User login
A 56-year-old Japanese man with a history of renal transplantation 20 years prior presented to the emergency department (ED) with two months of dyspnea on exertion and one day of fever and chills. The patient was in his usual state of health until two months prior to presentation, when he gradually noticed shortness of breath after sustained or effortful physical activities. The dyspnea improved with rest. Over the following two months, he noticed that the shortness of breath came on with lesser degrees of exertion, such as walking 100 meters. One day before presentation, he developed a fever of 39°C and chills at home, which prompted him to seek ED care. He denied chest pain, cough, leg swelling, or paroxysmal nocturnal dyspnea.
The differential diagnosis of exertional dyspnea progressing over several months includes cardiac, pulmonary, hematologic, and neuromuscular conditions. The patient’s history of renal transplantation prompts consideration of worsening indolent pneumonia (eg, Aspergillus, cytomegalovirus [CMV], or Pneumocystis pneumonia), allograft dysfunction with volume overload, recrudescence of the underlying disease that incited renal failure earlier in life (eg, vasculitis), or a late-onset posttransplantation lymphoproliferative disorder (PTLD). Additionally, acute fever in an immunocompromised patient immediately raises suspicion for infection (eg, pneumonia, enteritis, or urinary tract infection). At this point, it is difficult to know whether the subacute-to-chronic exertional dyspnea and the acute fever are consequences of the same disease or separate, potentially overlapping, processes.
His past medical history was significant for end-stage renal disease due to membranoproliferative glomerular nephropathy (MPGN), for which living, related-donor kidney transplantation was performed 20 years earlier. He also had type 2 diabetes mellitus, hypertension, and basal cell carcinoma of the face, which had been resected three years prior without spread or recurrence. He had no known allergies. Medications included prednisolone 15 mg daily, azathioprine 100 mg daily, and cyclosporine 100 mg daily, as well as amlodipine and candesartan. He lived in Japan with his wife and children. He denied any animal or environmental exposures. He did not smoke cigarettes or drink alcohol and had not traveled recently. His father had diabetes mellitus.
Recrudescence of an underlying autoimmune condition that may have incited MPGN earlier in life is unlikely while taking an immunosuppressive regimen consisting of prednisolone, azathioprine, and cyclosporine. However, these medications do increase susceptibility to infections, lymphoma, and skin cancers. Though he is immunocompromised, the patient is not on prophylaxis for Pneumocystis pneumonia (PCP). PCP in HIV-negative patients is associated with recent glucocorticoid exposure and typically follows an acute-to-subacute course with hypoxemia and respiratory distress. Though the risk of PCP infection is considered highest in the early posttransplantation period (when immunosuppression is most intense), many cases are diagnosed years after transplantation among patients no longer on prophylaxis. The patient has type 2 diabetes mellitus and hypertension, which are known complications of calcineurin inhibitor and steroid therapy and increase the risk of cardiovascular disease. Cardiovascular disease is a major cause of death among renal transplant recipients. Exertional dyspnea may be the presenting symptom of coronary artery disease.
On physical examination, the patient was alert, oriented, and in no acute distress. His temperature was 38.5°C, blood pressure 120/60 mm Hg, heart rate 146 beats per minute, respiratory rate 18 breaths per minute, and oxygen saturation 93% while breathing ambient air. The conjunctiva were normal without pallor or icterus. There was no cervical lymphadenopathy. Cardiac examination revealed tachycardia with a regular rhythm, normal S1 and S2, and no murmurs, rubs, or gallops. Jugular venous pressure was not elevated, and there was no lower extremity edema. Lungs were clear to auscultation bilaterally. The abdomen was soft, nontender, and nondistended. There was no tenderness over the transplanted kidney and no hepatosplenomegaly.
Dyspnea, fever, and tachycardia may be the sole manifestations of pneumonia in solid organ transplant recipients. The absence of cough or adventitious breath sounds does not eliminate concern for pneumonia. Pathogens that cause indolent pneumonia in immunocompromised patients include viruses (such as typical respiratory viruses and CMV), bacteria (typical organisms, Nocardia, Rhodococcus), and mycobacteria. Fungal causes include Aspergillus, Candida, Cryptococcus, Pneumocystis, and endemic mycoses. A detailed environmental history should be taken, and providers should ascertain which fungal diseases are endemic in the patient’s region of residence. There are no examination features suggesting hypervolemia or anemia. Although there is no hepatosplenomegaly or lymphadenopathy, PTLD often involves extranodal tissues, including the lungs. The incidence of PTLD is highest in the 12 months following transplantation, but it may occur at any time in the posttransplantation course. A complete blood count, comprehensive metabolic panel, lactate dehydrogenase (LDH), and blood and sputum cultures are indicated, along with computed tomography (CT) of the chest.
The leukocyte count was 3,500 cells/mm3, the hemoglobin level 9.0 g/dL, mean corpuscular volume 102 fL, and the platelet count 137,000 cells/mm3. The sodium level was 130 mEq/L, potassium 4.6 mEq/L, blood urea nitrogen 41 mg/dL, and creatinine 3.5 mg/dL. These complete blood count and serum electrolyte results were unchanged from the patient’s baseline values. The serum LDH level was 1,895 IU/L (normal range, 115-245 IU/L). The serum ferritin was 2,114 ng/mL (normal range, 13-277 ng/mL). A chest radiograph revealed diffuse, airspace-filling opacities in the bilateral lung bases. The urinalysis was normal. The patient was admitted and started empirically on intravenous ceftriaxone for potential bacterial pneumonia.
Chronic pancytopenia may result from azathioprine or cyclosporine use, marrow suppression or infiltration by a multisystem disease, or nutritional deficiency. Hemophagocytic lymphohistiocytosis (HLH) triggered by infection, a rheumatologic condition, acquired immunodeficiency, or malignancy can present with fevers, pancytopenia, and elevated ferritin, while splenomegaly may be absent. The euvolemic state, baseline creatinine level, and normal urinalysis argue against allograft dysfunction. The elevated serum ferritin nonspecifically confirms systemic inflammation. LDH, an intracellular enzyme involved in the bidirectional conversion of lactate to pyruvate, is expressed across tissue types. Elevated serum LDH attests to cell destruction, in this case potentially from lung infection (such as PCP) or malignancy (such as PTLD). At this point, the differential diagnosis of fever and pulmonary infiltrates in this patient remains broad.
Azathioprine and cyclosporine were stopped. The patient remained febrile despite the administration of intravenous antibiotics. His hypoxia worsened with an oxygen saturation of 90%-93% on 5 L/min of supplemental oxygen administered by nasal cannula. Noncontrast chest CT obtained on the second hospital day revealed ground-glass opacities in the bilateral lung bases. Blood, sputum, and urine cultures were sterile. As empiric therapies, ganciclovir was started for CMV infection, ciprofloxacin added for atypical pneumonia, and trimethoprim-sulfamethoxazole added for Pneumocystis infection.
