabolic association between HCC risk and HBV DNA levels up to 9 log10 IU/mL, with the highest risks found for moderate HBV DNA levels around 6 log10 IU/mL.
In this study, Lim and colleagues developed a prognostic model to integrate the nonlinear relationship and validated it externally, as well as compared it with the previous REACH-B model. The Revised REACH-B model incorporates six variables: age, sex, platelet count, HBV DNA level, ALT, and hepatitis B e-antigen (HBeAg).
The study included 14,378 treatment-naive, noncirrhotic adults with CHB and serum ALT levels < two times the upper limit of normal for at least 1 year and serum hepatitis B surface antigen for at least 6 months. The internal validation cohort included 6,949 patients from Asan Medical Center, and the external validation cohort included 7,429 patients from previous studies in Hong Kong, South Korea, and Taiwan.
Among the Asan cohort, the mean age was 45 years, 29.9% were HBeAg positive, median HBV DNA levels were 3.1 log10 IU/mL, and the median ALT level was 25 U/L. In the external cohort, the mean age was 46 years, 21% were HBeAg positive, median HBV DNA levels were 3.4 log10 IU/mL, and the median ALT level was 20 U/L.
In the Asan cohort, 435 patients (6.3%) developed HCC during a median follow-up of 10 years. The annual HCC incidence rate was 0.63 per 100 person-years, and the estimated cumulative probability of developing HCC at 10 years was 6.4%.
In the external cohort, 467 patients (6.3%) developed HCC during a median follow-up of 12 years. The annual HCC incidence rate was 0.42 per 100 person-years, and the estimated cumulative probability of developing HCC at 10 years was 3.1%.
Overall, the association between HBV viral load and HCC risk was linear in the HBeAg-negative groups and inverse in the HBeAg-positive groups, with the association between HBV viral load and HCC risk showing a nonlinear parabolic pattern.
Across both cohorts, patients with HBV DNA levels between 5 and 6 log10 IU/mL had the highest risk for HCC in both the HBeAg-negative and HBeAg-positive groups, which was more than eight times higher than those HBV DNA levels ≤ 3 log10 IU/mL.
For internal validation, the Revised REACH-B model had a c-statistic of 0.844 and 5-year area under the curve of 0.864. For external validation across the three external cohorts, the reREACH-B had c-statistics of 0.804, 0.808, and 0.813, and 5-year area under the curve of 0.839, 0.860, and 0.865.
In addition, the revised model yielded a greater positive net benefit than the REACH-B model in the threshold probability range between 0% and 18%.
“These analyses indicate the reREACH-B model can be a valuable tool in clinical practice, aiding in timely management decisions,” Lim said.
Considering Prognostic Models
This study highlights the importance of recognizing that the association between HBV DNA viral load and HCC risk isn’t linear, said Norah Terrault, MD, chief of Gastroenterology and Hepatology at the Keck School of Medicine at the University of Southern California, Los Angeles.
“In contrast to most chronic liver diseases where liver cancer develops only among those with advanced fibrosis/cirrhosis, people with chronic hepatitis B are at risk prior to the development of cirrhosis,” she said. “Risk prediction scores for HCC can be a useful means of identifying those without cirrhosis who should be enrolled in HCC surveillance programs.”
For instance, patients with HBV DNA levels < 3 log10 IU/mL or > 8 log10 IU/mL don’t have an increased risk, Terrault noted. However, the highest risk group appears to be around 5-6 log10 IU/mL.
“Future risk prediction models should acknowledge that relationship in modeling HCC risk,” she said. “The re-REACH-B provides modest improvement over the REACH-B, but further validation of this score in more diverse cohorts is essential.”
The study received financial support from the Korean government and grants from the Patient-Centered Clinical Research Coordinating Center of the National Evidence-based Healthcare Collaborating Agency and the National R&D Program for Cancer Control through the National Cancer Center, which is funded by Korea’s Ministry of Health and Welfare. Lim and Terrault reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The model, called Revised REACH-B or reREACH-B, stems from cohort studies in Hong Kong, South Korea, and Taiwan, and looks at the nonlinear parabolic association between serum hepatitis B virus (HBV) DNA levels and HCC risk.
“Current clinical practice guidelines don’t advocate antiviral treatment for patients with CHB who don’t show elevated alanine aminotransferase (ALT) levels, even in those with high HBV viral loads,” said coauthor Young-Suk Lim, MD, PhD, professor of gastroenterology at the University of Ulsan College of Medicine and Asan Medical Center in Seoul, South Korea.
“This stance is rooted in the notion that patients in the immune-tolerant phase are at very low risk for developing HCC,” Lim said. “However, the immune-tolerant phase includes patients with HBV DNA levels who face the highest risk for HCC, and many patients with moderate HBV viremia fall into an undefined gray zone.”
The study was published in Annals of Internal Medicine.
Validating reREACH-B
During a course of CHB, HBV viral loads and HCC risks evolve over time because of viral replication and host immune responses, Lim explained. Most patients typically move to seroclearance and an “inactive hepatitis” phase, but about 10%-20% can progress to a “reactivation” phase, where HBV DNA levels and ALT levels increase, which can increase HCC risk as well.
In a previous cohort study in Taiwan, a prognostic model called Risk Estimation for HCC in CHB — or REACH-B — found the risk for HCC increases tenfold with increasing levels of HBV DNA up to 5 log10IU/mL in noncirrhotic patients with CHB, regardless of ALT levels. Another cohort study in South Korea found a nonlinear parabolic association between HCC risk and HBV DNA levels up to 9 log10 IU/mL, with the highest risks found for moderate HBV DNA levels around 6 log10 IU/mL.
In this study, Lim and colleagues developed a prognostic model to integrate the nonlinear relationship and validated it externally, as well as compared it with the previous REACH-B model. The Revised REACH-B model incorporates six variables: age, sex, platelet count, HBV DNA level, ALT, and hepatitis B e-antigen (HBeAg).
The study included 14,378 treatment-naive, noncirrhotic adults with CHB and serum ALT levels < two times the upper limit of normal for at least 1 year and serum hepatitis B surface antigen for at least 6 months. The internal validation cohort included 6,949 patients from Asan Medical Center, and the external validation cohort included 7,429 patients from previous studies in Hong Kong, South Korea, and Taiwan.
Among the Asan cohort, the mean age was 45 years, 29.9% were HBeAg positive, median HBV DNA levels were 3.1 log10 IU/mL, and the median ALT level was 25 U/L. In the external cohort, the mean age was 46 years, 21% were HBeAg positive, median HBV DNA levels were 3.4 log10 IU/mL, and the median ALT level was 20 U/L.
In the Asan cohort, 435 patients (6.3%) developed HCC during a median follow-up of 10 years. The annual HCC incidence rate was 0.63 per 100 person-years, and the estimated cumulative probability of developing HCC at 10 years was 6.4%.
In the external cohort, 467 patients (6.3%) developed HCC during a median follow-up of 12 years. The annual HCC incidence rate was 0.42 per 100 person-years, and the estimated cumulative probability of developing HCC at 10 years was 3.1%.
Overall, the association between HBV viral load and HCC risk was linear in the HBeAg-negative groups and inverse in the HBeAg-positive groups, with the association between HBV viral load and HCC risk showing a nonlinear parabolic pattern.
Across both cohorts, patients with HBV DNA levels between 5 and 6 log10 IU/mL had the highest risk for HCC in both the HBeAg-negative and HBeAg-positive groups, which was more than eight times higher than those HBV DNA levels ≤ 3 log10 IU/mL.
For internal validation, the Revised REACH-B model had a c-statistic of 0.844 and 5-year area under the curve of 0.864. For external validation across the three external cohorts, the reREACH-B had c-statistics of 0.804, 0.808, and 0.813, and 5-year area under the curve of 0.839, 0.860, and 0.865.
In addition, the revised model yielded a greater positive net benefit than the REACH-B model in the threshold probability range between 0% and 18%.
“These analyses indicate the reREACH-B model can be a valuable tool in clinical practice, aiding in timely management decisions,” Lim said.
Considering Prognostic Models
This study highlights the importance of recognizing that the association between HBV DNA viral load and HCC risk isn’t linear, said Norah Terrault, MD, chief of Gastroenterology and Hepatology at the Keck School of Medicine at the University of Southern California, Los Angeles.
“In contrast to most chronic liver diseases where liver cancer develops only among those with advanced fibrosis/cirrhosis, people with chronic hepatitis B are at risk prior to the development of cirrhosis,” she said. “Risk prediction scores for HCC can be a useful means of identifying those without cirrhosis who should be enrolled in HCC surveillance programs.”
For instance, patients with HBV DNA levels < 3 log10 IU/mL or > 8 log10 IU/mL don’t have an increased risk, Terrault noted. However, the highest risk group appears to be around 5-6 log10 IU/mL.
“Future risk prediction models should acknowledge that relationship in modeling HCC risk,” she said. “The re-REACH-B provides modest improvement over the REACH-B, but further validation of this score in more diverse cohorts is essential.”
