User login
Chest pain is one of the most common concerns in patients presenting to the emergency department in the United States, accounting for approximately 7.6 million visits annually.1 Given the high mortality rate associated with acute coronary syndromes, prompt evaluation of chest pain is essential.2 Even in mild cases, recognition of newly onset or worsening coronary artery disease (CAD) is crucial to ensure that patients receive optimal medication therapy.
In symptomatic patients with risk factors for CAD, such as advanced age, hypertension, hyperlipidemia, obesity, and diabetes mellitus, myocardial perfusion imaging (MPI) is frequently used as a modality to assess the presence, location, and severity of ischemic or infarcted myocardium.2 MPI requires administration of a radiopharmaceutical before and after the patient undergoes a form of stress.2 This radiopharmaceutical is then detected in the myocardium with a nuclear camera, and images are obtained of the heart to assess myocardial blood flow.2
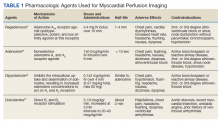
MPI can be performed using exercise-induced stress via a treadmill, or medication-induced stress (Table 1). In both strategies, healthy coronary arteries dilate to provide the myocardium with more blood flow to meet the increasing myocardial oxygen demand during this period of stress. While healthy vessels are able to dilate appropriately, coronary arteries with flow-limiting stenoses are unable to dilate to the same extent in response to stress.2 Because radioactive isotope uptake by the myocardium is directly related to arterial blood flow, MPI is able to demonstrate a mismatch in coronary blood flow between healthy and diseased coronary arteries indicated by differences in radioisotope uptake.2 The presence of such a mismatch, in conjunction with clinical history, potentially suggests the presence of CAD.
Prior to conducting MPI with a medication, certain substances should be avoided. For instance, methylxanthines, such as caffeine, aminophylline, and theophylline, antagonize adenosine receptors and can have major drug interactions with regadenoson, adenosine, and dipyridamole. Therefore, it is advised that these substances be stopped for at least 12 hours before testing.3 In some cases, other medications that can affect coronary blood flow, such as long-acting nitrates, β-blockers, and calcium channel blockers, are recommended to be avoided for 12 to 48 hours in order to obtain the most accurate depiction of underlying coronary disease.4
Because nicotine and nicotine replacement therapy (NRT) may have substantial effects on coronary circulation, a current area of controversy is whether these should be stopped prior to the use of a stress-inducing medication during MPI. To date, no formal drug interaction studies have been conducted between nicotine and regadenoson.5 Similarly, the ADVANCE MPI 2 Trial, which led to the US Food and Drug Administration approval of regadenoson, did not specify restrictions on the use of nicotine prior to stress testing in the protocol.6 However, as this trial was multicenter, investigators admit that individual study sites could have had their own restrictions on the use of nicotine prior to stress testing with regadenoson, but this information was not collected.6 The current review focuses on how the simultaneous use of nicotine or NRT during MPI with pharmacologic agents, such as regadenoson, may affect the accuracy of imaging results and the clinical impact of this interaction.
Nicotine Coronary Artery Effect
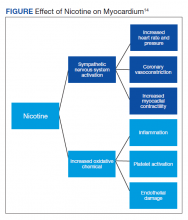
It is well documented that long-term cigarette smoking is a major risk factor for CAD.7 Compared with nonsmokers, cigarette smokers experience 2 times greater risk of morbidity and mortality from ischemic heart disease.7 There are several mechanisms by which nicotine induces damage to the myocardium (Figure). Nicotine has direct effects on both the sympathetic nervous system (SNS) and myocardial endothelium.8 Together, these factors result in reduced coronary blood flow, leading to less oxygen supply to meet an increased oxygen demand, resulting in myocardial ischemia.
Nicotine’s effect on coronary vasomotor tone occurs primarily through noradrenergic stimulation of α and β receptors associated with coronary vasoconstriction or vasodilation, respectively.9,10 These competing influences on coronary blood flow appear to manifest differently based on whether patients are at rest or in a stressed state. A study by Czerin and colleagues demonstrated that in healthy patients with relatively short smoking histories and in a healthy nonsmoker control group, coronary blood flow increased by 25% and 40%, respectively, with nicotine use at rest.9 However, when these patients were stressed with dipyramidole and while smoking during the examination, myocardial blood flow was reduced by 11% in the study group and 14% in the control group.9 This is likely because the patients studied had relatively healthy coronary arteries that were able to maximally dilate when stressed. In this scenario, nicotine’s dilatory effects are offset by nicotine’s α-receptor–mediated vasoconstriction effects.9 Of note, patients in the study group experienced a somewhat diminished increase in coronary blood flow at rest with nicotine use, suggesting that even a short smoking history may damage the myocardial endothelium, rendering it less responsive to nicotine’s vasodilatory effects.9
These principles similarly apply to patients with underlying moderate-to-severe cardiovascular disease (CVD). With nicotine use at rest, patients with significant CAD do not experience as dramatic of an increase in coronary blood flow, which typically decreases or remains the same despite increased myocardial work.10 This may be because patients with moderate-to-severe CAD often have flow-limiting stenoses and damaged endothelium that do not allow vessels to respond as efficiently to increased myocardial demand or to nicotine’s β-receptor–mediated vasodilatory effects.10,11 Moreover, when stressed, diseased coronary arteries are not able to further dilate and nicotine’s α-receptor–mediated vasoconstriction effects dominate.10,11
In a study by Quillen and colleagues of patients with moderate-to-severe CAD, the mean diameter of proximal coronary artery segments decreased by 5%, the distal coronary diameter decreased 8%, and the coronary vascular resistance increased by 21% while smoking at rest.12 The investigators did not analyze how parameters changed when these diseased coronary arteries were stressed using a medication during MPI. However, it can be predicted that coronary arteries would have constricted to a similar or greater degree than observed in Czerin and colleagues’ study, given that the underlying myocardium was diseased and more susceptible to nicotine’s vasoconstriction effects.9 Importantly, these studies have several limitations, most notably that they are older and have small sample sizes. Additionally, while statistically significant differences were found in the degree of changes in coronary circulation with nicotine use at rest and during stress, it is unclear whether this translates to a clinically significant and impactful finding.9-12
Nicotine Replacement Therapy and Stress Testing
Given the association between cigarette smoking and CAD, medical practitioners strongly encourage patients to quit smoking to reduce their risk of adverse cardiovascular outcomes. Various smoking cessation treatments are available for patients. Common, readily accessible forms of therapy include nicotine replacement products (Table 2).
