User login
Septic arthritis is a common orthopedic emergency. The most common causative organism is Staphylococcus aureus. Mycotic infections, such as those involving Candida organisms, are much less common but just as debilitating. Delayed diagnosis of septic arthritis caused by Candida infection may result in increased morbidity, making treatment more challenging. Here we report a case of Candida albicans septic arthritis of the ankle and subtalar joint in a patient with diabetes mellitus (DM) and rheumatoid arthritis (RA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
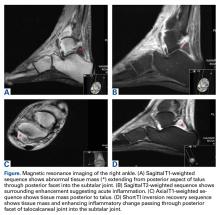
A 52-year-old woman with type 2 DM (requiring subcutaneous insulin analogue therapy) and RA presented to a local emergency department with a 3-day history of right ankle pain after having the subtalar joint injected with steroid by a rheumatologist 4 weeks earlier. For about 2 weeks, there was purulent discharge from the peroneal sheath. The patient’s RA was being treated with prednisolone (maintenance therapy). Physical examination revealed low-grade pyrexia (37.8°C) and difficulty bearing full weight on the ankle. Clinically, the joint was not erythematous, but active and passive movements were painful. Blood tests revealed a C-reactive protein level of 98 mg/dL and a white blood cell (WBC) count of 11.3 × 109/L. Erythrocyte sedimentation rate (ESR) was not checked. The ankle underwent magnetic resonance imaging (Figures A-D).
Mycotic screening of the fluid was positive for C albicans. The patient was referred to the orthopedic team, which performed urgent arthroscopic surgical débridement, biopsy, and washout of the subtalar joint. After surgery, a 6-week course of antifungal therapy with anidulafungin was started, per specialist microbiology advice.
The septic ankle was successfully managed with arthroscopic surgical débridement followed by treatment with anidulafungin. The patient continued to make good progress and was weight-bearing when discharged home from the orthopedic unit.
Discussion
Worldwide, about 1 in 6 people has arthritis, which affects daily lifestyle and reduces quality of life. Degenerative, inflammatory, and septic arthritis each has its management challenges.1
Septic arthritis is an acute infection of the joint, usually of bacterial etiology. It can present as a polyarticular arthropathy (~15% of cases),2,3 but a monoarthropathy of the hip, knee, or ankle is more common.4The Kocher criteria are often applied to cases of suspected septic arthritis of joints, even though they were initially used to distinguish septic arthritis from transient synovitis in pediatric hip joints.5 Kocher and colleagues5 reported 4 key clinical criteria: inability to bear weight, WBC count over 12 × 109/L, ESR over 40 mm/h, and temperature over 38.5°C. When all 4 criteria are met, the predictive value is 99.6%. These criteria are now widely applied to adult joints, and not only the hips.
In septic arthritis, the most common causative pathogen is S aureus.3,6Streptococcus, Neisseria, and Pseudomonas also are common.7 Although much rarer, Candida variants and other mycotic pathogens have been implicated as well.8C albicans is a well-known fungus that colonizes mucosal surfaces. Research indicates increased oral C albicans colonization in rheumatoid patients.9 Although most Candida septic arthritis cases are caused by C albicans, there is no large body of data showing the true incidence of fungal pathogens in septic arthritis.
Our literature search yielded 2 case reports on Candida septic arthritis involving the ankle, but the causative organisms were Candida parapsilosis and Candida glabrata.9,10 Cases of Candida septic arthritis involving the knee or shoulder have also been reported.11-15 Case reports demonstrate that Candida fungal arthritis is extremely rare.9 Etiology reportedly includes direct intra-articular inoculation by surgery or secondary to hematogenous seeding, particularly in immunocompromised patients.10 Risk factors include immunosuppression and joint suppression. DM and RA are common comorbidities in patients with septic arthritis.6,16 The pathophysiology of RA is inflammatory pannus formation of the periarticular surface with subsequent articular cartilage destruction and erosion, as well as progressive deformity and functional debilitation.1Patients with DM are at increased risk for developing fungal and other infections. Factors increasing this risk include disruption of skin-barrier integrity; reduced peripheral oxygen and blood supply, which also disrupts antibiotic delivery; and hyperglycemia-induced reduction in antibody function and disruption of phagocytosis and chemotaxis.17Fungi are eukaryotic, and infections caused by these organisms are difficult to treat.18 As fungal infections are more prevalent among immunosuppressed patients, they often result in prolonged treatment without guarantee of eradication, as spores may persist subclinically.
