User login
First described in 2000 in a case series of 15 patients, nephrogenic systemic fibrosis (NSF) is a rare scleroderma-like fibrosing skin condition associated with gadolinium exposure in end stage renal disease (ESRD).1 Patients with advanced chronic kidney disease (CKD) or ESRD are at the highest risk for this condition when exposed to gadolinium-based contrast dyes.
Nephrogenic systemic fibrosis is a devastating and rapidly progressive condition, making its prevention in at-risk populations of utmost importance. In this article, the authors describe a case of a patient who developed NSF in the setting of gadolinium exposure and multiple inflammatory dermatologic conditions. This case illustrates the possible role of a pro-inflammatory state in predisposing to NSF, which may help further elucidate its mechanism of action.
Case Presentation
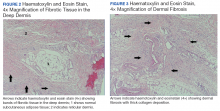
A 61-year-old Hispanic male with a history of IV heroin use with ESRD secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection presented to the West Los Angeles VAMC with fevers and night sweats that had persisted for 2 weeks. His physical examination was notable for diffuse tender palpable purpura and petechiae (including his palms and soles), altered mental status, and diffuse myoclonic jerks, which necessitated endotracheal intubation and mechanical ventilation for airway protection. Blood cultures were positive for methicillin-sensitive Staphylococcus aureus (MSSA). Laboratory results were notable for an elevated sedimentation rate of 53 mm/h (0-10 mm/h), C-reactive protein of 19.8 mg/L (< 0.744 mg/dL), and albumin of 1.2 g/dL (3.2-4.8 g/dL). An extensive rheumatologic workup was unrevealing, and a lumbar puncture was unremarkable. A biopsy of his skin lesions was consistent with leukocytoclastic vasculitis.
The patient’s prior hemodialysis access, a tunneled dialysis catheter in the right subclavian vein, was removed given concern for line infection and replaced with an internal jugular temporary hemodialysis line. Given his altered mental status and myoclonic jerks, the decision was made to pursue a magnetic resonance imaging (MRI) scan of the brain and spine with gadolinium contrast to evaluate for cerebral vasculitis and/or septic emboli to the brain.
The patient received 15 mL of gadoversetamide contrast in accordance with hospital imaging protocol. The MRI revealed only chronic ischemic changes. The patient underwent hemodialysis about 18 hours later. The patient was treated with a 6-week course of IV penicillin G. His altered mental status and myoclonic jerks resolved without intervention, and he was then discharged to an acute rehabilitation unit.
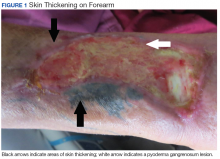
Eight weeks after his initial presentation the patient developed a purulent wound on his right forearm (Figure 1)
The patient was discharged to continue physical and occupational therapy to preserve his functional mobility, as no other treatment options were available.
Discussion
Nephrogenic systemic fibrosis is a poorly understood inflammatory condition that produces diffuse fibrosis of the skin. Typically, the disease begins with progressive skin induration of the extremities. Systemic involvement may occur, leading to fibrosis of skeletal muscle, fascia, and multiple organs. Flexion contractures may develop that limit physical function. Fibrosis can become apparent within days to months after exposure to gadolinium contrast.
Beyond renal insufficiency, it is unclear what other risk factors predispose patients to developing this condition. Only a minority of patients with CKD stages 1 through 4 will develop NSF on exposure to gadolinium contrast. However, the incidence of NSF among patients with CKD stage 5 who are exposed to gadolinium has been estimated to be about 13.4% in a prospective study involving 18 patients.2
In a 2015 meta-analysis by Zhang and colleagues, the only clear risk factor identified for the development of NSF, aside from gadolinium exposure, was severe renal insufficiency with a glomerular filtration rate of < 30 mL/min/1.75m2.3 Due to the limited number of patients identified with this disease, it is difficult to identify other risk factors associated with the development of NSF. Based on in vitro studies, it has been postulated that a pro-inflammatory state predisposes patients to develop NSF.4,5 The proposed mechanism for NSF involves extravasation of gadolinium in the setting of vascular endothelial permeability.5,6 Gadolinium then interacts with tissue macrophages, which induce the release of inflammatory cytokines and the secretion of smooth muscle actin by dermal fibroblasts.6,7
Treatment of NSF has been largely unsuccessful. Multiple modalities of treatment that included topical and oral steroids, immunosuppression, plasmapheresis, and ultraviolent therapy have been attempted, none of which have been proven to consistently limit progression of the disease.8 The most effective intervention is early physical therapy to preserve functionality and prevent contracture formation. For patients who are eligible, early renal transplantation may offer the best chance of improved mobility. In a case series review by Cuffy and colleagues, 5 of 6 patients who underwent renal transplantation after the development of NSF experienced softening of the involved skin, and 2 patients had improved mobility of joints.9