These chest imaging findings help prioritize the differential diagnosis. Bibasilar ground-glass opacities are evident, while pulmonary masses, nodules, cavitation, adenopathy, and pleural effusions are absent. The differential diagnosis of multifocal ground-glass opacities on chest imaging includes infectious pneumonia, chronic interstitial lung disease, acute alveolar conditions (eg, cardiogenic pulmonary edema, acute respiratory distress syndrome, diffuse alveolar hemorrhage), or other pathologies (eg, drug toxicity, bronchoalveolar carcinoma, cryptogenic organizing pneumonia).
Infectious pneumonia is the principal concern. A diagnosis of PCP could be unifying, given dyspnea, progressive respiratory failure with hypoxia, and elevated LDH in an immunocompromised patient who is not prescribed PCP prophylaxis. The bilateral lung infiltrates and the absence of thoracic adenopathy or pleural effusions are characteristic of PCP as well. However, caution should be exercised in making specific infectious diagnoses in immunocompromised hosts on the basis of clinical and imaging findings alone. There can be overlap in the radiologic appearance of various infections (eg, CMV pneumonia can also present with bilateral ground-glass infiltrates, with concurrent fever, hypoxia, and pancytopenia). Additionally, more than one pneumonic pathogen may be implicated (eg, acute viral pneumonia superimposed on indolent fungal pneumonia). Polymerase chain reaction (PCR) analysis of respiratory secretions for viruses, serum PCR and serologic testing for herpes viruses, and serum beta-D-glucan and galactomannan assays are indicated. Serum serologic testing for fungi and bacteria such as Nocardia can be helpful, though the negative predictive values of these tests may be reduced in patients with impaired humoral immunity. Timely bronchoalveolar lavage (BAL) with microbiologic and PCR analysis and cytology is advised.
Fever, elevated LDH, cytopenias, and pulmonary infiltrates also raise suspicion for an underlying hematologic malignancy, such as PTLD. However, pulmonary PTLD is seen more often in lung transplant recipients than in patients who have undergone transplantation of other solid organs. In kidney transplant recipients, PTLD most commonly manifests in the allograft itself, gastrointestinal tract, central nervous system, or lymph nodes; lung involvement is less common. Chest imaging in affected patients may reveal nodular or reticulonodular infiltrates of basilar predominance, solitary or multiple masses, cavitating or necrotic lesions, and/or lymphadenopathy. In this patient who has undergone renal transplantation, late-onset PTLD with isolated pulmonary involvement, with only ground-glass opacities on lung imaging, would be an atypical presentation of an uncommon syndrome.
Despite empiric treatment with antibiotics and antiviral agents, the patient’s fever persisted. His respiratory rate increased to 30 breaths per minute. His hypoxia worsened, and he required nasal cannula high-flow oxygen supplementation at 30 L/min with a fraction of inspired oxygen (FiO2) of 40%. On the fifth hospital day, contrast CT scan of the chest and abdomen showed new infiltrates in the bilateral upper lung fields as well as an area of low density in the tail of the pancreas without a focal mass (Figure 1). At this point, BAL was performed, and fluid PCR analysis returned positive for Pneumocystis jirovecii. CMV direct immunoperoxidase staining of leukocytes with peroxidase-labeled monoclonal antibody (C7-HRP test) was positive at five cells per 7.35 × 104 peripheral blood leukocytes. The serum Epstein-Barr virus (EBV) viral capsid antigen (VCA) IgG was positive, while VCA IgM and EBV nuclear antigen IgG were negative. A bone marrow biopsy revealed mild hemophagocytosis. His serum soluble interleukin-2 (sIL2R) level was elevated at 5,254 U/mL (normal range, 122-496 U/mL). Given the BAL Pneumocystis PCR result, the dose of prednisolone was increased to 30 mg/day, and the patient’s fever subsided. Supplemental oxygen was weaned to an FiO2 of 35%.
These studies should be interpreted carefully considering the biphasic clinical course. After two months of exertional dyspnea, the patient acutely developed persistent fever and progressive lung infiltrates. His clinical course, the positive PCR assay for Pneumocystis jirovecii in BAL fluid, and the compatible lung imaging findings make Pneumocystis jirovecii a likely pathogen. But PCP may only explain the second phase of this patient’s illness, considering its often-fulminant course in HIV-negative patients. To explain the two months of exertional dyspnea, marrow hemophagocytosis, pancreatic abnormality, and perhaps even the patient’s heightened susceptibility to PCP infection, an index of suspicion should be maintained for a separate, antecedent process. This could be either an indolent infection (eg, CMV or Aspergillus pneumonia) or a malignancy (eg, lymphoma or PTLD). Completion of serum serologic testing for viruses, bacteria, and fungi and comprehensive BAL fluid analysis (culture, viral PCR, and cytology) is recommended.
A CMV antigenemia assay returned positive, suggesting prior CMV infection. However, to diagnose CMV pneumonia, the virus must be detected in BAL fluid by PCR or cytologic analysis. CMV infection has been associated with cytopenias, HLH, pancreatic infiltration, and an increased risk for fungal infections and EBV-related PTLD. CMV infection could explain the first phase of this patient’s illness. Serum and BAL PCR for CMV are advised. Meanwhile, EBV testing indicates prior infection but does not distinguish between recent or more distant infection. EBV has been implicated in the pathophysiology of PTLD, as EBV-infected lymphoid tissue may proliferate in a variety of organs under reduced T-cell surveillance. EBV infection or PTLD with resulting immunomodulation may pose other explanations for this patient’s development of PCP infection. Cytologic analysis of the BAL fluid and marrow aspirate for evidence of PTLD is warranted. Finally, CMV, EBV, and PTLD have each been reported to trigger HLH. Though this patient has fevers, mild marrow hemophagocytosis, elevated serum ferritin, and elevated serum IL-2 receptor levels, he does not meet other diagnostic criteria for HLH (such as more pronounced cytopenias, splenomegaly, hypertriglyceridemia, hypofibrinogenemia, and low or absent natural killer T-cell activity). However, HLH may be muted in this patient because he was prescribed cyclosporine, which has been used in HLH treatment protocols.
On the 11th hospital day, the patient developed hemorrhagic shock due to massive hematemesis and was transferred to the intensive care unit. His hemoglobin level was 5.9 g/dL. A total of 18 units of packed red blood cells were transfused over the following week for ongoing gastrointestinal bleeding. The serum LDH level increased to 4,139 IU/L, and the ferritin level rose to 7,855 ng/mL. The EBV copy level by serum PCR returned at 1 × 106 copies/mL (normal range, less than 2 x 102 copies/mL). The patient was started on methylprednisolone (1 g/day for three days) and transitioned to dexamethasone and cyclosporine for possible EBV-related HLH. Ceftazidime, vancomycin, trimethoprim-sulfamethoxazole, and ciprofloxacin were administered. Amphotericin-B was initiated empirically for potential fungal pneumonia. Ganciclovir was continued. However, the patient remained in shock despite vasopressors and transfusions and died on the 22nd hospital day.