Early studies of NRT in patients with underlying CVD found an increased risk of cardiovascular events, such as myocardial infarction, presumably due to the nicotine content of these products.13,14 However, the concentration of nicotine in NRT is substantially lower than that found in cigarettes and in some formulations, such as transdermal patches, nicotine is delivered over a prolonged period of time.15 For this reason, NRT is thought to be safe in patients with underlying CVD and stable ischemic heart disease. A recent systematic review and meta-analysis found that while NRT may be associated with tachycardia, it did not increase the risk of more serious cardiovascular adverse effects (AEs).16,17
Given the lower nicotine concentration in NRT products, the associated hemodynamic effect of nicotine also is thought to be less pronounced. In a study conducted by Tzivoni and colleagues in patients with CAD using transdermal nicotine patches, no differences in blood pressure, heart rate, ischemia, or arrhythmias were found from baseline to 2 weeks.18 These findings were further confirmed in a small study by Lucini and colleagues, which found that nicotine patches produced slight hemodynamic effects, but to a lesser extent than cigarette smoking.19 For the NRT gum formulation, while a small study found that 4 mg produced coronary vasoconstriction in patients with underlying CAD, a study by Nitenberg and Antony demonstrated that healthy and diseased coronary arteries did not significantly constrict while patients were using nicotine gum both before and after a cold pressor test, suggesting a lesser degree of coronary vasoconstriction than nicotine from cigarette smoking.20,21 Similar findings have been described with the nicotine intranasal spray in a study by Keeley and colleagues, which showed no additional AEs on myocardial demand or vasoconstriction when an intranasal nicotine spray was added to cigarette smoking.22 Importantly, a review of the transdermal and gum formulations found that these less pronounced hemodynamic effects were observed across different doses of NRT; however, further studies are needed to clarify the relationship between NRT dose and cardiovascular effects.23
Overall, NRT does not seem to activate the SNS to the same degree as nicotine obtained via cigarette smoking and likely does not increase the myocardial oxygen demand as much. Additionally, by containing a lower concentration of nicotine, NRT may not impair the myocardium’s ability to supply oxygen to coronary arteries to the same extent as nicotine from cigarette smoking. Therefore, the effects of NRT on MPI using a stress-inducing medication may not be as pronounced. However, due to study limitations, results should be interpreted cautiously.18-23
Conclusions
Because of the close relationship between cigarette smoking and CAD, many patients with underlying CVD are either current smokers or may be using NRT for smoking cessation. Therefore, the question of whether to refrain from nicotine use prior to MPI is clinically relevant. Currently, there is a lack of high-quality studies demonstrating the effects of nicotine and NRT on coronary perfusion. Because of this, the impact of nicotine and NRT use on the accuracy of MPI using stress-inducing medications remains uncertain. Nevertheless, given that nicotine and NRT may largely affect the accuracy of imaging results, several institutions have adopted protocols that prohibit patients from using these drugs on the day of nuclear stress testing.
There are currently no data specifying the number of hours to hold nicotine products prior to cardiac stress testing. It is generally recommended that other medications that affect coronary blood flow be held for 5 half-lives before conducting MPI.4 Following the same guidance for nicotine and NRT may present a reasonable approach to ensure accurate imaging results. Based on the discussed literature, patients should be instructed to refrain from cigarette smoking for at least 5 to 10 hours prior to MPI, given nicotine’s half-life of about 1 to 2 hours.24
The data for NRT are less clear. While use of NRT may not be an absolute contraindication to conducting MPI, it is important to consider that this may affect the accuracy of results. Given this uncertainty, it is likely ideal to hold NRT prior to MPI, based on the specific formulation of NRT and that product's half-life. Further robust studies are needed to analyze the impact of nicotine and NRT on the accuracy of nuclear stress testing using a medication.