The study received financial support from the Korean government and grants from the Patient-Centered Clinical Research Coordinating Center of the National Evidence-based Healthcare Collaborating Agency and the National R&D Program for Cancer Control through the National Cancer Center, which is funded by Korea’s Ministry of Health and Welfare. Lim and Terrault reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Broken Sleep Linked to MASLD
TOPLINE:
Fragmented sleep — that is, increased wakefulness and reduced sleep efficiency — is a sign of metabolic dysfunction–associated steatotic liver disease (MASLD), a study using actigraphy showed.
METHODOLOGY:
- Researchers assessed sleep-wake rhythms in 35 patients with MASLD (median age, 58 years; 66% were men; 80% with metabolic syndrome) and 16 matched healthy controls (median age, 61 years; 50% were men) using data collected 24/7 via actigraphy for 4 weeks.
- Sub-analyses were conducted with MASLD comparator groups: 16 patients with MASH, 8 with MASH with cirrhosis, and 11 with non-MASH–related cirrhosis.
- All participants visited the clinic at baseline, week 2, and week 4 to undergo a clinical investigation and complete questionnaires about their sleep.
- A standardized sleep hygiene education session was conducted at week 2.
TAKEAWAY:
- Actigraphy data from patients with MASLD did not reveal significant differences in bedtime, sleep-onset latency, sleep duration, wake-up time, or time in bed compared with controls.
- However, compared with controls, those with MASLD woke 55% more often at night (8.5 vs 5.5), lay awake 113% longer after having first fallen asleep (45.4 minutes vs 21.3 minutes), and slept more often and longer during the day (decreased sleep efficiency).
- Subgroup analyses showed that actigraphy-measured sleep patterns and quality were similarly impaired in patients with MASH, MASH with cirrhosis, and non–MASH-related cirrhosis.
- Patients with MASLD self-reported their fragmented sleep as shorter sleep with a delayed onset. In sleep diaries, 32% of patients with MASLD reported sleep disturbances caused by psychological stress, compared with only 6.25% of controls and 9% of patients with cirrhosis.
- The sleep education session did not change the actigraphy measures or the sleep parameters assessed with sleep questionnaires at the end of the study.
IN PRACTICE:
“We concluded from our data that sleep fragmentation plays a role in the pathogenesis of human MASLD. Whether MASLD causes sleep disorders or vice versa remains unknown. The underlying mechanism presumably involves genetics, environmental factors, and the activation of immune responses — ultimately driven by obesity and metabolic syndrome,” said corresponding author.
SOURCE:
The study, led by Sofia Schaeffer, PhD, University of Basel, Switzerland, was published online in Frontiers in Network Physiology.
LIMITATIONS:
The study had several limitations. There was a significant difference in body mass index between patients with MASLD (median, 31) and controls (median, 23.5), representing a potential confounder that could explain the differences in sleep behavior. Undetected obstructive sleep apnea could also be a confounding factor. The small number of participants limited the interpretation and generalization of the data, especially in the MASLD subgroups.
DISCLOSURES:
This study was supported by a grant from the University of Basel. One coauthor received a research grant from the University Center for Gastrointestinal and Liver Diseases, Basel, Switzerland. Another coauthor was employed by NovoLytiX. Schaeffer and the remaining coauthors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Fragmented sleep — that is, increased wakefulness and reduced sleep efficiency — is a sign of metabolic dysfunction–associated steatotic liver disease (MASLD), a study using actigraphy showed.
METHODOLOGY:
- Researchers assessed sleep-wake rhythms in 35 patients with MASLD (median age, 58 years; 66% were men; 80% with metabolic syndrome) and 16 matched healthy controls (median age, 61 years; 50% were men) using data collected 24/7 via actigraphy for 4 weeks.
- Sub-analyses were conducted with MASLD comparator groups: 16 patients with MASH, 8 with MASH with cirrhosis, and 11 with non-MASH–related cirrhosis.
- All participants visited the clinic at baseline, week 2, and week 4 to undergo a clinical investigation and complete questionnaires about their sleep.
- A standardized sleep hygiene education session was conducted at week 2.
TAKEAWAY:
- Actigraphy data from patients with MASLD did not reveal significant differences in bedtime, sleep-onset latency, sleep duration, wake-up time, or time in bed compared with controls.
- However, compared with controls, those with MASLD woke 55% more often at night (8.5 vs 5.5), lay awake 113% longer after having first fallen asleep (45.4 minutes vs 21.3 minutes), and slept more often and longer during the day (decreased sleep efficiency).
- Subgroup analyses showed that actigraphy-measured sleep patterns and quality were similarly impaired in patients with MASH, MASH with cirrhosis, and non–MASH-related cirrhosis.
- Patients with MASLD self-reported their fragmented sleep as shorter sleep with a delayed onset. In sleep diaries, 32% of patients with MASLD reported sleep disturbances caused by psychological stress, compared with only 6.25% of controls and 9% of patients with cirrhosis.
- The sleep education session did not change the actigraphy measures or the sleep parameters assessed with sleep questionnaires at the end of the study.
IN PRACTICE:
“We concluded from our data that sleep fragmentation plays a role in the pathogenesis of human MASLD. Whether MASLD causes sleep disorders or vice versa remains unknown. The underlying mechanism presumably involves genetics, environmental factors, and the activation of immune responses — ultimately driven by obesity and metabolic syndrome,” said corresponding author.
SOURCE:
The study, led by Sofia Schaeffer, PhD, University of Basel, Switzerland, was published online in Frontiers in Network Physiology.
LIMITATIONS:
The study had several limitations. There was a significant difference in body mass index between patients with MASLD (median, 31) and controls (median, 23.5), representing a potential confounder that could explain the differences in sleep behavior. Undetected obstructive sleep apnea could also be a confounding factor. The small number of participants limited the interpretation and generalization of the data, especially in the MASLD subgroups.
DISCLOSURES:
This study was supported by a grant from the University of Basel. One coauthor received a research grant from the University Center for Gastrointestinal and Liver Diseases, Basel, Switzerland. Another coauthor was employed by NovoLytiX. Schaeffer and the remaining coauthors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Fragmented sleep — that is, increased wakefulness and reduced sleep efficiency — is a sign of metabolic dysfunction–associated steatotic liver disease (MASLD), a study using actigraphy showed.
METHODOLOGY:
- Researchers assessed sleep-wake rhythms in 35 patients with MASLD (median age, 58 years; 66% were men; 80% with metabolic syndrome) and 16 matched healthy controls (median age, 61 years; 50% were men) using data collected 24/7 via actigraphy for 4 weeks.
- Sub-analyses were conducted with MASLD comparator groups: 16 patients with MASH, 8 with MASH with cirrhosis, and 11 with non-MASH–related cirrhosis.
- All participants visited the clinic at baseline, week 2, and week 4 to undergo a clinical investigation and complete questionnaires about their sleep.
- A standardized sleep hygiene education session was conducted at week 2.
TAKEAWAY:
- Actigraphy data from patients with MASLD did not reveal significant differences in bedtime, sleep-onset latency, sleep duration, wake-up time, or time in bed compared with controls.
- However, compared with controls, those with MASLD woke 55% more often at night (8.5 vs 5.5), lay awake 113% longer after having first fallen asleep (45.4 minutes vs 21.3 minutes), and slept more often and longer during the day (decreased sleep efficiency).
- Subgroup analyses showed that actigraphy-measured sleep patterns and quality were similarly impaired in patients with MASH, MASH with cirrhosis, and non–MASH-related cirrhosis.
- Patients with MASLD self-reported their fragmented sleep as shorter sleep with a delayed onset. In sleep diaries, 32% of patients with MASLD reported sleep disturbances caused by psychological stress, compared with only 6.25% of controls and 9% of patients with cirrhosis.
- The sleep education session did not change the actigraphy measures or the sleep parameters assessed with sleep questionnaires at the end of the study.
IN PRACTICE:
“We concluded from our data that sleep fragmentation plays a role in the pathogenesis of human MASLD. Whether MASLD causes sleep disorders or vice versa remains unknown. The underlying mechanism presumably involves genetics, environmental factors, and the activation of immune responses — ultimately driven by obesity and metabolic syndrome,” said corresponding author.
SOURCE:
The study, led by Sofia Schaeffer, PhD, University of Basel, Switzerland, was published online in Frontiers in Network Physiology.
LIMITATIONS:
The study had several limitations. There was a significant difference in body mass index between patients with MASLD (median, 31) and controls (median, 23.5), representing a potential confounder that could explain the differences in sleep behavior. Undetected obstructive sleep apnea could also be a confounding factor. The small number of participants limited the interpretation and generalization of the data, especially in the MASLD subgroups.
DISCLOSURES:
This study was supported by a grant from the University of Basel. One coauthor received a research grant from the University Center for Gastrointestinal and Liver Diseases, Basel, Switzerland. Another coauthor was employed by NovoLytiX. Schaeffer and the remaining coauthors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
A version of this article first appeared on Medscape.com.
GLP-1 RAs: When Not to Prescribe
December 31, 2024
This transcript has been edited for clarity.
I’m Tamaan K. Osbourne-Roberts, family medicine physician and lifestyle medicine physician, here to discuss GLP-1 receptor agonist (RA) contraindications — the skinny on when not to prescribe.
