User login
MODULE 1: Historical Review of Evidence-Based Treatment of Hypertension
MODULE 2: Rethinking the Role of Thiazide-Type Diuretics in the Management of Hypertension: Which Diuretic Is Best?
MODULE 3: Using Thiazide-Type Diuretics in African Americans with Hypertension
Dr Cushman is a paid consultant to Daiichi-Sankyo, Inc; Merck & Co, Inc; Omron Healthcare, Inc; and Takeda Pharmaceuticals International, Inc. He has performed contracted research for Merck & Co, Inc.
Background
Despite the availability of 7 major classes of effective and safe antihypertensive medications and numerous combination drugs designed to reduce pill burden and improve adherence, just 50.1% of the estimated 76.4 million US adults with hypertension (33.5% of the population) have their condition under control.1
One of the greatest challenges for clinicians who manage patients with hypertension is choosing the most appropriate drug, whether as initial treatment or add-on therapy. Clinicians may be guided in this decision, however, by guidelines and algorithms that are provided for hypertension management. These algorithms are reviewed in the first article in this supplement by Dr William B. White.
National guidelines recommend thiazide-type diuretics as initial therapy for most patients with hypertension, regardless of the severity of the condition, either alone or in combination with 1 of the other classes of hypertension medications that have also been shown to reduce 1 or more hypertensive complications in randomized controlled outcome trials.2,3 These recommendations are based primarily on more than 50 years of data on the safety and efficacy of thiazide-type diuretics.
The first evidence of the benefits of thiazide-type diuretics came from publications of the VA (Veterans Administration) Cooperative Study in 1967 and 1970. It was the first trial to demonstrate reduced stroke, heart failure (HF), and progressive kidney damage in patients receiving antihypertensive treatment, including the then-newly released hydrochlorothiazide (HCTZ), a thiazide diuretic.4
Since then, hundreds of clinical trials have demonstrated the efficacy of thiazide-type diuretics. During that time, however, numerous other classes of antihypertensive medications were introduced, leading to the question of the appropriate place of thiazides within the antihypertensive arsenal. The seminal trial to answer this question was the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). This randomized, double-blind, multicenter, clinical trial was designed to determine whether the occurrence of fatal coronary heart disease (CHD) or nonfatal myocardial infarction (MI) was lower for high-risk hypertensive patients 55 years of age and older who were treated with the calcium channel blocker amlodipine, the angiotensin-converting enzyme inhibitor (ACEI) lisinopril, or the alpha-blocker doxazosin compared with the thiazide-type diuretic chlorthalidone (CTD).5 Investigators could add atenolol, clonidine, reserpine, and/or hydralazine as necessary to achieve blood pressure (BP) goal. The trial randomized 42,418 patients, 90% of whom had been previously treated.
At a mean follow-up of 4.9 years, there was no significant difference in the primary outcome or mortality between the 4 drugs.5 There was a 38% higher rate of HF with amlodipine, and a 10%, 15%, and 19% higher rate of cardiovascular disease (CVD), stroke, and HF, respectively, with lisinopril compared with CTD. For stroke, there was a statistically significant race-by-treatment interaction (40% higher stroke rate with lisinopril vs CTD in black participants). Participants in the doxazosin treatment group (n = 9061) were followed for a mean of 3.2 years. This arm was terminated early because of a 25% higher incidence of CVD events, including a nearly 2-fold higher risk of HF, accompanied by a low probability of reaching a statistically significant difference in the primary endpoint.5
Additional rationale for the use of diuretics in elderly populations came from the Systolic Hypertension in the Elderly Program (SHEP), a multicenter, randomized, double- blind, placebo-controlled trial of patients aged 60 years and older.6 Participants were randomized to either CTD 12.5 to 25 mg once daily±atenolol 25 to 50 mg once daily, or reserpine 0.05 mg once daily, or placebo. Treatment reduced the incidence of all fatal and nonfatal strokes by 36%, MI by 27%, all CHD by 27%, and all CVD by 32%.6
Underuse of diuretics
Despite trials such as SHEP and ALLHAT, and despite the long record of safety and efficacy in numerous patient populations, thiazide-type diuretics remain significantly under-used in clinical practice.7-10
Even intensive academic detailing designed to increase the use of thiazide-type diuretics found that the prescribing rates of 37.1% immediately before the intervention only increased to 39.6% overall after the intervention (46.5% in areas that received the most intensive intervention), reflecting what appears to be clinical resistance to this class of drugs (FIGURE 1).11 Even 4 years after the ALLHAT results were published, national use of thiazide-type drugs had not increased significantly.12
FIGURE 1
Proportion of visits by drug class among patients with drug-treated hypertension11
Data are from the IMS Health National Disease and Therapeutic Index, 2000 through 2008. “Other classes” indicates α-adrenergic receptor blockers, potassium-sparing diuretics, loop diuretics, and centrally acting agents.
Source: Archives of Internal Medicine by American Medical Association. Reproduced with permission of American Medical Association, in the format Journal via Copyright Clearance Center.
Hydrochlorothiazide and chlorthalidone: Similarities and differences
Underuse of thiazide-type diuretics is just 1 challenge. Others include which diuretic to use (HCTZ or CTD) and at what dosage.13-17 These 2 diuretics were approved at about the same time and, until recently, were considered equivalent and interchangeable despite differences in structure, pharmacokinetics, and pharmacodynamics.16,17
The publication of the VA Cooperative Morbidity Trial, the successful marketing and popularity of HCTZ and low-dose HCTZ/triamterene, the fear of hypokalemia (which was seen more often in the high doses of CTD initially used), and the subsequent inclusion of HCTZ as the primary diuretic in single-pill combination antihypertensives with ACEIs and angiotensin II receptor blockers (ARBs) led to HCTZ becoming the market leader for this class. Nonetheless, CTD was the diuretic chosen for many major randomized clinical trials, especially those sponsored by the National Heart, Lung, and Blood Institute (NHLBI).5,6,18-20
One reason for CTD’s relegation as a second-tier option to HCTZ could be the higher risk of hypokalemia observed at the higher dosages typically used in early studies.21-23 However, later studies found that substantially lower dosages of CTD could provide similar BP reductions with a significantly lower risk of hypokalemia.24 Materson et al,25 for instance, demonstrated that the 25-mg dosage of CTD was at least as effective for hypertension as the 50-mg and 75-mg dosages, while the 25-mg dosage was associated with less hypokalemia.
Increasingly, however, hypertension specialists, particularly those involved in research, have come to appreciate that CTD and HCTZ are, indeed, not interchangeable and do not have dosing equivalency. This understanding, together with the results of clinical trials like ALLHAT, has led to a resurgence of interest in the use of CTD.17,19,26
One assessment of outpatient prescription data from the VA from 2003 to 2008 found that although the proportion of patients receiving HCTZ during the period remained stable, the number of new users dropped 30% even as the proportion of thiazide users receiving CTD prescriptions doubled from 1.1% to 2.4% and the number of new prescriptions for CTD increased 40%.19
Chlorthalidone or hydrochlorothiazide: Study outcomes
The resurgence of interest in CTD has come with the publication of trials demonstrating its benefits in reducing CVD risk.5,6,27,28
The Multiple Risk Factor Intervention Trial (MRFIT) is the only large, long-term, randomized trial to directly compare HCTZ and CTD, although not in a randomized assignment. The primary endpoint was cardiovascular (CV) outcomes. The study launched in 1973 and enrolled 12,866 males aged 35 to 57 years who were in the upper 15% risk of death from chronic heart disease.29 Participants in the special care group were given HCTZ or CTD (investigator’s choice) at either 50 or 100 mg daily, depending on weight and sodium levels, and were given additional drugs as needed. The control group received usual care at that time from their health care provider. A 44% higher rate of CHD mortality in the HCTZ group observed towards the latter part of the trial led its Policy Advisory Board to change the option between the 2 diuretics and require CTD only. Following the change, the rate of CHD mortality decreased by 28% (P = .04 for comparison between the 2 time frames).29
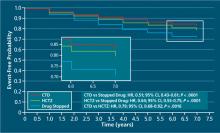
A recent retrospective analysis of MRFIT found significantly lower CV event rates in participants who received either CTD or HCTZ than in those receiving neither (CTD: adjusted hazard ratio [aHR], 0.51; 95% confidence interval [CI], 0.43-0.61; P < .0001; HCTZ: aHR, 0.65; 95% CI, 0.55- 0.75; P < .0001), but rates of nonfatal CV events were significantly lower in participants who received CTD than those who received HCTZ (aHR, 0.79; 95% CI, 0.68-0.92; P < .0016).30 The results are depicted in FIGURE 2.30
FIGURE 2
Adjusted event-free probability of cardiovascular events30
CI, confidence interval; CTD, chlorthalidone; HCTZ, hydrochlorothiazide; HR, hazard ratio.
