User login
LAS VEGAS – A technique that involves injecting tiny, closely placed amounts of botulinum toxin A to balance the actions of the muscles around the eyebrows yielded natural-looking outcomes without forehead paralysis, based on data from a 5-year study.
"Doctors have mistakenly adopted maximal forehead paralysis as a desirable treatment endpoint," Dr. Kenneth D. Steinsapir said at the annual meeting of the American Academy of Cosmetic Surgery. "Forehead paralysis as a result of cosmetic botulinum toxin is feared by the public and lampooned by the media."
Over the years, onabotulinumtoxinA treatments have evolved to create maximal frontalis brow lifts while minimizing the risk of eyelid ptosis. As a result, "the forehead is smooth, but the central forehead is also ptotic," said Dr. Steinsapir of the department of ophthalmology at the University of California, Los Angeles. "There can be recruitment lines on the side of the forehead, which can make for an undesirable treatment effect."
In 2006, Dr. Steinsapir first described the microdroplet botulinum toxin forehead lift, a technique he developed for treating eyebrow depressors that leaves the brow elevator untreated. "I hypothesized that very small quantities of botulinum toxic can be injected and effectively trapped between the skin and the underlying orbicularis oculi muscle in the brow, and in the crow’s feet area as well," said Dr. Steinsapir, who also maintains a private cosmetic surgery practice in Beverly Hills, Calif. "This weakens the eyebrow depressors, allowing the frontalis muscle to lift unopposed, lifting the brows. Forehead movement is preserved and unwanted diffusion responsible for eyelid ptosis is prevented."
Between August 2006 and July 2011, Dr. Steinsapir performed 574 consecutive microdroplet botulinum toxin forehead lift treatments on 175 women and 53 men with a mean age of 45 years. A typical treatment involves 10 mcL of injectable saline containing 0.33 U of botulinum toxin A using the product formulation of Botox or Xeomin. About 100 microinjections are needed to complete the pattern, and all patients in the study received 33 units of Botox exclusively.
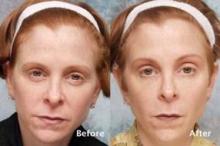
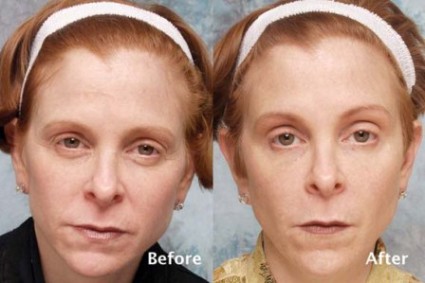
Dr. Steinsapir reported that there were no cases of treatment-induced upper eyelid or eyebrow ptosis or cases of diplopia, "which established that this treatment can be safely performed." Of the 574 patients, 49 returned for follow-up appointments between 10 and 45 days after treatment. Before and after images were used to assess the effect of treatment on the upper eyelid margin reflex distance, the tarsal platform show, and the brow position central to the cornea. Dr. Steinsapir used National Institutes of Health imaging software to perform quantitative image analysis and validated facial scales to assess the brow and forehead before and after treatment.
There was no significant change in the margin reflex distance after treatment in the 49 patients who returned for follow-up, Dr. Steinsapir said. "There was a slight trend to minimal brow elevation, and the tarsal platform show was essentially unchanged after treatment," he said. "It’s my clinical impression that the principal effect of the treatment is the softening of the brow pinch that commonly purses the brow and brings an unintentional negative affect to the face."
Dr. Steinsapir acknowledged that the procedure requires a learning curve "and a need to educate patients regarding the effect of treatment. This is more labor intensive than standard treatment methods." Dr. Steinsapir has developed a detailed training video that is available online on his website, and he said that he is working on a treatment atlas.
The microdroplet botulinum toxin forehead lift "presents the first alternative to standard periocular treatments that cause unwarranted forehead paralysis, brow flare, or muscle activation," Dr. Steinsapir said. "By controlling the depth, volume, and dose of agent, very controlled brow shaping and lifting can be performed to create aesthetic improvement with natural results, including preservation of forehead movement," he noted.
Dr. Steinsapir received a United States patent on the microdroplet method. He said that he hopes to license the technique to a drug company for the development of a Food and Drug Administration–approved indication, so the treatment can be directly marketed to consumers. He had no other relevant financial conflicts to disclose.