The patient deteriorated despite broad antimicrobial therapy. Laboratory studies revealed EBV viremia and rising serum LDH. Recent EBV infection may have induced PTLD in the gastrointestinal tract, which is a commonly involved site among affected renal transplant patients. Corticosteroids and stress from critical illness can contribute to intestinal mucosal erosion and bleeding from a luminal PTLD lesion. Overall, the patient’s condition was likely explained by EBV infection, which triggered HLH and gastrointestinal PTLD. The resulting immunomodulation increased his risk for PCP infection beyond that conferred by chronic immunosuppression. It is still possible that he had concomitant CMV pneumonia, Aspergillus pneumonia, or even pulmonary PTLD, in addition to the proven PCP diagnosis.
An autopsy was performed. Atypical lymphocytic infiltration and diffuse alveolar damage were shown in the right upper lobe (Figure 2). EBV RNA-positive atypical lymphocytes coexpressing CD20 were demonstrated in multiple organs including the bone marrow, lungs, heart, stomach, adrenal glands, duodenum, ileum, and mesentery (Figure 3). This confirmed the diagnosis of an underlying EBV-positive posttransplant lymphoproliferative disorder. Serum and BAL CMV PCR assays returned negative. Neither CMV nor Aspergillus was identified in autopsy specimens.
COMMENTARY
A broad differential diagnosis should be considered when acute fever develops in a patient who has undergone solid organ transplantation. Causes may include opportunistic and nonopportunistic infections as well as noninfectious etiologies such as malignancy, organ rejection, inflammatory conditions, and medication toxicity.1,2 As the discussant noted, more than one infection, or both infection and malignancy, can coexist in immunocompromised patients. For example, while viral pathogens such as EBV, CMV, and respiratory syncytial virus can cause illness due to direct tissue infection, they can also exert indirect effects in transplant recipients: acting as cofactors for and enabling other infections by causing immunosuppression (eg, Aspergillus or PCP developing after CMV infection), triggering graft rejection by upregulating proinflammatory cytokines, and inducing oncogenesis (eg, EBV-related PTLD).1,3-5
PTLD is a rare but serious complication of solid organ transplantation and immunosuppression. Most cases are driven by EBV infection and subsequent transformation of infected lymphoid tissue in a variety of organs in the context of reduced T-cell surveillance.6 The incidence of PTLD varies based on the organ transplanted, ranging from 0.8%-2.5% in those who have undergone renal transplantation to 1.0%-5.5% in liver transplant recipients and 3.0%-10% in lung transplant recipients.3 The incidence has increased over the past decade. This may be due to a greater number of solid organ transplantations being performed, aging of the transplant donor/recipient population, new immunosuppressive regimens, and improved PTLD diagnosis due to superior diagnostic tools and clinician awareness.3 However, the mortality rate among solid organ transplant recipients with PTLD remains high, ranging from 40% to 70%.6
Risk factors for PTLD include a greater intensity of T-cell immunosuppression,7 history of pretransplant malignancy, recipient EBV seronegativity and donor seropositivity, and younger age at the time of transplantation.8-10 EBV-related PTLD incidence in solid organ transplant recipients is greatest in the early posttransplantation course (the period of most intense immunosuppression) with over 80% of cases occurring in the first posttransplant year.11
A high index of suspicion for PTLD is warranted in any solid organ transplant recipient who presents with constitutional symptoms, adenopathy, or cytopenias. Clinical suspicion of PTLD can be informed by risk factors, constitutional symptoms, elevated serum LDH, a detectable or rising serum EBV viral load, and radiologic adenopathy or visceral tissue infiltration.12 The clinical presentation of PTLD is heterogeneous and varies in accordance with the organs affected. Extranodal involvement, such as pulmonary, gastrointestinal, and bone marrow involvement, is more common in PTLD than in other types of lymphoma.13 In this patient, the cytopenias, elevated serum LDH level, lung infiltrates, and radiologic pancreatic tail abnormality served as early clues to the presence of underlying PTLD.
The standard approach to diagnosing PTLD is biopsy of a suspicious lesion (adenopathy or an infiltrated visceral organ) with histopathological examination. Pathology may demonstrate distorted tissue architecture, clonal lymphocytes, or EBV-positive lymphocytes.14 Conventional CT is the most commonly used imaging modality to detect adenopathy or tissue infiltration related to PTLD,3 though 18F-fluorodeoxyglucose position-emission tomography (FDG-PET) is also used. Although FDG-PET has high diagnostic accuracy, with an overall sensitivity of 89% and specificity of 89%, false-negative results have been reported, particularly in cases of early PTLD lesions and diffuse large B-cell lymphoma.15 The majority of patients with EBV-associated PTLD demonstrate significant elevations in the serum EBV viral load compared with immunosuppressed controls without PTLD.16 An elevated EBV viral load can support a diagnosis of PTLD, though the absence of EBV viremia does not rule it out.17 Some transplant centers perform posttransplantation monitoring of the serum EBV viral load to aid in PTLD risk assessment and early diagnosis.
Management of PTLD is patient-specific and may involve reduction of immunosuppressive therapy, rituximab, chemotherapy, surgical excision, and/or radiation.13 Reduction of immunosuppression is the cornerstone of treatment.18 In patients who do not respond to the reduction of immunosuppression, rituximab and immunochemotherapy are second-line treatment options. A prospective, multicenter phase 2 trial (the PTLD-1 trial) demonstrated a complete response rate of 40% among patients with PTLD managed with rituximab.19
In summary, this case illustrates the importance of maintaining a broad differential diagnosis when acute fever develops in a patient who has undergone solid organ transplantation. The presence of more than one condition should be considered when the clinical presentation cannot be explained by a single diagnosis, as infections and malignancies can coexist in immunocompromised hosts. This case also highlights an unusual clinical presentation of PTLD, which was heralded mainly by its immunomodulatory effects rather than by compatible symptoms or obvious mass lesions.
Carefully reviewing the patient’s medical history and understanding how it sets the stage for the present illness is an essential step in clinical problem solving, because what is past is prologue.
TEACHING POINTS
- Fever in solid organ transplant recipients should prompt consideration of a broad differential diagnosis, including infection, malignancy, organ graft rejection, autoimmune disease, and medication toxicity.
- PTLD is a rare but serious complication of organ transplantation. Most cases are driven by EBV infection and transformation of infected lymphocytes in a variety of organs in the context of reduced T-cell surveillance. The clinical presentation can be heterogeneous and varies depending on the organs and tissues involved.
- More than one infection, or both infection and malignancy, can coexist in organ transplant recipients. Viral pathogens can exert direct pathologic effects on tissue but can also exert indirect effects, such as contributing to opportunistic infection susceptibility, graft rejection, and oncogenesis.
