User login
CASE: PATIENT OPTS FOR HYSTERECTOMY, ASKS ABOUT OOPHORECTOMY
Your 46-year-old patient reports increasingly severe dysmenorrhea at her annual visit, and a pelvic examination reveals an enlarged uterus. You order pelvic magnetic resonance imaging, which shows extensive adenomyosis.
After you counsel the patient about her options, she elects to undergo laparoscopic supracervical hysterectomy and asks whether she should have her ovaries removed at the time of surgery. She has no family history of ovarian or breast cancer.
What would you recommend for this woman, based on her situation and current medical research?
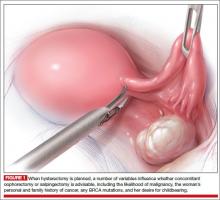
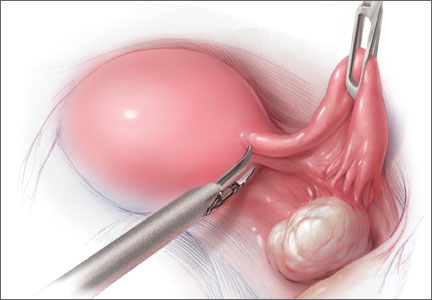
A prophylactic procedure should be considered only if 1) there is a reasonable expectation that it will benefit the patient and 2) there is evidence that, without it, the individual will be at high risk for disease.1 Bilateral oophorectomy at the time of hysterectomy for benign disease often has been recommended for women older than age 45 to prevent the subsequent development of ovarian cancer (FIGURES 1 and 2).
The 2002 Women’s Health Initiative report suggested that exogenous hormone use was associated with a slight increase in the risk of breast cancer.2 After its publication, the rate of oophorectomy at the time of hysterectomy declined slightly, likely reflecting women’s desire to preserve their own source of estrogen.3 For women younger than age 50, further slight declines in the rate of oophorectomy were seen from 2002 to 2010. However, in the United States, almost 300,000 women still undergo “prophylactic” bilateral salpingo-oophorectomy every year.4
The lifetime risk of ovarian cancer among women with a BRCA 1 mutation is 36% to 46%, and it is 10% to 27% among women with a BRCA 2 mutation. Annual screening for ovarian cancer using transvaginal ultrasound and CA 125 has not been effective even among this group of women and is not recommended.5 There is universal agreement that women with these mutations should strongly consider oophorectomy once they have completed childbearing.6 Genetic counseling and testing for these genetic mutations now are readily available.
In the general population of US women, the lifetime risk of ovarian cancer is 1.4%. The risk varies between populations, however. For white women with 3 or more term pregnancies and 4 or more years of oral contraceptive use, the lifetime risk is only 3 women in every 1,000 (0.3%).7
KNOW THE FULL RANGE OF RISKS ASSOCIATED WITH OOPHORECTOMY
After menopause and throughout a woman’s life, the ovaries continue to produce androgens, which are converted to estrone. Many studies suggest that endogenous estrogen is beneficial to the heart, bones, and brain.
A 2009 study from the Nurses’ Health Study (NHS) database found that, among women who underwent hysterectomy with oophorectomy, there were more cases of coronary heart disease (CHD), stroke, and lung cancer, compared with women who had hysterectomy with ovarian conservation.8
A subsequent NHS report focused on long-term mortality and found that, after 28 years of follow-up, women who had a hysterectomy and bilateral oophorectomy had a higher risk of dying from CHD (hazard ratio [HR], 1.23), colorectal cancer (HR, 1.49), lung cancer (HR, 1.29), and all causes (HR, 1.13) than did women who had hysterectomy and ovarian conservation.9 During the 28 years, 44 of 13,302 women (0.9%) died of ovarian cancer. At no age was there a survival advantage in the oophorectomy group. A Mayo Clinic study found similar results.10
Additional studies of the Mayo population found higher risks of anxiety, depression, dementia or cognitive impairment, and Parkinsonism in women who had their ovaries removed.11 Also, about 90% of premenopausal women experience vasomotor symptoms following oophorectomy; many women also experience mood changes, a decline in feelings of well-being, lower sexual desire, sleep disturbances, and headaches.