Source: Hypertension by American Heart Association; Council for High Blood Pressure Research (American Heart Association); InterAmerican Society of Hypertension. Reproduced with permission of Lippincott Williams & Wilkins in the format Journal via Copyright Clearance Center.A recent meta-analysis of 108 trials with HCTZ and 29 with CTD found that the 2 drugs did not provide equivalent reductions in systolic BP (SBP) within equivalent dosages. The study found that the median change in SBP with the median dose of HCTZ was –17 mm Hg, compared with -26 mm Hg for CTD. The slightly greater potassium loss observed with CTD was still nearly equivalent to that observed with HCTZ.31
Considerations for greater chlorthalidone efficacy
The differences between CTD and HCTZ, despite similar molecular structures, is a topic of much discussion.23 It is likely that these 2 drugs have different pharmacokinetic and pharmacodynamic properties, as shown in TABLE 1.17
TABLE 1
Pharmacokinetic/pharmacodynamic comparison of hydrochlorothiazide and chlorthalidone17
| Onset (h) | Peak (h) | Half-life (h) | Duration (h) | |
|---|---|---|---|---|
Hydrochlorothiazide
| 2 | 4-6 | 6-9 (single dose) 8-15 (long-term dosing) | 12 (single dose) 16-24 (long-term dosing) |
Chlorthalidone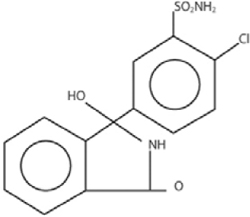
| 2-3 | 2-6 | 40 (single dose) 45-60 (long-term dosing) | 24-48 (single dose) 48-72 (long-term dosing) |
| Source: Hypertension by American Heart Association; Council for High Blood Pressure Research (American Heart Association); InterAmerican Society of Hypertension. Reproduced with permission of Lippincott Williams & Wilkins in the format Journal via Copyright Clearance Center | ||||
There is also evidence from an in vitro study that, compared with HCTZ, CTD has additional pleiotropic effects: reducing carbonic anhydrase activity, platelet aggregation, and vascular permeability while promoting angiogenesis.32
Another reason for the differences in efficacy between CTD and HCTZ could be the dosages of HCTZ used. Worldwide, nearly all prescriptions for HCTZ are for 12.5 to 25 mg/d, while most modern combination pills containing HCTZ incorporate these lower dosages.33 However, there is little evidence that such dosages lead to significantly improved outcomes.14,19,34-36
This was an issue in the Avoiding Cardiovascular Events Through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial. This study was designed to compare first-step therapy with benazepril/HCTZ 20/12.5 mg.37 Benazepril was force-titrated to 40 mg in each arm, whereas HCTZ and amlodipine were titrated to 25 mg and 10 mg, respectively, only if needed for BP control. The study was conducted in 11,506 high-risk patients 55 years of age and older. Other antihypertensive drugs could be added as needed for BP control. The study was stopped early after a mean follow-up of 36 months when the benazepril/amlodi-pine group demonstrated an HR of 0.8 (95% CI, 0.72-0.90) for the composite outcome of death from CV events, nonfatal MI or stroke, hospitalization for angina, resuscitation after sudden cardiac arrest, and coronary revascularization compared with the benazepril/HCTZ group.37
The ACCOMPLISH trial has been controversial for many reasons, with editorials suggesting that its design led to a “stacked deck” that favored amlodipine/benazepril over benazepril/HCTZ. Questions were raised as to why HCTZ was the diuretic of choice because CTD has been used in most thiazide-type trials. The dosages chosen were also questioned because outcome trials demonstrating reduced CV events with HCTZ used target doses of 50 mg/d or higher.14,21,22,38
Indeed, a meta-analysis published in 2011 found that despite the extensive use of HCTZ worldwide, the 12.5 to 25 mg dosage was inferior in reducing BP compared with standard doses of other antihypertensive agents (ACEIs, ARBs, beta blockers, and calcium channel blockers) in studies using 24-hour ambulatory BP monitoring.33 The efficacy of HCTZ closely mirrors that of the other drug classes at the 50-mg level, although that dose results in somewhat higher rates of hypokalemia. As the dose of HCTZ is increased to 100 mg, there is little or no further increase in antihypertensive efficacy, but hypokalemia becomes much more common.39
Thus, it can be clinically challenging to prescribe the optimum BP medication if practitioners prefer to use single-pill combinations that include HCTZ. Although the use of such single-pill combinations is warranted, particularly given the improved adherence with taking single-pill combinations compared with taking 2 or 3 pills, as noted in TABLE 1, most combinations include HCTZ dosages of 12.5 to 25 mg, which will often be less effective than full doses of 2 other medications.
Conclusion
Although thiazide-type diuretics are recommended as first-line therapy for most patients with hypertension, either alone or in combination with other classes of antihypertensives, they remain underused in clinical practice. In addition, HCTZ, which is the most commonly used diuretic (indeed, the most commonly prescribed antihypertensive) is prescribed at dosages too low to provide sufficient clinical efficacy in BP reduction and lower than what was proven to reduce CV events in clinical trials.
Chlorthalidone, a diuretic often considered a thiazide-type diuretic, has demonstrated superiority to HCTZ in reducing BP as evidenced in the MRFIT study and has been shown in numerous clinical trials to provide similar if not greater efficacy than other classes of antihypertensives in reducing BP, stroke, and CV events, with a good safety profile.
Clinicians need to manage patients with hypertension on an individual basis, selecting drugs and antihypertensive medication classes with the best outcomes in trials and then determining the most efficacious therapies with the lowest risk of adverse events for each patient. However, when prescribing a diuretic, they should also ensure that the drug used is prescribed at the appropriate therapeutic dosage level to enable patients to prevent the CV, thrombotic, and renal events that occur with long-term hypertension.
1. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303(20):2043-2050.
2. Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206-1252.
3. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114.
4. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213(7):1143-1152.
5. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) [published corrections appear in JAMA. 2003;289(2):178; JAMA. 2004;291(18):2196]. JAMA. 2002;288(23):2981-2997.
6. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265(24):3255-3264.
7. Petitti DB, Xie F, Barzilay JI. Prescribing patterns for thiazide diuretics in a large health maintenance organization: relationship to participation as an ALLHAT clinical center. Contemporary Clin Trials. 2006;27(5):397-403.
8. Dahlöf B, Devereux RB, Kjeldsen SE, et al. LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995-1003.
9. Psaty BM, Manolio TA, Smith NL, et al. Cardiovascular Health Study. Time trends in high blood pressure control and the use of antihypertensive medications in older adults: the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2325-2332.