Dr. Kenneth D. Steinsapir, American Academy of Cosmetic Surgery, cosmetic botulinum toxin, onabotulinumtoxinA, maximal frontalis brow lifts, eyelid ptosis, ptotic, microdroplet botulinum toxin forehead lift, eyebrow depressors,
LAS VEGAS – A technique that involves injecting tiny, closely placed amounts of botulinum toxin A to balance the actions of the muscles around the eyebrows yielded natural-looking outcomes without forehead paralysis, based on data from a 5-year study.
"Doctors have mistakenly adopted maximal forehead paralysis as a desirable treatment endpoint," Dr. Kenneth D. Steinsapir said at the annual meeting of the American Academy of Cosmetic Surgery. "Forehead paralysis as a result of cosmetic botulinum toxin is feared by the public and lampooned by the media."
Over the years, onabotulinumtoxinA treatments have evolved to create maximal frontalis brow lifts while minimizing the risk of eyelid ptosis. As a result, "the forehead is smooth, but the central forehead is also ptotic," said Dr. Steinsapir of the department of ophthalmology at the University of California, Los Angeles. "There can be recruitment lines on the side of the forehead, which can make for an undesirable treatment effect."
In 2006, Dr. Steinsapir first described the microdroplet botulinum toxin forehead lift, a technique he developed for treating eyebrow depressors that leaves the brow elevator untreated. "I hypothesized that very small quantities of botulinum toxic can be injected and effectively trapped between the skin and the underlying orbicularis oculi muscle in the brow, and in the crow’s feet area as well," said Dr. Steinsapir, who also maintains a private cosmetic surgery practice in Beverly Hills, Calif. "This weakens the eyebrow depressors, allowing the frontalis muscle to lift unopposed, lifting the brows. Forehead movement is preserved and unwanted diffusion responsible for eyelid ptosis is prevented."
Between August 2006 and July 2011, Dr. Steinsapir performed 574 consecutive microdroplet botulinum toxin forehead lift treatments on 175 women and 53 men with a mean age of 45 years. A typical treatment involves 10 mcL of injectable saline containing 0.33 U of botulinum toxin A using the product formulation of Botox or Xeomin. About 100 microinjections are needed to complete the pattern, and all patients in the study received 33 units of Botox exclusively.
Dr. Steinsapir reported that there were no cases of treatment-induced upper eyelid or eyebrow ptosis or cases of diplopia, "which established that this treatment can be safely performed." Of the 574 patients, 49 returned for follow-up appointments between 10 and 45 days after treatment. Before and after images were used to assess the effect of treatment on the upper eyelid margin reflex distance, the tarsal platform show, and the brow position central to the cornea. Dr. Steinsapir used National Institutes of Health imaging software to perform quantitative image analysis and validated facial scales to assess the brow and forehead before and after treatment.
There was no significant change in the margin reflex distance after treatment in the 49 patients who returned for follow-up, Dr. Steinsapir said. "There was a slight trend to minimal brow elevation, and the tarsal platform show was essentially unchanged after treatment," he said. "It’s my clinical impression that the principal effect of the treatment is the softening of the brow pinch that commonly purses the brow and brings an unintentional negative affect to the face."
Dr. Steinsapir acknowledged that the procedure requires a learning curve "and a need to educate patients regarding the effect of treatment. This is more labor intensive than standard treatment methods." Dr. Steinsapir has developed a detailed training video that is available online on his website, and he said that he is working on a treatment atlas.
The microdroplet botulinum toxin forehead lift "presents the first alternative to standard periocular treatments that cause unwarranted forehead paralysis, brow flare, or muscle activation," Dr. Steinsapir said. "By controlling the depth, volume, and dose of agent, very controlled brow shaping and lifting can be performed to create aesthetic improvement with natural results, including preservation of forehead movement," he noted.
Dr. Steinsapir received a United States patent on the microdroplet method. He said that he hopes to license the technique to a drug company for the development of a Food and Drug Administration–approved indication, so the treatment can be directly marketed to consumers. He had no other relevant financial conflicts to disclose.
LAS VEGAS – A technique that involves injecting tiny, closely placed amounts of botulinum toxin A to balance the actions of the muscles around the eyebrows yielded natural-looking outcomes without forehead paralysis, based on data from a 5-year study.
"Doctors have mistakenly adopted maximal forehead paralysis as a desirable treatment endpoint," Dr. Kenneth D. Steinsapir said at the annual meeting of the American Academy of Cosmetic Surgery. "Forehead paralysis as a result of cosmetic botulinum toxin is feared by the public and lampooned by the media."