1. Rui P, Kang K, Ashman JJ. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. Published 2016. Accessed March 30, 2020. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf
2. Lange RA. Cardiovascular testing. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 10th ed. McGraw Hill; 2017.
3. Mace S. Observation Medicine: Principles and Protocols. Cambridge University Press; 2017.
4. Currie GM. Pharmacology, part 4: nuclear cardiology. J Nucl Med Technol. 2019;47(2):97-110. doi:10.2967/jnmt.118.219675
5. Regadenoson; Package insert. Astellas Pharma US Inc; 2008.
6. Iskandrian AE, Bateman TM, Belardinelli L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14(5):645-658. doi:10.1016/j.nuclcard.2007.06.114
7. Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18(3):109-114. doi:10.4103/HEARTVIEWS.HEARTVIEWS_106_17
8. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26(6):515-523. doi:10.1016/j.tcm.2016.03.001
9. Czernin J, Sun K, Brunken R, Böttcher M, Phelps M, Schelbert H. Effect of acute and long-term smoking on myocardial blood flow and flow reserve. Circulation. 1995;91:2891-2897. doi:10.1161/01.CIR.91.12.2891
10. Winniford MD, Wheelan KR, Kremers MS, et al. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: evidence for adrenergically mediated alterations in coronary artery tone. Circulation. 1986;73(4):662-667. doi:10.1161/01.cir.73.4.662
11. Klein LW, Ambrose J, Pichard A, Holt J, Gorlin R, Teichholz LE. Acute coronary hemodynamic response to cigarette smoking in patients with coronary artery disease. J Am Coll Cardiol. 1984;3(4):879-886. doi:10.1016/s0735-1097(84)80344-7
12. Quillen JE, Rossen JD, Oskarsson HJ, Minor RL Jr, Lopez AG, Winniford MD. Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am Coll Cardiol. 1993;22(3):642-647. doi:10.1016/0735-1097(93)90170-6
13. Dacosta A, Guy JM, Tardy B, et al. Myocardial infarction and nicotine patch: a contributing or causative factor?. Eur Heart J. 1993;14(12):1709-1711. doi:10.1093/eurheartj/14.12.1709
14. Ottervanger JP, Festen JM, de Vries AG, Stricker BH. Acute myocardial infarction while using the nicotine patch. Chest. 1995;107(6):1765-1766. doi:10.1378/chest.107.6.1765
15. Dollerup J, Vestbo J, Murray-Thomas T, et al. Cardiovascular risks in smokers treated with nicotine replacement therapy: a historical cohort study. Clin Epidemiol. 2017;9:231-243. Published 2017 Apr 26. doi:10.2147/CLEP.S127775
16. Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. 2010;8(1):8. Published 2010 Jul 13. doi:10.1186/1617-9625-8-8
17. Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28-41. doi:10.1161/CIRCULATIONAHA.113.003961
18. Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther. 1998;12(3):239-244. doi:10.1023/a:1007757530765
19. Lucini D, Bertocchi F, Malliani A, Pagani M. Autonomic effects of nicotine patch administration in habitual cigarette smokers: a double-blind, placebo-controlled study using spectral analysis of RR interval and systolic arterial pressure variabilities. J Cardiovasc Pharmacol. 1998;31(5):714-720. doi:10.1097/00005344-199805000-00010
20. Kaijser L, Berglund B. Effect of nicotine on coronary blood-flow in man. Clin Physiol. 1985;5(6):541-552. doi:10.1111/j.1475-097x.1985.tb00767.x
21. Nitenberg A, Antony I. Effects of nicotine gum on coronary vasomotor responses during sympathetic stimulation in patients with coronary artery stenosis. J Cardiovasc Pharmacol. 1999;34(5):694-699. doi:10.1097/00005344-199911000-00011
22. Keeley EC, Pirwitz MJ, Landau C, et al. Intranasal nicotine spray does not augment the adverse effects of cigarette smoking on myocardial oxygen demand or coronary arterial dimensions. Am J Med. 1996;101(4):357-363. doi:10.1016/s0002-9343(96)00237-9
23. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29(7):1422-1431. doi:10.1016/s0735-1097(97)00079-x
24. Flowers L. Nicotine replacement therapy. Amer J Psych. 2017;11(6):4-7.
25. Adenosine; Package insert. Astellas Pharma US Inc; 1989.
26. Dipyridamole; Package insert. Boehringer Ingelheim Pharmaceuticals Inc; 2019.
27. Dobutamine; Package insert. Baxter Healthcare Corporation; 2012.
Chest pain is one of the most common concerns in patients presenting to the emergency department in the United States, accounting for approximately 7.6 million visits annually.1 Given the high mortality rate associated with acute coronary syndromes, prompt evaluation of chest pain is essential.2 Even in mild cases, recognition of newly onset or worsening coronary artery disease (CAD) is crucial to ensure that patients receive optimal medication therapy.
In symptomatic patients with risk factors for CAD, such as advanced age, hypertension, hyperlipidemia, obesity, and diabetes mellitus, myocardial perfusion imaging (MPI) is frequently used as a modality to assess the presence, location, and severity of ischemic or infarcted myocardium.2 MPI requires administration of a radiopharmaceutical before and after the patient undergoes a form of stress.2 This radiopharmaceutical is then detected in the myocardium with a nuclear camera, and images are obtained of the heart to assess myocardial blood flow.2
MPI can be performed using exercise-induced stress via a treadmill, or medication-induced stress (Table 1). In both strategies, healthy coronary arteries dilate to provide the myocardium with more blood flow to meet the increasing myocardial oxygen demand during this period of stress. While healthy vessels are able to dilate appropriately, coronary arteries with flow-limiting stenoses are unable to dilate to the same extent in response to stress.2 Because radioactive isotope uptake by the myocardium is directly related to arterial blood flow, MPI is able to demonstrate a mismatch in coronary blood flow between healthy and diseased coronary arteries indicated by differences in radioisotope uptake.2 The presence of such a mismatch, in conjunction with clinical history, potentially suggests the presence of CAD.
