User login
MADRID – Adding the oral Janus kinase (JAK) inhibitor baricitinib to standard atopic dermatitis therapy with low- and midpotency topical corticosteroids markedly improved disease severity and key patient-reported outcomes, compared with topical corticosteroids alone, in the phase 3, randomized, double-blind BREEZE-AD7 trial, Kristian Reich, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
in the phase 3 BREEZE-AD1 and -AD2 trials. But BREEZE-AD7 further advances the field because it’s the first phase 3 study testing the efficacy of a JAK inhibitor in combination with low- and midpotency topical steroids.
“I think this study is important because it looks into the situation that’s more like what happens in the real world, which is, as with dupilumab and other drugs, we use the systemic agent in combination with topical therapies and, in particular, with topical corticosteroids,” commented Dr. Reich, professor of dermatology at University Medical Center, Hamburg, and medical director at SCIderm, a scientific research company.
“This is what I think we can expect from existing and upcoming systemic therapies in atopic dermatitis: We will use them in combination with topical corticosteroids, and hopefully this will allow patients to dramatically reduce the concomitant use of topical corticosteroids, as shown here in BREEZE-AD7,” he added.
BREEZE-AD7 was a 16-week study that included 329 adults with moderate or severe atopic dermatitis who were randomized to low- and midpotency topical corticosteroids plus either baricitinib at 2 mg once daily, baricitinib at 4 mg once daily, or placebo. The group’s mean baseline Eczema Area and Severity Index (EASI) score was 29. Overall, 45% of participants had a baseline Investigator’s Global Assessment (IGA) of disease severity of 4 on a 0-4 scale.
The primary endpoint was achievement of an IGA of 0 or 1, meaning clear or almost clear, along with at least a 2-point IGA improvement from baseline at week 16. This was accomplished in 30.6% of those on 4 mg/day of baricitinib, 23.9% of patients in the 2-mg group, and 14.7% of controls.
The 4-mg dose of baricitinib was statistically superior to placebo; the 2-mg dose was not. However, Dr. Reich indicated he was untroubled by this because the primary endpoint was set at a high bar, and both doses of baricitinib proved to be significantly better than topical steroids plus placebo in terms of EASI 75 response rates, as well as reductions in itch, skin pain, and sleep problems, which aren’t captured in EASI scores (see graphic).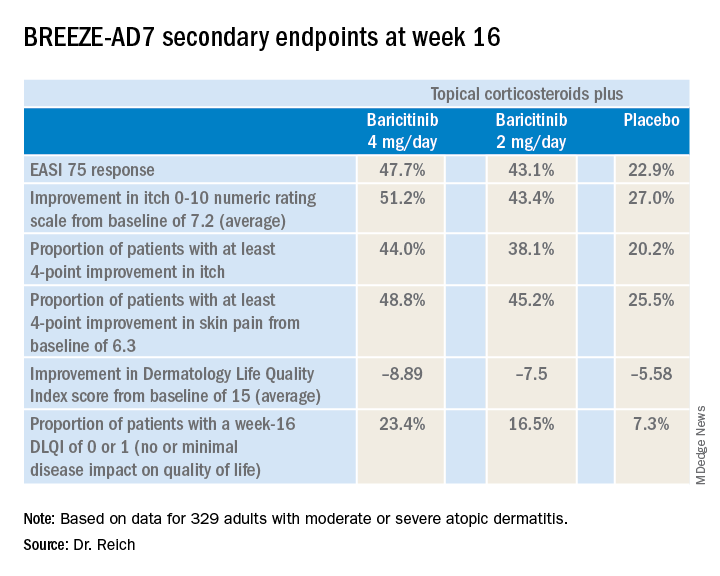
“One of my big learnings from this year’s EADV is that we have to rethink the dimensions of atopic dermatitis. I think we have underestimated the relevance of important symptoms such as itch, the impact atopic dermatitis has on pain, and the effect it has on sleeping problems,” the dermatologist said. “My feeling is that baricitinib is strongest in reducing itch, improving sleep, and reducing pain, but it also has good effects on the clinical signs of atopic dermatitis.”