Disclosures
The authors have nothing to disclose.
Previous Publication
This case was originally reported in the 121st Okinawa Association of Medical Sciences in 2015 in Okinawa, Japan, and the conference abstracts were covered in The Okinawa Medical Journal. The publication did not provide any detailed, step-by-step analysis of clinical decision-making.
1. Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601-2614. https://doi.org/10.1056/NEJMra064928.
2. Bouza E, Loeches B, Muñoz P. Fever of unknown origin in solid organ transplant recipients. Infect Dis Clin North Am. 2007;21(4):1033-1054, ix-x. https://doi.org/10.1016/j.idc.2007.09.001,
3. Kotton CN, Fishman JA. Viral infection in the renal transplant recipient. J Am Soc Nephrol. 2005;16(6):1758-1774. https://doi.org/10.1681/ASN.2004121113.
4. Arend SM, Westendorp RG, Kroon FP, et al. Rejection treatment and cytomegalovirus infection as risk factors for Pneumocystis carinii pneumonia in renal transplant recipients. Clin Infect Dis. 1996;22(6):920-925. https://doi.org/10.1093/clinids/22.6.920.
5. Reinke P, Fietze E, Ode-Hakim S, et al. Late-acute renal allograft rejection and symptomless cytomegalovirus infection. Lancet. 1994;344(8939-8940):1737-1738. https://doi.org/10.1016/S0140-6736(94)92887-8.
6. Tsai DE, Douglas L, Andreadis C, et al. EBV PCR in the diagnosis and monitoring of posttransplant lymphoproliferative disorder: results of a two-arm prospective trial. Am J Transplant. 2008;8(5):1016-1024. https://doi.org/10.1111/j.1600-6143.2008.02183.x.
7. Penn I. Cancers complicating organ transplantation. N Engl J Med. 1990;323(25):1767-1769. https://doi.org/10.1056/NEJM199012203232510
8. Walker RC, Marshall WF, Strickler JG, et al. Pretransplantation assessment of the risk of lymphoproliferative disorder. Clin Infect Dis. 1995;20(5):1346-1353. https://doi.org/10.1093/clinids/20.5.1346.
9. Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4(2):222-230. https://doi.org/10.1046/j.1600-6143.2003.00325.x.
10. Caillard S, Dharnidharka V, Agodoa L, Bohen E, Abbott K. Posttransplant lymphoproliferative disorders after renal transplantation in the United States in era of modern immunosuppression. Transplantation. 2005;80(9):1233-1243. doi: 10.1097/01.tp.0000179639.98338.39.
11. Opelz G, Henderson R. Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients. Lancet. 1993;342(8886-8887):1514-1516. https://doi.org/10.1016/S0140-6736(05)80084-4.
12. Samant H, Kothadia JP. Transplantation Posttransplantation Lymphoproliferative Disorders. Treasure Island, FL: StatPearls Publishing; 2018. PubMed
13. Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med. 2018;378(6):549-562. https://doi.org/10.1056/NEJMra1702693.
14. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. https://doi.org/10.1182/blood-2016-01-643569.
15. Dierickx D, Tousseyn T, Requilé A, et al. The accuracy of positron emission tomography in the detection of posttransplant lymphoproliferative disorder. Haematologica. 2013;98(5):771-775. https://doi.org/10.3324/haematol.2012.074500.
16. Wagner HJ, Wessel M, Jabs W, et al. Patients at risk for development of posttransplant lymphoproliferative disorder: plasma versus peripheral blood mononuclear cells as material for quantification of Epstein-Barr viral load by using real-time quantitative polymerase chain reaction. Transplantation. 2001;72(6):1012-1019. PubMed
17. Baldanti F, Rognoni V, Cascina A, Oggionni T, Tinelli C, Meloni F. Post-transplant lymphoproliferative disorders and Epstein-Barr virus DNAemia in a cohort of lung transplant recipients. Virol J. 2011;8:421. https://doi.org/10.1186/1743-422X-8-421.
18. Parker A, Bowles K, Bradley JA, et al. Management of post-transplant lymphoproliferative disorder in adult solid organ transplant recipients - BCSH and BTS Guidelines. Br J Haematol. 2010;149(5):693-705. https://doi.org/10.1111/j.1365-2141.2010.08160.x.
19. Trappe R, Oertel S, Leblond V, et al. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012;13(2):196-206. https://doi.org/10.1016/S1470-2045(11)70300-X.
A 56-year-old Japanese man with a history of renal transplantation 20 years prior presented to the emergency department (ED) with two months of dyspnea on exertion and one day of fever and chills. The patient was in his usual state of health until two months prior to presentation, when he gradually noticed shortness of breath after sustained or effortful physical activities. The dyspnea improved with rest. Over the following two months, he noticed that the shortness of breath came on with lesser degrees of exertion, such as walking 100 meters. One day before presentation, he developed a fever of 39°C and chills at home, which prompted him to seek ED care. He denied chest pain, cough, leg swelling, or paroxysmal nocturnal dyspnea.
The differential diagnosis of exertional dyspnea progressing over several months includes cardiac, pulmonary, hematologic, and neuromuscular conditions. The patient’s history of renal transplantation prompts consideration of worsening indolent pneumonia (eg, Aspergillus, cytomegalovirus [CMV], or Pneumocystis pneumonia), allograft dysfunction with volume overload, recrudescence of the underlying disease that incited renal failure earlier in life (eg, vasculitis), or a late-onset posttransplantation lymphoproliferative disorder (PTLD). Additionally, acute fever in an immunocompromised patient immediately raises suspicion for infection (eg, pneumonia, enteritis, or urinary tract infection). At this point, it is difficult to know whether the subacute-to-chronic exertional dyspnea and the acute fever are consequences of the same disease or separate, potentially overlapping, processes.
His past medical history was significant for end-stage renal disease due to membranoproliferative glomerular nephropathy (MPGN), for which living, related-donor kidney transplantation was performed 20 years earlier. He also had type 2 diabetes mellitus, hypertension, and basal cell carcinoma of the face, which had been resected three years prior without spread or recurrence. He had no known allergies. Medications included prednisolone 15 mg daily, azathioprine 100 mg daily, and cyclosporine 100 mg daily, as well as amlodipine and candesartan. He lived in Japan with his wife and children. He denied any animal or environmental exposures. He did not smoke cigarettes or drink alcohol and had not traveled recently. His father had diabetes mellitus.
Recrudescence of an underlying autoimmune condition that may have incited MPGN earlier in life is unlikely while taking an immunosuppressive regimen consisting of prednisolone, azathioprine, and cyclosporine. However, these medications do increase susceptibility to infections, lymphoma, and skin cancers. Though he is immunocompromised, the patient is not on prophylaxis for Pneumocystis pneumonia (PCP). PCP in HIV-negative patients is associated with recent glucocorticoid exposure and typically follows an acute-to-subacute course with hypoxemia and respiratory distress. Though the risk of PCP infection is considered highest in the early posttransplantation period (when immunosuppression is most intense), many cases are diagnosed years after transplantation among patients no longer on prophylaxis. The patient has type 2 diabetes mellitus and hypertension, which are known complications of calcineurin inhibitor and steroid therapy and increase the risk of cardiovascular disease. Cardiovascular disease is a major cause of death among renal transplant recipients. Exertional dyspnea may be the presenting symptom of coronary artery disease.
