User login
Granulomatosis with polyangiitis (GPA), is one of the most common types of small-vessel vasculitis, with an estimated prevalence in the United States of 3 per 100,000 people. It is distinguished from other necrotizing vasculitides by its tendency to affect the upper and lower respiratory system and the kidneys. Despite the success of induction and maintenance treatments with cyclophosphamide (CYC), glucocorticoids, and less toxic immunosuppressive alternative therapies in improving the disease course, significant treatment-related toxicities and frequent disease relapses demand stringent patient-specific monitoring in order to provide early treatment of relapses and prevent or decrease morbidity.
SMALL-VESSEL VASCULITIS MANAGEMENT OVERVIEW
Granulomatosis with polyangiitis (formerly Wegener’s granulomatosis, or WG) is an antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis that often affects the respiratory system and kidneys across a broad spectrum of clinical presentations, from mild through life-threatening disease. Patients with severe disease present with significant multisystem manifestations, which, in addition to the respiratory system and kidneys, may involve the joints, eyes, and other organs.
Managing patients diagnosed with systemic small-vessel vasculitides such as GPA and microscopic polyangiitis (MPA) is an inexact science. The goals of treatment are to increase survival, induce and maintain remission, reduce relapses, and minimize treatment-related toxicity. Inducing and maintaining remission have become realistic goals because of the availability of medications that prolong life. On the other hand, extended periods of treatment associated with prolonged life increase the risk of treatment-related toxicity in patients who are inadequately monitored.
MONITORING CONSIDERATIONS
Achieving treatment goals requires long-term monitoring of both disease activity and treatment-related toxicities, with constant adjustments to meet the needs of the individual patient and address the often rapidly changing disease and treatment course. The monitoring protocol consists of regularly scheduled follow-up office visits, urine sediment analyses at every office visit whether or not the patient has relapse symptoms, laboratory tests at regular intervals as indicated by the patient’s medication plan and disease presentation, additional tests such as lung computed tomography (CT), and patient education regarding new symptoms and the frequency of office visits. A consistent monitoring strategy will help detect a relapse before it can produce more severe morbidity, identify treatment-related complications, and—equally important—identify the achievement of remission. An example of the consequences of inconsistent monitoring is presented in “Relapse in a nonadherent patient.”
Because there is no definitive cure for small-vessel vasculitis, relapse is always a possibility. The early diagnosis and treatment of relapse may prevent or decrease morbidity from disease, but strict monitoring is needed to identify relapse and initiate treatment before morbidity occurs (see “Relapse in a patient with new symptoms”). Repeat induction therapy following a relapse introduces risk of drug toxicity and requires careful monitoring, as does long-term maintenance therapy.
In addition to induction and maintenance therapy, several other situations, including prior therapeutic complications, serum creatinine levels, and risk of cardiovascular disease, require special monitoring attention.

Induction therapy: monitor response

Response to treatment during induction must be monitored to identify whether remission is achieved. Induction monitoring requires complete assessment of organ-system involvement at every visit with tools such as the Birmingham Vasculitis Activity Score (BVAS) and, when appropriate, the BVAS/WG. If new or worsening symptoms develop during induction therapy, then the patient needs assessment for continued disease activity as well as treatment complications such as infections related to immunosuppressive therapy.
During induction therapy with daily oral CYC, monitoring should include weekly complete blood cell counts to ensure early identification of leukopenia and other cytopenias. The risk of morbidities increases with the cumulative dose, so a stable blood count for 2 months does not obviate the risk of leukopenia. If persistent hematuria is present without cellular casts, cystoscopy is indicated to look for signs of hemorrhagic cystitis. Prophylaxis against Pneumocystis jirovecii is recommended in all patients who receive immunosuppressive therapy. Finally, bone density measurements should be done at baseline.