Prior to conducting MPI with a medication, certain substances should be avoided. For instance, methylxanthines, such as caffeine, aminophylline, and theophylline, antagonize adenosine receptors and can have major drug interactions with regadenoson, adenosine, and dipyridamole. Therefore, it is advised that these substances be stopped for at least 12 hours before testing.3 In some cases, other medications that can affect coronary blood flow, such as long-acting nitrates, β-blockers, and calcium channel blockers, are recommended to be avoided for 12 to 48 hours in order to obtain the most accurate depiction of underlying coronary disease.4
Because nicotine and nicotine replacement therapy (NRT) may have substantial effects on coronary circulation, a current area of controversy is whether these should be stopped prior to the use of a stress-inducing medication during MPI. To date, no formal drug interaction studies have been conducted between nicotine and regadenoson.5 Similarly, the ADVANCE MPI 2 Trial, which led to the US Food and Drug Administration approval of regadenoson, did not specify restrictions on the use of nicotine prior to stress testing in the protocol.6 However, as this trial was multicenter, investigators admit that individual study sites could have had their own restrictions on the use of nicotine prior to stress testing with regadenoson, but this information was not collected.6 The current review focuses on how the simultaneous use of nicotine or NRT during MPI with pharmacologic agents, such as regadenoson, may affect the accuracy of imaging results and the clinical impact of this interaction.
Nicotine Coronary Artery Effect
It is well documented that long-term cigarette smoking is a major risk factor for CAD.7 Compared with nonsmokers, cigarette smokers experience 2 times greater risk of morbidity and mortality from ischemic heart disease.7 There are several mechanisms by which nicotine induces damage to the myocardium (Figure). Nicotine has direct effects on both the sympathetic nervous system (SNS) and myocardial endothelium.8 Together, these factors result in reduced coronary blood flow, leading to less oxygen supply to meet an increased oxygen demand, resulting in myocardial ischemia.
Nicotine’s effect on coronary vasomotor tone occurs primarily through noradrenergic stimulation of α and β receptors associated with coronary vasoconstriction or vasodilation, respectively.9,10 These competing influences on coronary blood flow appear to manifest differently based on whether patients are at rest or in a stressed state. A study by Czerin and colleagues demonstrated that in healthy patients with relatively short smoking histories and in a healthy nonsmoker control group, coronary blood flow increased by 25% and 40%, respectively, with nicotine use at rest.9 However, when these patients were stressed with dipyramidole and while smoking during the examination, myocardial blood flow was reduced by 11% in the study group and 14% in the control group.9 This is likely because the patients studied had relatively healthy coronary arteries that were able to maximally dilate when stressed. In this scenario, nicotine’s dilatory effects are offset by nicotine’s α-receptor–mediated vasoconstriction effects.9 Of note, patients in the study group experienced a somewhat diminished increase in coronary blood flow at rest with nicotine use, suggesting that even a short smoking history may damage the myocardial endothelium, rendering it less responsive to nicotine’s vasodilatory effects.9
These principles similarly apply to patients with underlying moderate-to-severe cardiovascular disease (CVD). With nicotine use at rest, patients with significant CAD do not experience as dramatic of an increase in coronary blood flow, which typically decreases or remains the same despite increased myocardial work.10 This may be because patients with moderate-to-severe CAD often have flow-limiting stenoses and damaged endothelium that do not allow vessels to respond as efficiently to increased myocardial demand or to nicotine’s β-receptor–mediated vasodilatory effects.10,11 Moreover, when stressed, diseased coronary arteries are not able to further dilate and nicotine’s α-receptor–mediated vasoconstriction effects dominate.10,11
In a study by Quillen and colleagues of patients with moderate-to-severe CAD, the mean diameter of proximal coronary artery segments decreased by 5%, the distal coronary diameter decreased 8%, and the coronary vascular resistance increased by 21% while smoking at rest.12 The investigators did not analyze how parameters changed when these diseased coronary arteries were stressed using a medication during MPI. However, it can be predicted that coronary arteries would have constricted to a similar or greater degree than observed in Czerin and colleagues’ study, given that the underlying myocardium was diseased and more susceptible to nicotine’s vasoconstriction effects.9 Importantly, these studies have several limitations, most notably that they are older and have small sample sizes. Additionally, while statistically significant differences were found in the degree of changes in coronary circulation with nicotine use at rest and during stress, it is unclear whether this translates to a clinically significant and impactful finding.9-12
Nicotine Replacement Therapy and Stress Testing
Given the association between cigarette smoking and CAD, medical practitioners strongly encourage patients to quit smoking to reduce their risk of adverse cardiovascular outcomes. Various smoking cessation treatments are available for patients. Common, readily accessible forms of therapy include nicotine replacement products (Table 2).