The baricitinib-treated patients’ rapidity of improvement in the various endpoints was particularly impressive. Both doses of the JAK 1/2 inhibitor showed significant separation from the control group in the first week, and the majority of improvement occurred by week 4.
A key finding was that patients on baricitinib at 2 mg/day and 4 mg/day used a mean total of 162 g and 137 g of midpotency topical steroids, respectively, during the 16 weeks, compared with 225 g in the control group. The higher-dose baricitinib group was topical corticosteroid-free on 33% of study days, compared with 25% of days for the baricitinib 2 mg patients and 17% of days for controls.
In terms of safety, there was a case of pulmonary embolism in the higher-dose baricitinib group and an opportunistic toxoplasmosis eye infection in the control population. The frequency of oral herpes and herpes simplex virus infections was 2.8% in controls, 4.6% in the baricitinib 2-mg group, and 6.3% in the 4-mg group. There was also a signal of a dose-dependent increased risk of new-onset acne, with rates of 0.9% in controls and patients on baricitinib 2 mg, climbing to 3.6% with baricitinib 4 mg.
“In phase 2 results with upadacitinib [another oral JAK inhibitor], we saw that more than 10% of patients in the highest-dose group developed what was classified as acne. I cannot explain this, but it’s something we will monitor in the future,” Dr. Reich promised.
A fuller picture of baricitinib’s safety profile in the setting of atopic dermatitis clearly requires larger and longer-term studies, he added.
Baricitinib at the 2 mg daily dose is already marketed as Olumiant for the treatment of rheumatoid arthritis, with labeling that includes a boxed warning about serious infections, malignancy, and thrombosis. The Food and Drug Administration did not approve the 4-mg dose after determining that its higher safety hazard outweighed the efficacy advantage over the lower dose.
The BREEZE-AD7 study was sponsored by Eli Lilly. Dr. Reich reported serving as an adviser to, paid speaker for, and recipient of research grants from that pharmaceutical company and more than two dozen others.
MADRID – Adding the oral Janus kinase (JAK) inhibitor baricitinib to standard atopic dermatitis therapy with low- and midpotency topical corticosteroids markedly improved disease severity and key patient-reported outcomes, compared with topical corticosteroids alone, in the phase 3, randomized, double-blind BREEZE-AD7 trial, Kristian Reich, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
in the phase 3 BREEZE-AD1 and -AD2 trials. But BREEZE-AD7 further advances the field because it’s the first phase 3 study testing the efficacy of a JAK inhibitor in combination with low- and midpotency topical steroids.
“I think this study is important because it looks into the situation that’s more like what happens in the real world, which is, as with dupilumab and other drugs, we use the systemic agent in combination with topical therapies and, in particular, with topical corticosteroids,” commented Dr. Reich, professor of dermatology at University Medical Center, Hamburg, and medical director at SCIderm, a scientific research company.
“This is what I think we can expect from existing and upcoming systemic therapies in atopic dermatitis: We will use them in combination with topical corticosteroids, and hopefully this will allow patients to dramatically reduce the concomitant use of topical corticosteroids, as shown here in BREEZE-AD7,” he added.
BREEZE-AD7 was a 16-week study that included 329 adults with moderate or severe atopic dermatitis who were randomized to low- and midpotency topical corticosteroids plus either baricitinib at 2 mg once daily, baricitinib at 4 mg once daily, or placebo. The group’s mean baseline Eczema Area and Severity Index (EASI) score was 29. Overall, 45% of participants had a baseline Investigator’s Global Assessment (IGA) of disease severity of 4 on a 0-4 scale.
The primary endpoint was achievement of an IGA of 0 or 1, meaning clear or almost clear, along with at least a 2-point IGA improvement from baseline at week 16. This was accomplished in 30.6% of those on 4 mg/day of baricitinib, 23.9% of patients in the 2-mg group, and 14.7% of controls.