Maintenance therapy: frequency can be extended
Monitoring during maintenance therapy is similar to induction monitoring; however, when the dosage of methotrexate or azathioprine is stabilized, the frequency of some tests can be extended to monthly rather than weekly. For example, a complete blood cell count, comprehensive metabolic panel, sedimentation rate, C-reactive protein measurement, and urinalysis should be performed monthly. Follow-up visits should include urine sediment analyses and monitoring for cardiovascular disease risk factors. Medication monitoring should include cystoscopy for persistent hematuria without cellular casts, bone density measurements, and ophthalmologic examinations as frequently as indicated for each individual’s needs. P jirovecii prophylaxis should continue as long as the patient receives immunosuppressive medication.

Therapy-related complications
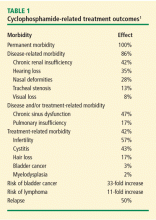
Bladder complications. In a retrospective analysis of 145 patients with GPA treated with CYC and followed for 0.5 to 27 years (median 8.5 years), nonglomerular hematuria developed in 50% of the patients and bladder carcinoma in 5%.2 The cumulative CYC dose (19 to 251 g) in this group was much higher than what is currently used. Cytologic examination of the urine showed 43% sensitivity for dysplasia (specificity 100%) and 29% sensitivity for atypia (specificity 89%). In contrast, in a retrospective outcomes analysis involving newly diagnosed patients with GPA treated with CYC or methotrexate, 82 patients followed for up to 12 years had no incidents of cystitis or bladder cancer.3 Patients in this study were treated with CYC for only 3 to 6 months and therefore received a lower cumulative dose.
To prevent cystitis during treatment with CYC, the patient should be well hydrated, especially in the morning when CYC should be taken. The bladder should be emptied frequently. The addition of mesna when administering intravenous CYC decreases the risk of cystitis. Serial cystoscopy and urine cytology should be used only in patients with nonglomerular hematuria.
Infertility. Preservation of ovarian function is a concern with CYC therapy in women of childbearing age. The cumulative dose threshold for gonadal failure is unknown, because data from cancer studies4 demonstrating gonadal failure involve higher cumulative CYC doses than are typical for vasculitis treatment. It is also unknown whether duration of amenorrhea predicts the recovery of menses or fertility. The primary option for preservation of ovarian function is the use of gonadotropin-releasing hormone agonists. Oral contraceptives also may be used, but the best prevention is to avoid CYC in these patients if possible.
Osteoporosis. At glucocorticoid dosages of 5 mg/day or greater, bone mineral density begins a rapid decline within the first 3 months and peaks at 6 months.5 The American College of Rheumatology has provided recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis.5 Table 2 presents recommendations for postmenopausal women and men aged 50 years and older who will use glucocorticoids for 3 months or more.5 Recommendations are also available for premenopausal women and men younger than 50 years of age who have a history of fragility fracture.
Leukopenia. Leukopenia should be avoided during CYC treatment. The target white blood cell count should be within the normal range. During treatment with daily oral CYC, the patient should be monitored with a weekly complete blood cell count and medication should be adjusted to maintain the target white blood cell count.
Upon completion of induction therapy, after 3 to 6 months, the patient is switched to maintenance therapy with an alternative immunosuppressive agent such as azathioprine or methotrexate, depending on the serum creatinine concentration and other factors. This transition, characterized by full-dose immunosuppressive therapy when the bone marrow has been previously suppressed by CYC treatment, may induce pancytopenia. Monitoring with weekly complete blood counts for at least 4 weeks after initiating maintenance therapy can help ensure stability during the transition period.

Monitor serum creatinine and adjust dosages
The serum creatinine concentration may increase as CYC treatment progresses; in some cases, the serum creatinine concentration increases before a response to treatment is seen. The CYC dosages should be adjusted as necessary in response to serum creatinine changes. Careful monitoring of serum creatinine is necessary during methotrexate therapy, as methotrexate treatment in the setting of renal insufficiency increases the risk of bone marrow suppression.