It can be hard not to think of GLP-1 RAs like Ozempic and Mounjaro as silver bullets, long-awaited miracle drugs that we should probably be putting in the water. And it’s true they have the potential to help a lot of people.
They include the following:
Patients with a family history of certain cancers. Given that GLP-1 RAs can increase the risk for thyroid cancer, patients with a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2 should not take these drugs.
Gut motility issues. Since one of the primary mechanisms of action for these drugs is to slow down the gut, patients with gastroparesis — diabetic or otherwise — or other gut motility issues should avoid these drugs. Patients with inflammatory bowel disease also should not use GLP-1 RAs.
Pancreatitis. These medications can increase the risk for serious pancreatitis on their own, so use in patients who have had pancreatitis already is not recommended.
Renal impairment. An eGFR [estimated glomerular filtrationrate] below threshold, typically around 30 mL/min per 1.73 m2, excludes GLP-1 RAs for some patients. Be certain to check the threshold for individual medications before prescribing.
And finally, pregnancy. These drugs generally should not be used in pregnancy, and people of childbearing age with the ability to become pregnant should use contraception while taking these medications.
GLP-1 RAs are great medications and have the potential to revolutionize obesity medicine, but like all drugs, it’s important to use them safely. Knowing when not to prescribe them is an important step in ensuring patient safety and will help ensure they are available for those who need them.
Tamaan K. Osbourne-Roberts, MD, MBA, Denver, Colorado, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
December 31, 2024
This transcript has been edited for clarity.
I’m Tamaan K. Osbourne-Roberts, family medicine physician and lifestyle medicine physician, here to discuss GLP-1 receptor agonist (RA) contraindications — the skinny on when not to prescribe.
It can be hard not to think of GLP-1 RAs like Ozempic and Mounjaro as silver bullets, long-awaited miracle drugs that we should probably be putting in the water. And it’s true they have the potential to help a lot of people.
They include the following:
Patients with a family history of certain cancers. Given that GLP-1 RAs can increase the risk for thyroid cancer, patients with a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2 should not take these drugs.
Gut motility issues. Since one of the primary mechanisms of action for these drugs is to slow down the gut, patients with gastroparesis — diabetic or otherwise — or other gut motility issues should avoid these drugs. Patients with inflammatory bowel disease also should not use GLP-1 RAs.
Pancreatitis. These medications can increase the risk for serious pancreatitis on their own, so use in patients who have had pancreatitis already is not recommended.
Renal impairment. An eGFR [estimated glomerular filtrationrate] below threshold, typically around 30 mL/min per 1.73 m2, excludes GLP-1 RAs for some patients. Be certain to check the threshold for individual medications before prescribing.
And finally, pregnancy. These drugs generally should not be used in pregnancy, and people of childbearing age with the ability to become pregnant should use contraception while taking these medications.
GLP-1 RAs are great medications and have the potential to revolutionize obesity medicine, but like all drugs, it’s important to use them safely. Knowing when not to prescribe them is an important step in ensuring patient safety and will help ensure they are available for those who need them.
Tamaan K. Osbourne-Roberts, MD, MBA, Denver, Colorado, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
December 31, 2024
This transcript has been edited for clarity.
I’m Tamaan K. Osbourne-Roberts, family medicine physician and lifestyle medicine physician, here to discuss GLP-1 receptor agonist (RA) contraindications — the skinny on when not to prescribe.
It can be hard not to think of GLP-1 RAs like Ozempic and Mounjaro as silver bullets, long-awaited miracle drugs that we should probably be putting in the water. And it’s true they have the potential to help a lot of people.
They include the following:
Patients with a family history of certain cancers. Given that GLP-1 RAs can increase the risk for thyroid cancer, patients with a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2 should not take these drugs.
Gut motility issues. Since one of the primary mechanisms of action for these drugs is to slow down the gut, patients with gastroparesis — diabetic or otherwise — or other gut motility issues should avoid these drugs. Patients with inflammatory bowel disease also should not use GLP-1 RAs.
Pancreatitis. These medications can increase the risk for serious pancreatitis on their own, so use in patients who have had pancreatitis already is not recommended.
Renal impairment. An eGFR [estimated glomerular filtrationrate] below threshold, typically around 30 mL/min per 1.73 m2, excludes GLP-1 RAs for some patients. Be certain to check the threshold for individual medications before prescribing.
And finally, pregnancy. These drugs generally should not be used in pregnancy, and people of childbearing age with the ability to become pregnant should use contraception while taking these medications.
GLP-1 RAs are great medications and have the potential to revolutionize obesity medicine, but like all drugs, it’s important to use them safely. Knowing when not to prescribe them is an important step in ensuring patient safety and will help ensure they are available for those who need them.
Tamaan K. Osbourne-Roberts, MD, MBA, Denver, Colorado, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Hepatocellular Carcinoma: Leading Causes of Mortality Predicted
TOPLINE:
Alcohol-associated liver disease (ALD) will likely become the leading cause of HCC-related mortality by 2026, and metabolic dysfunction–associated steatotic liver disease (MASLD) is projected to become the second leading cause by 2032, a new analysis found.
METHODOLOGY:
- HCC accounts for 75%-85% of primary liver cancers and most liver cancer deaths. Researchers have observed an upward trend in the incidence of and mortality from HCC in the past 2 decades.
- This cross-sectional study analyzed 188,280 HCC-related deaths among adults aged 25 and older to determine trends in mortality rates and project age-standardized mortality rates through 2040. Data came from the National Vital Statistics System database from 2006 to 2022.
- Researchers stratified mortality data by etiology of liver disease (ALD, hepatitis B virus, hepatitis C virus, and MASLD), age groups (25-64 or 65 and older years), sex, and race/ethnicity.
- Demographic data showed that 77.4% of deaths occurred in men, 55.6% in individuals aged 65 years or older, and 62.3% in White individuals.
TAKEAWAY:
- Overall, the age-standardized mortality rate for HCC-related deaths increased from 3.65 per 100,000 persons in 2006 to 5.03 in 2022 and was projected to increase to 6.39 per 100,000 persons by 2040.
- Sex- and age-related disparities were substantial. Men had much higher rates of HCC-related mortality than women (8.15 vs 2.33 per 100,000 persons), with a projected rate among men of 9.78 per 100,000 persons by 2040. HCC-related mortality rates for people aged 65 years or older were 10 times higher than for those aged 25-64 years (18.37 vs 1.79 per 100,000 persons) in 2022 and was projected to reach 32.81 per 100,000 persons by 2040 in the older group.
- Although hepatitis C virus–related deaths were projected to decline from 0.69 to 0.03 per 100,000 persons by 2034, ALD- and MASLD-related deaths showed increasing trends, with both projected to become the two leading causes of HCC-related mortality in the next few years.
- Racial disparities were also evident. By 2040, the American Indian/Alaska Native population showed the highest increase in projected HCC-related mortality rates, which went from 5.46 per 100,000 persons in 2006 to a project increase to 14.71 per 100,000 persons.
IN PRACTICE:
“HCC mortality was projected to continue increasing in the US, primarily due to rising rates of deaths attributable to ALD and MASLD,” the authors wrote.
This “study highlights the importance of addressing these conditions to decrease the burden of liver disease and liver disease mortality in the future,” Emad Qayed, MD, MPH, Emory University School of Medicine, Atlanta, wrote in an accompanying editorial.
SOURCE:
The study was led by Sikai Qiu, MM, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China, and was published online in JAMA Network Open.
LIMITATIONS:
The National Vital Statistics System database used in this study captured only mortality data without access to detailed clinical records or individual medical histories. Researchers could not analyze socioeconomic factors or individual-level risk factors owing to data anonymization requirements. Additionally, the inclusion of the COVID-19 pandemic period could have influenced observed trends and reliability of future projections.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China. Several authors reported receiving consulting fees, speaking fees, or research support from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Alcohol-associated liver disease (ALD) will likely become the leading cause of HCC-related mortality by 2026, and metabolic dysfunction–associated steatotic liver disease (MASLD) is projected to become the second leading cause by 2032, a new analysis found.
METHODOLOGY:
- HCC accounts for 75%-85% of primary liver cancers and most liver cancer deaths. Researchers have observed an upward trend in the incidence of and mortality from HCC in the past 2 decades.
- This cross-sectional study analyzed 188,280 HCC-related deaths among adults aged 25 and older to determine trends in mortality rates and project age-standardized mortality rates through 2040. Data came from the National Vital Statistics System database from 2006 to 2022.
- Researchers stratified mortality data by etiology of liver disease (ALD, hepatitis B virus, hepatitis C virus, and MASLD), age groups (25-64 or 65 and older years), sex, and race/ethnicity.
- Demographic data showed that 77.4% of deaths occurred in men, 55.6% in individuals aged 65 years or older, and 62.3% in White individuals.
TAKEAWAY:
- Overall, the age-standardized mortality rate for HCC-related deaths increased from 3.65 per 100,000 persons in 2006 to 5.03 in 2022 and was projected to increase to 6.39 per 100,000 persons by 2040.
