User login
A 48-year-old woman with a history of systemic lupus erythematosus (SLE) presented to the emergency department from the rheumatology clinic for digital ischemia. The clinical manifestations of her SLE consisted predominantly of arthralgias, which had been previously well controlled on hydroxychloroquine 300 mg/d PO. On presentation, she denied oral ulcers, alopecia, shortness of breath, chest pain/pressure, and history of blood clots or miscarriages.
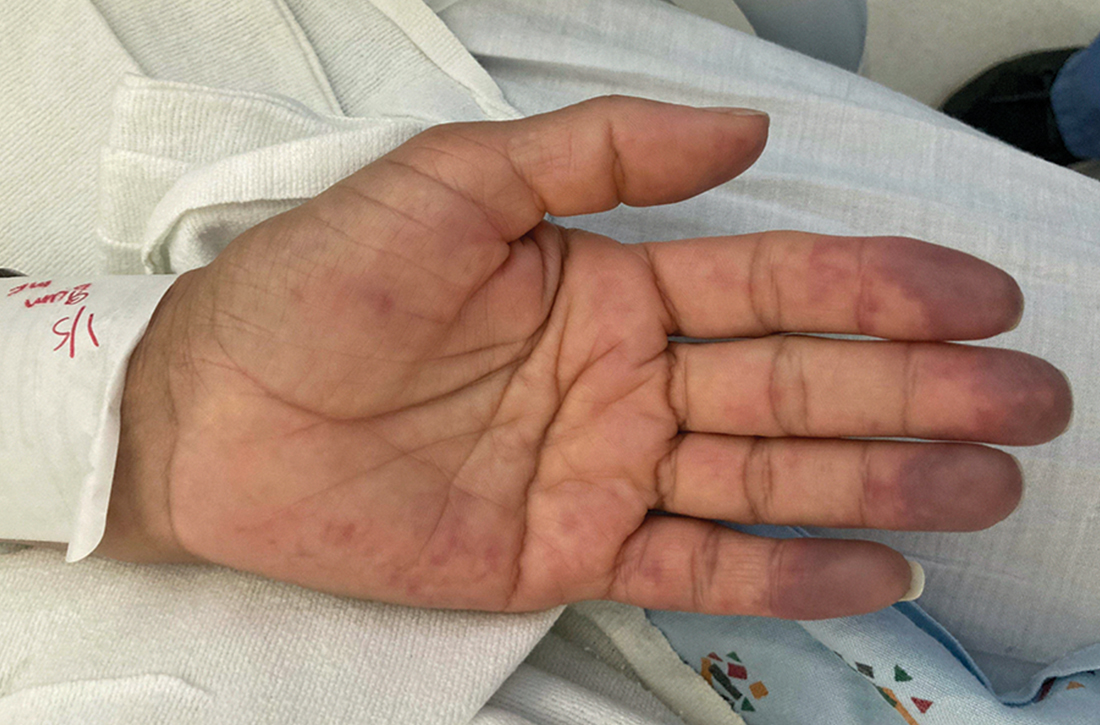
On exam, the patient was afebrile and had a heart rate of 74 bpm; blood pressure, 140/77 mm Hg; and respiratory rate, 18 breaths/min. The fingertips on her left hand were tender and cool to the touch, and the fingertips of her second through fifth digits were blue (FIGURE).
Laboratory workup was notable for the following: hemoglobin, 9.3 g/dL (normal range, 11.6-15.2 g/dL) and erythrocyte sedimentation rate, 44 mm/h (normal range, ≤ 25 mm/h). Double-stranded DNA and complement levels were normal.
Transthoracic echocardiogram did not show any valvular vegetations, and blood cultures from admission were negative. Computed tomography angiography (CTA) with contrast of her left upper extremity showed a filling defect in the origin of the left subclavian artery. Digital plethysmography showed dampened flow signals in the second through fifth digits of the left hand.
