User login
More than 5 million older Americans are living with Alzheimer’s disease and related dementias—and this number is estimated to rise to almost 14 million by 2050.1 Dementia is associated with high costs for the patient, family, and society. In 2017, nearly 16.1 million caregivers assisted older adults with dementia, devoting more than 18.2 billion hours per year in care.1 In the United States, the cost of caring for individuals with dementia is expected to reach $277 billion in 2018. Additionally, Medicare and Medicaid are expected to pay 67% of the estimated 2018 cost, and 22% is expected to come out of the pockets of patients and their caregivers.1
Although dementia is often viewed as a memory loss disease, neuropsychiatric symptoms (NPS) are common. NPS includes distressing behaviors, such as aggression and wandering, that increase caregiver burden, escalate the cost of care, and contribute to premature institutionalization. This article examines the evidence for the use of a combination of a cholinesterase inhibitor and memantine vs use of either medication alone for treating NPS of Alzheimer’s disease and other types of dementia.
First, rule out reversible causes of NPS
There are no disease-modifying treatments for dementia1; therefore, clinicians focus on decreasing patients’ suffering and improving their quality of life. Nearly all patients with dementia will develop at least one NPS. These commonly include auditory and visual hallucinations, delusions, depression, anxiety, psychosis, psychomotor agitation, aggression, apathy, repetitive questioning, wandering, socially or sexually inappropriate behaviors, and sleep disturbances.2 The underlying cause of these behaviors may be neurobiological,3 an acute medical condition, unmet needs or a pre-existing personality disorder, or other psychiatric illness.2 Because of this complexity, there is no specific treatment for NPS of dementia. Treatment should begin with an assessment to rule out potentially reversible causes of NPS, such as a urinary tract infection, environmental triggers, unmet needs, or untreated psychiatric illness. For mild to moderate NPS, short-term behavioral interventions, followed by pharmacologic interventions, are used. For moderate to severe NPS, pharmacologic interventions and behavioral interventions are often used simultaneously.
Pharmacologic options for treating NPS
The classes of medications frequently used to treat NPS include antidepressants, antipsychotics, mood stabilizers, and memory-enhancing, dementia-specific agents (cholinesterase inhibitors and the N-methyl-
Antipsychotic medications are typically reserved for treating specific non-cognitive NPS, such as psychosis and/or severe agitated behavior that causes significant distress. Atypical antipsychotics,
The mood stabilizers valproate
Continue to: Evidence for dementia-specific medications
Evidence for dementia-specific medications
An alternative to the above pharmacologic options is treatment with a cholinesterase inhibitor and/or memantine. Among cholinesterase inhibitors
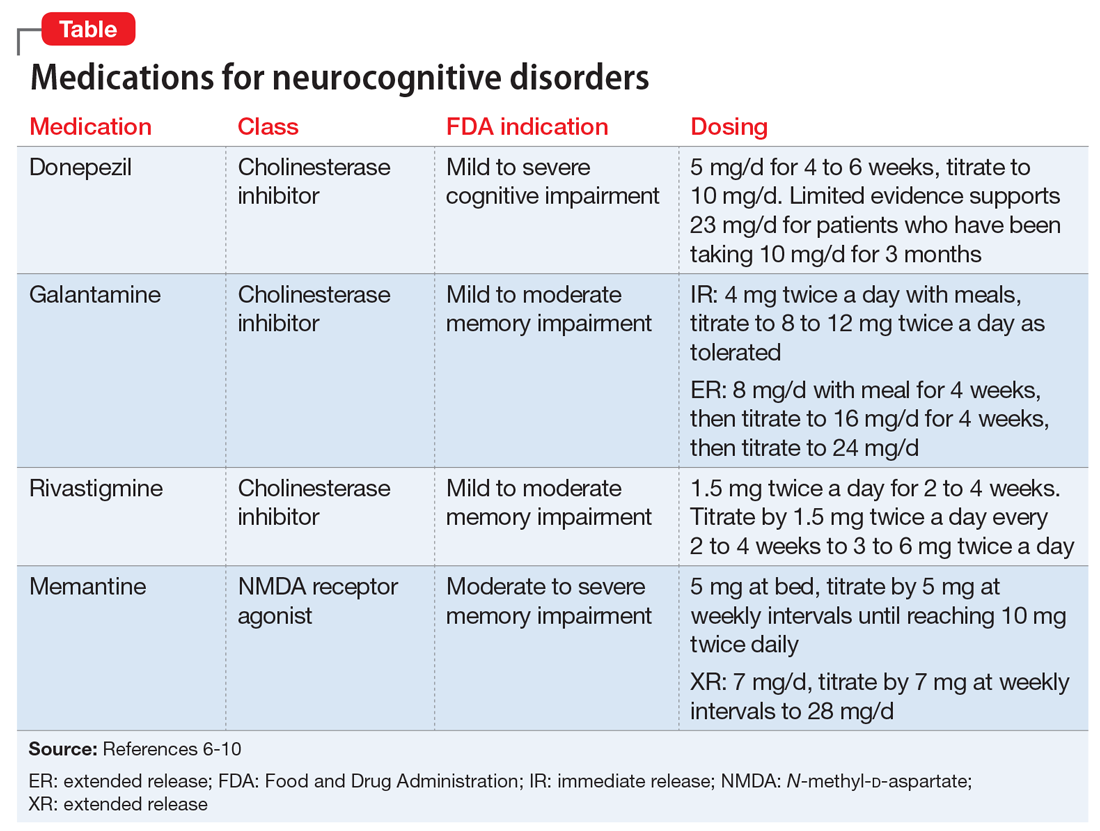
Few randomized controlled trials (RCTs) of cholinesterase inhibitors or memantine have focused on improvement of NPS as a primary outcome measure, but some RCTs have used treatment of NPS as a secondary outcome.4 Most RCT data for using medications for NPS have come from small studies that lasted 17 days to 28 weeks and had design limitations. Most meta-analyses and review articles exclude trials if they do not evaluate NPS as a primary outcome, and most RCTs have only included NPS as a secondary outcome. We hypothesize that this is because NPS is conceptualized as a psychiatric condition, while dementia is codified as a neurologic condition. The reality is that dementia is a neuropsychiatric condition. This artificial divergence complicates both the evaluation and treatment of patients with dementia, who almost always have NPS. Medication trials focused on the neurologic components for primary outcomes contribute to the confusion and difficulty of building an evidence base around the treatment of NPS in Alzheimer’s disease. Patients with severe NPS are seldom included in RCTs.