- Sex- and age-related disparities were substantial. Men had much higher rates of HCC-related mortality than women (8.15 vs 2.33 per 100,000 persons), with a projected rate among men of 9.78 per 100,000 persons by 2040. HCC-related mortality rates for people aged 65 years or older were 10 times higher than for those aged 25-64 years (18.37 vs 1.79 per 100,000 persons) in 2022 and was projected to reach 32.81 per 100,000 persons by 2040 in the older group.
- Although hepatitis C virus–related deaths were projected to decline from 0.69 to 0.03 per 100,000 persons by 2034, ALD- and MASLD-related deaths showed increasing trends, with both projected to become the two leading causes of HCC-related mortality in the next few years.
- Racial disparities were also evident. By 2040, the American Indian/Alaska Native population showed the highest increase in projected HCC-related mortality rates, which went from 5.46 per 100,000 persons in 2006 to a project increase to 14.71 per 100,000 persons.
IN PRACTICE:
“HCC mortality was projected to continue increasing in the US, primarily due to rising rates of deaths attributable to ALD and MASLD,” the authors wrote.
This “study highlights the importance of addressing these conditions to decrease the burden of liver disease and liver disease mortality in the future,” Emad Qayed, MD, MPH, Emory University School of Medicine, Atlanta, wrote in an accompanying editorial.
SOURCE:
The study was led by Sikai Qiu, MM, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China, and was published online in JAMA Network Open.
LIMITATIONS:
The National Vital Statistics System database used in this study captured only mortality data without access to detailed clinical records or individual medical histories. Researchers could not analyze socioeconomic factors or individual-level risk factors owing to data anonymization requirements. Additionally, the inclusion of the COVID-19 pandemic period could have influenced observed trends and reliability of future projections.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China. Several authors reported receiving consulting fees, speaking fees, or research support from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Alcohol-associated liver disease (ALD) will likely become the leading cause of HCC-related mortality by 2026, and metabolic dysfunction–associated steatotic liver disease (MASLD) is projected to become the second leading cause by 2032, a new analysis found.
METHODOLOGY:
- HCC accounts for 75%-85% of primary liver cancers and most liver cancer deaths. Researchers have observed an upward trend in the incidence of and mortality from HCC in the past 2 decades.
- This cross-sectional study analyzed 188,280 HCC-related deaths among adults aged 25 and older to determine trends in mortality rates and project age-standardized mortality rates through 2040. Data came from the National Vital Statistics System database from 2006 to 2022.
- Researchers stratified mortality data by etiology of liver disease (ALD, hepatitis B virus, hepatitis C virus, and MASLD), age groups (25-64 or 65 and older years), sex, and race/ethnicity.
- Demographic data showed that 77.4% of deaths occurred in men, 55.6% in individuals aged 65 years or older, and 62.3% in White individuals.
TAKEAWAY:
- Overall, the age-standardized mortality rate for HCC-related deaths increased from 3.65 per 100,000 persons in 2006 to 5.03 in 2022 and was projected to increase to 6.39 per 100,000 persons by 2040.
- Sex- and age-related disparities were substantial. Men had much higher rates of HCC-related mortality than women (8.15 vs 2.33 per 100,000 persons), with a projected rate among men of 9.78 per 100,000 persons by 2040. HCC-related mortality rates for people aged 65 years or older were 10 times higher than for those aged 25-64 years (18.37 vs 1.79 per 100,000 persons) in 2022 and was projected to reach 32.81 per 100,000 persons by 2040 in the older group.
- Although hepatitis C virus–related deaths were projected to decline from 0.69 to 0.03 per 100,000 persons by 2034, ALD- and MASLD-related deaths showed increasing trends, with both projected to become the two leading causes of HCC-related mortality in the next few years.
- Racial disparities were also evident. By 2040, the American Indian/Alaska Native population showed the highest increase in projected HCC-related mortality rates, which went from 5.46 per 100,000 persons in 2006 to a project increase to 14.71 per 100,000 persons.
IN PRACTICE:
“HCC mortality was projected to continue increasing in the US, primarily due to rising rates of deaths attributable to ALD and MASLD,” the authors wrote.
This “study highlights the importance of addressing these conditions to decrease the burden of liver disease and liver disease mortality in the future,” Emad Qayed, MD, MPH, Emory University School of Medicine, Atlanta, wrote in an accompanying editorial.
SOURCE:
The study was led by Sikai Qiu, MM, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China, and was published online in JAMA Network Open.
LIMITATIONS:
The National Vital Statistics System database used in this study captured only mortality data without access to detailed clinical records or individual medical histories. Researchers could not analyze socioeconomic factors or individual-level risk factors owing to data anonymization requirements. Additionally, the inclusion of the COVID-19 pandemic period could have influenced observed trends and reliability of future projections.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China. Several authors reported receiving consulting fees, speaking fees, or research support from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Health Impacts of Micro- and Nanoplastics
In preparation for a future international treaty aimed at reducing plastic pollution, the French Parliamentary Office for the Evaluation of Scientific and Technological Choices presented the conclusions of a public hearing on the impact of plastics on various aspects of human health.
Increased Global Plastic Production
Philippe Bolo, a member of the French Democratic Party and the rapporteur for the public mission on the health impacts of plastics, spoke about the latest round of treaty negotiations, held from November 25 to December 1 in South Korea, attended by leading French and global experts about the impact of plastics on human health.
The hearing highlighted a sharp increase in plastic production. “It has doubled in the last 20 years and is expected to exceed 500 million tons in 2024,” Bolo said. This is about 60 kg per person. According to projections from the Organization for Economic Co-operation and Development, on its current trajectory, plastic production will reach 750 million tons by 2040 and surpass 1 billion tons before 2050, he said.
Minimal Plastic Waste Recycling
Around one third (32%) of plastics are used for packaging. “Therefore, most plastic production is still intended for single-use purposes,” he said. Plastic waste follows a similar growth trajectory, with volumes expected to rise from 360 million tons in 2020 to 617 million tons by 2040 unless action is taken. Very little of this waste is recycled, even in the most countries that are most advanced in terms of collection, sorting, and processing.
In France, for example, in 2018, only 0.6 million tons of the 3.6 million tons of plastic waste produced was truly recycled. This is less than one fifth (17%). Globally, less than 10% of plastic waste is recycled. In 2020, plastic waste that ended up in the environment represented 81 million tons, or 22% of the total. “Beyond waste, this leads to pollution by microplastics and nanoplastics, resulting from their fragmentation. All environments are affected: Seas, rivers, soils, air, and even living organisms,” Bolo said.
Methodological Challenges
However, measuring the impact of plastics on health faces methodological difficulties due to the wide variety of composition, size, and shape of plastics. Nevertheless, the French Standardization Association (Association Française de Normalisation) has conducted work to establish a characterization standard for microplastics in water, which serves as an international reference.
“It is also very difficult to know what we are ingesting,” Bolo said. “A study conducted in 2019 estimated that the average human absorbs 5 grams of plastics per week, the equivalent of a credit card.» Since then, other studies have revised this estimate downward, but no consensus has been reached.
A recent study across 109 countries, both industrialized and developing, found significant exposure, estimated at 500 mg/d, particularly in Southeast Asian countries, where it was due mainly to seafood consumption.
A study concluded that plastic water bottles contain 240,000 particles per liter, 90% of which are nanoplastics. These nanoparticles can pass through the intestinal barrier to enter the bloodstream and reach several organs including the heart, brain, and placenta, as well as the fetus.
Changes to the Microbiome
Microplastics also accumulate in organs. Thus, the amount of plastic in the lungs increases with age, suggesting that particles may persist in the body without being eliminated. The health consequences of this are still poorly understood, but exposure to plastics appears to cause changes in the composition of the intestinal microbiota. Pathobionts (commensal bacteria with harmful potential) have been found in both adults and children, which could contribute to dysbiosis of the gut microbiome. Furthermore, a decrease in butyrate, a short-chain fatty acid beneficial to health, has been observed in children’s intestines.
Inhaled nanoplastics may disrupt the mucociliary clearance mechanisms of the respiratory system. The toxicity of inhaled plastic particles was demonstrated as early as the 1970s among workers in the flocking industry. Some developed lung function impairments, shortness of breath, inflammation, fibrosis, and even lung cancer. Similar symptoms have been observed in workers in the textile and polyvinyl chloride industries.
A study published recently in The New England Journal of Medicine measured the amount of microplastics collected from carotid plaque of more than 300 patients who had undergone carotid endarterectomy for asymptomatic carotid artery disease. It found a 4.53 times higher risk for the primary endpoint, a composite of myocardial infarction, stroke, and all-cause mortality, among individuals with microplastics and nanoplastics in plaque compared with those without.
Health Affects High
The danger of plastics is also directly linked to the chemical substances they contain. A general scientific review looked at the health impacts of three chemicals used almost exclusively in plastics: Polybromodiphenyl ethers (PBDEs), used as flame retardants in textiles or electronics; bisphenol A (BPA), used in the lining of cans and bottles; and phthalates, particularly diethylhexyl phthalate (DEHP), used to make plastics more flexible.