10. Glasser SP, Basile JN, Lackland DT. Does prehypertension represent an increased risk for incident hypertension and adverse cardiovascular outcome? Hypertension. 2009;54(5):954-955.
11. Stafford RS, Bartholomew LK, Cushman WC, et al. ALLHAT Collaborative Research Group. Impact of the ALLHAT/JNC7 Dissemination Project on thiazide-type diuretic use. Arch Intern Med. 2010;170(10):851-858.
12. Furmaga EM, Cunningham FE, Cushman WC, et al. National utilization of antihypertensive medications from 2000 to 2006 in the Veterans Health Administration: focus on thiazide diuretics. J Clin Hypertens (Greenwich). 2008;10(10):770-778.
13. Pitt B. The role of chlorthalidone in patients with high-risk hypertension. J Clin Hypertens (Greenwich). 2009;11(9):460-461.
14. Ernst ME, Carter BL, Basile JN. All thiazide-like diuretics are not chlorthalidone: putting the ACCOMPLISH study into perspective. J Clin Hypertens (Greenwich). 2009;11(1):5-10.
15. Sica DA. Chlorthalidone—a renaissance in use? Expert Opin Pharmacother. 2009;10(13):2037-2039.
16. Kaplan NM. Chlorthalidone versus hydrochlorothiazide: a tale of tortoises and a hare. Hypertension. 2011;58(6):994-995.
17. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43(1):4-9.
18. Carter BL, Malone DC, Ellis SL, Dombrowski RC. Antihypertensive drug utilization in hypertensive veterans with complex medication profiles. J Clin Hypertens (Greenwich). 2000;2(3):172-180.
19. Ernst ME, Lund BC. Renewed interest in chlorthalidone: evidence from the Veterans Health Administration. J Clin Hypertens (Greenwich). 2010;12(12):927-934.
20. Neaton JD, Grimm RH Jr, Prineas RJ, et al. Treatment of Mild Hypertension Study Research Group. Treatment of Mild Hypertension Study. Final results. JAMA. 1993;270(6):713-724.
21. Ford RV. Therapy of edema and hypertension, Comparative clinical effects of chlorothiazide and chlorthalidone. Tex State J Med. 1960;56:343-346.
22. Mach RS, Veyrat R. Clinical experiences with some of the newer diuretics, especially chlorthalidone. Ann N Y Acad Sci. 1960;88:841-863.
23. Dyrda I, Dufault C, Herbert JG, Tremblay G, Genest J. Studies on a new diuretic: chlorthalidone. Can Med Assoc J. 1962;86:475-482.
24. Kakaviatos N, Finnerty FA, Jr. Comparison of chlorthalidone, a long-acting antihypertensive and diuretic agent, with chlorothiazide. Analysis of one hundred twenty-three cases. Am J Cardiol. 1962;10:570-574.
25. Materson BJ, Oster JR, Michael UF, et al. Dose response to chlorthalidone in patients with mild hypertension. Efficacy of a lower dose. Clin Pharmacol Ther. 1978;24(2):192-198.
26. Ernst ME, Carter BL, Goerdt CJ, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47(3):352-358.
27. Dunbar-Jacob J, Erlen JA, Schlenk EA, Ryan CM, Sereika SM, Doswell WM. Adherence in chronic disease. Annu Rev Nurs Res. 2000;18:48-90.
28. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242(23):2562-2571.
29. Multiple Risk Factor Intervention Trial Research Group. Multiple risk factor intervention trial. Risk factor changes and mortality results. JAMA. 1982;248(12):1465-1477.
30. Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57(4):689-694.
31. Ernst ME, Carter BL, Zheng S, Grimm RH, Jr. Meta-analysis of dose-response characteristics of hydrochlorothiazide and chlorthalidone: effects on systolic blood pressure and potassium. Am J Hypertens. 2010;23(4):440-446.
32. Woodman R, Brown C, Lockette W. Chlorthalidone decreases platelet aggregation and vascular permeability and promotes angiogenesis. Hypertension. 2010;56(3):463-470.
33. Messerli FH, Makani H, Benjo A, Romero J, Alviar C, Bangalore S. Antihypertensive efficacy of hydrochlorothiazide as evaluated by ambulatory blood pressure monitoring: a meta-analysis of randomized trials. J Am Coll Cardiol. 2011;57(5):590-600.
34. Demirovic J, Prineas R, Rudolph M. Epidemiology of congestive heart failure in three ethnic groups. Congest Heart Fail. 2001;7(2):93-96.
35. Eide IK, Torjesen PA, Drolsum A, Babovic A, Lilledahl NP. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22(11):2217-2226.
36. Epstein M. Re-examining RAS-blocking treatment regimens for abrogating progression of chronic kidney disease. Nat Clin Pract Nephrol. 2008;5(1):12-13.
37. Jamerson K, Weber MA, Bakris GL, et al. ACCOMPLISH Trial Investigators. Ben-azepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417-2428.
38. Parra D, Rosenstein R. Benazepril plus amlodipine or hydrochlorothiazide for hypertension. N Engl J Med. 2009;360(11):1147-1150.
39. Messerli FH, Bangalore S, Julius S. Risk/benefit assessment of beta-blockers and diuretics precludes their use for first-line therapy in hypertension. Circulation. 2008;117(20):2706-2715.
MODULE 1: Historical Review of Evidence-Based Treatment of Hypertension
MODULE 2: Rethinking the Role of Thiazide-Type Diuretics in the Management of Hypertension: Which Diuretic Is Best?
MODULE 3: Using Thiazide-Type Diuretics in African Americans with Hypertension
Dr Cushman is a paid consultant to Daiichi-Sankyo, Inc; Merck & Co, Inc; Omron Healthcare, Inc; and Takeda Pharmaceuticals International, Inc. He has performed contracted research for Merck & Co, Inc.
Background
Despite the availability of 7 major classes of effective and safe antihypertensive medications and numerous combination drugs designed to reduce pill burden and improve adherence, just 50.1% of the estimated 76.4 million US adults with hypertension (33.5% of the population) have their condition under control.1
One of the greatest challenges for clinicians who manage patients with hypertension is choosing the most appropriate drug, whether as initial treatment or add-on therapy. Clinicians may be guided in this decision, however, by guidelines and algorithms that are provided for hypertension management. These algorithms are reviewed in the first article in this supplement by Dr William B. White.
National guidelines recommend thiazide-type diuretics as initial therapy for most patients with hypertension, regardless of the severity of the condition, either alone or in combination with 1 of the other classes of hypertension medications that have also been shown to reduce 1 or more hypertensive complications in randomized controlled outcome trials.2,3 These recommendations are based primarily on more than 50 years of data on the safety and efficacy of thiazide-type diuretics.
The first evidence of the benefits of thiazide-type diuretics came from publications of the VA (Veterans Administration) Cooperative Study in 1967 and 1970. It was the first trial to demonstrate reduced stroke, heart failure (HF), and progressive kidney damage in patients receiving antihypertensive treatment, including the then-newly released hydrochlorothiazide (HCTZ), a thiazide diuretic.4
Since then, hundreds of clinical trials have demonstrated the efficacy of thiazide-type diuretics. During that time, however, numerous other classes of antihypertensive medications were introduced, leading to the question of the appropriate place of thiazides within the antihypertensive arsenal. The seminal trial to answer this question was the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). This randomized, double-blind, multicenter, clinical trial was designed to determine whether the occurrence of fatal coronary heart disease (CHD) or nonfatal myocardial infarction (MI) was lower for high-risk hypertensive patients 55 years of age and older who were treated with the calcium channel blocker amlodipine, the angiotensin-converting enzyme inhibitor (ACEI) lisinopril, or the alpha-blocker doxazosin compared with the thiazide-type diuretic chlorthalidone (CTD).5 Investigators could add atenolol, clonidine, reserpine, and/or hydralazine as necessary to achieve blood pressure (BP) goal. The trial randomized 42,418 patients, 90% of whom had been previously treated.