Cardiovascular disease in GPA and MPA
Premature atherosclerosis has been well described in patients with GPA.6 Within 5 years of diagnosis of GPA or MPA, a cardiovascular event will occur in 14% of patients.7 In the absence of specific guidelines for prevention of cardiovascular disease in patients with vasculitis, it is essential to monitor patients and treat modifiable traditional risk factors aggressively, especially in younger patients. Suppiah et al found that independent determinants of cardiovascular outcome included older age, diastolic hypertension, and positive proteinase-3–ANCA status in patients without prior cardiovascular disease.7
In the Wegener’s Clinical Occurrence of Thrombosis (WeCLOT) study, Merkel et al showed an increased incidence of thrombosis in patients with active GPA8 (see “Relapse presenting as thrombosis,” left). As with cardiovascular disease, there are no specific guidelines for monitoring asymptomatic patients for thrombosis or for duration of anticoagulation in patients with GPA. It is recommended that patients be evaluated for active GPA or relapse in the setting of acute thrombosis whether or not symptoms of active GPA are present.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Talar-Williams C, Hijazi YM, Walther MM, et al. Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener granulomatosis. Ann Intern Med 1996; 124:477–484.
- Villa-Forte A, Clark TM, Gomes M, et al. Substitution of methotrexate for cyclophosphamide in Wegener granulomatosis: a 12-year single-practice experience. Medicine 2007; 86:269–277.
- Harel S, Fermé C, Poirot C. Management of fertility in patients treated for Hodgkin’s lymphoma [published online ahead of print August 9, 2011]. Haematologica 2011; 96:1692–1699. doi: 10.3324/haematol.2011.045856
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis [published online ahead of print July 26, 2010]. Arthritis Care Res (Hoboken) 2010; 62:1515–1526. doi: 10.1002/acr.20295
- Faurschou M, Mellemkjaer L, Sorensen IJ, Svalgaard Thomsen B, Dreyer L, Baslund B. Increased morbidity from ischemic heart disease in patients with Wegener’s granulomatosis. Arthritis Rheum 2009; 60:1187–1192.
- Suppiah R, Judge A, Batra R, et al. A model to predict cardiovascular events in patients with newly diagnosed Wegener’s granulomatosis and microscopic polyangiitis. Arthritis Care Res (Hoboken) 2011; 63:588–596.
- Merkel PA, Lo GH, Holbrook JT, et al; for Wegener’s Granulomatosis Etanercept Trial Research Group. Brief communication: high incidence of venous thrombotic events among patients with Wegener granulomatosis: the Wegener’s Clinical Occurrence of Thrombosis (WeCLOT) study. Ann Intern Med 2005; 142:620–626.
Granulomatosis with polyangiitis (GPA), is one of the most common types of small-vessel vasculitis, with an estimated prevalence in the United States of 3 per 100,000 people. It is distinguished from other necrotizing vasculitides by its tendency to affect the upper and lower respiratory system and the kidneys. Despite the success of induction and maintenance treatments with cyclophosphamide (CYC), glucocorticoids, and less toxic immunosuppressive alternative therapies in improving the disease course, significant treatment-related toxicities and frequent disease relapses demand stringent patient-specific monitoring in order to provide early treatment of relapses and prevent or decrease morbidity.
SMALL-VESSEL VASCULITIS MANAGEMENT OVERVIEW
Granulomatosis with polyangiitis (formerly Wegener’s granulomatosis, or WG) is an antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis that often affects the respiratory system and kidneys across a broad spectrum of clinical presentations, from mild through life-threatening disease. Patients with severe disease present with significant multisystem manifestations, which, in addition to the respiratory system and kidneys, may involve the joints, eyes, and other organs.