Overall, the evidence suggests that the removal of healthy ovaries does not meet the requirements for a prophylactic intervention.
EXOGENOUS ESTROGEN IS NOT A PRACTICAL STRATEGY AFTER OOPHORECTOMY
In the NHS studies, women who underwent hysterectomy and bilateral oophorectomy before age 50 but did not use subsequent estrogen therapy had a higher risk of all-cause mortality than women who did use estrogen (HR, 1.41).9 An early response to this finding was to advocate oophorectomy followed by the initiation of menopausal hormone therapy and statins to ward off any negative cardiovascular effects. However, data indicate that only 17% of women continue to take estrogen 5 years after the initial prescription, and only 18% of women still take statins 1 year after their first prescription.12 Even these figures are overstated because they do not include women who never see a doctor, those who see a doctor but don’t get a prescription, and those who never fill their first prescription.
Clearly, oophorectomy followed by initiation of estrogen and statins for women younger than 50 is unlikely to be effective.
THE LIKELIHOOD OF FUTURE ADNEXAL SURGERY IS LOW
Only about 6.2% of women who undergo hysterectomy with ovarian conservation require reoperation over the succeeding 20 years. The risk for age-matched women without hysterectomy is 4.8%, so the absolute difference is only 1.4% over 20 years.13
Although asymptomatic ovarian cysts are rather prevalent (6.6%) in postmenopausal women, they do not undergo transformation to cancer and usually resolve spontaneously.14 Therefore, the majority of these cysts do not need to be removed.
The suggestion that oophorectomy can avert the need for future adnexal surgery appears to be unfounded.
OVARIAN CANCER DOES NOT COME FROM THE OVARY
Seventy percent of epithelial ovarian cancers are of the serous high-grade and clinically aggressive type. The ovary contains no epithelial cells.15 Almost all high-grade cancers are associated with p53 mutations. Cancer precursor lesions called serous tubal intraepithelial cancer (STIC) have been found in the fallopian tubes of both BRCA-positive and BRCA-negative women, but no corresponding precursor lesions have ever been found in the ovary. Moreover, STIC precursor lesions have p53 mutations matching those found in high-grade serous “ovarian” cancers, but no similar p53 mutations have been found in low-grade, more indolent and treatable cancers found inside the ovary (ie, Stage 1). Therefore, the deadly form of ovarian cancer is, in fact, tubal cancer.
THE CASE FOR SALPINGECTOMY
Because convincing evidence points to the tubal origin of ovarian cancer, some experts have proposed salpingectomy for prophylaxis. Salpingectomy should remove the source of aggressive cancers and preserve functioning ovaries. However, some wondered whether salpingectomy would compromise collateral circulation to the ovaries and predispose women to early ovarian failure.
A recent study of 79 women found similar antral follicle counts and mean ovarian diameters (as measured sonographically) and similar serum levels of anti-Müllerian hormone and follicle-stimulating hormone at baseline (prior to salpingectomy) and 3 months following surgery.16 Therefore, bilateral salpingectomy may be a reasonable choice for women who have completed childbearing and who are considering pelvic surgery. As the Society of Gynecologic Oncologists stated in recent guidelines: “For women at average risk of ovarian cancer, salpingectomy should be discussed and considered prior to abdominal or pelvic surgery, hysterectomy, or in lieu of tubal ligation.”17
CASE: RESOLVED
After you review the risks and benefits of prophylactic oophorectomy versus prophylactic salpingectomy, the patient chooses the latter option and undergoes a successful surgery.
BOTTOM LINE: IN WOMEN WITH AN AVERAGE RISK OF OVARIAN CANCER, SALPINGECTOMY IS PREFERRED
Reasonable evidence now suggests that oophorectomy is associated with higher risks of CHD, colorectal and lung cancers, and overall mortality. Almost all high-grade serous cancers arise from the fallopian tubes, not the ovaries. Therefore, for women at average risk for ovarian cancer who have completed childbearing, salpingectomy should be considered at the time of pelvic surgery.