At a mean follow-up of 4.9 years, there was no significant difference in the primary outcome or mortality between the 4 drugs.5 There was a 38% higher rate of HF with amlodipine, and a 10%, 15%, and 19% higher rate of cardiovascular disease (CVD), stroke, and HF, respectively, with lisinopril compared with CTD. For stroke, there was a statistically significant race-by-treatment interaction (40% higher stroke rate with lisinopril vs CTD in black participants). Participants in the doxazosin treatment group (n = 9061) were followed for a mean of 3.2 years. This arm was terminated early because of a 25% higher incidence of CVD events, including a nearly 2-fold higher risk of HF, accompanied by a low probability of reaching a statistically significant difference in the primary endpoint.5
Additional rationale for the use of diuretics in elderly populations came from the Systolic Hypertension in the Elderly Program (SHEP), a multicenter, randomized, double- blind, placebo-controlled trial of patients aged 60 years and older.6 Participants were randomized to either CTD 12.5 to 25 mg once daily±atenolol 25 to 50 mg once daily, or reserpine 0.05 mg once daily, or placebo. Treatment reduced the incidence of all fatal and nonfatal strokes by 36%, MI by 27%, all CHD by 27%, and all CVD by 32%.6
Underuse of diuretics
Despite trials such as SHEP and ALLHAT, and despite the long record of safety and efficacy in numerous patient populations, thiazide-type diuretics remain significantly under-used in clinical practice.7-10
Even intensive academic detailing designed to increase the use of thiazide-type diuretics found that the prescribing rates of 37.1% immediately before the intervention only increased to 39.6% overall after the intervention (46.5% in areas that received the most intensive intervention), reflecting what appears to be clinical resistance to this class of drugs (FIGURE 1).11 Even 4 years after the ALLHAT results were published, national use of thiazide-type drugs had not increased significantly.12
FIGURE 1
Proportion of visits by drug class among patients with drug-treated hypertension11
Data are from the IMS Health National Disease and Therapeutic Index, 2000 through 2008. “Other classes” indicates α-adrenergic receptor blockers, potassium-sparing diuretics, loop diuretics, and centrally acting agents.
Source: Archives of Internal Medicine by American Medical Association. Reproduced with permission of American Medical Association, in the format Journal via Copyright Clearance Center.
Hydrochlorothiazide and chlorthalidone: Similarities and differences
Underuse of thiazide-type diuretics is just 1 challenge. Others include which diuretic to use (HCTZ or CTD) and at what dosage.13-17 These 2 diuretics were approved at about the same time and, until recently, were considered equivalent and interchangeable despite differences in structure, pharmacokinetics, and pharmacodynamics.16,17
The publication of the VA Cooperative Morbidity Trial, the successful marketing and popularity of HCTZ and low-dose HCTZ/triamterene, the fear of hypokalemia (which was seen more often in the high doses of CTD initially used), and the subsequent inclusion of HCTZ as the primary diuretic in single-pill combination antihypertensives with ACEIs and angiotensin II receptor blockers (ARBs) led to HCTZ becoming the market leader for this class. Nonetheless, CTD was the diuretic chosen for many major randomized clinical trials, especially those sponsored by the National Heart, Lung, and Blood Institute (NHLBI).5,6,18-20
One reason for CTD’s relegation as a second-tier option to HCTZ could be the higher risk of hypokalemia observed at the higher dosages typically used in early studies.21-23 However, later studies found that substantially lower dosages of CTD could provide similar BP reductions with a significantly lower risk of hypokalemia.24 Materson et al,25 for instance, demonstrated that the 25-mg dosage of CTD was at least as effective for hypertension as the 50-mg and 75-mg dosages, while the 25-mg dosage was associated with less hypokalemia.
Increasingly, however, hypertension specialists, particularly those involved in research, have come to appreciate that CTD and HCTZ are, indeed, not interchangeable and do not have dosing equivalency. This understanding, together with the results of clinical trials like ALLHAT, has led to a resurgence of interest in the use of CTD.17,19,26
One assessment of outpatient prescription data from the VA from 2003 to 2008 found that although the proportion of patients receiving HCTZ during the period remained stable, the number of new users dropped 30% even as the proportion of thiazide users receiving CTD prescriptions doubled from 1.1% to 2.4% and the number of new prescriptions for CTD increased 40%.19
Chlorthalidone or hydrochlorothiazide: Study outcomes
The resurgence of interest in CTD has come with the publication of trials demonstrating its benefits in reducing CVD risk.5,6,27,28
The Multiple Risk Factor Intervention Trial (MRFIT) is the only large, long-term, randomized trial to directly compare HCTZ and CTD, although not in a randomized assignment. The primary endpoint was cardiovascular (CV) outcomes. The study launched in 1973 and enrolled 12,866 males aged 35 to 57 years who were in the upper 15% risk of death from chronic heart disease.29 Participants in the special care group were given HCTZ or CTD (investigator’s choice) at either 50 or 100 mg daily, depending on weight and sodium levels, and were given additional drugs as needed. The control group received usual care at that time from their health care provider. A 44% higher rate of CHD mortality in the HCTZ group observed towards the latter part of the trial led its Policy Advisory Board to change the option between the 2 diuretics and require CTD only. Following the change, the rate of CHD mortality decreased by 28% (P = .04 for comparison between the 2 time frames).29
A recent retrospective analysis of MRFIT found significantly lower CV event rates in participants who received either CTD or HCTZ than in those receiving neither (CTD: adjusted hazard ratio [aHR], 0.51; 95% confidence interval [CI], 0.43-0.61; P < .0001; HCTZ: aHR, 0.65; 95% CI, 0.55- 0.75; P < .0001), but rates of nonfatal CV events were significantly lower in participants who received CTD than those who received HCTZ (aHR, 0.79; 95% CI, 0.68-0.92; P < .0016).30 The results are depicted in FIGURE 2.30
FIGURE 2
Adjusted event-free probability of cardiovascular events30
CI, confidence interval; CTD, chlorthalidone; HCTZ, hydrochlorothiazide; HR, hazard ratio.