A cholinesterase inhibitor, memantine, or both?
In the absence of extended RCTs, attention turns to the opinions of panels of experts examining available data.
The 2012 Fourth Canadian Consensus Conference on the Diagnosis and Treatment of Dementia12 recommended a trial of a cholinesterase inhibitor in most patients with Alzheimer’s disease or Alzheimer’s disease combined with another type of dementia. The panel did not find enough evidence to recommend for or against the use of cholinesterase inhibitors and/or memantine for the treatment of NPS as a primary indication. However, they warned of the risks of discontinuing a cholinesterase inhibitor and suggested a slow taper and monitoring, with consideration of restarting the medication if there is notable functional or behavioral decline.
Continue to: In 2015, the European Neurological Society and the European Federation of Neurological Societies...
In 2015, the European Neurological Society and the European Federation of Neurological Societies (now combined into the European Academy of Neurology) found a moderate benefit for using cholinesterase inhibitors to treat problematic behaviors in patients with Alzheimer’s disease.13 They found the evidence weak only when they included consideration of cognitive benefits. For patients with moderate to severe Alzheimer’s disease, the Academy endorsed the combination of cholinesterase inhibitors and memantine.13
The United Kingdom National Institute for Clinical Excellence (NICE) guideline on dementia is updated every 1 to 3 years based on evolving evidence for the treatment of Alzheimer’s disease and related symptoms. In 2016, NICE updated its guideline to recommend the use of a cholinesterase inhibitor for patients with mild to severe Alzheimer’s disease and memantine for those with severe Alzheimer’s disease.14 NICE specifically noted that it could not endorse the use of a cholinesterase inhibitor for severe dementia because that indication is not approved in the United Kingdom, even though there is evidence for this use. The NICE guidelines recommend use of cholinesterase inhibitors for the non-cognitive and/or behavioral symptoms of Alzheimer’s disease, vascular dementia, or mixed dementia after failure or intolerance of an antipsychotic medication. They recommend memantine if there is a failure to respond or intolerance of a cholinesterase inhibitor. The NICE guideline did not address concomitant use of a cholinesterase inhibitor with memantine.
The 2017 guideline published by the British Association for Psychopharmacology states that combination therapy (a cholinesterase inhibitor plus memantine) “may” be beneficial. The group noted that while studies were well-designed, sample sizes were small and not based on clinically representative samples.15
Both available evidence and published guidelines suggest that combination treatment for moderate to severe Alzheimer’s disease may slow the worsening of symptoms or prevent the emergence of NPS better than either medication could accomplish alone. Slowing symptom progression could potentially decrease the cost of in-home care and delay institutionalization.
For a patient prescribed combination therapy, the cost of treatment with generics (as of June 2018) could range from approximately $120 per year for donepezil, 10 mg/d, and approximately $180 per year for memantine, 10 mg twice daily, taken by mouth.16 The cost of a once-daily capsule that contains a combination pill of donepezil and memantine is much more because this product is not available generically.