Literature on C albicans septic arthritis is lacking in general but especially in rheumatoid patients. Delayed diagnosis and suboptimal treatment may result in fungal osteomyelitis. There is little evidence on treating this rare fungal complication, and outcomes historically have been poor.19In an animal model, Marijnissen and colleagues20 found that C albicans infection can increase destruction in an arthritic joint by cytokine environment modification. The result was advanced destruction of the joint and debilitation. For disease management, the authors considered these essential: early diagnosis, prompt treatment, and, as indicated, surgical débridement.
Treatment of Candida septic arthritis largely involves use of antifungal medication, either with surgical débridement, as in our patient’s case, or without. Which antifungal medication to use should be based on sensitivities, identified from wound aspirate, and microbiology advice about treatment duration. The antibiotic should be a broad-spectrum antifungal cover, in keeping with local antibiotic prescribing guidelines, which can be refined once definitive organism culture and sensitivity results are known. However, early aggressive treatment is essential. Periprosthetic fungal infection is rarely resolved without implant removal.21
Conclusion
This case reflects the complexities of septic arthritis caused by atypical pathogens and highlights the need for clinical vigilance in the setting of comorbidities, such as DM and RA. Failure to consider the diagnosis early on might result in delayed and inadequate treatment, increased joint destruction, and, potentially, osteomyelitis with subsequent increased morbidity. Early diagnosis (based on joint aspirate findings), surgical débridement, and prolonged aggressive treatment with antifungal medication are the mainstays of treatment.
Am J Orthop. 2016;45(7):E478-E480. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Auday BC, Buratovich MA, Marrocco, GF, Moglia P, eds. Magill’s Medical Guide. 7th ed. Ipswich, MA: Salem Press; 2014.
2. Dhaliwal S, LeBel ME. Rapidly progressing polyarticular septic arthritis in a patient with rheumatoid arthritis. Am J Orthop. 2012;41(7):E100-E101.
3. Mateo Soria L, Olivé Marqués A, García Casares E, García Melchor E, Holgado Pérez S, Tena Marsà X. Polyarticular septic arthritis: analysis of 19 cases [in Spanish]. Reumatol Clin. 2009;5(1):18-22.
4. Caksen H, Oztürk MK, Uzüm K, Yüksel S, Ustünbaş HB, Per H. Septic arthritis in childhood. Pediatr Int. 2000;42(5):534-540.
5. Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81(12):1662-1670.
6. Madruga Dias J, Costa MM, Pereira da Silva JA, Viana de Queiroz M. Septic arthritis: patients with or without isolated infectious agents have similar characteristics. Infection. 2014;42(2):385-391.
7. Louthrenoo W, Kasitanon N, Wangkaew S, Hongsongkiat S, Sukitawut W, Wichainun R. Streptococcus agalactiae: an emerging cause of septic arthritis. J Clin Rheumatol. 2014;20(2):74-78.
8. Zmierczak H, Goemaere S, Mielants H, Verbruggen G, Veys EM. Candida glabrata arthritis: case report and review of the literature of Candida arthritis. Clin Rheumatol. 1999;18(5):406-409.
9. Bishu S, Su EW, Wilkerson ER, et al. Rheumatoid arthritis patients exhibit impaired Candida albicans–specific Th17 responses. Arthritis Res Ther. 2014;16(1):R50.
10. Legout L, Assal M, Rohner P, Lew D, Bernard L, Hoffmeyer P. Successful treatment of Candida parapsilosis (fluconazole-resistant) osteomyelitis with caspofungin in a HIV patient. Scand J Infect Dis. 2006;38(8):728-730.
11. Sung J, Chun K. Candida parapsilosis arthritis involving the ankle in a diabetes patient. J Korean Soc Radiol. 2011;64:587-591.
12. Marmor L, Peter JB. Candida arthritis of the knee joint. Clin Orthop Relat Res. 1976;(118):133-135.
13. Turgut B, Vural O, Demir M, Kaldir M. Candida arthritis in a patient with chronic myelogenous leukemia (CML) in blastic transformation, unresponsive to fluconazole, but treated effectively with liposomal amphotericin B. Ann Hematol. 2002;81(9):529-531.