The 4-mg dose of baricitinib was statistically superior to placebo; the 2-mg dose was not. However, Dr. Reich indicated he was untroubled by this because the primary endpoint was set at a high bar, and both doses of baricitinib proved to be significantly better than topical steroids plus placebo in terms of EASI 75 response rates, as well as reductions in itch, skin pain, and sleep problems, which aren’t captured in EASI scores (see graphic).
“One of my big learnings from this year’s EADV is that we have to rethink the dimensions of atopic dermatitis. I think we have underestimated the relevance of important symptoms such as itch, the impact atopic dermatitis has on pain, and the effect it has on sleeping problems,” the dermatologist said. “My feeling is that baricitinib is strongest in reducing itch, improving sleep, and reducing pain, but it also has good effects on the clinical signs of atopic dermatitis.”
The baricitinib-treated patients’ rapidity of improvement in the various endpoints was particularly impressive. Both doses of the JAK 1/2 inhibitor showed significant separation from the control group in the first week, and the majority of improvement occurred by week 4.
A key finding was that patients on baricitinib at 2 mg/day and 4 mg/day used a mean total of 162 g and 137 g of midpotency topical steroids, respectively, during the 16 weeks, compared with 225 g in the control group. The higher-dose baricitinib group was topical corticosteroid-free on 33% of study days, compared with 25% of days for the baricitinib 2 mg patients and 17% of days for controls.
In terms of safety, there was a case of pulmonary embolism in the higher-dose baricitinib group and an opportunistic toxoplasmosis eye infection in the control population. The frequency of oral herpes and herpes simplex virus infections was 2.8% in controls, 4.6% in the baricitinib 2-mg group, and 6.3% in the 4-mg group. There was also a signal of a dose-dependent increased risk of new-onset acne, with rates of 0.9% in controls and patients on baricitinib 2 mg, climbing to 3.6% with baricitinib 4 mg.
“In phase 2 results with upadacitinib [another oral JAK inhibitor], we saw that more than 10% of patients in the highest-dose group developed what was classified as acne. I cannot explain this, but it’s something we will monitor in the future,” Dr. Reich promised.
A fuller picture of baricitinib’s safety profile in the setting of atopic dermatitis clearly requires larger and longer-term studies, he added.
Baricitinib at the 2 mg daily dose is already marketed as Olumiant for the treatment of rheumatoid arthritis, with labeling that includes a boxed warning about serious infections, malignancy, and thrombosis. The Food and Drug Administration did not approve the 4-mg dose after determining that its higher safety hazard outweighed the efficacy advantage over the lower dose.
The BREEZE-AD7 study was sponsored by Eli Lilly. Dr. Reich reported serving as an adviser to, paid speaker for, and recipient of research grants from that pharmaceutical company and more than two dozen others.
MADRID – Adding the oral Janus kinase (JAK) inhibitor baricitinib to standard atopic dermatitis therapy with low- and midpotency topical corticosteroids markedly improved disease severity and key patient-reported outcomes, compared with topical corticosteroids alone, in the phase 3, randomized, double-blind BREEZE-AD7 trial, Kristian Reich, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
in the phase 3 BREEZE-AD1 and -AD2 trials. But BREEZE-AD7 further advances the field because it’s the first phase 3 study testing the efficacy of a JAK inhibitor in combination with low- and midpotency topical steroids.
“I think this study is important because it looks into the situation that’s more like what happens in the real world, which is, as with dupilumab and other drugs, we use the systemic agent in combination with topical therapies and, in particular, with topical corticosteroids,” commented Dr. Reich, professor of dermatology at University Medical Center, Hamburg, and medical director at SCIderm, a scientific research company.
“This is what I think we can expect from existing and upcoming systemic therapies in atopic dermatitis: We will use them in combination with topical corticosteroids, and hopefully this will allow patients to dramatically reduce the concomitant use of topical corticosteroids, as shown here in BREEZE-AD7,” he added.
BREEZE-AD7 was a 16-week study that included 329 adults with moderate or severe atopic dermatitis who were randomized to low- and midpotency topical corticosteroids plus either baricitinib at 2 mg once daily, baricitinib at 4 mg once daily, or placebo. The group’s mean baseline Eczema Area and Severity Index (EASI) score was 29. Overall, 45% of participants had a baseline Investigator’s Global Assessment (IGA) of disease severity of 4 on a 0-4 scale.