Conclusion
The case presented here illustrates a possible association between a pro-inflammatory state and the development of NSF. This patient had multiple inflammatory conditions, including MSSA bacteremia, leukocytoclastic vasculitis, and pyoderma gangrenosum (the latter 2 conditions were thought to be associated with his underlying chronic hepatitis C infection), which the authors believe predisposed him to endothelial permeability and risk for developing NSF. The risk of developing NSF in at-risk patients with each episode of gadolinium exposure is estimated around 2.4%, or an incidence of 4.3 cases per 1,000 patient-years, leading the American College of Radiologists to recommend against the administration of gadolinium-based contrast except in cases in which benefits clearly outweigh risks.10 However, an MRI with gadolinium contrast can offer high diagnostic yield in cases such as the one presented here in which a diagnosis remains elusive. Moreover, the use of linear gadolinium-based contrast agents such as gadoversetamide, as in this case, has been reported to be associated with higher incidence of NSF.5 Since this case, the West Los Angeles VAMC has switched to gadobutrol contrast for its MRI protocol, which has been purported to be a lower risk agent compared with that of linear gadolinium-based contrast agents (although several cases of NSF have been reported with gadobutrol in the literature).11
Providers weighing the decision to administer gadolinium contrast to patients with ESRD should discuss the risks and benefits thoroughly, especially in patients with preexisting inflammatory conditions. In addition, although it has not been shown to effectively reduce the risk of NSF after administration of gadolinium, hemodialysis is recommended 2 hours after contrast administration for individuals at risk (the study patient received hemodialysis approximately 18 hours after).12 Given the lack of effective treatment options for NSF, prevention is key. A deeper understanding of the pathophysiology of NSF and identification of its risk factors is paramount to the prevention of this devastating disease.
1. Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356(9234):1000-1001.
2. Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis. Arthritis Rheum. 2007;56(10):3433-3441.
3. Zhang B, Liang L, Chen W, Liang C, Zhang S. An updated study to determine association between gadolinium-based contrast agents and nephrogenic systemic fibrosis. PLoS One. 2015;10(6):e0129720.
4. Wermuth PJ, Del Galdo F, Jiménez SA. Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis Rheum. 2009;60(5):1508-1518.
5. Daftari Besheli L, Aran S, Shaqdan K, Kay J, Abujudeh H. Current status of nephrogenic systemic fibrosis. Clin Radiol. 2014;69(7):661-668.
6. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;31(1):F1-F11.
7. Idée JM, Fretellier N, Robic C, Corot C. The role of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: a critical update. Crit Rev Toxicol. 2014;44(10):895-913.
8. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-249.
9. Cuffy MC, Singh M, Formica R, et al. Renal transplantation for nephrogenic systemic fibrosis: a case report and review of the literature. Nephrol Dial Transplant. 2011;26(3):1099-1109.
10. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development of gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267
11. Elmholdt TR, Jørgensen B, Ramsing M, Pedersen M, Olesen AB. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287.
12. Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis. 2011;18(3);188-198.
First described in 2000 in a case series of 15 patients, nephrogenic systemic fibrosis (NSF) is a rare scleroderma-like fibrosing skin condition associated with gadolinium exposure in end stage renal disease (ESRD).1 Patients with advanced chronic kidney disease (CKD) or ESRD are at the highest risk for this condition when exposed to gadolinium-based contrast dyes.
Nephrogenic systemic fibrosis is a devastating and rapidly progressive condition, making its prevention in at-risk populations of utmost importance. In this article, the authors describe a case of a patient who developed NSF in the setting of gadolinium exposure and multiple inflammatory dermatologic conditions. This case illustrates the possible role of a pro-inflammatory state in predisposing to NSF, which may help further elucidate its mechanism of action.
Case Presentation
A 61-year-old Hispanic male with a history of IV heroin use with ESRD secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection presented to the West Los Angeles VAMC with fevers and night sweats that had persisted for 2 weeks. His physical examination was notable for diffuse tender palpable purpura and petechiae (including his palms and soles), altered mental status, and diffuse myoclonic jerks, which necessitated endotracheal intubation and mechanical ventilation for airway protection. Blood cultures were positive for methicillin-sensitive Staphylococcus aureus (MSSA). Laboratory results were notable for an elevated sedimentation rate of 53 mm/h (0-10 mm/h), C-reactive protein of 19.8 mg/L (< 0.744 mg/dL), and albumin of 1.2 g/dL (3.2-4.8 g/dL). An extensive rheumatologic workup was unrevealing, and a lumbar puncture was unremarkable. A biopsy of his skin lesions was consistent with leukocytoclastic vasculitis.