The review highlighted strong epidemiological evidence linking fetal exposure to PBDEs during pregnancy to low birth weight and later exposure to delayed or impaired cognitive development in children and even a loss of IQ. Statistically significant evidence of disruption of thyroid function in adults was also found.
BPA is linked to genital malformations in female newborns exposed to BPA in utero, type 2 diabetes in adults, insulin resistance, and polycystic ovary syndrome in women. BPA exposure also increases the risk for obesity and hypertension in both children and adults, as well as the risk for cardiovascular disease in adults.
Finally, the review established links between exposure to DEHP and miscarriages, genital malformations in male newborns, delayed or impaired cognitive development in children, loss of IQ, delayed psychomotor development, early puberty in young girls, and endometriosis in young women. DEHP exposure also has multiple effects on cardiometabolic health, including insulin resistance, obesity, and elevated blood pressure.
The economic costs associated with the health impacts of these three substances have been estimated at $675 billion in the United States.
Bolo said that the solution to this plastic pollution is necessarily international. “We need an ambitious and legally binding treaty to reduce plastic production,” he said. “The damage is already done; we need to act to protect human health,” he concluded. The parliamentary office has made nine recommendations to the treaty negotiators.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
In preparation for a future international treaty aimed at reducing plastic pollution, the French Parliamentary Office for the Evaluation of Scientific and Technological Choices presented the conclusions of a public hearing on the impact of plastics on various aspects of human health.
Increased Global Plastic Production
Philippe Bolo, a member of the French Democratic Party and the rapporteur for the public mission on the health impacts of plastics, spoke about the latest round of treaty negotiations, held from November 25 to December 1 in South Korea, attended by leading French and global experts about the impact of plastics on human health.
The hearing highlighted a sharp increase in plastic production. “It has doubled in the last 20 years and is expected to exceed 500 million tons in 2024,” Bolo said. This is about 60 kg per person. According to projections from the Organization for Economic Co-operation and Development, on its current trajectory, plastic production will reach 750 million tons by 2040 and surpass 1 billion tons before 2050, he said.
Minimal Plastic Waste Recycling
Around one third (32%) of plastics are used for packaging. “Therefore, most plastic production is still intended for single-use purposes,” he said. Plastic waste follows a similar growth trajectory, with volumes expected to rise from 360 million tons in 2020 to 617 million tons by 2040 unless action is taken. Very little of this waste is recycled, even in the most countries that are most advanced in terms of collection, sorting, and processing.
In France, for example, in 2018, only 0.6 million tons of the 3.6 million tons of plastic waste produced was truly recycled. This is less than one fifth (17%). Globally, less than 10% of plastic waste is recycled. In 2020, plastic waste that ended up in the environment represented 81 million tons, or 22% of the total. “Beyond waste, this leads to pollution by microplastics and nanoplastics, resulting from their fragmentation. All environments are affected: Seas, rivers, soils, air, and even living organisms,” Bolo said.
Methodological Challenges
However, measuring the impact of plastics on health faces methodological difficulties due to the wide variety of composition, size, and shape of plastics. Nevertheless, the French Standardization Association (Association Française de Normalisation) has conducted work to establish a characterization standard for microplastics in water, which serves as an international reference.
“It is also very difficult to know what we are ingesting,” Bolo said. “A study conducted in 2019 estimated that the average human absorbs 5 grams of plastics per week, the equivalent of a credit card.» Since then, other studies have revised this estimate downward, but no consensus has been reached.
A recent study across 109 countries, both industrialized and developing, found significant exposure, estimated at 500 mg/d, particularly in Southeast Asian countries, where it was due mainly to seafood consumption.
A study concluded that plastic water bottles contain 240,000 particles per liter, 90% of which are nanoplastics. These nanoparticles can pass through the intestinal barrier to enter the bloodstream and reach several organs including the heart, brain, and placenta, as well as the fetus.
Changes to the Microbiome
Microplastics also accumulate in organs. Thus, the amount of plastic in the lungs increases with age, suggesting that particles may persist in the body without being eliminated. The health consequences of this are still poorly understood, but exposure to plastics appears to cause changes in the composition of the intestinal microbiota. Pathobionts (commensal bacteria with harmful potential) have been found in both adults and children, which could contribute to dysbiosis of the gut microbiome. Furthermore, a decrease in butyrate, a short-chain fatty acid beneficial to health, has been observed in children’s intestines.
Inhaled nanoplastics may disrupt the mucociliary clearance mechanisms of the respiratory system. The toxicity of inhaled plastic particles was demonstrated as early as the 1970s among workers in the flocking industry. Some developed lung function impairments, shortness of breath, inflammation, fibrosis, and even lung cancer. Similar symptoms have been observed in workers in the textile and polyvinyl chloride industries.
A study published recently in The New England Journal of Medicine measured the amount of microplastics collected from carotid plaque of more than 300 patients who had undergone carotid endarterectomy for asymptomatic carotid artery disease. It found a 4.53 times higher risk for the primary endpoint, a composite of myocardial infarction, stroke, and all-cause mortality, among individuals with microplastics and nanoplastics in plaque compared with those without.
Health Affects High
The danger of plastics is also directly linked to the chemical substances they contain. A general scientific review looked at the health impacts of three chemicals used almost exclusively in plastics: Polybromodiphenyl ethers (PBDEs), used as flame retardants in textiles or electronics; bisphenol A (BPA), used in the lining of cans and bottles; and phthalates, particularly diethylhexyl phthalate (DEHP), used to make plastics more flexible.
The review highlighted strong epidemiological evidence linking fetal exposure to PBDEs during pregnancy to low birth weight and later exposure to delayed or impaired cognitive development in children and even a loss of IQ. Statistically significant evidence of disruption of thyroid function in adults was also found.
BPA is linked to genital malformations in female newborns exposed to BPA in utero, type 2 diabetes in adults, insulin resistance, and polycystic ovary syndrome in women. BPA exposure also increases the risk for obesity and hypertension in both children and adults, as well as the risk for cardiovascular disease in adults.
Finally, the review established links between exposure to DEHP and miscarriages, genital malformations in male newborns, delayed or impaired cognitive development in children, loss of IQ, delayed psychomotor development, early puberty in young girls, and endometriosis in young women. DEHP exposure also has multiple effects on cardiometabolic health, including insulin resistance, obesity, and elevated blood pressure.
The economic costs associated with the health impacts of these three substances have been estimated at $675 billion in the United States.
Bolo said that the solution to this plastic pollution is necessarily international. “We need an ambitious and legally binding treaty to reduce plastic production,” he said. “The damage is already done; we need to act to protect human health,” he concluded. The parliamentary office has made nine recommendations to the treaty negotiators.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
In preparation for a future international treaty aimed at reducing plastic pollution, the French Parliamentary Office for the Evaluation of Scientific and Technological Choices presented the conclusions of a public hearing on the impact of plastics on various aspects of human health.
Increased Global Plastic Production
Philippe Bolo, a member of the French Democratic Party and the rapporteur for the public mission on the health impacts of plastics, spoke about the latest round of treaty negotiations, held from November 25 to December 1 in South Korea, attended by leading French and global experts about the impact of plastics on human health.
The hearing highlighted a sharp increase in plastic production. “It has doubled in the last 20 years and is expected to exceed 500 million tons in 2024,” Bolo said. This is about 60 kg per person. According to projections from the Organization for Economic Co-operation and Development, on its current trajectory, plastic production will reach 750 million tons by 2040 and surpass 1 billion tons before 2050, he said.
Minimal Plastic Waste Recycling
Around one third (32%) of plastics are used for packaging. “Therefore, most plastic production is still intended for single-use purposes,” he said. Plastic waste follows a similar growth trajectory, with volumes expected to rise from 360 million tons in 2020 to 617 million tons by 2040 unless action is taken. Very little of this waste is recycled, even in the most countries that are most advanced in terms of collection, sorting, and processing.
In France, for example, in 2018, only 0.6 million tons of the 3.6 million tons of plastic waste produced was truly recycled. This is less than one fifth (17%). Globally, less than 10% of plastic waste is recycled. In 2020, plastic waste that ended up in the environment represented 81 million tons, or 22% of the total. “Beyond waste, this leads to pollution by microplastics and nanoplastics, resulting from their fragmentation. All environments are affected: Seas, rivers, soils, air, and even living organisms,” Bolo said.
Methodological Challenges
However, measuring the impact of plastics on health faces methodological difficulties due to the wide variety of composition, size, and shape of plastics. Nevertheless, the French Standardization Association (Association Française de Normalisation) has conducted work to establish a characterization standard for microplastics in water, which serves as an international reference.
“It is also very difficult to know what we are ingesting,” Bolo said. “A study conducted in 2019 estimated that the average human absorbs 5 grams of plastics per week, the equivalent of a credit card.» Since then, other studies have revised this estimate downward, but no consensus has been reached.
A recent study across 109 countries, both industrialized and developing, found significant exposure, estimated at 500 mg/d, particularly in Southeast Asian countries, where it was due mainly to seafood consumption.
A study concluded that plastic water bottles contain 240,000 particles per liter, 90% of which are nanoplastics. These nanoparticles can pass through the intestinal barrier to enter the bloodstream and reach several organs including the heart, brain, and placenta, as well as the fetus.