The primary endpoint was achievement of an IGA of 0 or 1, meaning clear or almost clear, along with at least a 2-point IGA improvement from baseline at week 16. This was accomplished in 30.6% of those on 4 mg/day of baricitinib, 23.9% of patients in the 2-mg group, and 14.7% of controls.
The 4-mg dose of baricitinib was statistically superior to placebo; the 2-mg dose was not. However, Dr. Reich indicated he was untroubled by this because the primary endpoint was set at a high bar, and both doses of baricitinib proved to be significantly better than topical steroids plus placebo in terms of EASI 75 response rates, as well as reductions in itch, skin pain, and sleep problems, which aren’t captured in EASI scores (see graphic).
“One of my big learnings from this year’s EADV is that we have to rethink the dimensions of atopic dermatitis. I think we have underestimated the relevance of important symptoms such as itch, the impact atopic dermatitis has on pain, and the effect it has on sleeping problems,” the dermatologist said. “My feeling is that baricitinib is strongest in reducing itch, improving sleep, and reducing pain, but it also has good effects on the clinical signs of atopic dermatitis.”
The baricitinib-treated patients’ rapidity of improvement in the various endpoints was particularly impressive. Both doses of the JAK 1/2 inhibitor showed significant separation from the control group in the first week, and the majority of improvement occurred by week 4.
A key finding was that patients on baricitinib at 2 mg/day and 4 mg/day used a mean total of 162 g and 137 g of midpotency topical steroids, respectively, during the 16 weeks, compared with 225 g in the control group. The higher-dose baricitinib group was topical corticosteroid-free on 33% of study days, compared with 25% of days for the baricitinib 2 mg patients and 17% of days for controls.
In terms of safety, there was a case of pulmonary embolism in the higher-dose baricitinib group and an opportunistic toxoplasmosis eye infection in the control population. The frequency of oral herpes and herpes simplex virus infections was 2.8% in controls, 4.6% in the baricitinib 2-mg group, and 6.3% in the 4-mg group. There was also a signal of a dose-dependent increased risk of new-onset acne, with rates of 0.9% in controls and patients on baricitinib 2 mg, climbing to 3.6% with baricitinib 4 mg.
“In phase 2 results with upadacitinib [another oral JAK inhibitor], we saw that more than 10% of patients in the highest-dose group developed what was classified as acne. I cannot explain this, but it’s something we will monitor in the future,” Dr. Reich promised.
A fuller picture of baricitinib’s safety profile in the setting of atopic dermatitis clearly requires larger and longer-term studies, he added.
Baricitinib at the 2 mg daily dose is already marketed as Olumiant for the treatment of rheumatoid arthritis, with labeling that includes a boxed warning about serious infections, malignancy, and thrombosis. The Food and Drug Administration did not approve the 4-mg dose after determining that its higher safety hazard outweighed the efficacy advantage over the lower dose.
The BREEZE-AD7 study was sponsored by Eli Lilly. Dr. Reich reported serving as an adviser to, paid speaker for, and recipient of research grants from that pharmaceutical company and more than two dozen others.
REPORTING FROM EADV CONGRESS
Key clinical point: The Janus kinase 1/2 inhibitor baricitinib shows promise as a novel oral treatment for moderate or severe atopic dermatitis.
Major finding: Among atopic dermatitis patients on concomitant topical corticosteroids, a 75% improvement on Eczema Area and Severity Index at 16 weeks was achieved in 48% of those on baricitinib at 4 mg/day, 43% with baricitinib at 2 mg/day, and 23% on placebo.
Study details: BREEZE-AD7 was a phase 3, multicenter, 16-week, double-blind, three-arm study including 329 adults with moderate or severe atopic dermatitis.
Disclosures: The BREEZE-AD7 study was sponsored by Eli Lilly. The presenter reported serving as an adviser to, paid speaker for, and/or recipient of research grants from that pharmaceutical company and more than two dozen others.
Source: Reich K. EADV Congress, late breaker.