After decades of failure to achieve early diagnosis or curative treatment of “ovarian” cancer, we finally may have a way to reduce the incidence of this deadly disease.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
- Hodges F, Svoboda J, Van Howe RS. Prophylactic interventions on children: balancing human rights with public health. J Med Ethics. 2002;28(1):10–16.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Perera HK, Ananth CV, Richards CA, et al. Variation in ovarian conservation in women undergoing hysterectomy for benign indications. Obstet Gynecol. 2013;121(4):717–726.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1–e7.
- Evans GR, Gaarenstroom KN, Stirling D, et al. Screening for familial ovarian cancer: poor survival of BRCA1/2 related cancers. J Med Genet. 2009;46(9):593–597.
- Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: A multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–1337.
- Hartge P, Whittemore AS, Itnyre J, McGowan L, Cramer D. Rates and risks of ovarian cancer in subgroups of white women in the United States. The Collaborative Ovarian Cancer Group. Obstet Gynecol. 1994;84(5):760–764.
- Parker W, Broder M, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113(5):1027–1037.
- Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the Nurses’ Health Study. Obstet Gynecol. 2013;121(4):709–716.
- Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ III. Survival patterns after oophorectomy in premenopausal women: A population-based cohort study. Lancet Oncol. 2006;7(10):821–828.
- Rocca W, Bower J, Maraganore D, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083.
- Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: Results from the national health and nutrition examination survey, 1999–2010. Obstet Gynecol. 2012;120(3):595–603.
- Casiano ER, Trabuco EC, Bharucha AE, et al. Risk of oophorectomy after hysterectomy. Obstet Gynecol. 2013;121(5):1069–1074.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 Pt 1):210–217.
- Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443.
- Morelli M, Venturella R, Mocciaro R, et al. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: primum non nocere. Gynecol Oncol. 2013;129(6):448–451.
- SGO Clinical Practice Statement: Salpingectomy for Ovarian Cancer Prevention. Society of Gynecologic Oncology. November 2013. https://www.sgo.org/clinical-practice/guidelines/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention. Accessed February 10, 2014.
CASE: PATIENT OPTS FOR HYSTERECTOMY, ASKS ABOUT OOPHORECTOMY
Your 46-year-old patient reports increasingly severe dysmenorrhea at her annual visit, and a pelvic examination reveals an enlarged uterus. You order pelvic magnetic resonance imaging, which shows extensive adenomyosis.
After you counsel the patient about her options, she elects to undergo laparoscopic supracervical hysterectomy and asks whether she should have her ovaries removed at the time of surgery. She has no family history of ovarian or breast cancer.
What would you recommend for this woman, based on her situation and current medical research?
A prophylactic procedure should be considered only if 1) there is a reasonable expectation that it will benefit the patient and 2) there is evidence that, without it, the individual will be at high risk for disease.1 Bilateral oophorectomy at the time of hysterectomy for benign disease often has been recommended for women older than age 45 to prevent the subsequent development of ovarian cancer (FIGURES 1 and 2).
The 2002 Women’s Health Initiative report suggested that exogenous hormone use was associated with a slight increase in the risk of breast cancer.2 After its publication, the rate of oophorectomy at the time of hysterectomy declined slightly, likely reflecting women’s desire to preserve their own source of estrogen.3 For women younger than age 50, further slight declines in the rate of oophorectomy were seen from 2002 to 2010. However, in the United States, almost 300,000 women still undergo “prophylactic” bilateral salpingo-oophorectomy every year.4
The lifetime risk of ovarian cancer among women with a BRCA 1 mutation is 36% to 46%, and it is 10% to 27% among women with a BRCA 2 mutation. Annual screening for ovarian cancer using transvaginal ultrasound and CA 125 has not been effective even among this group of women and is not recommended.5 There is universal agreement that women with these mutations should strongly consider oophorectomy once they have completed childbearing.6 Genetic counseling and testing for these genetic mutations now are readily available.
In the general population of US women, the lifetime risk of ovarian cancer is 1.4%. The risk varies between populations, however. For white women with 3 or more term pregnancies and 4 or more years of oral contraceptive use, the lifetime risk is only 3 women in every 1,000 (0.3%).7
KNOW THE FULL RANGE OF RISKS ASSOCIATED WITH OOPHORECTOMY
After menopause and throughout a woman’s life, the ovaries continue to produce androgens, which are converted to estrone. Many studies suggest that endogenous estrogen is beneficial to the heart, bones, and brain.