The Donepezil and Memantine in Moderate to Severe Alzheimer’s disease (DOMINO-AD) trial assessed the effect of combination therapy on cognition, activities of daily living, and health-related quality of life, as well as the cost efficacy of the combined treatment.17 In the 52-week study, researchers found that combined donepezil and memantine was not more cost-effective than donepezil alone. However, a post hoc analysis of the DOMINO-AD data combined with the Memantine Clinical Trial Program data found benefits across multiple clinical domains.18
Continue to: Don't overlook nonpharmacologic interventions
Don’t overlook nonpharmacologic interventions
Families caring for a loved one with Alzheimer’s disease face many decisions. Regardless of when in the course of the disease the diagnosis occurs, its pronouncement is followed by a complex and often emotional negotiation process that includes identifying community resources, making care arrangements, and legal and financial planning. This work may take place concurrently with the exhausting physical care that often comes with the job of a caregiver. As the disease progresses, the physical, emotional, and financial stress on the family increases.
Because they may be pressed for time, have limited staff support, or have limited knowledge of community resources, physicians unfamiliar with the treatment of Alzheimer’s disease may focus on prescribing pharmacologic interventions rather than providing education, resources, and referrals. This approach may lead caregivers to unrealistic expectations of medications in lieu of beneficial environmental and behavioral interventions for NPS. For a family attempting to provide home care for a patient with Alzheimer’s disease, improved behavior may lead to improved quality of life—both for those with dementia and their caregivers. Further, environmental and behavioral interventions could also slow the speed of functional decline and decrease NPS.
Despite the quality of the small studies we examined, without replication in diverse populations that reflect patients seen in everyday clinical practice, it is difficult to know which patients will benefit from combination therapy. The goal of evidence-based medicine is to use evidence gathered from patients who are similar to those that the physician is treating. To evaluate the evidence base around the use of dementia-specific medications and the impact on patients with dementia, additional RCTs, longitudinal data, and secondary outcomes are needed. However, even without this evidence, currently available data should not be ignored. This is part of the evolution of the evidence base.
Bottom Line
For treatment of neuropsychiatric symptoms (NPS) in patients with dementia, evidence supports monotherapy with a cholinesterase inhibitor for patients with mild to moderate dementia, and memantine for those with moderate to severe dementia. The use of these agents results in moderate improvements in NPS. Combination of a cholinesterase inhibitor and memantine increasingly appears to offer benefit.
Related Resources
- Steffens DC, Blazer DG, Thakur ME. The American Psychiatric Publishing textbook of geriatric psychiatry, 5th ed. Arlington, Virginia: American Psychiatric Association; 2015.
- Jacobson SA. Clinical manual of geriatric psychopharmacology, 2nd ed. Washington, DC: American Psychiatric Publishing; 2014.
- Fitzpatrick JL. Cruising through caregiving. Austin, Texas: Greenleaf Book Press; 2016.
- Snow T. Positive approach to care. Techniques and training for families and professionals working with persons with cognitive impairment. www.teepasnow.com.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Donepezil • Aricept
Donepezil/memantine • Namzaric
Fluoxetine • Prozac, Sarafem
Galantamine • Razadyne
Lithium • Eskalith, Lithobid
Memantine • Namenda
Olanzapine • Zyprexa
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Valproate • Depakote
1. Alzheimer’s Association Report. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14(3):367-429.
2. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi:10.1136/bmj.h369.
3. Nowrangi MA, Lyketsos CG, Rosenberg PB. Principles and management of neuropsychiatric symptoms in Alzheimer’s dementia. Alzheimers Res Ther. 2015;7(1):12. doi: 10.1186/s13195-015-0096-3.
4. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia. JAMA. 2005;293(5):596-608.
5. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
6. Aricept [package insert]. Woodcliff Lak, NJ: Eisai Inc.; 2016.
7. Razadyne [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2016.
8. Exelon [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
9. Namenda [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
10. Namenda XR [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
11. Atri A, Hendrix SB, Pejovic
12. Gauthier S, Patterson C, Chertkow H, et al; CCCDTD4 participants. 4th Canadian consensus conference on the diagnosis and treatment of dementia. Can J Neurol Sci. 2012;39(6 suppl 5):S1-S8.
13. Schmidt R, Hofer E, Bouwman FH, et al. EFNS-ENS/EAN guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer’s disease. Eur J Neurol. 2015;22(6):889-898.
14. National Collaborating Centre for Mental Health (UK). Dementia: supporting people with dementia and their carers in health and social care. www.nice.org.uk/guidance/cg42. Updated September 2016. Accessed May 31, 2018.
15. O’Brien JT, Holmes C, Jones M, et al. Clinical practice with anti-dementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2017;31(2):147-168.
16. GoodRx. https://www.goodrx.com. Accessed May 31, 2018.
17. Knapp M, King D, Romeo R, et al. Cost-effectiveness of donepezil and memantine in moderate to severe Alzheimer’s disease (the DOMINO-AD trial). Int J Geriatr Psychiatry. 2017;32(12):1205-1216.