The patient’s prior hemodialysis access, a tunneled dialysis catheter in the right subclavian vein, was removed given concern for line infection and replaced with an internal jugular temporary hemodialysis line. Given his altered mental status and myoclonic jerks, the decision was made to pursue a magnetic resonance imaging (MRI) scan of the brain and spine with gadolinium contrast to evaluate for cerebral vasculitis and/or septic emboli to the brain.
The patient received 15 mL of gadoversetamide contrast in accordance with hospital imaging protocol. The MRI revealed only chronic ischemic changes. The patient underwent hemodialysis about 18 hours later. The patient was treated with a 6-week course of IV penicillin G. His altered mental status and myoclonic jerks resolved without intervention, and he was then discharged to an acute rehabilitation unit.
Eight weeks after his initial presentation the patient developed a purulent wound on his right forearm (Figure 1)
The patient was discharged to continue physical and occupational therapy to preserve his functional mobility, as no other treatment options were available.
Discussion
Nephrogenic systemic fibrosis is a poorly understood inflammatory condition that produces diffuse fibrosis of the skin. Typically, the disease begins with progressive skin induration of the extremities. Systemic involvement may occur, leading to fibrosis of skeletal muscle, fascia, and multiple organs. Flexion contractures may develop that limit physical function. Fibrosis can become apparent within days to months after exposure to gadolinium contrast.
Beyond renal insufficiency, it is unclear what other risk factors predispose patients to developing this condition. Only a minority of patients with CKD stages 1 through 4 will develop NSF on exposure to gadolinium contrast. However, the incidence of NSF among patients with CKD stage 5 who are exposed to gadolinium has been estimated to be about 13.4% in a prospective study involving 18 patients.2
In a 2015 meta-analysis by Zhang and colleagues, the only clear risk factor identified for the development of NSF, aside from gadolinium exposure, was severe renal insufficiency with a glomerular filtration rate of < 30 mL/min/1.75m2.3 Due to the limited number of patients identified with this disease, it is difficult to identify other risk factors associated with the development of NSF. Based on in vitro studies, it has been postulated that a pro-inflammatory state predisposes patients to develop NSF.4,5 The proposed mechanism for NSF involves extravasation of gadolinium in the setting of vascular endothelial permeability.5,6 Gadolinium then interacts with tissue macrophages, which induce the release of inflammatory cytokines and the secretion of smooth muscle actin by dermal fibroblasts.6,7
Treatment of NSF has been largely unsuccessful. Multiple modalities of treatment that included topical and oral steroids, immunosuppression, plasmapheresis, and ultraviolent therapy have been attempted, none of which have been proven to consistently limit progression of the disease.8 The most effective intervention is early physical therapy to preserve functionality and prevent contracture formation. For patients who are eligible, early renal transplantation may offer the best chance of improved mobility. In a case series review by Cuffy and colleagues, 5 of 6 patients who underwent renal transplantation after the development of NSF experienced softening of the involved skin, and 2 patients had improved mobility of joints.9
Conclusion
The case presented here illustrates a possible association between a pro-inflammatory state and the development of NSF. This patient had multiple inflammatory conditions, including MSSA bacteremia, leukocytoclastic vasculitis, and pyoderma gangrenosum (the latter 2 conditions were thought to be associated with his underlying chronic hepatitis C infection), which the authors believe predisposed him to endothelial permeability and risk for developing NSF. The risk of developing NSF in at-risk patients with each episode of gadolinium exposure is estimated around 2.4%, or an incidence of 4.3 cases per 1,000 patient-years, leading the American College of Radiologists to recommend against the administration of gadolinium-based contrast except in cases in which benefits clearly outweigh risks.10 However, an MRI with gadolinium contrast can offer high diagnostic yield in cases such as the one presented here in which a diagnosis remains elusive. Moreover, the use of linear gadolinium-based contrast agents such as gadoversetamide, as in this case, has been reported to be associated with higher incidence of NSF.5 Since this case, the West Los Angeles VAMC has switched to gadobutrol contrast for its MRI protocol, which has been purported to be a lower risk agent compared with that of linear gadolinium-based contrast agents (although several cases of NSF have been reported with gadobutrol in the literature).11
Providers weighing the decision to administer gadolinium contrast to patients with ESRD should discuss the risks and benefits thoroughly, especially in patients with preexisting inflammatory conditions. In addition, although it has not been shown to effectively reduce the risk of NSF after administration of gadolinium, hemodialysis is recommended 2 hours after contrast administration for individuals at risk (the study patient received hemodialysis approximately 18 hours after).12 Given the lack of effective treatment options for NSF, prevention is key. A deeper understanding of the pathophysiology of NSF and identification of its risk factors is paramount to the prevention of this devastating disease.