Source: Hypertension by American Heart Association; Council for High Blood Pressure Research (American Heart Association); InterAmerican Society of Hypertension. Reproduced with permission of Lippincott Williams & Wilkins in the format Journal via Copyright Clearance Center.A recent meta-analysis of 108 trials with HCTZ and 29 with CTD found that the 2 drugs did not provide equivalent reductions in systolic BP (SBP) within equivalent dosages. The study found that the median change in SBP with the median dose of HCTZ was –17 mm Hg, compared with -26 mm Hg for CTD. The slightly greater potassium loss observed with CTD was still nearly equivalent to that observed with HCTZ.31
Considerations for greater chlorthalidone efficacy
The differences between CTD and HCTZ, despite similar molecular structures, is a topic of much discussion.23 It is likely that these 2 drugs have different pharmacokinetic and pharmacodynamic properties, as shown in TABLE 1.17
TABLE 1
Pharmacokinetic/pharmacodynamic comparison of hydrochlorothiazide and chlorthalidone17
| Onset (h) | Peak (h) | Half-life (h) | Duration (h) | |
|---|---|---|---|---|
Hydrochlorothiazide
| 2 | 4-6 | 6-9 (single dose) 8-15 (long-term dosing) | 12 (single dose) 16-24 (long-term dosing) |
Chlorthalidone
| 2-3 | 2-6 | 40 (single dose) 45-60 (long-term dosing) | 24-48 (single dose) 48-72 (long-term dosing) |
| Source: Hypertension by American Heart Association; Council for High Blood Pressure Research (American Heart Association); InterAmerican Society of Hypertension. Reproduced with permission of Lippincott Williams & Wilkins in the format Journal via Copyright Clearance Center | ||||
There is also evidence from an in vitro study that, compared with HCTZ, CTD has additional pleiotropic effects: reducing carbonic anhydrase activity, platelet aggregation, and vascular permeability while promoting angiogenesis.32
Another reason for the differences in efficacy between CTD and HCTZ could be the dosages of HCTZ used. Worldwide, nearly all prescriptions for HCTZ are for 12.5 to 25 mg/d, while most modern combination pills containing HCTZ incorporate these lower dosages.33 However, there is little evidence that such dosages lead to significantly improved outcomes.14,19,34-36
This was an issue in the Avoiding Cardiovascular Events Through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial. This study was designed to compare first-step therapy with benazepril/HCTZ 20/12.5 mg.37 Benazepril was force-titrated to 40 mg in each arm, whereas HCTZ and amlodipine were titrated to 25 mg and 10 mg, respectively, only if needed for BP control. The study was conducted in 11,506 high-risk patients 55 years of age and older. Other antihypertensive drugs could be added as needed for BP control. The study was stopped early after a mean follow-up of 36 months when the benazepril/amlodi-pine group demonstrated an HR of 0.8 (95% CI, 0.72-0.90) for the composite outcome of death from CV events, nonfatal MI or stroke, hospitalization for angina, resuscitation after sudden cardiac arrest, and coronary revascularization compared with the benazepril/HCTZ group.37
The ACCOMPLISH trial has been controversial for many reasons, with editorials suggesting that its design led to a “stacked deck” that favored amlodipine/benazepril over benazepril/HCTZ. Questions were raised as to why HCTZ was the diuretic of choice because CTD has been used in most thiazide-type trials. The dosages chosen were also questioned because outcome trials demonstrating reduced CV events with HCTZ used target doses of 50 mg/d or higher.14,21,22,38
Indeed, a meta-analysis published in 2011 found that despite the extensive use of HCTZ worldwide, the 12.5 to 25 mg dosage was inferior in reducing BP compared with standard doses of other antihypertensive agents (ACEIs, ARBs, beta blockers, and calcium channel blockers) in studies using 24-hour ambulatory BP monitoring.33 The efficacy of HCTZ closely mirrors that of the other drug classes at the 50-mg level, although that dose results in somewhat higher rates of hypokalemia. As the dose of HCTZ is increased to 100 mg, there is little or no further increase in antihypertensive efficacy, but hypokalemia becomes much more common.39
Thus, it can be clinically challenging to prescribe the optimum BP medication if practitioners prefer to use single-pill combinations that include HCTZ. Although the use of such single-pill combinations is warranted, particularly given the improved adherence with taking single-pill combinations compared with taking 2 or 3 pills, as noted in TABLE 1, most combinations include HCTZ dosages of 12.5 to 25 mg, which will often be less effective than full doses of 2 other medications.
Conclusion
Although thiazide-type diuretics are recommended as first-line therapy for most patients with hypertension, either alone or in combination with other classes of antihypertensives, they remain underused in clinical practice. In addition, HCTZ, which is the most commonly used diuretic (indeed, the most commonly prescribed antihypertensive) is prescribed at dosages too low to provide sufficient clinical efficacy in BP reduction and lower than what was proven to reduce CV events in clinical trials.
Chlorthalidone, a diuretic often considered a thiazide-type diuretic, has demonstrated superiority to HCTZ in reducing BP as evidenced in the MRFIT study and has been shown in numerous clinical trials to provide similar if not greater efficacy than other classes of antihypertensives in reducing BP, stroke, and CV events, with a good safety profile.
Clinicians need to manage patients with hypertension on an individual basis, selecting drugs and antihypertensive medication classes with the best outcomes in trials and then determining the most efficacious therapies with the lowest risk of adverse events for each patient. However, when prescribing a diuretic, they should also ensure that the drug used is prescribed at the appropriate therapeutic dosage level to enable patients to prevent the CV, thrombotic, and renal events that occur with long-term hypertension.
MODULE 1: Historical Review of Evidence-Based Treatment of Hypertension
MODULE 2: Rethinking the Role of Thiazide-Type Diuretics in the Management of Hypertension: Which Diuretic Is Best?
MODULE 3: Using Thiazide-Type Diuretics in African Americans with Hypertension
Dr Cushman is a paid consultant to Daiichi-Sankyo, Inc; Merck & Co, Inc; Omron Healthcare, Inc; and Takeda Pharmaceuticals International, Inc. He has performed contracted research for Merck & Co, Inc.
Background
Despite the availability of 7 major classes of effective and safe antihypertensive medications and numerous combination drugs designed to reduce pill burden and improve adherence, just 50.1% of the estimated 76.4 million US adults with hypertension (33.5% of the population) have their condition under control.1
One of the greatest challenges for clinicians who manage patients with hypertension is choosing the most appropriate drug, whether as initial treatment or add-on therapy. Clinicians may be guided in this decision, however, by guidelines and algorithms that are provided for hypertension management. These algorithms are reviewed in the first article in this supplement by Dr William B. White.
National guidelines recommend thiazide-type diuretics as initial therapy for most patients with hypertension, regardless of the severity of the condition, either alone or in combination with 1 of the other classes of hypertension medications that have also been shown to reduce 1 or more hypertensive complications in randomized controlled outcome trials.2,3 These recommendations are based primarily on more than 50 years of data on the safety and efficacy of thiazide-type diuretics.
The first evidence of the benefits of thiazide-type diuretics came from publications of the VA (Veterans Administration) Cooperative Study in 1967 and 1970. It was the first trial to demonstrate reduced stroke, heart failure (HF), and progressive kidney damage in patients receiving antihypertensive treatment, including the then-newly released hydrochlorothiazide (HCTZ), a thiazide diuretic.4
Since then, hundreds of clinical trials have demonstrated the efficacy of thiazide-type diuretics. During that time, however, numerous other classes of antihypertensive medications were introduced, leading to the question of the appropriate place of thiazides within the antihypertensive arsenal. The seminal trial to answer this question was the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). This randomized, double-blind, multicenter, clinical trial was designed to determine whether the occurrence of fatal coronary heart disease (CHD) or nonfatal myocardial infarction (MI) was lower for high-risk hypertensive patients 55 years of age and older who were treated with the calcium channel blocker amlodipine, the angiotensin-converting enzyme inhibitor (ACEI) lisinopril, or the alpha-blocker doxazosin compared with the thiazide-type diuretic chlorthalidone (CTD).5 Investigators could add atenolol, clonidine, reserpine, and/or hydralazine as necessary to achieve blood pressure (BP) goal. The trial randomized 42,418 patients, 90% of whom had been previously treated.