14. Christensson B, Ryd L, Dahlberg L, Lohmander S. Candida albicans arthritis in a nonimmunocompromised patient. Complication of placebo intraarticular injections. Acta Orthop Scand. 1993;64(6):695-698.
15. Jeong YM, Cho HY, Lee SW, Hwang YM, Kim YK. Candida septic arthritis with rice body formation: a case report and review of literature. Korean J Radiol. 2013;14(3):465-469.
16. Favero M, Schiavon R, Riato L, Carraro V, Punzi L. Septic arthritis: a 12 years retrospective study in a rheumatological university clinic [in Italian]. Reumatismo. 2008;60(4):260-267.
17. Leslie D, Lansang C, Coppack S, Kennedy L. Diabetes: Clinician’s Desk Reference. Boca Raton, FL: CRC Press; 2012.
18. Silva PM, Gonçalves S, Santos NC. Defensins: antifungal lesions from eukaryotes. Front Microbiol. 2014;5:97.
19. Bariteau JT, Waryasz GR, McDonnell M, Fischer SA, Hayda RA, Born CT. Fungal osteomyelitis and septic arthritis. J Am Acad Orthop Surg. 2014;22(6):390-401.
20. Marijnissen RJ, Koenders MI, van de Veerdonk FL, et al. Exposure to Candida albicans polarizes a T-cell driven arthritis model towards Th17 responses, resulting in a more destructive arthritis. PLoS One. 2012;7(6):e38889.
21. International Consensus on Periprosthetic Joint Infection. Musculoskeletal Infection Society website. http://www.msis-na.org/international-consensus. Published August 1, 2013. Accessed October 16, 2016.
Septic arthritis is a common orthopedic emergency. The most common causative organism is Staphylococcus aureus. Mycotic infections, such as those involving Candida organisms, are much less common but just as debilitating. Delayed diagnosis of septic arthritis caused by Candida infection may result in increased morbidity, making treatment more challenging. Here we report a case of Candida albicans septic arthritis of the ankle and subtalar joint in a patient with diabetes mellitus (DM) and rheumatoid arthritis (RA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 52-year-old woman with type 2 DM (requiring subcutaneous insulin analogue therapy) and RA presented to a local emergency department with a 3-day history of right ankle pain after having the subtalar joint injected with steroid by a rheumatologist 4 weeks earlier. For about 2 weeks, there was purulent discharge from the peroneal sheath. The patient’s RA was being treated with prednisolone (maintenance therapy). Physical examination revealed low-grade pyrexia (37.8°C) and difficulty bearing full weight on the ankle. Clinically, the joint was not erythematous, but active and passive movements were painful. Blood tests revealed a C-reactive protein level of 98 mg/dL and a white blood cell (WBC) count of 11.3 × 109/L. Erythrocyte sedimentation rate (ESR) was not checked. The ankle underwent magnetic resonance imaging (Figures A-D).
Mycotic screening of the fluid was positive for C albicans. The patient was referred to the orthopedic team, which performed urgent arthroscopic surgical débridement, biopsy, and washout of the subtalar joint. After surgery, a 6-week course of antifungal therapy with anidulafungin was started, per specialist microbiology advice.
The septic ankle was successfully managed with arthroscopic surgical débridement followed by treatment with anidulafungin. The patient continued to make good progress and was weight-bearing when discharged home from the orthopedic unit.
Discussion
Worldwide, about 1 in 6 people has arthritis, which affects daily lifestyle and reduces quality of life. Degenerative, inflammatory, and septic arthritis each has its management challenges.1
Septic arthritis is an acute infection of the joint, usually of bacterial etiology. It can present as a polyarticular arthropathy (~15% of cases),2,3 but a monoarthropathy of the hip, knee, or ankle is more common.4The Kocher criteria are often applied to cases of suspected septic arthritis of joints, even though they were initially used to distinguish septic arthritis from transient synovitis in pediatric hip joints.5 Kocher and colleagues5 reported 4 key clinical criteria: inability to bear weight, WBC count over 12 × 109/L, ESR over 40 mm/h, and temperature over 38.5°C. When all 4 criteria are met, the predictive value is 99.6%. These criteria are now widely applied to adult joints, and not only the hips.