18. Hendrix S, Ellison N, Stanworth S, et al. Post hoc evidence for an additive effect of memantine and donepezil: consistent findings from DOMINO-AD study and Memantine Clinical Trial Program. J Prev Alzheimers Dis. 2015;2(3):165-171.
More than 5 million older Americans are living with Alzheimer’s disease and related dementias—and this number is estimated to rise to almost 14 million by 2050.1 Dementia is associated with high costs for the patient, family, and society. In 2017, nearly 16.1 million caregivers assisted older adults with dementia, devoting more than 18.2 billion hours per year in care.1 In the United States, the cost of caring for individuals with dementia is expected to reach $277 billion in 2018. Additionally, Medicare and Medicaid are expected to pay 67% of the estimated 2018 cost, and 22% is expected to come out of the pockets of patients and their caregivers.1
Although dementia is often viewed as a memory loss disease, neuropsychiatric symptoms (NPS) are common. NPS includes distressing behaviors, such as aggression and wandering, that increase caregiver burden, escalate the cost of care, and contribute to premature institutionalization. This article examines the evidence for the use of a combination of a cholinesterase inhibitor and memantine vs use of either medication alone for treating NPS of Alzheimer’s disease and other types of dementia.
First, rule out reversible causes of NPS
There are no disease-modifying treatments for dementia1; therefore, clinicians focus on decreasing patients’ suffering and improving their quality of life. Nearly all patients with dementia will develop at least one NPS. These commonly include auditory and visual hallucinations, delusions, depression, anxiety, psychosis, psychomotor agitation, aggression, apathy, repetitive questioning, wandering, socially or sexually inappropriate behaviors, and sleep disturbances.2 The underlying cause of these behaviors may be neurobiological,3 an acute medical condition, unmet needs or a pre-existing personality disorder, or other psychiatric illness.2 Because of this complexity, there is no specific treatment for NPS of dementia. Treatment should begin with an assessment to rule out potentially reversible causes of NPS, such as a urinary tract infection, environmental triggers, unmet needs, or untreated psychiatric illness. For mild to moderate NPS, short-term behavioral interventions, followed by pharmacologic interventions, are used. For moderate to severe NPS, pharmacologic interventions and behavioral interventions are often used simultaneously.
Pharmacologic options for treating NPS
The classes of medications frequently used to treat NPS include antidepressants, antipsychotics, mood stabilizers, and memory-enhancing, dementia-specific agents (cholinesterase inhibitors and the N-methyl-
Antipsychotic medications are typically reserved for treating specific non-cognitive NPS, such as psychosis and/or severe agitated behavior that causes significant distress. Atypical antipsychotics,
The mood stabilizers valproate
Continue to: Evidence for dementia-specific medications
Evidence for dementia-specific medications
An alternative to the above pharmacologic options is treatment with a cholinesterase inhibitor and/or memantine. Among cholinesterase inhibitors

Few randomized controlled trials (RCTs) of cholinesterase inhibitors or memantine have focused on improvement of NPS as a primary outcome measure, but some RCTs have used treatment of NPS as a secondary outcome.4 Most RCT data for using medications for NPS have come from small studies that lasted 17 days to 28 weeks and had design limitations. Most meta-analyses and review articles exclude trials if they do not evaluate NPS as a primary outcome, and most RCTs have only included NPS as a secondary outcome. We hypothesize that this is because NPS is conceptualized as a psychiatric condition, while dementia is codified as a neurologic condition. The reality is that dementia is a neuropsychiatric condition. This artificial divergence complicates both the evaluation and treatment of patients with dementia, who almost always have NPS. Medication trials focused on the neurologic components for primary outcomes contribute to the confusion and difficulty of building an evidence base around the treatment of NPS in Alzheimer’s disease. Patients with severe NPS are seldom included in RCTs.
A cholinesterase inhibitor, memantine, or both?
In the absence of extended RCTs, attention turns to the opinions of panels of experts examining available data.
The 2012 Fourth Canadian Consensus Conference on the Diagnosis and Treatment of Dementia12 recommended a trial of a cholinesterase inhibitor in most patients with Alzheimer’s disease or Alzheimer’s disease combined with another type of dementia. The panel did not find enough evidence to recommend for or against the use of cholinesterase inhibitors and/or memantine for the treatment of NPS as a primary indication. However, they warned of the risks of discontinuing a cholinesterase inhibitor and suggested a slow taper and monitoring, with consideration of restarting the medication if there is notable functional or behavioral decline.
Continue to: In 2015, the European Neurological Society and the European Federation of Neurological Societies...