Changes to the Microbiome
Microplastics also accumulate in organs. Thus, the amount of plastic in the lungs increases with age, suggesting that particles may persist in the body without being eliminated. The health consequences of this are still poorly understood, but exposure to plastics appears to cause changes in the composition of the intestinal microbiota. Pathobionts (commensal bacteria with harmful potential) have been found in both adults and children, which could contribute to dysbiosis of the gut microbiome. Furthermore, a decrease in butyrate, a short-chain fatty acid beneficial to health, has been observed in children’s intestines.
Inhaled nanoplastics may disrupt the mucociliary clearance mechanisms of the respiratory system. The toxicity of inhaled plastic particles was demonstrated as early as the 1970s among workers in the flocking industry. Some developed lung function impairments, shortness of breath, inflammation, fibrosis, and even lung cancer. Similar symptoms have been observed in workers in the textile and polyvinyl chloride industries.
A study published recently in The New England Journal of Medicine measured the amount of microplastics collected from carotid plaque of more than 300 patients who had undergone carotid endarterectomy for asymptomatic carotid artery disease. It found a 4.53 times higher risk for the primary endpoint, a composite of myocardial infarction, stroke, and all-cause mortality, among individuals with microplastics and nanoplastics in plaque compared with those without.
Health Affects High
The danger of plastics is also directly linked to the chemical substances they contain. A general scientific review looked at the health impacts of three chemicals used almost exclusively in plastics: Polybromodiphenyl ethers (PBDEs), used as flame retardants in textiles or electronics; bisphenol A (BPA), used in the lining of cans and bottles; and phthalates, particularly diethylhexyl phthalate (DEHP), used to make plastics more flexible.
The review highlighted strong epidemiological evidence linking fetal exposure to PBDEs during pregnancy to low birth weight and later exposure to delayed or impaired cognitive development in children and even a loss of IQ. Statistically significant evidence of disruption of thyroid function in adults was also found.
BPA is linked to genital malformations in female newborns exposed to BPA in utero, type 2 diabetes in adults, insulin resistance, and polycystic ovary syndrome in women. BPA exposure also increases the risk for obesity and hypertension in both children and adults, as well as the risk for cardiovascular disease in adults.
Finally, the review established links between exposure to DEHP and miscarriages, genital malformations in male newborns, delayed or impaired cognitive development in children, loss of IQ, delayed psychomotor development, early puberty in young girls, and endometriosis in young women. DEHP exposure also has multiple effects on cardiometabolic health, including insulin resistance, obesity, and elevated blood pressure.
The economic costs associated with the health impacts of these three substances have been estimated at $675 billion in the United States.
Bolo said that the solution to this plastic pollution is necessarily international. “We need an ambitious and legally binding treaty to reduce plastic production,” he said. “The damage is already done; we need to act to protect human health,” he concluded. The parliamentary office has made nine recommendations to the treaty negotiators.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Some Antihypertensives Linked to HCC Risk in Patients With MASLD and Cirrhosis
SAN DIEGO — according to new research.
In particular, the use of calcium channel blockers (CCBs), angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) was associated with a higher risk of developing HCC, compared with not using these medications.
About half of patients with MASLD have hypertension, and the use of antihypertensives in these patients is beneficial to reduce the risk for cardiovascular disease and complications related to MASLD, said lead author Ahmed Elhariri, MD, a research fellow at the University of Texas MD Anderson Cancer Center, Houston, who conducted the study as a research assistant in gastroenterology and hepatology at the Baylor College of Medicine, also in Houston.
However, previous studies have suggested a possible link between these medications and cancer development, “especially CCBs and breast and lung cancer,” said Elhariri, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Analyzing Potential Risks
In a case-control study, Elhariri and colleagues analyzed antihypertensive medication use among patients with MASLD-induced HCC, as defined by histology or radiology based on the Liver Imaging Reporting & Data System, and control patients with MASLD but without HCC.
Between 2020 and 2024, the research team recruited 153 newly diagnosed HCC cases with different etiologies and 170 patients with MASLD but without HCC from Baylor College of Medicine’s outpatient clinics. For this study, they selected 47 age- and sex-matched pairs, all of whom had cirrhosis. Only those with a history of hypertension were included, however. Data on risk factors of metabolic syndrome (including diabetes) and HCC were collected, along with details about medication use such as metformin and statins.
A total of 42 patients with MASLD and HCC and 39 MASLD control individuals had a history of hypertension and were treated with antihypertensive medications. The mean age was 66.5 years for the HCC group and 63.5 years for the control group, and the mean body mass index (BMI) was 31.1 for the HCC group and 31.7 for the control group.
After adjusting for age, sex, BMI, Hispanic ethnicity, and use of other medications, patients taking CCBs had an increased HCC risk (odds ratio [OR], 2.76), compared with those not taking CCBs. Patients taking ACE inhibitors or ARBs also had an increased HCC risk (OR, 2.54), compared with those not taking ACE inhibitors or ARBs.
However, there wasn’t a statistically significant difference in HCC risk among patients taking beta-blockers (OR, 0.87).
“Patients with fatty liver in the presence of metabolic syndrome, especially in the presence of cirrhosis and antihypertensives, need to have stricter surveillance for liver cancer,” Elhariri said.
“We need to carefully review blood pressure medications in patients with MASLD and cirrhosis,” he said. CCBs, ACE inhibitors, and ARBs can be replaced with beta-blockers, “which have been shown to reduce progression of cirrhosis-related complications.”
Considering Clinical Implications
“Although our study showed some association between the use of some commonly used antihypertensives and the risk for HCC in this high-risk population, it is based on data collected retrospectively on a small number of selected patients with advanced liver disease,” Elhariri noted.
The associations and underlying mechanisms should be studied in larger populations and prospective trials, he said. “Until we have more data with a significantly larger sample size, it’s premature to raise the concern in the general population.”
“The cardiovascular benefits of controlling blood pressure far outweigh the risk of liver cancer in patients with metabolic syndrome,” Elhariri added.
In ongoing studies, researchers are investigating ways to improve patient outcomes and reduce the negative effects of cirrhosis-associated complications among patients with MASLD and metabolic dysfunction–associated steatohepatitis (MASH), Muhammad Ali Butt, MD, a hepatology fellow at Beth Israel Lahey Hospital & Medical Center in Burlington, Massachusetts, said in an interview.
Butt, who wasn’t involved with this study, presented separate research on statins in MASH patients with cirrhosis, which indicated statistically significant decreases in portal hypertension, thrombosis, hepatorenal syndrome, hepatic encephalopathy, and mortality.
“We know patients with MASLD- and MASH-associated cirrhosis commonly have other comorbidities, including high cardiovascular risks, diabetes, and hyperlipidemia,” he said. “All of these conditions indicate patients to be on other medications such as antihypertensives or statins. It’s important to know the role these medications play, especially given the high-risk profile of these patients.”
Elhariri and Butt reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
SAN DIEGO — according to new research.
In particular, the use of calcium channel blockers (CCBs), angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) was associated with a higher risk of developing HCC, compared with not using these medications.
About half of patients with MASLD have hypertension, and the use of antihypertensives in these patients is beneficial to reduce the risk for cardiovascular disease and complications related to MASLD, said lead author Ahmed Elhariri, MD, a research fellow at the University of Texas MD Anderson Cancer Center, Houston, who conducted the study as a research assistant in gastroenterology and hepatology at the Baylor College of Medicine, also in Houston.
However, previous studies have suggested a possible link between these medications and cancer development, “especially CCBs and breast and lung cancer,” said Elhariri, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Analyzing Potential Risks
In a case-control study, Elhariri and colleagues analyzed antihypertensive medication use among patients with MASLD-induced HCC, as defined by histology or radiology based on the Liver Imaging Reporting & Data System, and control patients with MASLD but without HCC.
Between 2020 and 2024, the research team recruited 153 newly diagnosed HCC cases with different etiologies and 170 patients with MASLD but without HCC from Baylor College of Medicine’s outpatient clinics. For this study, they selected 47 age- and sex-matched pairs, all of whom had cirrhosis. Only those with a history of hypertension were included, however. Data on risk factors of metabolic syndrome (including diabetes) and HCC were collected, along with details about medication use such as metformin and statins.
A total of 42 patients with MASLD and HCC and 39 MASLD control individuals had a history of hypertension and were treated with antihypertensive medications. The mean age was 66.5 years for the HCC group and 63.5 years for the control group, and the mean body mass index (BMI) was 31.1 for the HCC group and 31.7 for the control group.
After adjusting for age, sex, BMI, Hispanic ethnicity, and use of other medications, patients taking CCBs had an increased HCC risk (odds ratio [OR], 2.76), compared with those not taking CCBs. Patients taking ACE inhibitors or ARBs also had an increased HCC risk (OR, 2.54), compared with those not taking ACE inhibitors or ARBs.
However, there wasn’t a statistically significant difference in HCC risk among patients taking beta-blockers (OR, 0.87).
“Patients with fatty liver in the presence of metabolic syndrome, especially in the presence of cirrhosis and antihypertensives, need to have stricter surveillance for liver cancer,” Elhariri said.