At a mean follow-up of 4.9 years, there was no significant difference in the primary outcome or mortality between the 4 drugs.5 There was a 38% higher rate of HF with amlodipine, and a 10%, 15%, and 19% higher rate of cardiovascular disease (CVD), stroke, and HF, respectively, with lisinopril compared with CTD. For stroke, there was a statistically significant race-by-treatment interaction (40% higher stroke rate with lisinopril vs CTD in black participants). Participants in the doxazosin treatment group (n = 9061) were followed for a mean of 3.2 years. This arm was terminated early because of a 25% higher incidence of CVD events, including a nearly 2-fold higher risk of HF, accompanied by a low probability of reaching a statistically significant difference in the primary endpoint.5
Additional rationale for the use of diuretics in elderly populations came from the Systolic Hypertension in the Elderly Program (SHEP), a multicenter, randomized, double- blind, placebo-controlled trial of patients aged 60 years and older.6 Participants were randomized to either CTD 12.5 to 25 mg once daily±atenolol 25 to 50 mg once daily, or reserpine 0.05 mg once daily, or placebo. Treatment reduced the incidence of all fatal and nonfatal strokes by 36%, MI by 27%, all CHD by 27%, and all CVD by 32%.6
Underuse of diuretics
Despite trials such as SHEP and ALLHAT, and despite the long record of safety and efficacy in numerous patient populations, thiazide-type diuretics remain significantly under-used in clinical practice.7-10
Even intensive academic detailing designed to increase the use of thiazide-type diuretics found that the prescribing rates of 37.1% immediately before the intervention only increased to 39.6% overall after the intervention (46.5% in areas that received the most intensive intervention), reflecting what appears to be clinical resistance to this class of drugs (FIGURE 1).11 Even 4 years after the ALLHAT results were published, national use of thiazide-type drugs had not increased significantly.12
FIGURE 1
Proportion of visits by drug class among patients with drug-treated hypertension11
Data are from the IMS Health National Disease and Therapeutic Index, 2000 through 2008. “Other classes” indicates α-adrenergic receptor blockers, potassium-sparing diuretics, loop diuretics, and centrally acting agents.
Source: Archives of Internal Medicine by American Medical Association. Reproduced with permission of American Medical Association, in the format Journal via Copyright Clearance Center.
Hydrochlorothiazide and chlorthalidone: Similarities and differences
Underuse of thiazide-type diuretics is just 1 challenge. Others include which diuretic to use (HCTZ or CTD) and at what dosage.13-17 These 2 diuretics were approved at about the same time and, until recently, were considered equivalent and interchangeable despite differences in structure, pharmacokinetics, and pharmacodynamics.16,17
The publication of the VA Cooperative Morbidity Trial, the successful marketing and popularity of HCTZ and low-dose HCTZ/triamterene, the fear of hypokalemia (which was seen more often in the high doses of CTD initially used), and the subsequent inclusion of HCTZ as the primary diuretic in single-pill combination antihypertensives with ACEIs and angiotensin II receptor blockers (ARBs) led to HCTZ becoming the market leader for this class. Nonetheless, CTD was the diuretic chosen for many major randomized clinical trials, especially those sponsored by the National Heart, Lung, and Blood Institute (NHLBI).5,6,18-20
One reason for CTD’s relegation as a second-tier option to HCTZ could be the higher risk of hypokalemia observed at the higher dosages typically used in early studies.21-23 However, later studies found that substantially lower dosages of CTD could provide similar BP reductions with a significantly lower risk of hypokalemia.24 Materson et al,25 for instance, demonstrated that the 25-mg dosage of CTD was at least as effective for hypertension as the 50-mg and 75-mg dosages, while the 25-mg dosage was associated with less hypokalemia.
Increasingly, however, hypertension specialists, particularly those involved in research, have come to appreciate that CTD and HCTZ are, indeed, not interchangeable and do not have dosing equivalency. This understanding, together with the results of clinical trials like ALLHAT, has led to a resurgence of interest in the use of CTD.17,19,26
One assessment of outpatient prescription data from the VA from 2003 to 2008 found that although the proportion of patients receiving HCTZ during the period remained stable, the number of new users dropped 30% even as the proportion of thiazide users receiving CTD prescriptions doubled from 1.1% to 2.4% and the number of new prescriptions for CTD increased 40%.19
Chlorthalidone or hydrochlorothiazide: Study outcomes
The resurgence of interest in CTD has come with the publication of trials demonstrating its benefits in reducing CVD risk.5,6,27,28
The Multiple Risk Factor Intervention Trial (MRFIT) is the only large, long-term, randomized trial to directly compare HCTZ and CTD, although not in a randomized assignment. The primary endpoint was cardiovascular (CV) outcomes. The study launched in 1973 and enrolled 12,866 males aged 35 to 57 years who were in the upper 15% risk of death from chronic heart disease.29 Participants in the special care group were given HCTZ or CTD (investigator’s choice) at either 50 or 100 mg daily, depending on weight and sodium levels, and were given additional drugs as needed. The control group received usual care at that time from their health care provider. A 44% higher rate of CHD mortality in the HCTZ group observed towards the latter part of the trial led its Policy Advisory Board to change the option between the 2 diuretics and require CTD only. Following the change, the rate of CHD mortality decreased by 28% (P = .04 for comparison between the 2 time frames).29
A recent retrospective analysis of MRFIT found significantly lower CV event rates in participants who received either CTD or HCTZ than in those receiving neither (CTD: adjusted hazard ratio [aHR], 0.51; 95% confidence interval [CI], 0.43-0.61; P < .0001; HCTZ: aHR, 0.65; 95% CI, 0.55- 0.75; P < .0001), but rates of nonfatal CV events were significantly lower in participants who received CTD than those who received HCTZ (aHR, 0.79; 95% CI, 0.68-0.92; P < .0016).30 The results are depicted in FIGURE 2.30
FIGURE 2
Adjusted event-free probability of cardiovascular events30
CI, confidence interval; CTD, chlorthalidone; HCTZ, hydrochlorothiazide; HR, hazard ratio.
Source: Hypertension by American Heart Association; Council for High Blood Pressure Research (American Heart Association); InterAmerican Society of Hypertension. Reproduced with permission of Lippincott Williams & Wilkins in the format Journal via Copyright Clearance Center.A recent meta-analysis of 108 trials with HCTZ and 29 with CTD found that the 2 drugs did not provide equivalent reductions in systolic BP (SBP) within equivalent dosages. The study found that the median change in SBP with the median dose of HCTZ was –17 mm Hg, compared with -26 mm Hg for CTD. The slightly greater potassium loss observed with CTD was still nearly equivalent to that observed with HCTZ.31
Considerations for greater chlorthalidone efficacy
The differences between CTD and HCTZ, despite similar molecular structures, is a topic of much discussion.23 It is likely that these 2 drugs have different pharmacokinetic and pharmacodynamic properties, as shown in TABLE 1.17
TABLE 1
Pharmacokinetic/pharmacodynamic comparison of hydrochlorothiazide and chlorthalidone17
| Onset (h) | Peak (h) | Half-life (h) | Duration (h) | |
|---|---|---|---|---|
Hydrochlorothiazide
| 2 | 4-6 | 6-9 (single dose) 8-15 (long-term dosing) | 12 (single dose) 16-24 (long-term dosing) |
Chlorthalidone
| 2-3 | 2-6 | 40 (single dose) 45-60 (long-term dosing) | 24-48 (single dose) 48-72 (long-term dosing) |
| Source: Hypertension by American Heart Association; Council for High Blood Pressure Research (American Heart Association); InterAmerican Society of Hypertension. Reproduced with permission of Lippincott Williams & Wilkins in the format Journal via Copyright Clearance Center | ||||
There is also evidence from an in vitro study that, compared with HCTZ, CTD has additional pleiotropic effects: reducing carbonic anhydrase activity, platelet aggregation, and vascular permeability while promoting angiogenesis.32
Another reason for the differences in efficacy between CTD and HCTZ could be the dosages of HCTZ used. Worldwide, nearly all prescriptions for HCTZ are for 12.5 to 25 mg/d, while most modern combination pills containing HCTZ incorporate these lower dosages.33 However, there is little evidence that such dosages lead to significantly improved outcomes.14,19,34-36
This was an issue in the Avoiding Cardiovascular Events Through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial. This study was designed to compare first-step therapy with benazepril/HCTZ 20/12.5 mg.37 Benazepril was force-titrated to 40 mg in each arm, whereas HCTZ and amlodipine were titrated to 25 mg and 10 mg, respectively, only if needed for BP control. The study was conducted in 11,506 high-risk patients 55 years of age and older. Other antihypertensive drugs could be added as needed for BP control. The study was stopped early after a mean follow-up of 36 months when the benazepril/amlodi-pine group demonstrated an HR of 0.8 (95% CI, 0.72-0.90) for the composite outcome of death from CV events, nonfatal MI or stroke, hospitalization for angina, resuscitation after sudden cardiac arrest, and coronary revascularization compared with the benazepril/HCTZ group.37
The ACCOMPLISH trial has been controversial for many reasons, with editorials suggesting that its design led to a “stacked deck” that favored amlodipine/benazepril over benazepril/HCTZ. Questions were raised as to why HCTZ was the diuretic of choice because CTD has been used in most thiazide-type trials. The dosages chosen were also questioned because outcome trials demonstrating reduced CV events with HCTZ used target doses of 50 mg/d or higher.14,21,22,38
Indeed, a meta-analysis published in 2011 found that despite the extensive use of HCTZ worldwide, the 12.5 to 25 mg dosage was inferior in reducing BP compared with standard doses of other antihypertensive agents (ACEIs, ARBs, beta blockers, and calcium channel blockers) in studies using 24-hour ambulatory BP monitoring.33 The efficacy of HCTZ closely mirrors that of the other drug classes at the 50-mg level, although that dose results in somewhat higher rates of hypokalemia. As the dose of HCTZ is increased to 100 mg, there is little or no further increase in antihypertensive efficacy, but hypokalemia becomes much more common.39
Thus, it can be clinically challenging to prescribe the optimum BP medication if practitioners prefer to use single-pill combinations that include HCTZ. Although the use of such single-pill combinations is warranted, particularly given the improved adherence with taking single-pill combinations compared with taking 2 or 3 pills, as noted in TABLE 1, most combinations include HCTZ dosages of 12.5 to 25 mg, which will often be less effective than full doses of 2 other medications.