In 2015, the European Neurological Society and the European Federation of Neurological Societies (now combined into the European Academy of Neurology) found a moderate benefit for using cholinesterase inhibitors to treat problematic behaviors in patients with Alzheimer’s disease.13 They found the evidence weak only when they included consideration of cognitive benefits. For patients with moderate to severe Alzheimer’s disease, the Academy endorsed the combination of cholinesterase inhibitors and memantine.13
The United Kingdom National Institute for Clinical Excellence (NICE) guideline on dementia is updated every 1 to 3 years based on evolving evidence for the treatment of Alzheimer’s disease and related symptoms. In 2016, NICE updated its guideline to recommend the use of a cholinesterase inhibitor for patients with mild to severe Alzheimer’s disease and memantine for those with severe Alzheimer’s disease.14 NICE specifically noted that it could not endorse the use of a cholinesterase inhibitor for severe dementia because that indication is not approved in the United Kingdom, even though there is evidence for this use. The NICE guidelines recommend use of cholinesterase inhibitors for the non-cognitive and/or behavioral symptoms of Alzheimer’s disease, vascular dementia, or mixed dementia after failure or intolerance of an antipsychotic medication. They recommend memantine if there is a failure to respond or intolerance of a cholinesterase inhibitor. The NICE guideline did not address concomitant use of a cholinesterase inhibitor with memantine.
The 2017 guideline published by the British Association for Psychopharmacology states that combination therapy (a cholinesterase inhibitor plus memantine) “may” be beneficial. The group noted that while studies were well-designed, sample sizes were small and not based on clinically representative samples.15
Both available evidence and published guidelines suggest that combination treatment for moderate to severe Alzheimer’s disease may slow the worsening of symptoms or prevent the emergence of NPS better than either medication could accomplish alone. Slowing symptom progression could potentially decrease the cost of in-home care and delay institutionalization.
For a patient prescribed combination therapy, the cost of treatment with generics (as of June 2018) could range from approximately $120 per year for donepezil, 10 mg/d, and approximately $180 per year for memantine, 10 mg twice daily, taken by mouth.16 The cost of a once-daily capsule that contains a combination pill of donepezil and memantine is much more because this product is not available generically.
The Donepezil and Memantine in Moderate to Severe Alzheimer’s disease (DOMINO-AD) trial assessed the effect of combination therapy on cognition, activities of daily living, and health-related quality of life, as well as the cost efficacy of the combined treatment.17 In the 52-week study, researchers found that combined donepezil and memantine was not more cost-effective than donepezil alone. However, a post hoc analysis of the DOMINO-AD data combined with the Memantine Clinical Trial Program data found benefits across multiple clinical domains.18
Continue to: Don't overlook nonpharmacologic interventions
Don’t overlook nonpharmacologic interventions
Families caring for a loved one with Alzheimer’s disease face many decisions. Regardless of when in the course of the disease the diagnosis occurs, its pronouncement is followed by a complex and often emotional negotiation process that includes identifying community resources, making care arrangements, and legal and financial planning. This work may take place concurrently with the exhausting physical care that often comes with the job of a caregiver. As the disease progresses, the physical, emotional, and financial stress on the family increases.
Because they may be pressed for time, have limited staff support, or have limited knowledge of community resources, physicians unfamiliar with the treatment of Alzheimer’s disease may focus on prescribing pharmacologic interventions rather than providing education, resources, and referrals. This approach may lead caregivers to unrealistic expectations of medications in lieu of beneficial environmental and behavioral interventions for NPS. For a family attempting to provide home care for a patient with Alzheimer’s disease, improved behavior may lead to improved quality of life—both for those with dementia and their caregivers. Further, environmental and behavioral interventions could also slow the speed of functional decline and decrease NPS.
Despite the quality of the small studies we examined, without replication in diverse populations that reflect patients seen in everyday clinical practice, it is difficult to know which patients will benefit from combination therapy. The goal of evidence-based medicine is to use evidence gathered from patients who are similar to those that the physician is treating. To evaluate the evidence base around the use of dementia-specific medications and the impact on patients with dementia, additional RCTs, longitudinal data, and secondary outcomes are needed. However, even without this evidence, currently available data should not be ignored. This is part of the evolution of the evidence base.
Bottom Line
For treatment of neuropsychiatric symptoms (NPS) in patients with dementia, evidence supports monotherapy with a cholinesterase inhibitor for patients with mild to moderate dementia, and memantine for those with moderate to severe dementia. The use of these agents results in moderate improvements in NPS. Combination of a cholinesterase inhibitor and memantine increasingly appears to offer benefit.
Related Resources
- Steffens DC, Blazer DG, Thakur ME. The American Psychiatric Publishing textbook of geriatric psychiatry, 5th ed. Arlington, Virginia: American Psychiatric Association; 2015.
- Jacobson SA. Clinical manual of geriatric psychopharmacology, 2nd ed. Washington, DC: American Psychiatric Publishing; 2014.