“We need to carefully review blood pressure medications in patients with MASLD and cirrhosis,” he said. CCBs, ACE inhibitors, and ARBs can be replaced with beta-blockers, “which have been shown to reduce progression of cirrhosis-related complications.”
Considering Clinical Implications
“Although our study showed some association between the use of some commonly used antihypertensives and the risk for HCC in this high-risk population, it is based on data collected retrospectively on a small number of selected patients with advanced liver disease,” Elhariri noted.
The associations and underlying mechanisms should be studied in larger populations and prospective trials, he said. “Until we have more data with a significantly larger sample size, it’s premature to raise the concern in the general population.”
“The cardiovascular benefits of controlling blood pressure far outweigh the risk of liver cancer in patients with metabolic syndrome,” Elhariri added.
In ongoing studies, researchers are investigating ways to improve patient outcomes and reduce the negative effects of cirrhosis-associated complications among patients with MASLD and metabolic dysfunction–associated steatohepatitis (MASH), Muhammad Ali Butt, MD, a hepatology fellow at Beth Israel Lahey Hospital & Medical Center in Burlington, Massachusetts, said in an interview.
Butt, who wasn’t involved with this study, presented separate research on statins in MASH patients with cirrhosis, which indicated statistically significant decreases in portal hypertension, thrombosis, hepatorenal syndrome, hepatic encephalopathy, and mortality.
“We know patients with MASLD- and MASH-associated cirrhosis commonly have other comorbidities, including high cardiovascular risks, diabetes, and hyperlipidemia,” he said. “All of these conditions indicate patients to be on other medications such as antihypertensives or statins. It’s important to know the role these medications play, especially given the high-risk profile of these patients.”
Elhariri and Butt reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
SAN DIEGO — according to new research.
In particular, the use of calcium channel blockers (CCBs), angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) was associated with a higher risk of developing HCC, compared with not using these medications.
About half of patients with MASLD have hypertension, and the use of antihypertensives in these patients is beneficial to reduce the risk for cardiovascular disease and complications related to MASLD, said lead author Ahmed Elhariri, MD, a research fellow at the University of Texas MD Anderson Cancer Center, Houston, who conducted the study as a research assistant in gastroenterology and hepatology at the Baylor College of Medicine, also in Houston.
However, previous studies have suggested a possible link between these medications and cancer development, “especially CCBs and breast and lung cancer,” said Elhariri, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Analyzing Potential Risks
In a case-control study, Elhariri and colleagues analyzed antihypertensive medication use among patients with MASLD-induced HCC, as defined by histology or radiology based on the Liver Imaging Reporting & Data System, and control patients with MASLD but without HCC.
Between 2020 and 2024, the research team recruited 153 newly diagnosed HCC cases with different etiologies and 170 patients with MASLD but without HCC from Baylor College of Medicine’s outpatient clinics. For this study, they selected 47 age- and sex-matched pairs, all of whom had cirrhosis. Only those with a history of hypertension were included, however. Data on risk factors of metabolic syndrome (including diabetes) and HCC were collected, along with details about medication use such as metformin and statins.
A total of 42 patients with MASLD and HCC and 39 MASLD control individuals had a history of hypertension and were treated with antihypertensive medications. The mean age was 66.5 years for the HCC group and 63.5 years for the control group, and the mean body mass index (BMI) was 31.1 for the HCC group and 31.7 for the control group.
After adjusting for age, sex, BMI, Hispanic ethnicity, and use of other medications, patients taking CCBs had an increased HCC risk (odds ratio [OR], 2.76), compared with those not taking CCBs. Patients taking ACE inhibitors or ARBs also had an increased HCC risk (OR, 2.54), compared with those not taking ACE inhibitors or ARBs.
However, there wasn’t a statistically significant difference in HCC risk among patients taking beta-blockers (OR, 0.87).
“Patients with fatty liver in the presence of metabolic syndrome, especially in the presence of cirrhosis and antihypertensives, need to have stricter surveillance for liver cancer,” Elhariri said.
“We need to carefully review blood pressure medications in patients with MASLD and cirrhosis,” he said. CCBs, ACE inhibitors, and ARBs can be replaced with beta-blockers, “which have been shown to reduce progression of cirrhosis-related complications.”
Considering Clinical Implications
“Although our study showed some association between the use of some commonly used antihypertensives and the risk for HCC in this high-risk population, it is based on data collected retrospectively on a small number of selected patients with advanced liver disease,” Elhariri noted.
The associations and underlying mechanisms should be studied in larger populations and prospective trials, he said. “Until we have more data with a significantly larger sample size, it’s premature to raise the concern in the general population.”
“The cardiovascular benefits of controlling blood pressure far outweigh the risk of liver cancer in patients with metabolic syndrome,” Elhariri added.
In ongoing studies, researchers are investigating ways to improve patient outcomes and reduce the negative effects of cirrhosis-associated complications among patients with MASLD and metabolic dysfunction–associated steatohepatitis (MASH), Muhammad Ali Butt, MD, a hepatology fellow at Beth Israel Lahey Hospital & Medical Center in Burlington, Massachusetts, said in an interview.
Butt, who wasn’t involved with this study, presented separate research on statins in MASH patients with cirrhosis, which indicated statistically significant decreases in portal hypertension, thrombosis, hepatorenal syndrome, hepatic encephalopathy, and mortality.
“We know patients with MASLD- and MASH-associated cirrhosis commonly have other comorbidities, including high cardiovascular risks, diabetes, and hyperlipidemia,” he said. “All of these conditions indicate patients to be on other medications such as antihypertensives or statins. It’s important to know the role these medications play, especially given the high-risk profile of these patients.”
Elhariri and Butt reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM AASLD 2024
Tapering Corticosteroids in Severe Alcohol-Associated Hepatitis Appears Safe
SAN DIEGO — , according to new research.
“Although several drugs have been evaluated for severe alcohol-associated hepatitis, none have succeeded in practice. Corticosteroids remain the mainstay of treatment; however, infections remain a major concern in 25%-40% of cases,” said Anand Kulkarni, MD, senior consultant and director of critical care hepatology at the Asian Institute of Gastroenterology in Hyderabad, India.
“There are no standard society guidelines for steroid dosing, and our current practices stem from studies in the 1970s, so there’s a major knowledge gap around optimal dosing and if stepwise tapering helps,” said Kulkarni, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Assessing Tapered Doses
In a multicenter, open-label randomized controlled trial, 254 patients with SAH from four Indian centers and one Canadian center were randomized to receive either a fixed or tapering dose of 40 mg prednisolone daily for 4 weeks. The patients in the tapering group received a starting dose of 40 mg, which was reduced by 10 mg weekly over 4 weeks.
While taking corticosteroids, 66% of those in the fixed dose group and 55% of those in the tapering group also received prophylactic antibiotics.
The mean age of participants was 41.1 years, the median Model For End-Stage Liver Disease score was 25.6, and 98.4% were men.
The primary objective was to compare the incidence of drug-related adverse events, infections, hospitalization, and mortality through day 90.
The duration of corticosteroid therapy was 22 days in the fixed dose group and 23 days in the tapering dose group.
Overall, the proportion of steroid responders was similar in both groups, at 80.3% in the fixed dose group and 82.5% in the tapering dose group.
However, the incidence of drug-related adverse events was significantly higher in the fixed dose group (52%) than in the tapering dose group (36.2%). The most common adverse events in both groups were infection, hyperglycemia, and hematochezia.
At 90 days, the incidence of infection was significantly lower in the tapering group (19.7%) than in the fixed dose group (33.1%). In both groups, the most common infection sites were the lungs (28.3%) and urinary tract (22.4%).
In terms of liver-related outcomes, some patients developed hepatic encephalopathy (11.8% in fixed dose vs 6.3% in tapering dose) and acute variceal bleed (3.1% in each group), as well as acute kidney injury (26.8% in fixed dose vs 18.9% in tapering dose).
Hospitalization within 90 days was required in 44.1% of the fixed dose group and 33.1% of the tapering dose group.
Survival at day 90 was 83.5% in the fixed dose group and 86.6% in the tapering dose group. Four patients in the fixed dose group and three patients in the tapering dose group underwent living donor liver transplantation by day 90.
Relapse of alcohol use by day 90 occurred in 13.4% of the fixed dose group and 12.6% of the tapering dose group.
“Rapid tapering in severe alcohol-associated hepatitis reduces infections and hospitalizations but doesn’t have a significant impact on survival,” Kulkarni concluded.
Considering Alternative Therapies
Given the high risk for infection in patients with SAH and limited certainty around benefits, the data may also call into question whether to give steroids to these patients at all, said session co-moderator Aleksander Krag, MD, professor of clinical medicine at the University of Southern Denmark, Odense, Denmark, and secretary general of the European Association for the Study of Liver 2023-2025.
“Since there are no other treatments available as of now, we’ll still continue to give steroids,” Kulkarni noted. But “tapering the dose should be beneficial.”