Managing patients diagnosed with systemic small-vessel vasculitides such as GPA and microscopic polyangiitis (MPA) is an inexact science. The goals of treatment are to increase survival, induce and maintain remission, reduce relapses, and minimize treatment-related toxicity. Inducing and maintaining remission have become realistic goals because of the availability of medications that prolong life. On the other hand, extended periods of treatment associated with prolonged life increase the risk of treatment-related toxicity in patients who are inadequately monitored.
MONITORING CONSIDERATIONS
Achieving treatment goals requires long-term monitoring of both disease activity and treatment-related toxicities, with constant adjustments to meet the needs of the individual patient and address the often rapidly changing disease and treatment course. The monitoring protocol consists of regularly scheduled follow-up office visits, urine sediment analyses at every office visit whether or not the patient has relapse symptoms, laboratory tests at regular intervals as indicated by the patient’s medication plan and disease presentation, additional tests such as lung computed tomography (CT), and patient education regarding new symptoms and the frequency of office visits. A consistent monitoring strategy will help detect a relapse before it can produce more severe morbidity, identify treatment-related complications, and—equally important—identify the achievement of remission. An example of the consequences of inconsistent monitoring is presented in “Relapse in a nonadherent patient.”
Because there is no definitive cure for small-vessel vasculitis, relapse is always a possibility. The early diagnosis and treatment of relapse may prevent or decrease morbidity from disease, but strict monitoring is needed to identify relapse and initiate treatment before morbidity occurs (see “Relapse in a patient with new symptoms”). Repeat induction therapy following a relapse introduces risk of drug toxicity and requires careful monitoring, as does long-term maintenance therapy.
In addition to induction and maintenance therapy, several other situations, including prior therapeutic complications, serum creatinine levels, and risk of cardiovascular disease, require special monitoring attention.

Induction therapy: monitor response

Response to treatment during induction must be monitored to identify whether remission is achieved. Induction monitoring requires complete assessment of organ-system involvement at every visit with tools such as the Birmingham Vasculitis Activity Score (BVAS) and, when appropriate, the BVAS/WG. If new or worsening symptoms develop during induction therapy, then the patient needs assessment for continued disease activity as well as treatment complications such as infections related to immunosuppressive therapy.
During induction therapy with daily oral CYC, monitoring should include weekly complete blood cell counts to ensure early identification of leukopenia and other cytopenias. The risk of morbidities increases with the cumulative dose, so a stable blood count for 2 months does not obviate the risk of leukopenia. If persistent hematuria is present without cellular casts, cystoscopy is indicated to look for signs of hemorrhagic cystitis. Prophylaxis against Pneumocystis jirovecii is recommended in all patients who receive immunosuppressive therapy. Finally, bone density measurements should be done at baseline.
Maintenance therapy: frequency can be extended
Monitoring during maintenance therapy is similar to induction monitoring; however, when the dosage of methotrexate or azathioprine is stabilized, the frequency of some tests can be extended to monthly rather than weekly. For example, a complete blood cell count, comprehensive metabolic panel, sedimentation rate, C-reactive protein measurement, and urinalysis should be performed monthly. Follow-up visits should include urine sediment analyses and monitoring for cardiovascular disease risk factors. Medication monitoring should include cystoscopy for persistent hematuria without cellular casts, bone density measurements, and ophthalmologic examinations as frequently as indicated for each individual’s needs. P jirovecii prophylaxis should continue as long as the patient receives immunosuppressive medication.

Therapy-related complications
Bladder complications. In a retrospective analysis of 145 patients with GPA treated with CYC and followed for 0.5 to 27 years (median 8.5 years), nonglomerular hematuria developed in 50% of the patients and bladder carcinoma in 5%.2 The cumulative CYC dose (19 to 251 g) in this group was much higher than what is currently used. Cytologic examination of the urine showed 43% sensitivity for dysplasia (specificity 100%) and 29% sensitivity for atypia (specificity 89%). In contrast, in a retrospective outcomes analysis involving newly diagnosed patients with GPA treated with CYC or methotrexate, 82 patients followed for up to 12 years had no incidents of cystitis or bladder cancer.3 Patients in this study were treated with CYC for only 3 to 6 months and therefore received a lower cumulative dose.