Early studies of NRT in patients with underlying CVD found an increased risk of cardiovascular events, such as myocardial infarction, presumably due to the nicotine content of these products.13,14 However, the concentration of nicotine in NRT is substantially lower than that found in cigarettes and in some formulations, such as transdermal patches, nicotine is delivered over a prolonged period of time.15 For this reason, NRT is thought to be safe in patients with underlying CVD and stable ischemic heart disease. A recent systematic review and meta-analysis found that while NRT may be associated with tachycardia, it did not increase the risk of more serious cardiovascular adverse effects (AEs).16,17
Given the lower nicotine concentration in NRT products, the associated hemodynamic effect of nicotine also is thought to be less pronounced. In a study conducted by Tzivoni and colleagues in patients with CAD using transdermal nicotine patches, no differences in blood pressure, heart rate, ischemia, or arrhythmias were found from baseline to 2 weeks.18 These findings were further confirmed in a small study by Lucini and colleagues, which found that nicotine patches produced slight hemodynamic effects, but to a lesser extent than cigarette smoking.19 For the NRT gum formulation, while a small study found that 4 mg produced coronary vasoconstriction in patients with underlying CAD, a study by Nitenberg and Antony demonstrated that healthy and diseased coronary arteries did not significantly constrict while patients were using nicotine gum both before and after a cold pressor test, suggesting a lesser degree of coronary vasoconstriction than nicotine from cigarette smoking.20,21 Similar findings have been described with the nicotine intranasal spray in a study by Keeley and colleagues, which showed no additional AEs on myocardial demand or vasoconstriction when an intranasal nicotine spray was added to cigarette smoking.22 Importantly, a review of the transdermal and gum formulations found that these less pronounced hemodynamic effects were observed across different doses of NRT; however, further studies are needed to clarify the relationship between NRT dose and cardiovascular effects.23
Overall, NRT does not seem to activate the SNS to the same degree as nicotine obtained via cigarette smoking and likely does not increase the myocardial oxygen demand as much. Additionally, by containing a lower concentration of nicotine, NRT may not impair the myocardium’s ability to supply oxygen to coronary arteries to the same extent as nicotine from cigarette smoking. Therefore, the effects of NRT on MPI using a stress-inducing medication may not be as pronounced. However, due to study limitations, results should be interpreted cautiously.18-23
Conclusions
Because of the close relationship between cigarette smoking and CAD, many patients with underlying CVD are either current smokers or may be using NRT for smoking cessation. Therefore, the question of whether to refrain from nicotine use prior to MPI is clinically relevant. Currently, there is a lack of high-quality studies demonstrating the effects of nicotine and NRT on coronary perfusion. Because of this, the impact of nicotine and NRT use on the accuracy of MPI using stress-inducing medications remains uncertain. Nevertheless, given that nicotine and NRT may largely affect the accuracy of imaging results, several institutions have adopted protocols that prohibit patients from using these drugs on the day of nuclear stress testing.
There are currently no data specifying the number of hours to hold nicotine products prior to cardiac stress testing. It is generally recommended that other medications that affect coronary blood flow be held for 5 half-lives before conducting MPI.4 Following the same guidance for nicotine and NRT may present a reasonable approach to ensure accurate imaging results. Based on the discussed literature, patients should be instructed to refrain from cigarette smoking for at least 5 to 10 hours prior to MPI, given nicotine’s half-life of about 1 to 2 hours.24
The data for NRT are less clear. While use of NRT may not be an absolute contraindication to conducting MPI, it is important to consider that this may affect the accuracy of results. Given this uncertainty, it is likely ideal to hold NRT prior to MPI, based on the specific formulation of NRT and that product's half-life. Further robust studies are needed to analyze the impact of nicotine and NRT on the accuracy of nuclear stress testing using a medication.
Chest pain is one of the most common concerns in patients presenting to the emergency department in the United States, accounting for approximately 7.6 million visits annually.1 Given the high mortality rate associated with acute coronary syndromes, prompt evaluation of chest pain is essential.2 Even in mild cases, recognition of newly onset or worsening coronary artery disease (CAD) is crucial to ensure that patients receive optimal medication therapy.
In symptomatic patients with risk factors for CAD, such as advanced age, hypertension, hyperlipidemia, obesity, and diabetes mellitus, myocardial perfusion imaging (MPI) is frequently used as a modality to assess the presence, location, and severity of ischemic or infarcted myocardium.2 MPI requires administration of a radiopharmaceutical before and after the patient undergoes a form of stress.2 This radiopharmaceutical is then detected in the myocardium with a nuclear camera, and images are obtained of the heart to assess myocardial blood flow.2
MPI can be performed using exercise-induced stress via a treadmill, or medication-induced stress (Table 1). In both strategies, healthy coronary arteries dilate to provide the myocardium with more blood flow to meet the increasing myocardial oxygen demand during this period of stress. While healthy vessels are able to dilate appropriately, coronary arteries with flow-limiting stenoses are unable to dilate to the same extent in response to stress.2 Because radioactive isotope uptake by the myocardium is directly related to arterial blood flow, MPI is able to demonstrate a mismatch in coronary blood flow between healthy and diseased coronary arteries indicated by differences in radioisotope uptake.2 The presence of such a mismatch, in conjunction with clinical history, potentially suggests the presence of CAD.
Prior to conducting MPI with a medication, certain substances should be avoided. For instance, methylxanthines, such as caffeine, aminophylline, and theophylline, antagonize adenosine receptors and can have major drug interactions with regadenoson, adenosine, and dipyridamole. Therefore, it is advised that these substances be stopped for at least 12 hours before testing.3 In some cases, other medications that can affect coronary blood flow, such as long-acting nitrates, β-blockers, and calcium channel blockers, are recommended to be avoided for 12 to 48 hours in order to obtain the most accurate depiction of underlying coronary disease.4
Because nicotine and nicotine replacement therapy (NRT) may have substantial effects on coronary circulation, a current area of controversy is whether these should be stopped prior to the use of a stress-inducing medication during MPI. To date, no formal drug interaction studies have been conducted between nicotine and regadenoson.5 Similarly, the ADVANCE MPI 2 Trial, which led to the US Food and Drug Administration approval of regadenoson, did not specify restrictions on the use of nicotine prior to stress testing in the protocol.6 However, as this trial was multicenter, investigators admit that individual study sites could have had their own restrictions on the use of nicotine prior to stress testing with regadenoson, but this information was not collected.6 The current review focuses on how the simultaneous use of nicotine or NRT during MPI with pharmacologic agents, such as regadenoson, may affect the accuracy of imaging results and the clinical impact of this interaction.