On physical examination, the patient was alert, oriented, and in no acute distress. His temperature was 38.5°C, blood pressure 120/60 mm Hg, heart rate 146 beats per minute, respiratory rate 18 breaths per minute, and oxygen saturation 93% while breathing ambient air. The conjunctiva were normal without pallor or icterus. There was no cervical lymphadenopathy. Cardiac examination revealed tachycardia with a regular rhythm, normal S1 and S2, and no murmurs, rubs, or gallops. Jugular venous pressure was not elevated, and there was no lower extremity edema. Lungs were clear to auscultation bilaterally. The abdomen was soft, nontender, and nondistended. There was no tenderness over the transplanted kidney and no hepatosplenomegaly.
Dyspnea, fever, and tachycardia may be the sole manifestations of pneumonia in solid organ transplant recipients. The absence of cough or adventitious breath sounds does not eliminate concern for pneumonia. Pathogens that cause indolent pneumonia in immunocompromised patients include viruses (such as typical respiratory viruses and CMV), bacteria (typical organisms, Nocardia, Rhodococcus), and mycobacteria. Fungal causes include Aspergillus, Candida, Cryptococcus, Pneumocystis, and endemic mycoses. A detailed environmental history should be taken, and providers should ascertain which fungal diseases are endemic in the patient’s region of residence. There are no examination features suggesting hypervolemia or anemia. Although there is no hepatosplenomegaly or lymphadenopathy, PTLD often involves extranodal tissues, including the lungs. The incidence of PTLD is highest in the 12 months following transplantation, but it may occur at any time in the posttransplantation course. A complete blood count, comprehensive metabolic panel, lactate dehydrogenase (LDH), and blood and sputum cultures are indicated, along with computed tomography (CT) of the chest.
The leukocyte count was 3,500 cells/mm3, the hemoglobin level 9.0 g/dL, mean corpuscular volume 102 fL, and the platelet count 137,000 cells/mm3. The sodium level was 130 mEq/L, potassium 4.6 mEq/L, blood urea nitrogen 41 mg/dL, and creatinine 3.5 mg/dL. These complete blood count and serum electrolyte results were unchanged from the patient’s baseline values. The serum LDH level was 1,895 IU/L (normal range, 115-245 IU/L). The serum ferritin was 2,114 ng/mL (normal range, 13-277 ng/mL). A chest radiograph revealed diffuse, airspace-filling opacities in the bilateral lung bases. The urinalysis was normal. The patient was admitted and started empirically on intravenous ceftriaxone for potential bacterial pneumonia.
Chronic pancytopenia may result from azathioprine or cyclosporine use, marrow suppression or infiltration by a multisystem disease, or nutritional deficiency. Hemophagocytic lymphohistiocytosis (HLH) triggered by infection, a rheumatologic condition, acquired immunodeficiency, or malignancy can present with fevers, pancytopenia, and elevated ferritin, while splenomegaly may be absent. The euvolemic state, baseline creatinine level, and normal urinalysis argue against allograft dysfunction. The elevated serum ferritin nonspecifically confirms systemic inflammation. LDH, an intracellular enzyme involved in the bidirectional conversion of lactate to pyruvate, is expressed across tissue types. Elevated serum LDH attests to cell destruction, in this case potentially from lung infection (such as PCP) or malignancy (such as PTLD). At this point, the differential diagnosis of fever and pulmonary infiltrates in this patient remains broad.
Azathioprine and cyclosporine were stopped. The patient remained febrile despite the administration of intravenous antibiotics. His hypoxia worsened with an oxygen saturation of 90%-93% on 5 L/min of supplemental oxygen administered by nasal cannula. Noncontrast chest CT obtained on the second hospital day revealed ground-glass opacities in the bilateral lung bases. Blood, sputum, and urine cultures were sterile. As empiric therapies, ganciclovir was started for CMV infection, ciprofloxacin added for atypical pneumonia, and trimethoprim-sulfamethoxazole added for Pneumocystis infection.
These chest imaging findings help prioritize the differential diagnosis. Bibasilar ground-glass opacities are evident, while pulmonary masses, nodules, cavitation, adenopathy, and pleural effusions are absent. The differential diagnosis of multifocal ground-glass opacities on chest imaging includes infectious pneumonia, chronic interstitial lung disease, acute alveolar conditions (eg, cardiogenic pulmonary edema, acute respiratory distress syndrome, diffuse alveolar hemorrhage), or other pathologies (eg, drug toxicity, bronchoalveolar carcinoma, cryptogenic organizing pneumonia).
Infectious pneumonia is the principal concern. A diagnosis of PCP could be unifying, given dyspnea, progressive respiratory failure with hypoxia, and elevated LDH in an immunocompromised patient who is not prescribed PCP prophylaxis. The bilateral lung infiltrates and the absence of thoracic adenopathy or pleural effusions are characteristic of PCP as well. However, caution should be exercised in making specific infectious diagnoses in immunocompromised hosts on the basis of clinical and imaging findings alone. There can be overlap in the radiologic appearance of various infections (eg, CMV pneumonia can also present with bilateral ground-glass infiltrates, with concurrent fever, hypoxia, and pancytopenia). Additionally, more than one pneumonic pathogen may be implicated (eg, acute viral pneumonia superimposed on indolent fungal pneumonia). Polymerase chain reaction (PCR) analysis of respiratory secretions for viruses, serum PCR and serologic testing for herpes viruses, and serum beta-D-glucan and galactomannan assays are indicated. Serum serologic testing for fungi and bacteria such as Nocardia can be helpful, though the negative predictive values of these tests may be reduced in patients with impaired humoral immunity. Timely bronchoalveolar lavage (BAL) with microbiologic and PCR analysis and cytology is advised.
Fever, elevated LDH, cytopenias, and pulmonary infiltrates also raise suspicion for an underlying hematologic malignancy, such as PTLD. However, pulmonary PTLD is seen more often in lung transplant recipients than in patients who have undergone transplantation of other solid organs. In kidney transplant recipients, PTLD most commonly manifests in the allograft itself, gastrointestinal tract, central nervous system, or lymph nodes; lung involvement is less common. Chest imaging in affected patients may reveal nodular or reticulonodular infiltrates of basilar predominance, solitary or multiple masses, cavitating or necrotic lesions, and/or lymphadenopathy. In this patient who has undergone renal transplantation, late-onset PTLD with isolated pulmonary involvement, with only ground-glass opacities on lung imaging, would be an atypical presentation of an uncommon syndrome.