A 2009 study from the Nurses’ Health Study (NHS) database found that, among women who underwent hysterectomy with oophorectomy, there were more cases of coronary heart disease (CHD), stroke, and lung cancer, compared with women who had hysterectomy with ovarian conservation.8
A subsequent NHS report focused on long-term mortality and found that, after 28 years of follow-up, women who had a hysterectomy and bilateral oophorectomy had a higher risk of dying from CHD (hazard ratio [HR], 1.23), colorectal cancer (HR, 1.49), lung cancer (HR, 1.29), and all causes (HR, 1.13) than did women who had hysterectomy and ovarian conservation.9 During the 28 years, 44 of 13,302 women (0.9%) died of ovarian cancer. At no age was there a survival advantage in the oophorectomy group. A Mayo Clinic study found similar results.10
Additional studies of the Mayo population found higher risks of anxiety, depression, dementia or cognitive impairment, and Parkinsonism in women who had their ovaries removed.11 Also, about 90% of premenopausal women experience vasomotor symptoms following oophorectomy; many women also experience mood changes, a decline in feelings of well-being, lower sexual desire, sleep disturbances, and headaches.
Overall, the evidence suggests that the removal of healthy ovaries does not meet the requirements for a prophylactic intervention.
EXOGENOUS ESTROGEN IS NOT A PRACTICAL STRATEGY AFTER OOPHORECTOMY
In the NHS studies, women who underwent hysterectomy and bilateral oophorectomy before age 50 but did not use subsequent estrogen therapy had a higher risk of all-cause mortality than women who did use estrogen (HR, 1.41).9 An early response to this finding was to advocate oophorectomy followed by the initiation of menopausal hormone therapy and statins to ward off any negative cardiovascular effects. However, data indicate that only 17% of women continue to take estrogen 5 years after the initial prescription, and only 18% of women still take statins 1 year after their first prescription.12 Even these figures are overstated because they do not include women who never see a doctor, those who see a doctor but don’t get a prescription, and those who never fill their first prescription.
Clearly, oophorectomy followed by initiation of estrogen and statins for women younger than 50 is unlikely to be effective.
THE LIKELIHOOD OF FUTURE ADNEXAL SURGERY IS LOW
Only about 6.2% of women who undergo hysterectomy with ovarian conservation require reoperation over the succeeding 20 years. The risk for age-matched women without hysterectomy is 4.8%, so the absolute difference is only 1.4% over 20 years.13
Although asymptomatic ovarian cysts are rather prevalent (6.6%) in postmenopausal women, they do not undergo transformation to cancer and usually resolve spontaneously.14 Therefore, the majority of these cysts do not need to be removed.
The suggestion that oophorectomy can avert the need for future adnexal surgery appears to be unfounded.
OVARIAN CANCER DOES NOT COME FROM THE OVARY
Seventy percent of epithelial ovarian cancers are of the serous high-grade and clinically aggressive type. The ovary contains no epithelial cells.15 Almost all high-grade cancers are associated with p53 mutations. Cancer precursor lesions called serous tubal intraepithelial cancer (STIC) have been found in the fallopian tubes of both BRCA-positive and BRCA-negative women, but no corresponding precursor lesions have ever been found in the ovary. Moreover, STIC precursor lesions have p53 mutations matching those found in high-grade serous “ovarian” cancers, but no similar p53 mutations have been found in low-grade, more indolent and treatable cancers found inside the ovary (ie, Stage 1). Therefore, the deadly form of ovarian cancer is, in fact, tubal cancer.
THE CASE FOR SALPINGECTOMY
Because convincing evidence points to the tubal origin of ovarian cancer, some experts have proposed salpingectomy for prophylaxis. Salpingectomy should remove the source of aggressive cancers and preserve functioning ovaries. However, some wondered whether salpingectomy would compromise collateral circulation to the ovaries and predispose women to early ovarian failure.