In septic arthritis, the most common causative pathogen is S aureus.3,6Streptococcus, Neisseria, and Pseudomonas also are common.7 Although much rarer, Candida variants and other mycotic pathogens have been implicated as well.8C albicans is a well-known fungus that colonizes mucosal surfaces. Research indicates increased oral C albicans colonization in rheumatoid patients.9 Although most Candida septic arthritis cases are caused by C albicans, there is no large body of data showing the true incidence of fungal pathogens in septic arthritis.
Our literature search yielded 2 case reports on Candida septic arthritis involving the ankle, but the causative organisms were Candida parapsilosis and Candida glabrata.9,10 Cases of Candida septic arthritis involving the knee or shoulder have also been reported.11-15 Case reports demonstrate that Candida fungal arthritis is extremely rare.9 Etiology reportedly includes direct intra-articular inoculation by surgery or secondary to hematogenous seeding, particularly in immunocompromised patients.10 Risk factors include immunosuppression and joint suppression. DM and RA are common comorbidities in patients with septic arthritis.6,16 The pathophysiology of RA is inflammatory pannus formation of the periarticular surface with subsequent articular cartilage destruction and erosion, as well as progressive deformity and functional debilitation.1Patients with DM are at increased risk for developing fungal and other infections. Factors increasing this risk include disruption of skin-barrier integrity; reduced peripheral oxygen and blood supply, which also disrupts antibiotic delivery; and hyperglycemia-induced reduction in antibody function and disruption of phagocytosis and chemotaxis.17Fungi are eukaryotic, and infections caused by these organisms are difficult to treat.18 As fungal infections are more prevalent among immunosuppressed patients, they often result in prolonged treatment without guarantee of eradication, as spores may persist subclinically.
Literature on C albicans septic arthritis is lacking in general but especially in rheumatoid patients. Delayed diagnosis and suboptimal treatment may result in fungal osteomyelitis. There is little evidence on treating this rare fungal complication, and outcomes historically have been poor.19In an animal model, Marijnissen and colleagues20 found that C albicans infection can increase destruction in an arthritic joint by cytokine environment modification. The result was advanced destruction of the joint and debilitation. For disease management, the authors considered these essential: early diagnosis, prompt treatment, and, as indicated, surgical débridement.
Treatment of Candida septic arthritis largely involves use of antifungal medication, either with surgical débridement, as in our patient’s case, or without. Which antifungal medication to use should be based on sensitivities, identified from wound aspirate, and microbiology advice about treatment duration. The antibiotic should be a broad-spectrum antifungal cover, in keeping with local antibiotic prescribing guidelines, which can be refined once definitive organism culture and sensitivity results are known. However, early aggressive treatment is essential. Periprosthetic fungal infection is rarely resolved without implant removal.21
Conclusion
This case reflects the complexities of septic arthritis caused by atypical pathogens and highlights the need for clinical vigilance in the setting of comorbidities, such as DM and RA. Failure to consider the diagnosis early on might result in delayed and inadequate treatment, increased joint destruction, and, potentially, osteomyelitis with subsequent increased morbidity. Early diagnosis (based on joint aspirate findings), surgical débridement, and prolonged aggressive treatment with antifungal medication are the mainstays of treatment.
Am J Orthop. 2016;45(7):E478-E480. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Septic arthritis is a common orthopedic emergency. The most common causative organism is Staphylococcus aureus. Mycotic infections, such as those involving Candida organisms, are much less common but just as debilitating. Delayed diagnosis of septic arthritis caused by Candida infection may result in increased morbidity, making treatment more challenging. Here we report a case of Candida albicans septic arthritis of the ankle and subtalar joint in a patient with diabetes mellitus (DM) and rheumatoid arthritis (RA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 52-year-old woman with type 2 DM (requiring subcutaneous insulin analogue therapy) and RA presented to a local emergency department with a 3-day history of right ankle pain after having the subtalar joint injected with steroid by a rheumatologist 4 weeks earlier. For about 2 weeks, there was purulent discharge from the peroneal sheath. The patient’s RA was being treated with prednisolone (maintenance therapy). Physical examination revealed low-grade pyrexia (37.8°C) and difficulty bearing full weight on the ankle. Clinically, the joint was not erythematous, but active and passive movements were painful. Blood tests revealed a C-reactive protein level of 98 mg/dL and a white blood cell (WBC) count of 11.3 × 109/L. Erythrocyte sedimentation rate (ESR) was not checked. The ankle underwent magnetic resonance imaging (Figures A-D).