- Fitzpatrick JL. Cruising through caregiving. Austin, Texas: Greenleaf Book Press; 2016.
- Snow T. Positive approach to care. Techniques and training for families and professionals working with persons with cognitive impairment. www.teepasnow.com.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Donepezil • Aricept
Donepezil/memantine • Namzaric
Fluoxetine • Prozac, Sarafem
Galantamine • Razadyne
Lithium • Eskalith, Lithobid
Memantine • Namenda
Olanzapine • Zyprexa
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Valproate • Depakote
More than 5 million older Americans are living with Alzheimer’s disease and related dementias—and this number is estimated to rise to almost 14 million by 2050.1 Dementia is associated with high costs for the patient, family, and society. In 2017, nearly 16.1 million caregivers assisted older adults with dementia, devoting more than 18.2 billion hours per year in care.1 In the United States, the cost of caring for individuals with dementia is expected to reach $277 billion in 2018. Additionally, Medicare and Medicaid are expected to pay 67% of the estimated 2018 cost, and 22% is expected to come out of the pockets of patients and their caregivers.1
Although dementia is often viewed as a memory loss disease, neuropsychiatric symptoms (NPS) are common. NPS includes distressing behaviors, such as aggression and wandering, that increase caregiver burden, escalate the cost of care, and contribute to premature institutionalization. This article examines the evidence for the use of a combination of a cholinesterase inhibitor and memantine vs use of either medication alone for treating NPS of Alzheimer’s disease and other types of dementia.
First, rule out reversible causes of NPS
There are no disease-modifying treatments for dementia1; therefore, clinicians focus on decreasing patients’ suffering and improving their quality of life. Nearly all patients with dementia will develop at least one NPS. These commonly include auditory and visual hallucinations, delusions, depression, anxiety, psychosis, psychomotor agitation, aggression, apathy, repetitive questioning, wandering, socially or sexually inappropriate behaviors, and sleep disturbances.2 The underlying cause of these behaviors may be neurobiological,3 an acute medical condition, unmet needs or a pre-existing personality disorder, or other psychiatric illness.2 Because of this complexity, there is no specific treatment for NPS of dementia. Treatment should begin with an assessment to rule out potentially reversible causes of NPS, such as a urinary tract infection, environmental triggers, unmet needs, or untreated psychiatric illness. For mild to moderate NPS, short-term behavioral interventions, followed by pharmacologic interventions, are used. For moderate to severe NPS, pharmacologic interventions and behavioral interventions are often used simultaneously.
Pharmacologic options for treating NPS
The classes of medications frequently used to treat NPS include antidepressants, antipsychotics, mood stabilizers, and memory-enhancing, dementia-specific agents (cholinesterase inhibitors and the N-methyl-
Antipsychotic medications are typically reserved for treating specific non-cognitive NPS, such as psychosis and/or severe agitated behavior that causes significant distress. Atypical antipsychotics,
The mood stabilizers valproate
Continue to: Evidence for dementia-specific medications
Evidence for dementia-specific medications
An alternative to the above pharmacologic options is treatment with a cholinesterase inhibitor and/or memantine. Among cholinesterase inhibitors

Few randomized controlled trials (RCTs) of cholinesterase inhibitors or memantine have focused on improvement of NPS as a primary outcome measure, but some RCTs have used treatment of NPS as a secondary outcome.4 Most RCT data for using medications for NPS have come from small studies that lasted 17 days to 28 weeks and had design limitations. Most meta-analyses and review articles exclude trials if they do not evaluate NPS as a primary outcome, and most RCTs have only included NPS as a secondary outcome. We hypothesize that this is because NPS is conceptualized as a psychiatric condition, while dementia is codified as a neurologic condition. The reality is that dementia is a neuropsychiatric condition. This artificial divergence complicates both the evaluation and treatment of patients with dementia, who almost always have NPS. Medication trials focused on the neurologic components for primary outcomes contribute to the confusion and difficulty of building an evidence base around the treatment of NPS in Alzheimer’s disease. Patients with severe NPS are seldom included in RCTs.
A cholinesterase inhibitor, memantine, or both?
In the absence of extended RCTs, attention turns to the opinions of panels of experts examining available data.
The 2012 Fourth Canadian Consensus Conference on the Diagnosis and Treatment of Dementia12 recommended a trial of a cholinesterase inhibitor in most patients with Alzheimer’s disease or Alzheimer’s disease combined with another type of dementia. The panel did not find enough evidence to recommend for or against the use of cholinesterase inhibitors and/or memantine for the treatment of NPS as a primary indication. However, they warned of the risks of discontinuing a cholinesterase inhibitor and suggested a slow taper and monitoring, with consideration of restarting the medication if there is notable functional or behavioral decline.