A recent study of 79 women found similar antral follicle counts and mean ovarian diameters (as measured sonographically) and similar serum levels of anti-Müllerian hormone and follicle-stimulating hormone at baseline (prior to salpingectomy) and 3 months following surgery.16 Therefore, bilateral salpingectomy may be a reasonable choice for women who have completed childbearing and who are considering pelvic surgery. As the Society of Gynecologic Oncologists stated in recent guidelines: “For women at average risk of ovarian cancer, salpingectomy should be discussed and considered prior to abdominal or pelvic surgery, hysterectomy, or in lieu of tubal ligation.”17
CASE: RESOLVED
After you review the risks and benefits of prophylactic oophorectomy versus prophylactic salpingectomy, the patient chooses the latter option and undergoes a successful surgery.
BOTTOM LINE: IN WOMEN WITH AN AVERAGE RISK OF OVARIAN CANCER, SALPINGECTOMY IS PREFERRED
Reasonable evidence now suggests that oophorectomy is associated with higher risks of CHD, colorectal and lung cancers, and overall mortality. Almost all high-grade serous cancers arise from the fallopian tubes, not the ovaries. Therefore, for women at average risk for ovarian cancer who have completed childbearing, salpingectomy should be considered at the time of pelvic surgery.
After decades of failure to achieve early diagnosis or curative treatment of “ovarian” cancer, we finally may have a way to reduce the incidence of this deadly disease.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
CASE: PATIENT OPTS FOR HYSTERECTOMY, ASKS ABOUT OOPHORECTOMY
Your 46-year-old patient reports increasingly severe dysmenorrhea at her annual visit, and a pelvic examination reveals an enlarged uterus. You order pelvic magnetic resonance imaging, which shows extensive adenomyosis.
After you counsel the patient about her options, she elects to undergo laparoscopic supracervical hysterectomy and asks whether she should have her ovaries removed at the time of surgery. She has no family history of ovarian or breast cancer.
What would you recommend for this woman, based on her situation and current medical research?
A prophylactic procedure should be considered only if 1) there is a reasonable expectation that it will benefit the patient and 2) there is evidence that, without it, the individual will be at high risk for disease.1 Bilateral oophorectomy at the time of hysterectomy for benign disease often has been recommended for women older than age 45 to prevent the subsequent development of ovarian cancer (FIGURES 1 and 2).
The 2002 Women’s Health Initiative report suggested that exogenous hormone use was associated with a slight increase in the risk of breast cancer.2 After its publication, the rate of oophorectomy at the time of hysterectomy declined slightly, likely reflecting women’s desire to preserve their own source of estrogen.3 For women younger than age 50, further slight declines in the rate of oophorectomy were seen from 2002 to 2010. However, in the United States, almost 300,000 women still undergo “prophylactic” bilateral salpingo-oophorectomy every year.4
The lifetime risk of ovarian cancer among women with a BRCA 1 mutation is 36% to 46%, and it is 10% to 27% among women with a BRCA 2 mutation. Annual screening for ovarian cancer using transvaginal ultrasound and CA 125 has not been effective even among this group of women and is not recommended.5 There is universal agreement that women with these mutations should strongly consider oophorectomy once they have completed childbearing.6 Genetic counseling and testing for these genetic mutations now are readily available.
In the general population of US women, the lifetime risk of ovarian cancer is 1.4%. The risk varies between populations, however. For white women with 3 or more term pregnancies and 4 or more years of oral contraceptive use, the lifetime risk is only 3 women in every 1,000 (0.3%).7
KNOW THE FULL RANGE OF RISKS ASSOCIATED WITH OOPHORECTOMY
After menopause and throughout a woman’s life, the ovaries continue to produce androgens, which are converted to estrone. Many studies suggest that endogenous estrogen is beneficial to the heart, bones, and brain.
A 2009 study from the Nurses’ Health Study (NHS) database found that, among women who underwent hysterectomy with oophorectomy, there were more cases of coronary heart disease (CHD), stroke, and lung cancer, compared with women who had hysterectomy with ovarian conservation.8
A subsequent NHS report focused on long-term mortality and found that, after 28 years of follow-up, women who had a hysterectomy and bilateral oophorectomy had a higher risk of dying from CHD (hazard ratio [HR], 1.23), colorectal cancer (HR, 1.49), lung cancer (HR, 1.29), and all causes (HR, 1.13) than did women who had hysterectomy and ovarian conservation.9 During the 28 years, 44 of 13,302 women (0.9%) died of ovarian cancer. At no age was there a survival advantage in the oophorectomy group. A Mayo Clinic study found similar results.10
Additional studies of the Mayo population found higher risks of anxiety, depression, dementia or cognitive impairment, and Parkinsonism in women who had their ovaries removed.11 Also, about 90% of premenopausal women experience vasomotor symptoms following oophorectomy; many women also experience mood changes, a decline in feelings of well-being, lower sexual desire, sleep disturbances, and headaches.