Mycotic screening of the fluid was positive for C albicans. The patient was referred to the orthopedic team, which performed urgent arthroscopic surgical débridement, biopsy, and washout of the subtalar joint. After surgery, a 6-week course of antifungal therapy with anidulafungin was started, per specialist microbiology advice.
The septic ankle was successfully managed with arthroscopic surgical débridement followed by treatment with anidulafungin. The patient continued to make good progress and was weight-bearing when discharged home from the orthopedic unit.
Discussion
Worldwide, about 1 in 6 people has arthritis, which affects daily lifestyle and reduces quality of life. Degenerative, inflammatory, and septic arthritis each has its management challenges.1
Septic arthritis is an acute infection of the joint, usually of bacterial etiology. It can present as a polyarticular arthropathy (~15% of cases),2,3 but a monoarthropathy of the hip, knee, or ankle is more common.4The Kocher criteria are often applied to cases of suspected septic arthritis of joints, even though they were initially used to distinguish septic arthritis from transient synovitis in pediatric hip joints.5 Kocher and colleagues5 reported 4 key clinical criteria: inability to bear weight, WBC count over 12 × 109/L, ESR over 40 mm/h, and temperature over 38.5°C. When all 4 criteria are met, the predictive value is 99.6%. These criteria are now widely applied to adult joints, and not only the hips.
In septic arthritis, the most common causative pathogen is S aureus.3,6Streptococcus, Neisseria, and Pseudomonas also are common.7 Although much rarer, Candida variants and other mycotic pathogens have been implicated as well.8C albicans is a well-known fungus that colonizes mucosal surfaces. Research indicates increased oral C albicans colonization in rheumatoid patients.9 Although most Candida septic arthritis cases are caused by C albicans, there is no large body of data showing the true incidence of fungal pathogens in septic arthritis.
Our literature search yielded 2 case reports on Candida septic arthritis involving the ankle, but the causative organisms were Candida parapsilosis and Candida glabrata.9,10 Cases of Candida septic arthritis involving the knee or shoulder have also been reported.11-15 Case reports demonstrate that Candida fungal arthritis is extremely rare.9 Etiology reportedly includes direct intra-articular inoculation by surgery or secondary to hematogenous seeding, particularly in immunocompromised patients.10 Risk factors include immunosuppression and joint suppression. DM and RA are common comorbidities in patients with septic arthritis.6,16 The pathophysiology of RA is inflammatory pannus formation of the periarticular surface with subsequent articular cartilage destruction and erosion, as well as progressive deformity and functional debilitation.1Patients with DM are at increased risk for developing fungal and other infections. Factors increasing this risk include disruption of skin-barrier integrity; reduced peripheral oxygen and blood supply, which also disrupts antibiotic delivery; and hyperglycemia-induced reduction in antibody function and disruption of phagocytosis and chemotaxis.17Fungi are eukaryotic, and infections caused by these organisms are difficult to treat.18 As fungal infections are more prevalent among immunosuppressed patients, they often result in prolonged treatment without guarantee of eradication, as spores may persist subclinically.
Literature on C albicans septic arthritis is lacking in general but especially in rheumatoid patients. Delayed diagnosis and suboptimal treatment may result in fungal osteomyelitis. There is little evidence on treating this rare fungal complication, and outcomes historically have been poor.19In an animal model, Marijnissen and colleagues20 found that C albicans infection can increase destruction in an arthritic joint by cytokine environment modification. The result was advanced destruction of the joint and debilitation. For disease management, the authors considered these essential: early diagnosis, prompt treatment, and, as indicated, surgical débridement.
Treatment of Candida septic arthritis largely involves use of antifungal medication, either with surgical débridement, as in our patient’s case, or without. Which antifungal medication to use should be based on sensitivities, identified from wound aspirate, and microbiology advice about treatment duration. The antibiotic should be a broad-spectrum antifungal cover, in keeping with local antibiotic prescribing guidelines, which can be refined once definitive organism culture and sensitivity results are known. However, early aggressive treatment is essential. Periprosthetic fungal infection is rarely resolved without implant removal.21
Conclusion
This case reflects the complexities of septic arthritis caused by atypical pathogens and highlights the need for clinical vigilance in the setting of comorbidities, such as DM and RA. Failure to consider the diagnosis early on might result in delayed and inadequate treatment, increased joint destruction, and, potentially, osteomyelitis with subsequent increased morbidity. Early diagnosis (based on joint aspirate findings), surgical débridement, and prolonged aggressive treatment with antifungal medication are the mainstays of treatment.