Nicotine Coronary Artery Effect
It is well documented that long-term cigarette smoking is a major risk factor for CAD.7 Compared with nonsmokers, cigarette smokers experience 2 times greater risk of morbidity and mortality from ischemic heart disease.7 There are several mechanisms by which nicotine induces damage to the myocardium (Figure). Nicotine has direct effects on both the sympathetic nervous system (SNS) and myocardial endothelium.8 Together, these factors result in reduced coronary blood flow, leading to less oxygen supply to meet an increased oxygen demand, resulting in myocardial ischemia.
Nicotine’s effect on coronary vasomotor tone occurs primarily through noradrenergic stimulation of α and β receptors associated with coronary vasoconstriction or vasodilation, respectively.9,10 These competing influences on coronary blood flow appear to manifest differently based on whether patients are at rest or in a stressed state. A study by Czerin and colleagues demonstrated that in healthy patients with relatively short smoking histories and in a healthy nonsmoker control group, coronary blood flow increased by 25% and 40%, respectively, with nicotine use at rest.9 However, when these patients were stressed with dipyramidole and while smoking during the examination, myocardial blood flow was reduced by 11% in the study group and 14% in the control group.9 This is likely because the patients studied had relatively healthy coronary arteries that were able to maximally dilate when stressed. In this scenario, nicotine’s dilatory effects are offset by nicotine’s α-receptor–mediated vasoconstriction effects.9 Of note, patients in the study group experienced a somewhat diminished increase in coronary blood flow at rest with nicotine use, suggesting that even a short smoking history may damage the myocardial endothelium, rendering it less responsive to nicotine’s vasodilatory effects.9
These principles similarly apply to patients with underlying moderate-to-severe cardiovascular disease (CVD). With nicotine use at rest, patients with significant CAD do not experience as dramatic of an increase in coronary blood flow, which typically decreases or remains the same despite increased myocardial work.10 This may be because patients with moderate-to-severe CAD often have flow-limiting stenoses and damaged endothelium that do not allow vessels to respond as efficiently to increased myocardial demand or to nicotine’s β-receptor–mediated vasodilatory effects.10,11 Moreover, when stressed, diseased coronary arteries are not able to further dilate and nicotine’s α-receptor–mediated vasoconstriction effects dominate.10,11
In a study by Quillen and colleagues of patients with moderate-to-severe CAD, the mean diameter of proximal coronary artery segments decreased by 5%, the distal coronary diameter decreased 8%, and the coronary vascular resistance increased by 21% while smoking at rest.12 The investigators did not analyze how parameters changed when these diseased coronary arteries were stressed using a medication during MPI. However, it can be predicted that coronary arteries would have constricted to a similar or greater degree than observed in Czerin and colleagues’ study, given that the underlying myocardium was diseased and more susceptible to nicotine’s vasoconstriction effects.9 Importantly, these studies have several limitations, most notably that they are older and have small sample sizes. Additionally, while statistically significant differences were found in the degree of changes in coronary circulation with nicotine use at rest and during stress, it is unclear whether this translates to a clinically significant and impactful finding.9-12
Nicotine Replacement Therapy and Stress Testing
Given the association between cigarette smoking and CAD, medical practitioners strongly encourage patients to quit smoking to reduce their risk of adverse cardiovascular outcomes. Various smoking cessation treatments are available for patients. Common, readily accessible forms of therapy include nicotine replacement products (Table 2).
Early studies of NRT in patients with underlying CVD found an increased risk of cardiovascular events, such as myocardial infarction, presumably due to the nicotine content of these products.13,14 However, the concentration of nicotine in NRT is substantially lower than that found in cigarettes and in some formulations, such as transdermal patches, nicotine is delivered over a prolonged period of time.15 For this reason, NRT is thought to be safe in patients with underlying CVD and stable ischemic heart disease. A recent systematic review and meta-analysis found that while NRT may be associated with tachycardia, it did not increase the risk of more serious cardiovascular adverse effects (AEs).16,17
Given the lower nicotine concentration in NRT products, the associated hemodynamic effect of nicotine also is thought to be less pronounced. In a study conducted by Tzivoni and colleagues in patients with CAD using transdermal nicotine patches, no differences in blood pressure, heart rate, ischemia, or arrhythmias were found from baseline to 2 weeks.18 These findings were further confirmed in a small study by Lucini and colleagues, which found that nicotine patches produced slight hemodynamic effects, but to a lesser extent than cigarette smoking.19 For the NRT gum formulation, while a small study found that 4 mg produced coronary vasoconstriction in patients with underlying CAD, a study by Nitenberg and Antony demonstrated that healthy and diseased coronary arteries did not significantly constrict while patients were using nicotine gum both before and after a cold pressor test, suggesting a lesser degree of coronary vasoconstriction than nicotine from cigarette smoking.20,21 Similar findings have been described with the nicotine intranasal spray in a study by Keeley and colleagues, which showed no additional AEs on myocardial demand or vasoconstriction when an intranasal nicotine spray was added to cigarette smoking.22 Importantly, a review of the transdermal and gum formulations found that these less pronounced hemodynamic effects were observed across different doses of NRT; however, further studies are needed to clarify the relationship between NRT dose and cardiovascular effects.23
Overall, NRT does not seem to activate the SNS to the same degree as nicotine obtained via cigarette smoking and likely does not increase the myocardial oxygen demand as much. Additionally, by containing a lower concentration of nicotine, NRT may not impair the myocardium’s ability to supply oxygen to coronary arteries to the same extent as nicotine from cigarette smoking. Therefore, the effects of NRT on MPI using a stress-inducing medication may not be as pronounced. However, due to study limitations, results should be interpreted cautiously.18-23
Conclusions
Because of the close relationship between cigarette smoking and CAD, many patients with underlying CVD are either current smokers or may be using NRT for smoking cessation. Therefore, the question of whether to refrain from nicotine use prior to MPI is clinically relevant. Currently, there is a lack of high-quality studies demonstrating the effects of nicotine and NRT on coronary perfusion. Because of this, the impact of nicotine and NRT use on the accuracy of MPI using stress-inducing medications remains uncertain. Nevertheless, given that nicotine and NRT may largely affect the accuracy of imaging results, several institutions have adopted protocols that prohibit patients from using these drugs on the day of nuclear stress testing.