Although steroid therapy has been considered the “mainstay treatment” for SAH for 50 years, it doesn’t always lead to long-term improvement in liver values or survival, said Prasun Jalal, MD, the Stan and Sue Partee Endowed Chair in Hepatology at Baylor College of Medicine, Houston, who wasn’t involved with the study.
Researchers are looking to other connections, such as the gut microbiome, to find treatments for advanced alcoholic liver disease, Jalal said in an interview. In a small pilot study, he and colleagues found that intestinal microbiota transplantation (IMT) appears to be safe and effective for these patients.
“Early analyses suggest that IMT has a favorable outcome on the prognosis of patients with severe alcohol-associated hepatitis and is safe,” Jalal said. “A longer follow-up study with a larger sample size is in progress.”
Kulkarni and Krag reported no relevant disclosures. Jalal has speaking and teaching relationships with AbbVie and Madrigal.
A version of this article appeared on Medscape.com.
SAN DIEGO — , according to new research.
“Although several drugs have been evaluated for severe alcohol-associated hepatitis, none have succeeded in practice. Corticosteroids remain the mainstay of treatment; however, infections remain a major concern in 25%-40% of cases,” said Anand Kulkarni, MD, senior consultant and director of critical care hepatology at the Asian Institute of Gastroenterology in Hyderabad, India.
“There are no standard society guidelines for steroid dosing, and our current practices stem from studies in the 1970s, so there’s a major knowledge gap around optimal dosing and if stepwise tapering helps,” said Kulkarni, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Assessing Tapered Doses
In a multicenter, open-label randomized controlled trial, 254 patients with SAH from four Indian centers and one Canadian center were randomized to receive either a fixed or tapering dose of 40 mg prednisolone daily for 4 weeks. The patients in the tapering group received a starting dose of 40 mg, which was reduced by 10 mg weekly over 4 weeks.
While taking corticosteroids, 66% of those in the fixed dose group and 55% of those in the tapering group also received prophylactic antibiotics.
The mean age of participants was 41.1 years, the median Model For End-Stage Liver Disease score was 25.6, and 98.4% were men.
The primary objective was to compare the incidence of drug-related adverse events, infections, hospitalization, and mortality through day 90.
The duration of corticosteroid therapy was 22 days in the fixed dose group and 23 days in the tapering dose group.
Overall, the proportion of steroid responders was similar in both groups, at 80.3% in the fixed dose group and 82.5% in the tapering dose group.
However, the incidence of drug-related adverse events was significantly higher in the fixed dose group (52%) than in the tapering dose group (36.2%). The most common adverse events in both groups were infection, hyperglycemia, and hematochezia.
At 90 days, the incidence of infection was significantly lower in the tapering group (19.7%) than in the fixed dose group (33.1%). In both groups, the most common infection sites were the lungs (28.3%) and urinary tract (22.4%).
In terms of liver-related outcomes, some patients developed hepatic encephalopathy (11.8% in fixed dose vs 6.3% in tapering dose) and acute variceal bleed (3.1% in each group), as well as acute kidney injury (26.8% in fixed dose vs 18.9% in tapering dose).
Hospitalization within 90 days was required in 44.1% of the fixed dose group and 33.1% of the tapering dose group.
Survival at day 90 was 83.5% in the fixed dose group and 86.6% in the tapering dose group. Four patients in the fixed dose group and three patients in the tapering dose group underwent living donor liver transplantation by day 90.
Relapse of alcohol use by day 90 occurred in 13.4% of the fixed dose group and 12.6% of the tapering dose group.
“Rapid tapering in severe alcohol-associated hepatitis reduces infections and hospitalizations but doesn’t have a significant impact on survival,” Kulkarni concluded.
Considering Alternative Therapies
Given the high risk for infection in patients with SAH and limited certainty around benefits, the data may also call into question whether to give steroids to these patients at all, said session co-moderator Aleksander Krag, MD, professor of clinical medicine at the University of Southern Denmark, Odense, Denmark, and secretary general of the European Association for the Study of Liver 2023-2025.
“Since there are no other treatments available as of now, we’ll still continue to give steroids,” Kulkarni noted. But “tapering the dose should be beneficial.”
Although steroid therapy has been considered the “mainstay treatment” for SAH for 50 years, it doesn’t always lead to long-term improvement in liver values or survival, said Prasun Jalal, MD, the Stan and Sue Partee Endowed Chair in Hepatology at Baylor College of Medicine, Houston, who wasn’t involved with the study.
Researchers are looking to other connections, such as the gut microbiome, to find treatments for advanced alcoholic liver disease, Jalal said in an interview. In a small pilot study, he and colleagues found that intestinal microbiota transplantation (IMT) appears to be safe and effective for these patients.
“Early analyses suggest that IMT has a favorable outcome on the prognosis of patients with severe alcohol-associated hepatitis and is safe,” Jalal said. “A longer follow-up study with a larger sample size is in progress.”
Kulkarni and Krag reported no relevant disclosures. Jalal has speaking and teaching relationships with AbbVie and Madrigal.
A version of this article appeared on Medscape.com.
SAN DIEGO — , according to new research.
“Although several drugs have been evaluated for severe alcohol-associated hepatitis, none have succeeded in practice. Corticosteroids remain the mainstay of treatment; however, infections remain a major concern in 25%-40% of cases,” said Anand Kulkarni, MD, senior consultant and director of critical care hepatology at the Asian Institute of Gastroenterology in Hyderabad, India.
“There are no standard society guidelines for steroid dosing, and our current practices stem from studies in the 1970s, so there’s a major knowledge gap around optimal dosing and if stepwise tapering helps,” said Kulkarni, who presented the findings at The Liver Meeting 2024: American Association for the Study of Liver Diseases (AASLD).
Assessing Tapered Doses
In a multicenter, open-label randomized controlled trial, 254 patients with SAH from four Indian centers and one Canadian center were randomized to receive either a fixed or tapering dose of 40 mg prednisolone daily for 4 weeks. The patients in the tapering group received a starting dose of 40 mg, which was reduced by 10 mg weekly over 4 weeks.
While taking corticosteroids, 66% of those in the fixed dose group and 55% of those in the tapering group also received prophylactic antibiotics.
The mean age of participants was 41.1 years, the median Model For End-Stage Liver Disease score was 25.6, and 98.4% were men.
The primary objective was to compare the incidence of drug-related adverse events, infections, hospitalization, and mortality through day 90.
The duration of corticosteroid therapy was 22 days in the fixed dose group and 23 days in the tapering dose group.
Overall, the proportion of steroid responders was similar in both groups, at 80.3% in the fixed dose group and 82.5% in the tapering dose group.
However, the incidence of drug-related adverse events was significantly higher in the fixed dose group (52%) than in the tapering dose group (36.2%). The most common adverse events in both groups were infection, hyperglycemia, and hematochezia.
At 90 days, the incidence of infection was significantly lower in the tapering group (19.7%) than in the fixed dose group (33.1%). In both groups, the most common infection sites were the lungs (28.3%) and urinary tract (22.4%).
In terms of liver-related outcomes, some patients developed hepatic encephalopathy (11.8% in fixed dose vs 6.3% in tapering dose) and acute variceal bleed (3.1% in each group), as well as acute kidney injury (26.8% in fixed dose vs 18.9% in tapering dose).
Hospitalization within 90 days was required in 44.1% of the fixed dose group and 33.1% of the tapering dose group.
Survival at day 90 was 83.5% in the fixed dose group and 86.6% in the tapering dose group. Four patients in the fixed dose group and three patients in the tapering dose group underwent living donor liver transplantation by day 90.
Relapse of alcohol use by day 90 occurred in 13.4% of the fixed dose group and 12.6% of the tapering dose group.
“Rapid tapering in severe alcohol-associated hepatitis reduces infections and hospitalizations but doesn’t have a significant impact on survival,” Kulkarni concluded.
Considering Alternative Therapies
Given the high risk for infection in patients with SAH and limited certainty around benefits, the data may also call into question whether to give steroids to these patients at all, said session co-moderator Aleksander Krag, MD, professor of clinical medicine at the University of Southern Denmark, Odense, Denmark, and secretary general of the European Association for the Study of Liver 2023-2025.
“Since there are no other treatments available as of now, we’ll still continue to give steroids,” Kulkarni noted. But “tapering the dose should be beneficial.”
Although steroid therapy has been considered the “mainstay treatment” for SAH for 50 years, it doesn’t always lead to long-term improvement in liver values or survival, said Prasun Jalal, MD, the Stan and Sue Partee Endowed Chair in Hepatology at Baylor College of Medicine, Houston, who wasn’t involved with the study.
Researchers are looking to other connections, such as the gut microbiome, to find treatments for advanced alcoholic liver disease, Jalal said in an interview. In a small pilot study, he and colleagues found that intestinal microbiota transplantation (IMT) appears to be safe and effective for these patients.
“Early analyses suggest that IMT has a favorable outcome on the prognosis of patients with severe alcohol-associated hepatitis and is safe,” Jalal said. “A longer follow-up study with a larger sample size is in progress.”
Kulkarni and Krag reported no relevant disclosures. Jalal has speaking and teaching relationships with AbbVie and Madrigal.
A version of this article appeared on Medscape.com.
FROM AASLD 2024