Am J Orthop. 2016;45(7):E478-E480. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Auday BC, Buratovich MA, Marrocco, GF, Moglia P, eds. Magill’s Medical Guide. 7th ed. Ipswich, MA: Salem Press; 2014.
2. Dhaliwal S, LeBel ME. Rapidly progressing polyarticular septic arthritis in a patient with rheumatoid arthritis. Am J Orthop. 2012;41(7):E100-E101.
3. Mateo Soria L, Olivé Marqués A, García Casares E, García Melchor E, Holgado Pérez S, Tena Marsà X. Polyarticular septic arthritis: analysis of 19 cases [in Spanish]. Reumatol Clin. 2009;5(1):18-22.
4. Caksen H, Oztürk MK, Uzüm K, Yüksel S, Ustünbaş HB, Per H. Septic arthritis in childhood. Pediatr Int. 2000;42(5):534-540.
5. Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81(12):1662-1670.
6. Madruga Dias J, Costa MM, Pereira da Silva JA, Viana de Queiroz M. Septic arthritis: patients with or without isolated infectious agents have similar characteristics. Infection. 2014;42(2):385-391.
7. Louthrenoo W, Kasitanon N, Wangkaew S, Hongsongkiat S, Sukitawut W, Wichainun R. Streptococcus agalactiae: an emerging cause of septic arthritis. J Clin Rheumatol. 2014;20(2):74-78.
8. Zmierczak H, Goemaere S, Mielants H, Verbruggen G, Veys EM. Candida glabrata arthritis: case report and review of the literature of Candida arthritis. Clin Rheumatol. 1999;18(5):406-409.
9. Bishu S, Su EW, Wilkerson ER, et al. Rheumatoid arthritis patients exhibit impaired Candida albicans–specific Th17 responses. Arthritis Res Ther. 2014;16(1):R50.
10. Legout L, Assal M, Rohner P, Lew D, Bernard L, Hoffmeyer P. Successful treatment of Candida parapsilosis (fluconazole-resistant) osteomyelitis with caspofungin in a HIV patient. Scand J Infect Dis. 2006;38(8):728-730.
11. Sung J, Chun K. Candida parapsilosis arthritis involving the ankle in a diabetes patient. J Korean Soc Radiol. 2011;64:587-591.
12. Marmor L, Peter JB. Candida arthritis of the knee joint. Clin Orthop Relat Res. 1976;(118):133-135.
13. Turgut B, Vural O, Demir M, Kaldir M. Candida arthritis in a patient with chronic myelogenous leukemia (CML) in blastic transformation, unresponsive to fluconazole, but treated effectively with liposomal amphotericin B. Ann Hematol. 2002;81(9):529-531.
14. Christensson B, Ryd L, Dahlberg L, Lohmander S. Candida albicans arthritis in a nonimmunocompromised patient. Complication of placebo intraarticular injections. Acta Orthop Scand. 1993;64(6):695-698.
15. Jeong YM, Cho HY, Lee SW, Hwang YM, Kim YK. Candida septic arthritis with rice body formation: a case report and review of literature. Korean J Radiol. 2013;14(3):465-469.
16. Favero M, Schiavon R, Riato L, Carraro V, Punzi L. Septic arthritis: a 12 years retrospective study in a rheumatological university clinic [in Italian]. Reumatismo. 2008;60(4):260-267.
17. Leslie D, Lansang C, Coppack S, Kennedy L. Diabetes: Clinician’s Desk Reference. Boca Raton, FL: CRC Press; 2012.
18. Silva PM, Gonçalves S, Santos NC. Defensins: antifungal lesions from eukaryotes. Front Microbiol. 2014;5:97.
19. Bariteau JT, Waryasz GR, McDonnell M, Fischer SA, Hayda RA, Born CT. Fungal osteomyelitis and septic arthritis. J Am Acad Orthop Surg. 2014;22(6):390-401.
20. Marijnissen RJ, Koenders MI, van de Veerdonk FL, et al. Exposure to Candida albicans polarizes a T-cell driven arthritis model towards Th17 responses, resulting in a more destructive arthritis. PLoS One. 2012;7(6):e38889.
21. International Consensus on Periprosthetic Joint Infection. Musculoskeletal Infection Society website. http://www.msis-na.org/international-consensus. Published August 1, 2013. Accessed October 16, 2016.