First described in 2000 in a case series of 15 patients, nephrogenic systemic fibrosis (NSF) is a rare scleroderma-like fibrosing skin condition associated with gadolinium exposure in end stage renal disease (ESRD).1 Patients with advanced chronic kidney disease (CKD) or ESRD are at the highest risk for this condition when exposed to gadolinium-based contrast dyes.
Nephrogenic systemic fibrosis is a devastating and rapidly progressive condition, making its prevention in at-risk populations of utmost importance. In this article, the authors describe a case of a patient who developed NSF in the setting of gadolinium exposure and multiple inflammatory dermatologic conditions. This case illustrates the possible role of a pro-inflammatory state in predisposing to NSF, which may help further elucidate its mechanism of action.
Case Presentation
A 61-year-old Hispanic male with a history of IV heroin use with ESRD secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection presented to the West Los Angeles VAMC with fevers and night sweats that had persisted for 2 weeks. His physical examination was notable for diffuse tender palpable purpura and petechiae (including his palms and soles), altered mental status, and diffuse myoclonic jerks, which necessitated endotracheal intubation and mechanical ventilation for airway protection. Blood cultures were positive for methicillin-sensitive Staphylococcus aureus (MSSA). Laboratory results were notable for an elevated sedimentation rate of 53 mm/h (0-10 mm/h), C-reactive protein of 19.8 mg/L (< 0.744 mg/dL), and albumin of 1.2 g/dL (3.2-4.8 g/dL). An extensive rheumatologic workup was unrevealing, and a lumbar puncture was unremarkable. A biopsy of his skin lesions was consistent with leukocytoclastic vasculitis.
The patient’s prior hemodialysis access, a tunneled dialysis catheter in the right subclavian vein, was removed given concern for line infection and replaced with an internal jugular temporary hemodialysis line. Given his altered mental status and myoclonic jerks, the decision was made to pursue a magnetic resonance imaging (MRI) scan of the brain and spine with gadolinium contrast to evaluate for cerebral vasculitis and/or septic emboli to the brain.
The patient received 15 mL of gadoversetamide contrast in accordance with hospital imaging protocol. The MRI revealed only chronic ischemic changes. The patient underwent hemodialysis about 18 hours later. The patient was treated with a 6-week course of IV penicillin G. His altered mental status and myoclonic jerks resolved without intervention, and he was then discharged to an acute rehabilitation unit.
Eight weeks after his initial presentation the patient developed a purulent wound on his right forearm (Figure 1)
The patient was discharged to continue physical and occupational therapy to preserve his functional mobility, as no other treatment options were available.
Discussion
Nephrogenic systemic fibrosis is a poorly understood inflammatory condition that produces diffuse fibrosis of the skin. Typically, the disease begins with progressive skin induration of the extremities. Systemic involvement may occur, leading to fibrosis of skeletal muscle, fascia, and multiple organs. Flexion contractures may develop that limit physical function. Fibrosis can become apparent within days to months after exposure to gadolinium contrast.