To prevent cystitis during treatment with CYC, the patient should be well hydrated, especially in the morning when CYC should be taken. The bladder should be emptied frequently. The addition of mesna when administering intravenous CYC decreases the risk of cystitis. Serial cystoscopy and urine cytology should be used only in patients with nonglomerular hematuria.
Infertility. Preservation of ovarian function is a concern with CYC therapy in women of childbearing age. The cumulative dose threshold for gonadal failure is unknown, because data from cancer studies4 demonstrating gonadal failure involve higher cumulative CYC doses than are typical for vasculitis treatment. It is also unknown whether duration of amenorrhea predicts the recovery of menses or fertility. The primary option for preservation of ovarian function is the use of gonadotropin-releasing hormone agonists. Oral contraceptives also may be used, but the best prevention is to avoid CYC in these patients if possible.
Osteoporosis. At glucocorticoid dosages of 5 mg/day or greater, bone mineral density begins a rapid decline within the first 3 months and peaks at 6 months.5 The American College of Rheumatology has provided recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis.5 Table 2 presents recommendations for postmenopausal women and men aged 50 years and older who will use glucocorticoids for 3 months or more.5 Recommendations are also available for premenopausal women and men younger than 50 years of age who have a history of fragility fracture.
Leukopenia. Leukopenia should be avoided during CYC treatment. The target white blood cell count should be within the normal range. During treatment with daily oral CYC, the patient should be monitored with a weekly complete blood cell count and medication should be adjusted to maintain the target white blood cell count.
Upon completion of induction therapy, after 3 to 6 months, the patient is switched to maintenance therapy with an alternative immunosuppressive agent such as azathioprine or methotrexate, depending on the serum creatinine concentration and other factors. This transition, characterized by full-dose immunosuppressive therapy when the bone marrow has been previously suppressed by CYC treatment, may induce pancytopenia. Monitoring with weekly complete blood counts for at least 4 weeks after initiating maintenance therapy can help ensure stability during the transition period.

Monitor serum creatinine and adjust dosages
The serum creatinine concentration may increase as CYC treatment progresses; in some cases, the serum creatinine concentration increases before a response to treatment is seen. The CYC dosages should be adjusted as necessary in response to serum creatinine changes. Careful monitoring of serum creatinine is necessary during methotrexate therapy, as methotrexate treatment in the setting of renal insufficiency increases the risk of bone marrow suppression.
Cardiovascular disease in GPA and MPA
Premature atherosclerosis has been well described in patients with GPA.6 Within 5 years of diagnosis of GPA or MPA, a cardiovascular event will occur in 14% of patients.7 In the absence of specific guidelines for prevention of cardiovascular disease in patients with vasculitis, it is essential to monitor patients and treat modifiable traditional risk factors aggressively, especially in younger patients. Suppiah et al found that independent determinants of cardiovascular outcome included older age, diastolic hypertension, and positive proteinase-3–ANCA status in patients without prior cardiovascular disease.7
In the Wegener’s Clinical Occurrence of Thrombosis (WeCLOT) study, Merkel et al showed an increased incidence of thrombosis in patients with active GPA8 (see “Relapse presenting as thrombosis,” left). As with cardiovascular disease, there are no specific guidelines for monitoring asymptomatic patients for thrombosis or for duration of anticoagulation in patients with GPA. It is recommended that patients be evaluated for active GPA or relapse in the setting of acute thrombosis whether or not symptoms of active GPA are present.