1. Auday BC, Buratovich MA, Marrocco, GF, Moglia P, eds. Magill’s Medical Guide. 7th ed. Ipswich, MA: Salem Press; 2014.
2. Dhaliwal S, LeBel ME. Rapidly progressing polyarticular septic arthritis in a patient with rheumatoid arthritis. Am J Orthop. 2012;41(7):E100-E101.
3. Mateo Soria L, Olivé Marqués A, García Casares E, García Melchor E, Holgado Pérez S, Tena Marsà X. Polyarticular septic arthritis: analysis of 19 cases [in Spanish]. Reumatol Clin. 2009;5(1):18-22.
4. Caksen H, Oztürk MK, Uzüm K, Yüksel S, Ustünbaş HB, Per H. Septic arthritis in childhood. Pediatr Int. 2000;42(5):534-540.
5. Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81(12):1662-1670.
6. Madruga Dias J, Costa MM, Pereira da Silva JA, Viana de Queiroz M. Septic arthritis: patients with or without isolated infectious agents have similar characteristics. Infection. 2014;42(2):385-391.
7. Louthrenoo W, Kasitanon N, Wangkaew S, Hongsongkiat S, Sukitawut W, Wichainun R. Streptococcus agalactiae: an emerging cause of septic arthritis. J Clin Rheumatol. 2014;20(2):74-78.
8. Zmierczak H, Goemaere S, Mielants H, Verbruggen G, Veys EM. Candida glabrata arthritis: case report and review of the literature of Candida arthritis. Clin Rheumatol. 1999;18(5):406-409.
9. Bishu S, Su EW, Wilkerson ER, et al. Rheumatoid arthritis patients exhibit impaired Candida albicans–specific Th17 responses. Arthritis Res Ther. 2014;16(1):R50.
10. Legout L, Assal M, Rohner P, Lew D, Bernard L, Hoffmeyer P. Successful treatment of Candida parapsilosis (fluconazole-resistant) osteomyelitis with caspofungin in a HIV patient. Scand J Infect Dis. 2006;38(8):728-730.
11. Sung J, Chun K. Candida parapsilosis arthritis involving the ankle in a diabetes patient. J Korean Soc Radiol. 2011;64:587-591.
12. Marmor L, Peter JB. Candida arthritis of the knee joint. Clin Orthop Relat Res. 1976;(118):133-135.
13. Turgut B, Vural O, Demir M, Kaldir M. Candida arthritis in a patient with chronic myelogenous leukemia (CML) in blastic transformation, unresponsive to fluconazole, but treated effectively with liposomal amphotericin B. Ann Hematol. 2002;81(9):529-531.
14. Christensson B, Ryd L, Dahlberg L, Lohmander S. Candida albicans arthritis in a nonimmunocompromised patient. Complication of placebo intraarticular injections. Acta Orthop Scand. 1993;64(6):695-698.
15. Jeong YM, Cho HY, Lee SW, Hwang YM, Kim YK. Candida septic arthritis with rice body formation: a case report and review of literature. Korean J Radiol. 2013;14(3):465-469.
16. Favero M, Schiavon R, Riato L, Carraro V, Punzi L. Septic arthritis: a 12 years retrospective study in a rheumatological university clinic [in Italian]. Reumatismo. 2008;60(4):260-267.
17. Leslie D, Lansang C, Coppack S, Kennedy L. Diabetes: Clinician’s Desk Reference. Boca Raton, FL: CRC Press; 2012.
18. Silva PM, Gonçalves S, Santos NC. Defensins: antifungal lesions from eukaryotes. Front Microbiol. 2014;5:97.
19. Bariteau JT, Waryasz GR, McDonnell M, Fischer SA, Hayda RA, Born CT. Fungal osteomyelitis and septic arthritis. J Am Acad Orthop Surg. 2014;22(6):390-401.
20. Marijnissen RJ, Koenders MI, van de Veerdonk FL, et al. Exposure to Candida albicans polarizes a T-cell driven arthritis model towards Th17 responses, resulting in a more destructive arthritis. PLoS One. 2012;7(6):e38889.
21. International Consensus on Periprosthetic Joint Infection. Musculoskeletal Infection Society website. http://www.msis-na.org/international-consensus. Published August 1, 2013. Accessed October 16, 2016.