Overall, the evidence suggests that the removal of healthy ovaries does not meet the requirements for a prophylactic intervention.
EXOGENOUS ESTROGEN IS NOT A PRACTICAL STRATEGY AFTER OOPHORECTOMY
In the NHS studies, women who underwent hysterectomy and bilateral oophorectomy before age 50 but did not use subsequent estrogen therapy had a higher risk of all-cause mortality than women who did use estrogen (HR, 1.41).9 An early response to this finding was to advocate oophorectomy followed by the initiation of menopausal hormone therapy and statins to ward off any negative cardiovascular effects. However, data indicate that only 17% of women continue to take estrogen 5 years after the initial prescription, and only 18% of women still take statins 1 year after their first prescription.12 Even these figures are overstated because they do not include women who never see a doctor, those who see a doctor but don’t get a prescription, and those who never fill their first prescription.
Clearly, oophorectomy followed by initiation of estrogen and statins for women younger than 50 is unlikely to be effective.
THE LIKELIHOOD OF FUTURE ADNEXAL SURGERY IS LOW
Only about 6.2% of women who undergo hysterectomy with ovarian conservation require reoperation over the succeeding 20 years. The risk for age-matched women without hysterectomy is 4.8%, so the absolute difference is only 1.4% over 20 years.13
Although asymptomatic ovarian cysts are rather prevalent (6.6%) in postmenopausal women, they do not undergo transformation to cancer and usually resolve spontaneously.14 Therefore, the majority of these cysts do not need to be removed.
The suggestion that oophorectomy can avert the need for future adnexal surgery appears to be unfounded.
OVARIAN CANCER DOES NOT COME FROM THE OVARY
Seventy percent of epithelial ovarian cancers are of the serous high-grade and clinically aggressive type. The ovary contains no epithelial cells.15 Almost all high-grade cancers are associated with p53 mutations. Cancer precursor lesions called serous tubal intraepithelial cancer (STIC) have been found in the fallopian tubes of both BRCA-positive and BRCA-negative women, but no corresponding precursor lesions have ever been found in the ovary. Moreover, STIC precursor lesions have p53 mutations matching those found in high-grade serous “ovarian” cancers, but no similar p53 mutations have been found in low-grade, more indolent and treatable cancers found inside the ovary (ie, Stage 1). Therefore, the deadly form of ovarian cancer is, in fact, tubal cancer.
THE CASE FOR SALPINGECTOMY
Because convincing evidence points to the tubal origin of ovarian cancer, some experts have proposed salpingectomy for prophylaxis. Salpingectomy should remove the source of aggressive cancers and preserve functioning ovaries. However, some wondered whether salpingectomy would compromise collateral circulation to the ovaries and predispose women to early ovarian failure.
A recent study of 79 women found similar antral follicle counts and mean ovarian diameters (as measured sonographically) and similar serum levels of anti-Müllerian hormone and follicle-stimulating hormone at baseline (prior to salpingectomy) and 3 months following surgery.16 Therefore, bilateral salpingectomy may be a reasonable choice for women who have completed childbearing and who are considering pelvic surgery. As the Society of Gynecologic Oncologists stated in recent guidelines: “For women at average risk of ovarian cancer, salpingectomy should be discussed and considered prior to abdominal or pelvic surgery, hysterectomy, or in lieu of tubal ligation.”17
CASE: RESOLVED
After you review the risks and benefits of prophylactic oophorectomy versus prophylactic salpingectomy, the patient chooses the latter option and undergoes a successful surgery.
BOTTOM LINE: IN WOMEN WITH AN AVERAGE RISK OF OVARIAN CANCER, SALPINGECTOMY IS PREFERRED
Reasonable evidence now suggests that oophorectomy is associated with higher risks of CHD, colorectal and lung cancers, and overall mortality. Almost all high-grade serous cancers arise from the fallopian tubes, not the ovaries. Therefore, for women at average risk for ovarian cancer who have completed childbearing, salpingectomy should be considered at the time of pelvic surgery.