Granulomatosis with polyangiitis (GPA), is one of the most common types of small-vessel vasculitis, with an estimated prevalence in the United States of 3 per 100,000 people. It is distinguished from other necrotizing vasculitides by its tendency to affect the upper and lower respiratory system and the kidneys. Despite the success of induction and maintenance treatments with cyclophosphamide (CYC), glucocorticoids, and less toxic immunosuppressive alternative therapies in improving the disease course, significant treatment-related toxicities and frequent disease relapses demand stringent patient-specific monitoring in order to provide early treatment of relapses and prevent or decrease morbidity.
SMALL-VESSEL VASCULITIS MANAGEMENT OVERVIEW
Granulomatosis with polyangiitis (formerly Wegener’s granulomatosis, or WG) is an antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis that often affects the respiratory system and kidneys across a broad spectrum of clinical presentations, from mild through life-threatening disease. Patients with severe disease present with significant multisystem manifestations, which, in addition to the respiratory system and kidneys, may involve the joints, eyes, and other organs.
Managing patients diagnosed with systemic small-vessel vasculitides such as GPA and microscopic polyangiitis (MPA) is an inexact science. The goals of treatment are to increase survival, induce and maintain remission, reduce relapses, and minimize treatment-related toxicity. Inducing and maintaining remission have become realistic goals because of the availability of medications that prolong life. On the other hand, extended periods of treatment associated with prolonged life increase the risk of treatment-related toxicity in patients who are inadequately monitored.
MONITORING CONSIDERATIONS
Achieving treatment goals requires long-term monitoring of both disease activity and treatment-related toxicities, with constant adjustments to meet the needs of the individual patient and address the often rapidly changing disease and treatment course. The monitoring protocol consists of regularly scheduled follow-up office visits, urine sediment analyses at every office visit whether or not the patient has relapse symptoms, laboratory tests at regular intervals as indicated by the patient’s medication plan and disease presentation, additional tests such as lung computed tomography (CT), and patient education regarding new symptoms and the frequency of office visits. A consistent monitoring strategy will help detect a relapse before it can produce more severe morbidity, identify treatment-related complications, and—equally important—identify the achievement of remission. An example of the consequences of inconsistent monitoring is presented in “Relapse in a nonadherent patient.”
Because there is no definitive cure for small-vessel vasculitis, relapse is always a possibility. The early diagnosis and treatment of relapse may prevent or decrease morbidity from disease, but strict monitoring is needed to identify relapse and initiate treatment before morbidity occurs (see “Relapse in a patient with new symptoms”). Repeat induction therapy following a relapse introduces risk of drug toxicity and requires careful monitoring, as does long-term maintenance therapy.
In addition to induction and maintenance therapy, several other situations, including prior therapeutic complications, serum creatinine levels, and risk of cardiovascular disease, require special monitoring attention.

Induction therapy: monitor response

Response to treatment during induction must be monitored to identify whether remission is achieved. Induction monitoring requires complete assessment of organ-system involvement at every visit with tools such as the Birmingham Vasculitis Activity Score (BVAS) and, when appropriate, the BVAS/WG. If new or worsening symptoms develop during induction therapy, then the patient needs assessment for continued disease activity as well as treatment complications such as infections related to immunosuppressive therapy.
During induction therapy with daily oral CYC, monitoring should include weekly complete blood cell counts to ensure early identification of leukopenia and other cytopenias. The risk of morbidities increases with the cumulative dose, so a stable blood count for 2 months does not obviate the risk of leukopenia. If persistent hematuria is present without cellular casts, cystoscopy is indicated to look for signs of hemorrhagic cystitis. Prophylaxis against Pneumocystis jirovecii is recommended in all patients who receive immunosuppressive therapy. Finally, bone density measurements should be done at baseline.
Maintenance therapy: frequency can be extended
Monitoring during maintenance therapy is similar to induction monitoring; however, when the dosage of methotrexate or azathioprine is stabilized, the frequency of some tests can be extended to monthly rather than weekly. For example, a complete blood cell count, comprehensive metabolic panel, sedimentation rate, C-reactive protein measurement, and urinalysis should be performed monthly. Follow-up visits should include urine sediment analyses and monitoring for cardiovascular disease risk factors. Medication monitoring should include cystoscopy for persistent hematuria without cellular casts, bone density measurements, and ophthalmologic examinations as frequently as indicated for each individual’s needs. P jirovecii prophylaxis should continue as long as the patient receives immunosuppressive medication.