There are currently no data specifying the number of hours to hold nicotine products prior to cardiac stress testing. It is generally recommended that other medications that affect coronary blood flow be held for 5 half-lives before conducting MPI.4 Following the same guidance for nicotine and NRT may present a reasonable approach to ensure accurate imaging results. Based on the discussed literature, patients should be instructed to refrain from cigarette smoking for at least 5 to 10 hours prior to MPI, given nicotine’s half-life of about 1 to 2 hours.24
The data for NRT are less clear. While use of NRT may not be an absolute contraindication to conducting MPI, it is important to consider that this may affect the accuracy of results. Given this uncertainty, it is likely ideal to hold NRT prior to MPI, based on the specific formulation of NRT and that product's half-life. Further robust studies are needed to analyze the impact of nicotine and NRT on the accuracy of nuclear stress testing using a medication.
1. Rui P, Kang K, Ashman JJ. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. Published 2016. Accessed March 30, 2020. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf
2. Lange RA. Cardiovascular testing. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 10th ed. McGraw Hill; 2017.
3. Mace S. Observation Medicine: Principles and Protocols. Cambridge University Press; 2017.
4. Currie GM. Pharmacology, part 4: nuclear cardiology. J Nucl Med Technol. 2019;47(2):97-110. doi:10.2967/jnmt.118.219675
5. Regadenoson; Package insert. Astellas Pharma US Inc; 2008.
6. Iskandrian AE, Bateman TM, Belardinelli L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14(5):645-658. doi:10.1016/j.nuclcard.2007.06.114
7. Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18(3):109-114. doi:10.4103/HEARTVIEWS.HEARTVIEWS_106_17
8. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26(6):515-523. doi:10.1016/j.tcm.2016.03.001
9. Czernin J, Sun K, Brunken R, Böttcher M, Phelps M, Schelbert H. Effect of acute and long-term smoking on myocardial blood flow and flow reserve. Circulation. 1995;91:2891-2897. doi:10.1161/01.CIR.91.12.2891
10. Winniford MD, Wheelan KR, Kremers MS, et al. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: evidence for adrenergically mediated alterations in coronary artery tone. Circulation. 1986;73(4):662-667. doi:10.1161/01.cir.73.4.662
11. Klein LW, Ambrose J, Pichard A, Holt J, Gorlin R, Teichholz LE. Acute coronary hemodynamic response to cigarette smoking in patients with coronary artery disease. J Am Coll Cardiol. 1984;3(4):879-886. doi:10.1016/s0735-1097(84)80344-7
12. Quillen JE, Rossen JD, Oskarsson HJ, Minor RL Jr, Lopez AG, Winniford MD. Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am Coll Cardiol. 1993;22(3):642-647. doi:10.1016/0735-1097(93)90170-6
13. Dacosta A, Guy JM, Tardy B, et al. Myocardial infarction and nicotine patch: a contributing or causative factor?. Eur Heart J. 1993;14(12):1709-1711. doi:10.1093/eurheartj/14.12.1709
14. Ottervanger JP, Festen JM, de Vries AG, Stricker BH. Acute myocardial infarction while using the nicotine patch. Chest. 1995;107(6):1765-1766. doi:10.1378/chest.107.6.1765
15. Dollerup J, Vestbo J, Murray-Thomas T, et al. Cardiovascular risks in smokers treated with nicotine replacement therapy: a historical cohort study. Clin Epidemiol. 2017;9:231-243. Published 2017 Apr 26. doi:10.2147/CLEP.S127775
16. Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. 2010;8(1):8. Published 2010 Jul 13. doi:10.1186/1617-9625-8-8
17. Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28-41. doi:10.1161/CIRCULATIONAHA.113.003961
18. Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther. 1998;12(3):239-244. doi:10.1023/a:1007757530765
19. Lucini D, Bertocchi F, Malliani A, Pagani M. Autonomic effects of nicotine patch administration in habitual cigarette smokers: a double-blind, placebo-controlled study using spectral analysis of RR interval and systolic arterial pressure variabilities. J Cardiovasc Pharmacol. 1998;31(5):714-720. doi:10.1097/00005344-199805000-00010
20. Kaijser L, Berglund B. Effect of nicotine on coronary blood-flow in man. Clin Physiol. 1985;5(6):541-552. doi:10.1111/j.1475-097x.1985.tb00767.x
21. Nitenberg A, Antony I. Effects of nicotine gum on coronary vasomotor responses during sympathetic stimulation in patients with coronary artery stenosis. J Cardiovasc Pharmacol. 1999;34(5):694-699. doi:10.1097/00005344-199911000-00011
22. Keeley EC, Pirwitz MJ, Landau C, et al. Intranasal nicotine spray does not augment the adverse effects of cigarette smoking on myocardial oxygen demand or coronary arterial dimensions. Am J Med. 1996;101(4):357-363. doi:10.1016/s0002-9343(96)00237-9
23. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29(7):1422-1431. doi:10.1016/s0735-1097(97)00079-x