After decades of failure to achieve early diagnosis or curative treatment of “ovarian” cancer, we finally may have a way to reduce the incidence of this deadly disease.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
- Hodges F, Svoboda J, Van Howe RS. Prophylactic interventions on children: balancing human rights with public health. J Med Ethics. 2002;28(1):10–16.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Perera HK, Ananth CV, Richards CA, et al. Variation in ovarian conservation in women undergoing hysterectomy for benign indications. Obstet Gynecol. 2013;121(4):717–726.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1–e7.
- Evans GR, Gaarenstroom KN, Stirling D, et al. Screening for familial ovarian cancer: poor survival of BRCA1/2 related cancers. J Med Genet. 2009;46(9):593–597.
- Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: A multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–1337.
- Hartge P, Whittemore AS, Itnyre J, McGowan L, Cramer D. Rates and risks of ovarian cancer in subgroups of white women in the United States. The Collaborative Ovarian Cancer Group. Obstet Gynecol. 1994;84(5):760–764.
- Parker W, Broder M, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113(5):1027–1037.
- Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the Nurses’ Health Study. Obstet Gynecol. 2013;121(4):709–716.
- Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ III. Survival patterns after oophorectomy in premenopausal women: A population-based cohort study. Lancet Oncol. 2006;7(10):821–828.
- Rocca W, Bower J, Maraganore D, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083.
- Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: Results from the national health and nutrition examination survey, 1999–2010. Obstet Gynecol. 2012;120(3):595–603.
- Casiano ER, Trabuco EC, Bharucha AE, et al. Risk of oophorectomy after hysterectomy. Obstet Gynecol. 2013;121(5):1069–1074.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 Pt 1):210–217.
- Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443.
- Morelli M, Venturella R, Mocciaro R, et al. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: primum non nocere. Gynecol Oncol. 2013;129(6):448–451.
- SGO Clinical Practice Statement: Salpingectomy for Ovarian Cancer Prevention. Society of Gynecologic Oncology. November 2013. https://www.sgo.org/clinical-practice/guidelines/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention. Accessed February 10, 2014.
- Hodges F, Svoboda J, Van Howe RS. Prophylactic interventions on children: balancing human rights with public health. J Med Ethics. 2002;28(1):10–16.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Perera HK, Ananth CV, Richards CA, et al. Variation in ovarian conservation in women undergoing hysterectomy for benign indications. Obstet Gynecol. 2013;121(4):717–726.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1–e7.
- Evans GR, Gaarenstroom KN, Stirling D, et al. Screening for familial ovarian cancer: poor survival of BRCA1/2 related cancers. J Med Genet. 2009;46(9):593–597.
- Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: A multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–1337.
- Hartge P, Whittemore AS, Itnyre J, McGowan L, Cramer D. Rates and risks of ovarian cancer in subgroups of white women in the United States. The Collaborative Ovarian Cancer Group. Obstet Gynecol. 1994;84(5):760–764.
- Parker W, Broder M, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113(5):1027–1037.
- Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the Nurses’ Health Study. Obstet Gynecol. 2013;121(4):709–716.
- Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ III. Survival patterns after oophorectomy in premenopausal women: A population-based cohort study. Lancet Oncol. 2006;7(10):821–828.
- Rocca W, Bower J, Maraganore D, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083.
- Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: Results from the national health and nutrition examination survey, 1999–2010. Obstet Gynecol. 2012;120(3):595–603.
- Casiano ER, Trabuco EC, Bharucha AE, et al. Risk of oophorectomy after hysterectomy. Obstet Gynecol. 2013;121(5):1069–1074.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 Pt 1):210–217.
- Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443.
- Morelli M, Venturella R, Mocciaro R, et al. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: primum non nocere. Gynecol Oncol. 2013;129(6):448–451.
- SGO Clinical Practice Statement: Salpingectomy for Ovarian Cancer Prevention. Society of Gynecologic Oncology. November 2013. https://www.sgo.org/clinical-practice/guidelines/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention. Accessed February 10, 2014.