Conclusion
Although thiazide-type diuretics are recommended as first-line therapy for most patients with hypertension, either alone or in combination with other classes of antihypertensives, they remain underused in clinical practice. In addition, HCTZ, which is the most commonly used diuretic (indeed, the most commonly prescribed antihypertensive) is prescribed at dosages too low to provide sufficient clinical efficacy in BP reduction and lower than what was proven to reduce CV events in clinical trials.
Chlorthalidone, a diuretic often considered a thiazide-type diuretic, has demonstrated superiority to HCTZ in reducing BP as evidenced in the MRFIT study and has been shown in numerous clinical trials to provide similar if not greater efficacy than other classes of antihypertensives in reducing BP, stroke, and CV events, with a good safety profile.
Clinicians need to manage patients with hypertension on an individual basis, selecting drugs and antihypertensive medication classes with the best outcomes in trials and then determining the most efficacious therapies with the lowest risk of adverse events for each patient. However, when prescribing a diuretic, they should also ensure that the drug used is prescribed at the appropriate therapeutic dosage level to enable patients to prevent the CV, thrombotic, and renal events that occur with long-term hypertension.
1. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303(20):2043-2050.
2. Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206-1252.
3. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114.
4. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213(7):1143-1152.
5. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) [published corrections appear in JAMA. 2003;289(2):178; JAMA. 2004;291(18):2196]. JAMA. 2002;288(23):2981-2997.
6. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265(24):3255-3264.
7. Petitti DB, Xie F, Barzilay JI. Prescribing patterns for thiazide diuretics in a large health maintenance organization: relationship to participation as an ALLHAT clinical center. Contemporary Clin Trials. 2006;27(5):397-403.
8. Dahlöf B, Devereux RB, Kjeldsen SE, et al. LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995-1003.
9. Psaty BM, Manolio TA, Smith NL, et al. Cardiovascular Health Study. Time trends in high blood pressure control and the use of antihypertensive medications in older adults: the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2325-2332.
10. Glasser SP, Basile JN, Lackland DT. Does prehypertension represent an increased risk for incident hypertension and adverse cardiovascular outcome? Hypertension. 2009;54(5):954-955.
11. Stafford RS, Bartholomew LK, Cushman WC, et al. ALLHAT Collaborative Research Group. Impact of the ALLHAT/JNC7 Dissemination Project on thiazide-type diuretic use. Arch Intern Med. 2010;170(10):851-858.
12. Furmaga EM, Cunningham FE, Cushman WC, et al. National utilization of antihypertensive medications from 2000 to 2006 in the Veterans Health Administration: focus on thiazide diuretics. J Clin Hypertens (Greenwich). 2008;10(10):770-778.
13. Pitt B. The role of chlorthalidone in patients with high-risk hypertension. J Clin Hypertens (Greenwich). 2009;11(9):460-461.
14. Ernst ME, Carter BL, Basile JN. All thiazide-like diuretics are not chlorthalidone: putting the ACCOMPLISH study into perspective. J Clin Hypertens (Greenwich). 2009;11(1):5-10.
15. Sica DA. Chlorthalidone—a renaissance in use? Expert Opin Pharmacother. 2009;10(13):2037-2039.
16. Kaplan NM. Chlorthalidone versus hydrochlorothiazide: a tale of tortoises and a hare. Hypertension. 2011;58(6):994-995.
17. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43(1):4-9.
18. Carter BL, Malone DC, Ellis SL, Dombrowski RC. Antihypertensive drug utilization in hypertensive veterans with complex medication profiles. J Clin Hypertens (Greenwich). 2000;2(3):172-180.
19. Ernst ME, Lund BC. Renewed interest in chlorthalidone: evidence from the Veterans Health Administration. J Clin Hypertens (Greenwich). 2010;12(12):927-934.
20. Neaton JD, Grimm RH Jr, Prineas RJ, et al. Treatment of Mild Hypertension Study Research Group. Treatment of Mild Hypertension Study. Final results. JAMA. 1993;270(6):713-724.
21. Ford RV. Therapy of edema and hypertension, Comparative clinical effects of chlorothiazide and chlorthalidone. Tex State J Med. 1960;56:343-346.
22. Mach RS, Veyrat R. Clinical experiences with some of the newer diuretics, especially chlorthalidone. Ann N Y Acad Sci. 1960;88:841-863.
23. Dyrda I, Dufault C, Herbert JG, Tremblay G, Genest J. Studies on a new diuretic: chlorthalidone. Can Med Assoc J. 1962;86:475-482.
24. Kakaviatos N, Finnerty FA, Jr. Comparison of chlorthalidone, a long-acting antihypertensive and diuretic agent, with chlorothiazide. Analysis of one hundred twenty-three cases. Am J Cardiol. 1962;10:570-574.
25. Materson BJ, Oster JR, Michael UF, et al. Dose response to chlorthalidone in patients with mild hypertension. Efficacy of a lower dose. Clin Pharmacol Ther. 1978;24(2):192-198.
26. Ernst ME, Carter BL, Goerdt CJ, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47(3):352-358.
27. Dunbar-Jacob J, Erlen JA, Schlenk EA, Ryan CM, Sereika SM, Doswell WM. Adherence in chronic disease. Annu Rev Nurs Res. 2000;18:48-90.
28. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242(23):2562-2571.
29. Multiple Risk Factor Intervention Trial Research Group. Multiple risk factor intervention trial. Risk factor changes and mortality results. JAMA. 1982;248(12):1465-1477.
30. Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57(4):689-694.