Tests for antiphospholipid antibodies were positive for lupus anticoagulant; there were also high titers of anti-β-2-glycoprotein immunoglobulin (Ig) G (58 SGU; normal, ≤ 20 SGU) and anticardiolipin IgG (242.4 CU; normal, ≤ 20 CU).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Antiphospholipid syndrome
Given our patient’s SLE, left subclavian artery thrombosis, digital ischemia, and high-titer antiphospholipid antibodies, we had significant concern for antiphospholipid syndrome (APS). The diagnosis of APS is most often based on the fulfillment of the revised Sapporo classification criteria. These criteria include both clinical criteria (vascular thrombosis or pregnancy morbidity) and laboratory criteria (the presence of antiphospholipid antibodies on at least 2 separate occasions separated by 12 weeks).1 Our patient met clinical criteria given the evidence of subclavian artery thrombosis on CTA as well as digital plethysmography findings consistent with digital emboli. To meet laboratory criteria, she would have needed to have persistent high-titer antiphospholipid antibodies 12 weeks apart.
APS is an autoimmune disease in which the presence of antiphospholipid antibodies is associated with thrombosis; it can be divided into primary and secondary APS. The estimated prevalence of APS is 50 per 100,000 people in the United States.2 Primary APS occurs in the absence of an underlying autoimmune disease, while secondary APS occurs in the presence of an underlying autoimmune disease.
The autoimmune disease most often associated with APS is SLE.3 Among patients with SLE, 15% to 34% have positive lupus anticoagulant and 12% to 30% have anticardiolipin antibodies.4-6 This is compared with young healthy subjects among whom only 1% to 4% have positive lupus anticoagulant and 1% to 5% have anticardiolipin antibodies.7 Previous studies have estimated that 30% to 50% of patients with SLE who test positive for antiphospholipid antibodies will develop thrombosis.5,7
Differential includes Raynaud phenomenon, vasculitis
The differential diagnosis for digital ischemia in a patient with SLE includes APS, Raynaud phenomenon, vasculitis, and septic emboli.
Raynaud phenomenon can manifest in patients with SLE, but the presence of thrombosis on CTA and high-titer positive antiphospholipid antibodies make the diagnosis of APS more likely. Additionally, Raynaud phenomenon is typically temperature dependent with vasospasm in the digital arteries, occurring in cold temperatures and resolving with warming.
Systemic vasculitis may develop in patients with SLE, but in our case was less likely given the patient did not have any evidence of vasculitis on CTA, such as blood vessel wall thickening and/or enhancement.8
Septic emboli from endocarditis can cause digital ischemia but is typically associated with positive blood cultures, fever, and other systemic signs of infection, and/or vegetations on an echocardiogram.
Continue to: Thrombosis determines intensity of lifelong antiocagulation Tx
Thrombosis determines intensity of lifelong anticoagulation Tx
The mainstay of therapy for patients with APS is lifelong anticoagulation with a vitamin K antagonist. The intensity of anticoagulation is determined based on the presence of venous or arterial thrombosis. In patients who present with arterial thrombosis, a higher intensity vitamin K antagonist (ie, international normalized ratio [INR] goal > 3) or the addition of low-dose aspirin should be considered.9,10
Factor Xa inhibitors are generally not recommended at this time due to the lack of evidence to support their use.10 Additionally, a randomized clinical trial comparing rivaroxaban and warfarin in patients with triple antiphospholipid antibody positivity was terminated prematurely due to increased thromboembolic events in the rivaroxaban arm.11
For patients with secondary APS in the setting of SLE, hydroxychloroquine in combination with a vitamin K antagonist has been shown to decrease the risk for recurrent thrombosis compared with treatment with a vitamin K antagonist alone.12
Our patient was started on a heparin drip and transitioned to an oral vitamin K antagonist with an INR goal of 2 to 3. Lifelong anticoagulation was planned. The pain and discoloration in her hands improved on anticoagulation and had nearly resolved by the time of discharge. Given her history of arterial thrombosis, the addition of aspirin was also considered, but this decision was ultimately deferred to her outpatient rheumatologist and hematologist.
1. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. doi: 10.1111/j.1538-7836.2006.01753.x
2. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
3. Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346:752-763. doi: 10.1056/NEJMra002974
4. Cervera R, Khamashta MA, Font J, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. Medicine (Baltimore). 1993;72:113-124.
5. Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders: prevalence and clinical significance. Ann Intern Med. 1990;112:682-698. doi: 10.7326/0003-4819-112-9-682
6. Merkel PA, Chang YC, Pierangeli SS, et al. The prevalence and clinical associations of anticardiolipin antibodies in a large inception cohort of patients with connective tissue diseases. Am J Med. 1996;101:576-583. doi: 10.1016/s0002-9343(96)00335-x
7. Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15:145-151. doi: 10.1006/jaut. 2000.0409
8. Bozlar U, Ogur T, Khaja MS, et al. CT angiography of the upper extremity arterial system: Part 2—Clinical applications beyond trauma patients. AJR Am J Roentgenol. 2013;201:753-763. doi: 10.2214/AJR.13.11208
9. Ruiz-Irastorza G, Hunt BJ, Khamashta MA. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum. 2007;7:1487-1495. doi: 10.1002/art.23109
10. Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296-1304. doi: 10.1136/annrheumdis-2019-215213
Efficacy and safety of rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome: rationale and design of the Trial on Rivaroxaban in AntiPhospholipid Syndrome (TRAPS) trial. Lupus. 2016;25:301-306. doi: 10.1177/0961203315611495
12. Schmidt-Tanguy A, Voswinkel J, Henrion D, et al. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J Thromb Haemost. 2013;11:1927-1929. doi: 10.1111/jth.12363
A 48-year-old woman with a history of systemic lupus erythematosus (SLE) presented to the emergency department from the rheumatology clinic for digital ischemia. The clinical manifestations of her SLE consisted predominantly of arthralgias, which had been previously well controlled on hydroxychloroquine 300 mg/d PO. On presentation, she denied oral ulcers, alopecia, shortness of breath, chest pain/pressure, and history of blood clots or miscarriages.
On exam, the patient was afebrile and had a heart rate of 74 bpm; blood pressure, 140/77 mm Hg; and respiratory rate, 18 breaths/min. The fingertips on her left hand were tender and cool to the touch, and the fingertips of her second through fifth digits were blue (FIGURE).
Laboratory workup was notable for the following: hemoglobin, 9.3 g/dL (normal range, 11.6-15.2 g/dL) and erythrocyte sedimentation rate, 44 mm/h (normal range, ≤ 25 mm/h). Double-stranded DNA and complement levels were normal.
Transthoracic echocardiogram did not show any valvular vegetations, and blood cultures from admission were negative. Computed tomography angiography (CTA) with contrast of her left upper extremity showed a filling defect in the origin of the left subclavian artery. Digital plethysmography showed dampened flow signals in the second through fifth digits of the left hand.
Tests for antiphospholipid antibodies were positive for lupus anticoagulant; there were also high titers of anti-β-2-glycoprotein immunoglobulin (Ig) G (58 SGU; normal, ≤ 20 SGU) and anticardiolipin IgG (242.4 CU; normal, ≤ 20 CU).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Antiphospholipid syndrome
Given our patient’s SLE, left subclavian artery thrombosis, digital ischemia, and high-titer antiphospholipid antibodies, we had significant concern for antiphospholipid syndrome (APS). The diagnosis of APS is most often based on the fulfillment of the revised Sapporo classification criteria. These criteria include both clinical criteria (vascular thrombosis or pregnancy morbidity) and laboratory criteria (the presence of antiphospholipid antibodies on at least 2 separate occasions separated by 12 weeks).1 Our patient met clinical criteria given the evidence of subclavian artery thrombosis on CTA as well as digital plethysmography findings consistent with digital emboli. To meet laboratory criteria, she would have needed to have persistent high-titer antiphospholipid antibodies 12 weeks apart.