Continue to: In 2015, the European Neurological Society and the European Federation of Neurological Societies...
In 2015, the European Neurological Society and the European Federation of Neurological Societies (now combined into the European Academy of Neurology) found a moderate benefit for using cholinesterase inhibitors to treat problematic behaviors in patients with Alzheimer’s disease.13 They found the evidence weak only when they included consideration of cognitive benefits. For patients with moderate to severe Alzheimer’s disease, the Academy endorsed the combination of cholinesterase inhibitors and memantine.13
The United Kingdom National Institute for Clinical Excellence (NICE) guideline on dementia is updated every 1 to 3 years based on evolving evidence for the treatment of Alzheimer’s disease and related symptoms. In 2016, NICE updated its guideline to recommend the use of a cholinesterase inhibitor for patients with mild to severe Alzheimer’s disease and memantine for those with severe Alzheimer’s disease.14 NICE specifically noted that it could not endorse the use of a cholinesterase inhibitor for severe dementia because that indication is not approved in the United Kingdom, even though there is evidence for this use. The NICE guidelines recommend use of cholinesterase inhibitors for the non-cognitive and/or behavioral symptoms of Alzheimer’s disease, vascular dementia, or mixed dementia after failure or intolerance of an antipsychotic medication. They recommend memantine if there is a failure to respond or intolerance of a cholinesterase inhibitor. The NICE guideline did not address concomitant use of a cholinesterase inhibitor with memantine.
The 2017 guideline published by the British Association for Psychopharmacology states that combination therapy (a cholinesterase inhibitor plus memantine) “may” be beneficial. The group noted that while studies were well-designed, sample sizes were small and not based on clinically representative samples.15
Both available evidence and published guidelines suggest that combination treatment for moderate to severe Alzheimer’s disease may slow the worsening of symptoms or prevent the emergence of NPS better than either medication could accomplish alone. Slowing symptom progression could potentially decrease the cost of in-home care and delay institutionalization.
For a patient prescribed combination therapy, the cost of treatment with generics (as of June 2018) could range from approximately $120 per year for donepezil, 10 mg/d, and approximately $180 per year for memantine, 10 mg twice daily, taken by mouth.16 The cost of a once-daily capsule that contains a combination pill of donepezil and memantine is much more because this product is not available generically.
The Donepezil and Memantine in Moderate to Severe Alzheimer’s disease (DOMINO-AD) trial assessed the effect of combination therapy on cognition, activities of daily living, and health-related quality of life, as well as the cost efficacy of the combined treatment.17 In the 52-week study, researchers found that combined donepezil and memantine was not more cost-effective than donepezil alone. However, a post hoc analysis of the DOMINO-AD data combined with the Memantine Clinical Trial Program data found benefits across multiple clinical domains.18
Continue to: Don't overlook nonpharmacologic interventions
Don’t overlook nonpharmacologic interventions
Families caring for a loved one with Alzheimer’s disease face many decisions. Regardless of when in the course of the disease the diagnosis occurs, its pronouncement is followed by a complex and often emotional negotiation process that includes identifying community resources, making care arrangements, and legal and financial planning. This work may take place concurrently with the exhausting physical care that often comes with the job of a caregiver. As the disease progresses, the physical, emotional, and financial stress on the family increases.
Because they may be pressed for time, have limited staff support, or have limited knowledge of community resources, physicians unfamiliar with the treatment of Alzheimer’s disease may focus on prescribing pharmacologic interventions rather than providing education, resources, and referrals. This approach may lead caregivers to unrealistic expectations of medications in lieu of beneficial environmental and behavioral interventions for NPS. For a family attempting to provide home care for a patient with Alzheimer’s disease, improved behavior may lead to improved quality of life—both for those with dementia and their caregivers. Further, environmental and behavioral interventions could also slow the speed of functional decline and decrease NPS.
Despite the quality of the small studies we examined, without replication in diverse populations that reflect patients seen in everyday clinical practice, it is difficult to know which patients will benefit from combination therapy. The goal of evidence-based medicine is to use evidence gathered from patients who are similar to those that the physician is treating. To evaluate the evidence base around the use of dementia-specific medications and the impact on patients with dementia, additional RCTs, longitudinal data, and secondary outcomes are needed. However, even without this evidence, currently available data should not be ignored. This is part of the evolution of the evidence base.
Bottom Line
For treatment of neuropsychiatric symptoms (NPS) in patients with dementia, evidence supports monotherapy with a cholinesterase inhibitor for patients with mild to moderate dementia, and memantine for those with moderate to severe dementia. The use of these agents results in moderate improvements in NPS. Combination of a cholinesterase inhibitor and memantine increasingly appears to offer benefit.
Related Resources
- Steffens DC, Blazer DG, Thakur ME. The American Psychiatric Publishing textbook of geriatric psychiatry, 5th ed. Arlington, Virginia: American Psychiatric Association; 2015.