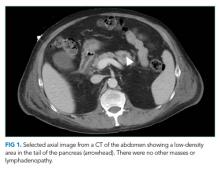
Despite empiric treatment with antibiotics and antiviral agents, the patient’s fever persisted. His respiratory rate increased to 30 breaths per minute. His hypoxia worsened, and he required nasal cannula high-flow oxygen supplementation at 30 L/min with a fraction of inspired oxygen (FiO2) of 40%. On the fifth hospital day, contrast CT scan of the chest and abdomen showed new infiltrates in the bilateral upper lung fields as well as an area of low density in the tail of the pancreas without a focal mass (Figure 1). At this point, BAL was performed, and fluid PCR analysis returned positive for Pneumocystis jirovecii. CMV direct immunoperoxidase staining of leukocytes with peroxidase-labeled monoclonal antibody (C7-HRP test) was positive at five cells per 7.35 × 104 peripheral blood leukocytes. The serum Epstein-Barr virus (EBV) viral capsid antigen (VCA) IgG was positive, while VCA IgM and EBV nuclear antigen IgG were negative. A bone marrow biopsy revealed mild hemophagocytosis. His serum soluble interleukin-2 (sIL2R) level was elevated at 5,254 U/mL (normal range, 122-496 U/mL). Given the BAL Pneumocystis PCR result, the dose of prednisolone was increased to 30 mg/day, and the patient’s fever subsided. Supplemental oxygen was weaned to an FiO2 of 35%.
These studies should be interpreted carefully considering the biphasic clinical course. After two months of exertional dyspnea, the patient acutely developed persistent fever and progressive lung infiltrates. His clinical course, the positive PCR assay for Pneumocystis jirovecii in BAL fluid, and the compatible lung imaging findings make Pneumocystis jirovecii a likely pathogen. But PCP may only explain the second phase of this patient’s illness, considering its often-fulminant course in HIV-negative patients. To explain the two months of exertional dyspnea, marrow hemophagocytosis, pancreatic abnormality, and perhaps even the patient’s heightened susceptibility to PCP infection, an index of suspicion should be maintained for a separate, antecedent process. This could be either an indolent infection (eg, CMV or Aspergillus pneumonia) or a malignancy (eg, lymphoma or PTLD). Completion of serum serologic testing for viruses, bacteria, and fungi and comprehensive BAL fluid analysis (culture, viral PCR, and cytology) is recommended.
A CMV antigenemia assay returned positive, suggesting prior CMV infection. However, to diagnose CMV pneumonia, the virus must be detected in BAL fluid by PCR or cytologic analysis. CMV infection has been associated with cytopenias, HLH, pancreatic infiltration, and an increased risk for fungal infections and EBV-related PTLD. CMV infection could explain the first phase of this patient’s illness. Serum and BAL PCR for CMV are advised. Meanwhile, EBV testing indicates prior infection but does not distinguish between recent or more distant infection. EBV has been implicated in the pathophysiology of PTLD, as EBV-infected lymphoid tissue may proliferate in a variety of organs under reduced T-cell surveillance. EBV infection or PTLD with resulting immunomodulation may pose other explanations for this patient’s development of PCP infection. Cytologic analysis of the BAL fluid and marrow aspirate for evidence of PTLD is warranted. Finally, CMV, EBV, and PTLD have each been reported to trigger HLH. Though this patient has fevers, mild marrow hemophagocytosis, elevated serum ferritin, and elevated serum IL-2 receptor levels, he does not meet other diagnostic criteria for HLH (such as more pronounced cytopenias, splenomegaly, hypertriglyceridemia, hypofibrinogenemia, and low or absent natural killer T-cell activity). However, HLH may be muted in this patient because he was prescribed cyclosporine, which has been used in HLH treatment protocols.
On the 11th hospital day, the patient developed hemorrhagic shock due to massive hematemesis and was transferred to the intensive care unit. His hemoglobin level was 5.9 g/dL. A total of 18 units of packed red blood cells were transfused over the following week for ongoing gastrointestinal bleeding. The serum LDH level increased to 4,139 IU/L, and the ferritin level rose to 7,855 ng/mL. The EBV copy level by serum PCR returned at 1 × 106 copies/mL (normal range, less than 2 x 102 copies/mL). The patient was started on methylprednisolone (1 g/day for three days) and transitioned to dexamethasone and cyclosporine for possible EBV-related HLH. Ceftazidime, vancomycin, trimethoprim-sulfamethoxazole, and ciprofloxacin were administered. Amphotericin-B was initiated empirically for potential fungal pneumonia. Ganciclovir was continued. However, the patient remained in shock despite vasopressors and transfusions and died on the 22nd hospital day.
The patient deteriorated despite broad antimicrobial therapy. Laboratory studies revealed EBV viremia and rising serum LDH. Recent EBV infection may have induced PTLD in the gastrointestinal tract, which is a commonly involved site among affected renal transplant patients. Corticosteroids and stress from critical illness can contribute to intestinal mucosal erosion and bleeding from a luminal PTLD lesion. Overall, the patient’s condition was likely explained by EBV infection, which triggered HLH and gastrointestinal PTLD. The resulting immunomodulation increased his risk for PCP infection beyond that conferred by chronic immunosuppression. It is still possible that he had concomitant CMV pneumonia, Aspergillus pneumonia, or even pulmonary PTLD, in addition to the proven PCP diagnosis.
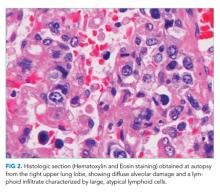
An autopsy was performed. Atypical lymphocytic infiltration and diffuse alveolar damage were shown in the right upper lobe (Figure 2). EBV RNA-positive atypical lymphocytes coexpressing CD20 were demonstrated in multiple organs including the bone marrow, lungs, heart, stomach, adrenal glands, duodenum, ileum, and mesentery (Figure 3). This confirmed the diagnosis of an underlying EBV-positive posttransplant lymphoproliferative disorder. Serum and BAL CMV PCR assays returned negative. Neither CMV nor Aspergillus was identified in autopsy specimens.