Therapy-related complications
Bladder complications. In a retrospective analysis of 145 patients with GPA treated with CYC and followed for 0.5 to 27 years (median 8.5 years), nonglomerular hematuria developed in 50% of the patients and bladder carcinoma in 5%.2 The cumulative CYC dose (19 to 251 g) in this group was much higher than what is currently used. Cytologic examination of the urine showed 43% sensitivity for dysplasia (specificity 100%) and 29% sensitivity for atypia (specificity 89%). In contrast, in a retrospective outcomes analysis involving newly diagnosed patients with GPA treated with CYC or methotrexate, 82 patients followed for up to 12 years had no incidents of cystitis or bladder cancer.3 Patients in this study were treated with CYC for only 3 to 6 months and therefore received a lower cumulative dose.
To prevent cystitis during treatment with CYC, the patient should be well hydrated, especially in the morning when CYC should be taken. The bladder should be emptied frequently. The addition of mesna when administering intravenous CYC decreases the risk of cystitis. Serial cystoscopy and urine cytology should be used only in patients with nonglomerular hematuria.
Infertility. Preservation of ovarian function is a concern with CYC therapy in women of childbearing age. The cumulative dose threshold for gonadal failure is unknown, because data from cancer studies4 demonstrating gonadal failure involve higher cumulative CYC doses than are typical for vasculitis treatment. It is also unknown whether duration of amenorrhea predicts the recovery of menses or fertility. The primary option for preservation of ovarian function is the use of gonadotropin-releasing hormone agonists. Oral contraceptives also may be used, but the best prevention is to avoid CYC in these patients if possible.
Osteoporosis. At glucocorticoid dosages of 5 mg/day or greater, bone mineral density begins a rapid decline within the first 3 months and peaks at 6 months.5 The American College of Rheumatology has provided recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis.5 Table 2 presents recommendations for postmenopausal women and men aged 50 years and older who will use glucocorticoids for 3 months or more.5 Recommendations are also available for premenopausal women and men younger than 50 years of age who have a history of fragility fracture.
Leukopenia. Leukopenia should be avoided during CYC treatment. The target white blood cell count should be within the normal range. During treatment with daily oral CYC, the patient should be monitored with a weekly complete blood cell count and medication should be adjusted to maintain the target white blood cell count.
Upon completion of induction therapy, after 3 to 6 months, the patient is switched to maintenance therapy with an alternative immunosuppressive agent such as azathioprine or methotrexate, depending on the serum creatinine concentration and other factors. This transition, characterized by full-dose immunosuppressive therapy when the bone marrow has been previously suppressed by CYC treatment, may induce pancytopenia. Monitoring with weekly complete blood counts for at least 4 weeks after initiating maintenance therapy can help ensure stability during the transition period.

Monitor serum creatinine and adjust dosages
The serum creatinine concentration may increase as CYC treatment progresses; in some cases, the serum creatinine concentration increases before a response to treatment is seen. The CYC dosages should be adjusted as necessary in response to serum creatinine changes. Careful monitoring of serum creatinine is necessary during methotrexate therapy, as methotrexate treatment in the setting of renal insufficiency increases the risk of bone marrow suppression.