24. Flowers L. Nicotine replacement therapy. Amer J Psych. 2017;11(6):4-7.
25. Adenosine; Package insert. Astellas Pharma US Inc; 1989.
26. Dipyridamole; Package insert. Boehringer Ingelheim Pharmaceuticals Inc; 2019.
27. Dobutamine; Package insert. Baxter Healthcare Corporation; 2012.
1. Rui P, Kang K, Ashman JJ. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. Published 2016. Accessed March 30, 2020. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf
2. Lange RA. Cardiovascular testing. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 10th ed. McGraw Hill; 2017.
3. Mace S. Observation Medicine: Principles and Protocols. Cambridge University Press; 2017.
4. Currie GM. Pharmacology, part 4: nuclear cardiology. J Nucl Med Technol. 2019;47(2):97-110. doi:10.2967/jnmt.118.219675
5. Regadenoson; Package insert. Astellas Pharma US Inc; 2008.
6. Iskandrian AE, Bateman TM, Belardinelli L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14(5):645-658. doi:10.1016/j.nuclcard.2007.06.114
7. Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18(3):109-114. doi:10.4103/HEARTVIEWS.HEARTVIEWS_106_17
8. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26(6):515-523. doi:10.1016/j.tcm.2016.03.001
9. Czernin J, Sun K, Brunken R, Böttcher M, Phelps M, Schelbert H. Effect of acute and long-term smoking on myocardial blood flow and flow reserve. Circulation. 1995;91:2891-2897. doi:10.1161/01.CIR.91.12.2891
10. Winniford MD, Wheelan KR, Kremers MS, et al. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: evidence for adrenergically mediated alterations in coronary artery tone. Circulation. 1986;73(4):662-667. doi:10.1161/01.cir.73.4.662
11. Klein LW, Ambrose J, Pichard A, Holt J, Gorlin R, Teichholz LE. Acute coronary hemodynamic response to cigarette smoking in patients with coronary artery disease. J Am Coll Cardiol. 1984;3(4):879-886. doi:10.1016/s0735-1097(84)80344-7
12. Quillen JE, Rossen JD, Oskarsson HJ, Minor RL Jr, Lopez AG, Winniford MD. Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am Coll Cardiol. 1993;22(3):642-647. doi:10.1016/0735-1097(93)90170-6
13. Dacosta A, Guy JM, Tardy B, et al. Myocardial infarction and nicotine patch: a contributing or causative factor?. Eur Heart J. 1993;14(12):1709-1711. doi:10.1093/eurheartj/14.12.1709
14. Ottervanger JP, Festen JM, de Vries AG, Stricker BH. Acute myocardial infarction while using the nicotine patch. Chest. 1995;107(6):1765-1766. doi:10.1378/chest.107.6.1765
15. Dollerup J, Vestbo J, Murray-Thomas T, et al. Cardiovascular risks in smokers treated with nicotine replacement therapy: a historical cohort study. Clin Epidemiol. 2017;9:231-243. Published 2017 Apr 26. doi:10.2147/CLEP.S127775
16. Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. 2010;8(1):8. Published 2010 Jul 13. doi:10.1186/1617-9625-8-8
17. Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28-41. doi:10.1161/CIRCULATIONAHA.113.003961
18. Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther. 1998;12(3):239-244. doi:10.1023/a:1007757530765
19. Lucini D, Bertocchi F, Malliani A, Pagani M. Autonomic effects of nicotine patch administration in habitual cigarette smokers: a double-blind, placebo-controlled study using spectral analysis of RR interval and systolic arterial pressure variabilities. J Cardiovasc Pharmacol. 1998;31(5):714-720. doi:10.1097/00005344-199805000-00010
20. Kaijser L, Berglund B. Effect of nicotine on coronary blood-flow in man. Clin Physiol. 1985;5(6):541-552. doi:10.1111/j.1475-097x.1985.tb00767.x
21. Nitenberg A, Antony I. Effects of nicotine gum on coronary vasomotor responses during sympathetic stimulation in patients with coronary artery stenosis. J Cardiovasc Pharmacol. 1999;34(5):694-699. doi:10.1097/00005344-199911000-00011
22. Keeley EC, Pirwitz MJ, Landau C, et al. Intranasal nicotine spray does not augment the adverse effects of cigarette smoking on myocardial oxygen demand or coronary arterial dimensions. Am J Med. 1996;101(4):357-363. doi:10.1016/s0002-9343(96)00237-9
23. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29(7):1422-1431. doi:10.1016/s0735-1097(97)00079-x
24. Flowers L. Nicotine replacement therapy. Amer J Psych. 2017;11(6):4-7.
25. Adenosine; Package insert. Astellas Pharma US Inc; 1989.
26. Dipyridamole; Package insert. Boehringer Ingelheim Pharmaceuticals Inc; 2019.
27. Dobutamine; Package insert. Baxter Healthcare Corporation; 2012.