- Jacobson SA. Clinical manual of geriatric psychopharmacology, 2nd ed. Washington, DC: American Psychiatric Publishing; 2014.
- Fitzpatrick JL. Cruising through caregiving. Austin, Texas: Greenleaf Book Press; 2016.
- Snow T. Positive approach to care. Techniques and training for families and professionals working with persons with cognitive impairment. www.teepasnow.com.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Donepezil • Aricept
Donepezil/memantine • Namzaric
Fluoxetine • Prozac, Sarafem
Galantamine • Razadyne
Lithium • Eskalith, Lithobid
Memantine • Namenda
Olanzapine • Zyprexa
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Valproate • Depakote
1. Alzheimer’s Association Report. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14(3):367-429.
2. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi:10.1136/bmj.h369.
3. Nowrangi MA, Lyketsos CG, Rosenberg PB. Principles and management of neuropsychiatric symptoms in Alzheimer’s dementia. Alzheimers Res Ther. 2015;7(1):12. doi: 10.1186/s13195-015-0096-3.
4. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia. JAMA. 2005;293(5):596-608.
5. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
6. Aricept [package insert]. Woodcliff Lak, NJ: Eisai Inc.; 2016.
7. Razadyne [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2016.
8. Exelon [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
9. Namenda [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
10. Namenda XR [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
11. Atri A, Hendrix SB, Pejovic
12. Gauthier S, Patterson C, Chertkow H, et al; CCCDTD4 participants. 4th Canadian consensus conference on the diagnosis and treatment of dementia. Can J Neurol Sci. 2012;39(6 suppl 5):S1-S8.
13. Schmidt R, Hofer E, Bouwman FH, et al. EFNS-ENS/EAN guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer’s disease. Eur J Neurol. 2015;22(6):889-898.
14. National Collaborating Centre for Mental Health (UK). Dementia: supporting people with dementia and their carers in health and social care. www.nice.org.uk/guidance/cg42. Updated September 2016. Accessed May 31, 2018.
15. O’Brien JT, Holmes C, Jones M, et al. Clinical practice with anti-dementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2017;31(2):147-168.
16. GoodRx. https://www.goodrx.com. Accessed May 31, 2018.
17. Knapp M, King D, Romeo R, et al. Cost-effectiveness of donepezil and memantine in moderate to severe Alzheimer’s disease (the DOMINO-AD trial). Int J Geriatr Psychiatry. 2017;32(12):1205-1216.
18. Hendrix S, Ellison N, Stanworth S, et al. Post hoc evidence for an additive effect of memantine and donepezil: consistent findings from DOMINO-AD study and Memantine Clinical Trial Program. J Prev Alzheimers Dis. 2015;2(3):165-171.
1. Alzheimer’s Association Report. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14(3):367-429.
2. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi:10.1136/bmj.h369.
3. Nowrangi MA, Lyketsos CG, Rosenberg PB. Principles and management of neuropsychiatric symptoms in Alzheimer’s dementia. Alzheimers Res Ther. 2015;7(1):12. doi: 10.1186/s13195-015-0096-3.
4. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia. JAMA. 2005;293(5):596-608.
5. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
6. Aricept [package insert]. Woodcliff Lak, NJ: Eisai Inc.; 2016.
7. Razadyne [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2016.
8. Exelon [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
9. Namenda [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
10. Namenda XR [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
11. Atri A, Hendrix SB, Pejovic
12. Gauthier S, Patterson C, Chertkow H, et al; CCCDTD4 participants. 4th Canadian consensus conference on the diagnosis and treatment of dementia. Can J Neurol Sci. 2012;39(6 suppl 5):S1-S8.
13. Schmidt R, Hofer E, Bouwman FH, et al. EFNS-ENS/EAN guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer’s disease. Eur J Neurol. 2015;22(6):889-898.
14. National Collaborating Centre for Mental Health (UK). Dementia: supporting people with dementia and their carers in health and social care. www.nice.org.uk/guidance/cg42. Updated September 2016. Accessed May 31, 2018.
15. O’Brien JT, Holmes C, Jones M, et al. Clinical practice with anti-dementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2017;31(2):147-168.
16. GoodRx. https://www.goodrx.com. Accessed May 31, 2018.
17. Knapp M, King D, Romeo R, et al. Cost-effectiveness of donepezil and memantine in moderate to severe Alzheimer’s disease (the DOMINO-AD trial). Int J Geriatr Psychiatry. 2017;32(12):1205-1216.
18. Hendrix S, Ellison N, Stanworth S, et al. Post hoc evidence for an additive effect of memantine and donepezil: consistent findings from DOMINO-AD study and Memantine Clinical Trial Program. J Prev Alzheimers Dis. 2015;2(3):165-171.