COMMENTARY
A broad differential diagnosis should be considered when acute fever develops in a patient who has undergone solid organ transplantation. Causes may include opportunistic and nonopportunistic infections as well as noninfectious etiologies such as malignancy, organ rejection, inflammatory conditions, and medication toxicity.1,2 As the discussant noted, more than one infection, or both infection and malignancy, can coexist in immunocompromised patients. For example, while viral pathogens such as EBV, CMV, and respiratory syncytial virus can cause illness due to direct tissue infection, they can also exert indirect effects in transplant recipients: acting as cofactors for and enabling other infections by causing immunosuppression (eg, Aspergillus or PCP developing after CMV infection), triggering graft rejection by upregulating proinflammatory cytokines, and inducing oncogenesis (eg, EBV-related PTLD).1,3-5
PTLD is a rare but serious complication of solid organ transplantation and immunosuppression. Most cases are driven by EBV infection and subsequent transformation of infected lymphoid tissue in a variety of organs in the context of reduced T-cell surveillance.6 The incidence of PTLD varies based on the organ transplanted, ranging from 0.8%-2.5% in those who have undergone renal transplantation to 1.0%-5.5% in liver transplant recipients and 3.0%-10% in lung transplant recipients.3 The incidence has increased over the past decade. This may be due to a greater number of solid organ transplantations being performed, aging of the transplant donor/recipient population, new immunosuppressive regimens, and improved PTLD diagnosis due to superior diagnostic tools and clinician awareness.3 However, the mortality rate among solid organ transplant recipients with PTLD remains high, ranging from 40% to 70%.6
Risk factors for PTLD include a greater intensity of T-cell immunosuppression,7 history of pretransplant malignancy, recipient EBV seronegativity and donor seropositivity, and younger age at the time of transplantation.8-10 EBV-related PTLD incidence in solid organ transplant recipients is greatest in the early posttransplantation course (the period of most intense immunosuppression) with over 80% of cases occurring in the first posttransplant year.11
A high index of suspicion for PTLD is warranted in any solid organ transplant recipient who presents with constitutional symptoms, adenopathy, or cytopenias. Clinical suspicion of PTLD can be informed by risk factors, constitutional symptoms, elevated serum LDH, a detectable or rising serum EBV viral load, and radiologic adenopathy or visceral tissue infiltration.12 The clinical presentation of PTLD is heterogeneous and varies in accordance with the organs affected. Extranodal involvement, such as pulmonary, gastrointestinal, and bone marrow involvement, is more common in PTLD than in other types of lymphoma.13 In this patient, the cytopenias, elevated serum LDH level, lung infiltrates, and radiologic pancreatic tail abnormality served as early clues to the presence of underlying PTLD.
The standard approach to diagnosing PTLD is biopsy of a suspicious lesion (adenopathy or an infiltrated visceral organ) with histopathological examination. Pathology may demonstrate distorted tissue architecture, clonal lymphocytes, or EBV-positive lymphocytes.14 Conventional CT is the most commonly used imaging modality to detect adenopathy or tissue infiltration related to PTLD,3 though 18F-fluorodeoxyglucose position-emission tomography (FDG-PET) is also used. Although FDG-PET has high diagnostic accuracy, with an overall sensitivity of 89% and specificity of 89%, false-negative results have been reported, particularly in cases of early PTLD lesions and diffuse large B-cell lymphoma.15 The majority of patients with EBV-associated PTLD demonstrate significant elevations in the serum EBV viral load compared with immunosuppressed controls without PTLD.16 An elevated EBV viral load can support a diagnosis of PTLD, though the absence of EBV viremia does not rule it out.17 Some transplant centers perform posttransplantation monitoring of the serum EBV viral load to aid in PTLD risk assessment and early diagnosis.
Management of PTLD is patient-specific and may involve reduction of immunosuppressive therapy, rituximab, chemotherapy, surgical excision, and/or radiation.13 Reduction of immunosuppression is the cornerstone of treatment.18 In patients who do not respond to the reduction of immunosuppression, rituximab and immunochemotherapy are second-line treatment options. A prospective, multicenter phase 2 trial (the PTLD-1 trial) demonstrated a complete response rate of 40% among patients with PTLD managed with rituximab.19
In summary, this case illustrates the importance of maintaining a broad differential diagnosis when acute fever develops in a patient who has undergone solid organ transplantation. The presence of more than one condition should be considered when the clinical presentation cannot be explained by a single diagnosis, as infections and malignancies can coexist in immunocompromised hosts. This case also highlights an unusual clinical presentation of PTLD, which was heralded mainly by its immunomodulatory effects rather than by compatible symptoms or obvious mass lesions.
Carefully reviewing the patient’s medical history and understanding how it sets the stage for the present illness is an essential step in clinical problem solving, because what is past is prologue.
TEACHING POINTS
- Fever in solid organ transplant recipients should prompt consideration of a broad differential diagnosis, including infection, malignancy, organ graft rejection, autoimmune disease, and medication toxicity.
- PTLD is a rare but serious complication of organ transplantation. Most cases are driven by EBV infection and transformation of infected lymphocytes in a variety of organs in the context of reduced T-cell surveillance. The clinical presentation can be heterogeneous and varies depending on the organs and tissues involved.
- More than one infection, or both infection and malignancy, can coexist in organ transplant recipients. Viral pathogens can exert direct pathologic effects on tissue but can also exert indirect effects, such as contributing to opportunistic infection susceptibility, graft rejection, and oncogenesis.
Disclosures
The authors have nothing to disclose.
Previous Publication
This case was originally reported in the 121st Okinawa Association of Medical Sciences in 2015 in Okinawa, Japan, and the conference abstracts were covered in The Okinawa Medical Journal. The publication did not provide any detailed, step-by-step analysis of clinical decision-making.
A 56-year-old Japanese man with a history of renal transplantation 20 years prior presented to the emergency department (ED) with two months of dyspnea on exertion and one day of fever and chills. The patient was in his usual state of health until two months prior to presentation, when he gradually noticed shortness of breath after sustained or effortful physical activities. The dyspnea improved with rest. Over the following two months, he noticed that the shortness of breath came on with lesser degrees of exertion, such as walking 100 meters. One day before presentation, he developed a fever of 39°C and chills at home, which prompted him to seek ED care. He denied chest pain, cough, leg swelling, or paroxysmal nocturnal dyspnea.
The differential diagnosis of exertional dyspnea progressing over several months includes cardiac, pulmonary, hematologic, and neuromuscular conditions. The patient’s history of renal transplantation prompts consideration of worsening indolent pneumonia (eg, Aspergillus, cytomegalovirus [CMV], or Pneumocystis pneumonia), allograft dysfunction with volume overload, recrudescence of the underlying disease that incited renal failure earlier in life (eg, vasculitis), or a late-onset posttransplantation lymphoproliferative disorder (PTLD). Additionally, acute fever in an immunocompromised patient immediately raises suspicion for infection (eg, pneumonia, enteritis, or urinary tract infection). At this point, it is difficult to know whether the subacute-to-chronic exertional dyspnea and the acute fever are consequences of the same disease or separate, potentially overlapping, processes.
His past medical history was significant for end-stage renal disease due to membranoproliferative glomerular nephropathy (MPGN), for which living, related-donor kidney transplantation was performed 20 years earlier. He also had type 2 diabetes mellitus, hypertension, and basal cell carcinoma of the face, which had been resected three years prior without spread or recurrence. He had no known allergies. Medications included prednisolone 15 mg daily, azathioprine 100 mg daily, and cyclosporine 100 mg daily, as well as amlodipine and candesartan. He lived in Japan with his wife and children. He denied any animal or environmental exposures. He did not smoke cigarettes or drink alcohol and had not traveled recently. His father had diabetes mellitus.