Over the years, onabotulinumtoxinA treatments have evolved to create maximal frontalis brow lifts while minimizing the risk of eyelid ptosis. As a result, "the forehead is smooth, but the central forehead is also ptotic," said Dr. Steinsapir of the department of ophthalmology at the University of California, Los Angeles. "There can be recruitment lines on the side of the forehead, which can make for an undesirable treatment effect."
In 2006, Dr. Steinsapir first described the microdroplet botulinum toxin forehead lift, a technique he developed for treating eyebrow depressors that leaves the brow elevator untreated. "I hypothesized that very small quantities of botulinum toxic can be injected and effectively trapped between the skin and the underlying orbicularis oculi muscle in the brow, and in the crow’s feet area as well," said Dr. Steinsapir, who also maintains a private cosmetic surgery practice in Beverly Hills, Calif. "This weakens the eyebrow depressors, allowing the frontalis muscle to lift unopposed, lifting the brows. Forehead movement is preserved and unwanted diffusion responsible for eyelid ptosis is prevented."
Between August 2006 and July 2011, Dr. Steinsapir performed 574 consecutive microdroplet botulinum toxin forehead lift treatments on 175 women and 53 men with a mean age of 45 years. A typical treatment involves 10 mcL of injectable saline containing 0.33 U of botulinum toxin A using the product formulation of Botox or Xeomin. About 100 microinjections are needed to complete the pattern, and all patients in the study received 33 units of Botox exclusively.
Dr. Steinsapir reported that there were no cases of treatment-induced upper eyelid or eyebrow ptosis or cases of diplopia, "which established that this treatment can be safely performed." Of the 574 patients, 49 returned for follow-up appointments between 10 and 45 days after treatment. Before and after images were used to assess the effect of treatment on the upper eyelid margin reflex distance, the tarsal platform show, and the brow position central to the cornea. Dr. Steinsapir used National Institutes of Health imaging software to perform quantitative image analysis and validated facial scales to assess the brow and forehead before and after treatment.
There was no significant change in the margin reflex distance after treatment in the 49 patients who returned for follow-up, Dr. Steinsapir said. "There was a slight trend to minimal brow elevation, and the tarsal platform show was essentially unchanged after treatment," he said. "It’s my clinical impression that the principal effect of the treatment is the softening of the brow pinch that commonly purses the brow and brings an unintentional negative affect to the face."
Dr. Steinsapir acknowledged that the procedure requires a learning curve "and a need to educate patients regarding the effect of treatment. This is more labor intensive than standard treatment methods." Dr. Steinsapir has developed a detailed training video that is available online on his website, and he said that he is working on a treatment atlas.
The microdroplet botulinum toxin forehead lift "presents the first alternative to standard periocular treatments that cause unwarranted forehead paralysis, brow flare, or muscle activation," Dr. Steinsapir said. "By controlling the depth, volume, and dose of agent, very controlled brow shaping and lifting can be performed to create aesthetic improvement with natural results, including preservation of forehead movement," he noted.
Dr. Steinsapir received a United States patent on the microdroplet method. He said that he hopes to license the technique to a drug company for the development of a Food and Drug Administration–approved indication, so the treatment can be directly marketed to consumers. He had no other relevant financial conflicts to disclose.
Dr. Kenneth D. Steinsapir, American Academy of Cosmetic Surgery, cosmetic botulinum toxin, onabotulinumtoxinA, maximal frontalis brow lifts, eyelid ptosis, ptotic, microdroplet botulinum toxin forehead lift, eyebrow depressors,
Dr. Kenneth D. Steinsapir, American Academy of Cosmetic Surgery, cosmetic botulinum toxin, onabotulinumtoxinA, maximal frontalis brow lifts, eyelid ptosis, ptotic, microdroplet botulinum toxin forehead lift, eyebrow depressors,
AT THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF COSMETIC SURGERY
Major Finding: Patients who underwent the microdroplet botulinum toxin forehead lift experienced no cases of treatment-induced upper eyelid or eyebrow ptosis or cases of diplopia.
Data Source: A 5-year study of the technique performed on 574 consecutive patients with a mean age of 45 years.
Disclosures: Dr. Steinsapir received a United States patent on the microdroplet method. He said that he hopes to license the technique to a drug company for the development of an FDA-approved indication so the treatment can be directly marketed to consumers. He had no other relevant financial conflicts to disclose.