Beyond renal insufficiency, it is unclear what other risk factors predispose patients to developing this condition. Only a minority of patients with CKD stages 1 through 4 will develop NSF on exposure to gadolinium contrast. However, the incidence of NSF among patients with CKD stage 5 who are exposed to gadolinium has been estimated to be about 13.4% in a prospective study involving 18 patients.2
In a 2015 meta-analysis by Zhang and colleagues, the only clear risk factor identified for the development of NSF, aside from gadolinium exposure, was severe renal insufficiency with a glomerular filtration rate of < 30 mL/min/1.75m2.3 Due to the limited number of patients identified with this disease, it is difficult to identify other risk factors associated with the development of NSF. Based on in vitro studies, it has been postulated that a pro-inflammatory state predisposes patients to develop NSF.4,5 The proposed mechanism for NSF involves extravasation of gadolinium in the setting of vascular endothelial permeability.5,6 Gadolinium then interacts with tissue macrophages, which induce the release of inflammatory cytokines and the secretion of smooth muscle actin by dermal fibroblasts.6,7
Treatment of NSF has been largely unsuccessful. Multiple modalities of treatment that included topical and oral steroids, immunosuppression, plasmapheresis, and ultraviolent therapy have been attempted, none of which have been proven to consistently limit progression of the disease.8 The most effective intervention is early physical therapy to preserve functionality and prevent contracture formation. For patients who are eligible, early renal transplantation may offer the best chance of improved mobility. In a case series review by Cuffy and colleagues, 5 of 6 patients who underwent renal transplantation after the development of NSF experienced softening of the involved skin, and 2 patients had improved mobility of joints.9
Conclusion
The case presented here illustrates a possible association between a pro-inflammatory state and the development of NSF. This patient had multiple inflammatory conditions, including MSSA bacteremia, leukocytoclastic vasculitis, and pyoderma gangrenosum (the latter 2 conditions were thought to be associated with his underlying chronic hepatitis C infection), which the authors believe predisposed him to endothelial permeability and risk for developing NSF. The risk of developing NSF in at-risk patients with each episode of gadolinium exposure is estimated around 2.4%, or an incidence of 4.3 cases per 1,000 patient-years, leading the American College of Radiologists to recommend against the administration of gadolinium-based contrast except in cases in which benefits clearly outweigh risks.10 However, an MRI with gadolinium contrast can offer high diagnostic yield in cases such as the one presented here in which a diagnosis remains elusive. Moreover, the use of linear gadolinium-based contrast agents such as gadoversetamide, as in this case, has been reported to be associated with higher incidence of NSF.5 Since this case, the West Los Angeles VAMC has switched to gadobutrol contrast for its MRI protocol, which has been purported to be a lower risk agent compared with that of linear gadolinium-based contrast agents (although several cases of NSF have been reported with gadobutrol in the literature).11
Providers weighing the decision to administer gadolinium contrast to patients with ESRD should discuss the risks and benefits thoroughly, especially in patients with preexisting inflammatory conditions. In addition, although it has not been shown to effectively reduce the risk of NSF after administration of gadolinium, hemodialysis is recommended 2 hours after contrast administration for individuals at risk (the study patient received hemodialysis approximately 18 hours after).12 Given the lack of effective treatment options for NSF, prevention is key. A deeper understanding of the pathophysiology of NSF and identification of its risk factors is paramount to the prevention of this devastating disease.
1. Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356(9234):1000-1001.
2. Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis. Arthritis Rheum. 2007;56(10):3433-3441.
3. Zhang B, Liang L, Chen W, Liang C, Zhang S. An updated study to determine association between gadolinium-based contrast agents and nephrogenic systemic fibrosis. PLoS One. 2015;10(6):e0129720.
4. Wermuth PJ, Del Galdo F, Jiménez SA. Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis Rheum. 2009;60(5):1508-1518.
5. Daftari Besheli L, Aran S, Shaqdan K, Kay J, Abujudeh H. Current status of nephrogenic systemic fibrosis. Clin Radiol. 2014;69(7):661-668.
6. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;31(1):F1-F11.
7. Idée JM, Fretellier N, Robic C, Corot C. The role of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: a critical update. Crit Rev Toxicol. 2014;44(10):895-913.
8. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-249.
9. Cuffy MC, Singh M, Formica R, et al. Renal transplantation for nephrogenic systemic fibrosis: a case report and review of the literature. Nephrol Dial Transplant. 2011;26(3):1099-1109.
10. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development of gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267
11. Elmholdt TR, Jørgensen B, Ramsing M, Pedersen M, Olesen AB. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287.
12. Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis. 2011;18(3);188-198.
1. Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356(9234):1000-1001.
2. Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis. Arthritis Rheum. 2007;56(10):3433-3441.
3. Zhang B, Liang L, Chen W, Liang C, Zhang S. An updated study to determine association between gadolinium-based contrast agents and nephrogenic systemic fibrosis. PLoS One. 2015;10(6):e0129720.
4. Wermuth PJ, Del Galdo F, Jiménez SA. Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis Rheum. 2009;60(5):1508-1518.
5. Daftari Besheli L, Aran S, Shaqdan K, Kay J, Abujudeh H. Current status of nephrogenic systemic fibrosis. Clin Radiol. 2014;69(7):661-668.
6. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;31(1):F1-F11.
7. Idée JM, Fretellier N, Robic C, Corot C. The role of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: a critical update. Crit Rev Toxicol. 2014;44(10):895-913.
8. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-249.
9. Cuffy MC, Singh M, Formica R, et al. Renal transplantation for nephrogenic systemic fibrosis: a case report and review of the literature. Nephrol Dial Transplant. 2011;26(3):1099-1109.
10. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development of gadolinium exposure. Clin J Am Soc Nephrol. 2007;2(2):264-267
11. Elmholdt TR, Jørgensen B, Ramsing M, Pedersen M, Olesen AB. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287.
12. Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis. 2011;18(3);188-198.