APS is an autoimmune disease in which the presence of antiphospholipid antibodies is associated with thrombosis; it can be divided into primary and secondary APS. The estimated prevalence of APS is 50 per 100,000 people in the United States.2 Primary APS occurs in the absence of an underlying autoimmune disease, while secondary APS occurs in the presence of an underlying autoimmune disease.
The autoimmune disease most often associated with APS is SLE.3 Among patients with SLE, 15% to 34% have positive lupus anticoagulant and 12% to 30% have anticardiolipin antibodies.4-6 This is compared with young healthy subjects among whom only 1% to 4% have positive lupus anticoagulant and 1% to 5% have anticardiolipin antibodies.7 Previous studies have estimated that 30% to 50% of patients with SLE who test positive for antiphospholipid antibodies will develop thrombosis.5,7
Differential includes Raynaud phenomenon, vasculitis
The differential diagnosis for digital ischemia in a patient with SLE includes APS, Raynaud phenomenon, vasculitis, and septic emboli.
Raynaud phenomenon can manifest in patients with SLE, but the presence of thrombosis on CTA and high-titer positive antiphospholipid antibodies make the diagnosis of APS more likely. Additionally, Raynaud phenomenon is typically temperature dependent with vasospasm in the digital arteries, occurring in cold temperatures and resolving with warming.
Systemic vasculitis may develop in patients with SLE, but in our case was less likely given the patient did not have any evidence of vasculitis on CTA, such as blood vessel wall thickening and/or enhancement.8
Septic emboli from endocarditis can cause digital ischemia but is typically associated with positive blood cultures, fever, and other systemic signs of infection, and/or vegetations on an echocardiogram.
Continue to: Thrombosis determines intensity of lifelong antiocagulation Tx
Thrombosis determines intensity of lifelong anticoagulation Tx
The mainstay of therapy for patients with APS is lifelong anticoagulation with a vitamin K antagonist. The intensity of anticoagulation is determined based on the presence of venous or arterial thrombosis. In patients who present with arterial thrombosis, a higher intensity vitamin K antagonist (ie, international normalized ratio [INR] goal > 3) or the addition of low-dose aspirin should be considered.9,10
Factor Xa inhibitors are generally not recommended at this time due to the lack of evidence to support their use.10 Additionally, a randomized clinical trial comparing rivaroxaban and warfarin in patients with triple antiphospholipid antibody positivity was terminated prematurely due to increased thromboembolic events in the rivaroxaban arm.11
For patients with secondary APS in the setting of SLE, hydroxychloroquine in combination with a vitamin K antagonist has been shown to decrease the risk for recurrent thrombosis compared with treatment with a vitamin K antagonist alone.12
Our patient was started on a heparin drip and transitioned to an oral vitamin K antagonist with an INR goal of 2 to 3. Lifelong anticoagulation was planned. The pain and discoloration in her hands improved on anticoagulation and had nearly resolved by the time of discharge. Given her history of arterial thrombosis, the addition of aspirin was also considered, but this decision was ultimately deferred to her outpatient rheumatologist and hematologist.
A 48-year-old woman with a history of systemic lupus erythematosus (SLE) presented to the emergency department from the rheumatology clinic for digital ischemia. The clinical manifestations of her SLE consisted predominantly of arthralgias, which had been previously well controlled on hydroxychloroquine 300 mg/d PO. On presentation, she denied oral ulcers, alopecia, shortness of breath, chest pain/pressure, and history of blood clots or miscarriages.
On exam, the patient was afebrile and had a heart rate of 74 bpm; blood pressure, 140/77 mm Hg; and respiratory rate, 18 breaths/min. The fingertips on her left hand were tender and cool to the touch, and the fingertips of her second through fifth digits were blue (FIGURE).