31. Ernst ME, Carter BL, Zheng S, Grimm RH, Jr. Meta-analysis of dose-response characteristics of hydrochlorothiazide and chlorthalidone: effects on systolic blood pressure and potassium. Am J Hypertens. 2010;23(4):440-446.
32. Woodman R, Brown C, Lockette W. Chlorthalidone decreases platelet aggregation and vascular permeability and promotes angiogenesis. Hypertension. 2010;56(3):463-470.
33. Messerli FH, Makani H, Benjo A, Romero J, Alviar C, Bangalore S. Antihypertensive efficacy of hydrochlorothiazide as evaluated by ambulatory blood pressure monitoring: a meta-analysis of randomized trials. J Am Coll Cardiol. 2011;57(5):590-600.
34. Demirovic J, Prineas R, Rudolph M. Epidemiology of congestive heart failure in three ethnic groups. Congest Heart Fail. 2001;7(2):93-96.
35. Eide IK, Torjesen PA, Drolsum A, Babovic A, Lilledahl NP. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22(11):2217-2226.
36. Epstein M. Re-examining RAS-blocking treatment regimens for abrogating progression of chronic kidney disease. Nat Clin Pract Nephrol. 2008;5(1):12-13.
37. Jamerson K, Weber MA, Bakris GL, et al. ACCOMPLISH Trial Investigators. Ben-azepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417-2428.
38. Parra D, Rosenstein R. Benazepril plus amlodipine or hydrochlorothiazide for hypertension. N Engl J Med. 2009;360(11):1147-1150.
39. Messerli FH, Bangalore S, Julius S. Risk/benefit assessment of beta-blockers and diuretics precludes their use for first-line therapy in hypertension. Circulation. 2008;117(20):2706-2715.
1. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303(20):2043-2050.
2. Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206-1252.
3. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114.
4. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213(7):1143-1152.
5. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) [published corrections appear in JAMA. 2003;289(2):178; JAMA. 2004;291(18):2196]. JAMA. 2002;288(23):2981-2997.
6. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265(24):3255-3264.
7. Petitti DB, Xie F, Barzilay JI. Prescribing patterns for thiazide diuretics in a large health maintenance organization: relationship to participation as an ALLHAT clinical center. Contemporary Clin Trials. 2006;27(5):397-403.
8. Dahlöf B, Devereux RB, Kjeldsen SE, et al. LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995-1003.
9. Psaty BM, Manolio TA, Smith NL, et al. Cardiovascular Health Study. Time trends in high blood pressure control and the use of antihypertensive medications in older adults: the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2325-2332.
10. Glasser SP, Basile JN, Lackland DT. Does prehypertension represent an increased risk for incident hypertension and adverse cardiovascular outcome? Hypertension. 2009;54(5):954-955.
11. Stafford RS, Bartholomew LK, Cushman WC, et al. ALLHAT Collaborative Research Group. Impact of the ALLHAT/JNC7 Dissemination Project on thiazide-type diuretic use. Arch Intern Med. 2010;170(10):851-858.
12. Furmaga EM, Cunningham FE, Cushman WC, et al. National utilization of antihypertensive medications from 2000 to 2006 in the Veterans Health Administration: focus on thiazide diuretics. J Clin Hypertens (Greenwich). 2008;10(10):770-778.
13. Pitt B. The role of chlorthalidone in patients with high-risk hypertension. J Clin Hypertens (Greenwich). 2009;11(9):460-461.
14. Ernst ME, Carter BL, Basile JN. All thiazide-like diuretics are not chlorthalidone: putting the ACCOMPLISH study into perspective. J Clin Hypertens (Greenwich). 2009;11(1):5-10.
15. Sica DA. Chlorthalidone—a renaissance in use? Expert Opin Pharmacother. 2009;10(13):2037-2039.
16. Kaplan NM. Chlorthalidone versus hydrochlorothiazide: a tale of tortoises and a hare. Hypertension. 2011;58(6):994-995.
17. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43(1):4-9.
18. Carter BL, Malone DC, Ellis SL, Dombrowski RC. Antihypertensive drug utilization in hypertensive veterans with complex medication profiles. J Clin Hypertens (Greenwich). 2000;2(3):172-180.
19. Ernst ME, Lund BC. Renewed interest in chlorthalidone: evidence from the Veterans Health Administration. J Clin Hypertens (Greenwich). 2010;12(12):927-934.
20. Neaton JD, Grimm RH Jr, Prineas RJ, et al. Treatment of Mild Hypertension Study Research Group. Treatment of Mild Hypertension Study. Final results. JAMA. 1993;270(6):713-724.
21. Ford RV. Therapy of edema and hypertension, Comparative clinical effects of chlorothiazide and chlorthalidone. Tex State J Med. 1960;56:343-346.
22. Mach RS, Veyrat R. Clinical experiences with some of the newer diuretics, especially chlorthalidone. Ann N Y Acad Sci. 1960;88:841-863.
23. Dyrda I, Dufault C, Herbert JG, Tremblay G, Genest J. Studies on a new diuretic: chlorthalidone. Can Med Assoc J. 1962;86:475-482.
24. Kakaviatos N, Finnerty FA, Jr. Comparison of chlorthalidone, a long-acting antihypertensive and diuretic agent, with chlorothiazide. Analysis of one hundred twenty-three cases. Am J Cardiol. 1962;10:570-574.
25. Materson BJ, Oster JR, Michael UF, et al. Dose response to chlorthalidone in patients with mild hypertension. Efficacy of a lower dose. Clin Pharmacol Ther. 1978;24(2):192-198.
26. Ernst ME, Carter BL, Goerdt CJ, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47(3):352-358.
27. Dunbar-Jacob J, Erlen JA, Schlenk EA, Ryan CM, Sereika SM, Doswell WM. Adherence in chronic disease. Annu Rev Nurs Res. 2000;18:48-90.
28. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242(23):2562-2571.
29. Multiple Risk Factor Intervention Trial Research Group. Multiple risk factor intervention trial. Risk factor changes and mortality results. JAMA. 1982;248(12):1465-1477.
30. Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57(4):689-694.
31. Ernst ME, Carter BL, Zheng S, Grimm RH, Jr. Meta-analysis of dose-response characteristics of hydrochlorothiazide and chlorthalidone: effects on systolic blood pressure and potassium. Am J Hypertens. 2010;23(4):440-446.
32. Woodman R, Brown C, Lockette W. Chlorthalidone decreases platelet aggregation and vascular permeability and promotes angiogenesis. Hypertension. 2010;56(3):463-470.
33. Messerli FH, Makani H, Benjo A, Romero J, Alviar C, Bangalore S. Antihypertensive efficacy of hydrochlorothiazide as evaluated by ambulatory blood pressure monitoring: a meta-analysis of randomized trials. J Am Coll Cardiol. 2011;57(5):590-600.
34. Demirovic J, Prineas R, Rudolph M. Epidemiology of congestive heart failure in three ethnic groups. Congest Heart Fail. 2001;7(2):93-96.
35. Eide IK, Torjesen PA, Drolsum A, Babovic A, Lilledahl NP. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22(11):2217-2226.
36. Epstein M. Re-examining RAS-blocking treatment regimens for abrogating progression of chronic kidney disease. Nat Clin Pract Nephrol. 2008;5(1):12-13.
37. Jamerson K, Weber MA, Bakris GL, et al. ACCOMPLISH Trial Investigators. Ben-azepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417-2428.
38. Parra D, Rosenstein R. Benazepril plus amlodipine or hydrochlorothiazide for hypertension. N Engl J Med. 2009;360(11):1147-1150.
39. Messerli FH, Bangalore S, Julius S. Risk/benefit assessment of beta-blockers and diuretics precludes their use for first-line therapy in hypertension. Circulation. 2008;117(20):2706-2715.