Cardiovascular disease in GPA and MPA
Premature atherosclerosis has been well described in patients with GPA.6 Within 5 years of diagnosis of GPA or MPA, a cardiovascular event will occur in 14% of patients.7 In the absence of specific guidelines for prevention of cardiovascular disease in patients with vasculitis, it is essential to monitor patients and treat modifiable traditional risk factors aggressively, especially in younger patients. Suppiah et al found that independent determinants of cardiovascular outcome included older age, diastolic hypertension, and positive proteinase-3–ANCA status in patients without prior cardiovascular disease.7
In the Wegener’s Clinical Occurrence of Thrombosis (WeCLOT) study, Merkel et al showed an increased incidence of thrombosis in patients with active GPA8 (see “Relapse presenting as thrombosis,” left). As with cardiovascular disease, there are no specific guidelines for monitoring asymptomatic patients for thrombosis or for duration of anticoagulation in patients with GPA. It is recommended that patients be evaluated for active GPA or relapse in the setting of acute thrombosis whether or not symptoms of active GPA are present.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Talar-Williams C, Hijazi YM, Walther MM, et al. Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener granulomatosis. Ann Intern Med 1996; 124:477–484.
- Villa-Forte A, Clark TM, Gomes M, et al. Substitution of methotrexate for cyclophosphamide in Wegener granulomatosis: a 12-year single-practice experience. Medicine 2007; 86:269–277.
- Harel S, Fermé C, Poirot C. Management of fertility in patients treated for Hodgkin’s lymphoma [published online ahead of print August 9, 2011]. Haematologica 2011; 96:1692–1699. doi: 10.3324/haematol.2011.045856
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis [published online ahead of print July 26, 2010]. Arthritis Care Res (Hoboken) 2010; 62:1515–1526. doi: 10.1002/acr.20295
- Faurschou M, Mellemkjaer L, Sorensen IJ, Svalgaard Thomsen B, Dreyer L, Baslund B. Increased morbidity from ischemic heart disease in patients with Wegener’s granulomatosis. Arthritis Rheum 2009; 60:1187–1192.
- Suppiah R, Judge A, Batra R, et al. A model to predict cardiovascular events in patients with newly diagnosed Wegener’s granulomatosis and microscopic polyangiitis. Arthritis Care Res (Hoboken) 2011; 63:588–596.
- Merkel PA, Lo GH, Holbrook JT, et al; for Wegener’s Granulomatosis Etanercept Trial Research Group. Brief communication: high incidence of venous thrombotic events among patients with Wegener granulomatosis: the Wegener’s Clinical Occurrence of Thrombosis (WeCLOT) study. Ann Intern Med 2005; 142:620–626.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Talar-Williams C, Hijazi YM, Walther MM, et al. Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener granulomatosis. Ann Intern Med 1996; 124:477–484.
- Villa-Forte A, Clark TM, Gomes M, et al. Substitution of methotrexate for cyclophosphamide in Wegener granulomatosis: a 12-year single-practice experience. Medicine 2007; 86:269–277.
- Harel S, Fermé C, Poirot C. Management of fertility in patients treated for Hodgkin’s lymphoma [published online ahead of print August 9, 2011]. Haematologica 2011; 96:1692–1699. doi: 10.3324/haematol.2011.045856
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis [published online ahead of print July 26, 2010]. Arthritis Care Res (Hoboken) 2010; 62:1515–1526. doi: 10.1002/acr.20295
- Faurschou M, Mellemkjaer L, Sorensen IJ, Svalgaard Thomsen B, Dreyer L, Baslund B. Increased morbidity from ischemic heart disease in patients with Wegener’s granulomatosis. Arthritis Rheum 2009; 60:1187–1192.
- Suppiah R, Judge A, Batra R, et al. A model to predict cardiovascular events in patients with newly diagnosed Wegener’s granulomatosis and microscopic polyangiitis. Arthritis Care Res (Hoboken) 2011; 63:588–596.
- Merkel PA, Lo GH, Holbrook JT, et al; for Wegener’s Granulomatosis Etanercept Trial Research Group. Brief communication: high incidence of venous thrombotic events among patients with Wegener granulomatosis: the Wegener’s Clinical Occurrence of Thrombosis (WeCLOT) study. Ann Intern Med 2005; 142:620–626.