Laboratory workup was notable for the following: hemoglobin, 9.3 g/dL (normal range, 11.6-15.2 g/dL) and erythrocyte sedimentation rate, 44 mm/h (normal range, ≤ 25 mm/h). Double-stranded DNA and complement levels were normal.
Transthoracic echocardiogram did not show any valvular vegetations, and blood cultures from admission were negative. Computed tomography angiography (CTA) with contrast of her left upper extremity showed a filling defect in the origin of the left subclavian artery. Digital plethysmography showed dampened flow signals in the second through fifth digits of the left hand.
Tests for antiphospholipid antibodies were positive for lupus anticoagulant; there were also high titers of anti-β-2-glycoprotein immunoglobulin (Ig) G (58 SGU; normal, ≤ 20 SGU) and anticardiolipin IgG (242.4 CU; normal, ≤ 20 CU).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Antiphospholipid syndrome
Given our patient’s SLE, left subclavian artery thrombosis, digital ischemia, and high-titer antiphospholipid antibodies, we had significant concern for antiphospholipid syndrome (APS). The diagnosis of APS is most often based on the fulfillment of the revised Sapporo classification criteria. These criteria include both clinical criteria (vascular thrombosis or pregnancy morbidity) and laboratory criteria (the presence of antiphospholipid antibodies on at least 2 separate occasions separated by 12 weeks).1 Our patient met clinical criteria given the evidence of subclavian artery thrombosis on CTA as well as digital plethysmography findings consistent with digital emboli. To meet laboratory criteria, she would have needed to have persistent high-titer antiphospholipid antibodies 12 weeks apart.
APS is an autoimmune disease in which the presence of antiphospholipid antibodies is associated with thrombosis; it can be divided into primary and secondary APS. The estimated prevalence of APS is 50 per 100,000 people in the United States.2 Primary APS occurs in the absence of an underlying autoimmune disease, while secondary APS occurs in the presence of an underlying autoimmune disease.
The autoimmune disease most often associated with APS is SLE.3 Among patients with SLE, 15% to 34% have positive lupus anticoagulant and 12% to 30% have anticardiolipin antibodies.4-6 This is compared with young healthy subjects among whom only 1% to 4% have positive lupus anticoagulant and 1% to 5% have anticardiolipin antibodies.7 Previous studies have estimated that 30% to 50% of patients with SLE who test positive for antiphospholipid antibodies will develop thrombosis.5,7
Differential includes Raynaud phenomenon, vasculitis
The differential diagnosis for digital ischemia in a patient with SLE includes APS, Raynaud phenomenon, vasculitis, and septic emboli.
Raynaud phenomenon can manifest in patients with SLE, but the presence of thrombosis on CTA and high-titer positive antiphospholipid antibodies make the diagnosis of APS more likely. Additionally, Raynaud phenomenon is typically temperature dependent with vasospasm in the digital arteries, occurring in cold temperatures and resolving with warming.
Systemic vasculitis may develop in patients with SLE, but in our case was less likely given the patient did not have any evidence of vasculitis on CTA, such as blood vessel wall thickening and/or enhancement.8
Septic emboli from endocarditis can cause digital ischemia but is typically associated with positive blood cultures, fever, and other systemic signs of infection, and/or vegetations on an echocardiogram.
Continue to: Thrombosis determines intensity of lifelong antiocagulation Tx
Thrombosis determines intensity of lifelong anticoagulation Tx
The mainstay of therapy for patients with APS is lifelong anticoagulation with a vitamin K antagonist. The intensity of anticoagulation is determined based on the presence of venous or arterial thrombosis. In patients who present with arterial thrombosis, a higher intensity vitamin K antagonist (ie, international normalized ratio [INR] goal > 3) or the addition of low-dose aspirin should be considered.9,10
Factor Xa inhibitors are generally not recommended at this time due to the lack of evidence to support their use.10 Additionally, a randomized clinical trial comparing rivaroxaban and warfarin in patients with triple antiphospholipid antibody positivity was terminated prematurely due to increased thromboembolic events in the rivaroxaban arm.11
For patients with secondary APS in the setting of SLE, hydroxychloroquine in combination with a vitamin K antagonist has been shown to decrease the risk for recurrent thrombosis compared with treatment with a vitamin K antagonist alone.12
Our patient was started on a heparin drip and transitioned to an oral vitamin K antagonist with an INR goal of 2 to 3. Lifelong anticoagulation was planned. The pain and discoloration in her hands improved on anticoagulation and had nearly resolved by the time of discharge. Given her history of arterial thrombosis, the addition of aspirin was also considered, but this decision was ultimately deferred to her outpatient rheumatologist and hematologist.
1. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. doi: 10.1111/j.1538-7836.2006.01753.x
2. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
3. Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346:752-763. doi: 10.1056/NEJMra002974
4. Cervera R, Khamashta MA, Font J, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. Medicine (Baltimore). 1993;72:113-124.
5. Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders: prevalence and clinical significance. Ann Intern Med. 1990;112:682-698. doi: 10.7326/0003-4819-112-9-682
6. Merkel PA, Chang YC, Pierangeli SS, et al. The prevalence and clinical associations of anticardiolipin antibodies in a large inception cohort of patients with connective tissue diseases. Am J Med. 1996;101:576-583. doi: 10.1016/s0002-9343(96)00335-x
7. Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15:145-151. doi: 10.1006/jaut. 2000.0409
8. Bozlar U, Ogur T, Khaja MS, et al. CT angiography of the upper extremity arterial system: Part 2—Clinical applications beyond trauma patients. AJR Am J Roentgenol. 2013;201:753-763. doi: 10.2214/AJR.13.11208
9. Ruiz-Irastorza G, Hunt BJ, Khamashta MA. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum. 2007;7:1487-1495. doi: 10.1002/art.23109
10. Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296-1304. doi: 10.1136/annrheumdis-2019-215213
Efficacy and safety of rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome: rationale and design of the Trial on Rivaroxaban in AntiPhospholipid Syndrome (TRAPS) trial. Lupus. 2016;25:301-306. doi: 10.1177/0961203315611495
12. Schmidt-Tanguy A, Voswinkel J, Henrion D, et al. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J Thromb Haemost. 2013;11:1927-1929. doi: 10.1111/jth.12363
1. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. doi: 10.1111/j.1538-7836.2006.01753.x
2. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
3. Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346:752-763. doi: 10.1056/NEJMra002974
4. Cervera R, Khamashta MA, Font J, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. Medicine (Baltimore). 1993;72:113-124.
5. Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders: prevalence and clinical significance. Ann Intern Med. 1990;112:682-698. doi: 10.7326/0003-4819-112-9-682
6. Merkel PA, Chang YC, Pierangeli SS, et al. The prevalence and clinical associations of anticardiolipin antibodies in a large inception cohort of patients with connective tissue diseases. Am J Med. 1996;101:576-583. doi: 10.1016/s0002-9343(96)00335-x
7. Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15:145-151. doi: 10.1006/jaut. 2000.0409
8. Bozlar U, Ogur T, Khaja MS, et al. CT angiography of the upper extremity arterial system: Part 2—Clinical applications beyond trauma patients. AJR Am J Roentgenol. 2013;201:753-763. doi: 10.2214/AJR.13.11208
9. Ruiz-Irastorza G, Hunt BJ, Khamashta MA. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum. 2007;7:1487-1495. doi: 10.1002/art.23109
10. Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296-1304. doi: 10.1136/annrheumdis-2019-215213
Efficacy and safety of rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome: rationale and design of the Trial on Rivaroxaban in AntiPhospholipid Syndrome (TRAPS) trial. Lupus. 2016;25:301-306. doi: 10.1177/0961203315611495
12. Schmidt-Tanguy A, Voswinkel J, Henrion D, et al. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J Thromb Haemost. 2013;11:1927-1929. doi: 10.1111/jth.12363