Recrudescence of an underlying autoimmune condition that may have incited MPGN earlier in life is unlikely while taking an immunosuppressive regimen consisting of prednisolone, azathioprine, and cyclosporine. However, these medications do increase susceptibility to infections, lymphoma, and skin cancers. Though he is immunocompromised, the patient is not on prophylaxis for Pneumocystis pneumonia (PCP). PCP in HIV-negative patients is associated with recent glucocorticoid exposure and typically follows an acute-to-subacute course with hypoxemia and respiratory distress. Though the risk of PCP infection is considered highest in the early posttransplantation period (when immunosuppression is most intense), many cases are diagnosed years after transplantation among patients no longer on prophylaxis. The patient has type 2 diabetes mellitus and hypertension, which are known complications of calcineurin inhibitor and steroid therapy and increase the risk of cardiovascular disease. Cardiovascular disease is a major cause of death among renal transplant recipients. Exertional dyspnea may be the presenting symptom of coronary artery disease.
On physical examination, the patient was alert, oriented, and in no acute distress. His temperature was 38.5°C, blood pressure 120/60 mm Hg, heart rate 146 beats per minute, respiratory rate 18 breaths per minute, and oxygen saturation 93% while breathing ambient air. The conjunctiva were normal without pallor or icterus. There was no cervical lymphadenopathy. Cardiac examination revealed tachycardia with a regular rhythm, normal S1 and S2, and no murmurs, rubs, or gallops. Jugular venous pressure was not elevated, and there was no lower extremity edema. Lungs were clear to auscultation bilaterally. The abdomen was soft, nontender, and nondistended. There was no tenderness over the transplanted kidney and no hepatosplenomegaly.
Dyspnea, fever, and tachycardia may be the sole manifestations of pneumonia in solid organ transplant recipients. The absence of cough or adventitious breath sounds does not eliminate concern for pneumonia. Pathogens that cause indolent pneumonia in immunocompromised patients include viruses (such as typical respiratory viruses and CMV), bacteria (typical organisms, Nocardia, Rhodococcus), and mycobacteria. Fungal causes include Aspergillus, Candida, Cryptococcus, Pneumocystis, and endemic mycoses. A detailed environmental history should be taken, and providers should ascertain which fungal diseases are endemic in the patient’s region of residence. There are no examination features suggesting hypervolemia or anemia. Although there is no hepatosplenomegaly or lymphadenopathy, PTLD often involves extranodal tissues, including the lungs. The incidence of PTLD is highest in the 12 months following transplantation, but it may occur at any time in the posttransplantation course. A complete blood count, comprehensive metabolic panel, lactate dehydrogenase (LDH), and blood and sputum cultures are indicated, along with computed tomography (CT) of the chest.
The leukocyte count was 3,500 cells/mm3, the hemoglobin level 9.0 g/dL, mean corpuscular volume 102 fL, and the platelet count 137,000 cells/mm3. The sodium level was 130 mEq/L, potassium 4.6 mEq/L, blood urea nitrogen 41 mg/dL, and creatinine 3.5 mg/dL. These complete blood count and serum electrolyte results were unchanged from the patient’s baseline values. The serum LDH level was 1,895 IU/L (normal range, 115-245 IU/L). The serum ferritin was 2,114 ng/mL (normal range, 13-277 ng/mL). A chest radiograph revealed diffuse, airspace-filling opacities in the bilateral lung bases. The urinalysis was normal. The patient was admitted and started empirically on intravenous ceftriaxone for potential bacterial pneumonia.
Chronic pancytopenia may result from azathioprine or cyclosporine use, marrow suppression or infiltration by a multisystem disease, or nutritional deficiency. Hemophagocytic lymphohistiocytosis (HLH) triggered by infection, a rheumatologic condition, acquired immunodeficiency, or malignancy can present with fevers, pancytopenia, and elevated ferritin, while splenomegaly may be absent. The euvolemic state, baseline creatinine level, and normal urinalysis argue against allograft dysfunction. The elevated serum ferritin nonspecifically confirms systemic inflammation. LDH, an intracellular enzyme involved in the bidirectional conversion of lactate to pyruvate, is expressed across tissue types. Elevated serum LDH attests to cell destruction, in this case potentially from lung infection (such as PCP) or malignancy (such as PTLD). At this point, the differential diagnosis of fever and pulmonary infiltrates in this patient remains broad.
Azathioprine and cyclosporine were stopped. The patient remained febrile despite the administration of intravenous antibiotics. His hypoxia worsened with an oxygen saturation of 90%-93% on 5 L/min of supplemental oxygen administered by nasal cannula. Noncontrast chest CT obtained on the second hospital day revealed ground-glass opacities in the bilateral lung bases. Blood, sputum, and urine cultures were sterile. As empiric therapies, ganciclovir was started for CMV infection, ciprofloxacin added for atypical pneumonia, and trimethoprim-sulfamethoxazole added for Pneumocystis infection.
These chest imaging findings help prioritize the differential diagnosis. Bibasilar ground-glass opacities are evident, while pulmonary masses, nodules, cavitation, adenopathy, and pleural effusions are absent. The differential diagnosis of multifocal ground-glass opacities on chest imaging includes infectious pneumonia, chronic interstitial lung disease, acute alveolar conditions (eg, cardiogenic pulmonary edema, acute respiratory distress syndrome, diffuse alveolar hemorrhage), or other pathologies (eg, drug toxicity, bronchoalveolar carcinoma, cryptogenic organizing pneumonia).
Infectious pneumonia is the principal concern. A diagnosis of PCP could be unifying, given dyspnea, progressive respiratory failure with hypoxia, and elevated LDH in an immunocompromised patient who is not prescribed PCP prophylaxis. The bilateral lung infiltrates and the absence of thoracic adenopathy or pleural effusions are characteristic of PCP as well. However, caution should be exercised in making specific infectious diagnoses in immunocompromised hosts on the basis of clinical and imaging findings alone. There can be overlap in the radiologic appearance of various infections (eg, CMV pneumonia can also present with bilateral ground-glass infiltrates, with concurrent fever, hypoxia, and pancytopenia). Additionally, more than one pneumonic pathogen may be implicated (eg, acute viral pneumonia superimposed on indolent fungal pneumonia). Polymerase chain reaction (PCR) analysis of respiratory secretions for viruses, serum PCR and serologic testing for herpes viruses, and serum beta-D-glucan and galactomannan assays are indicated. Serum serologic testing for fungi and bacteria such as Nocardia can be helpful, though the negative predictive values of these tests may be reduced in patients with impaired humoral immunity. Timely bronchoalveolar lavage (BAL) with microbiologic and PCR analysis and cytology is advised.
Fever, elevated LDH, cytopenias, and pulmonary infiltrates also raise suspicion for an underlying hematologic malignancy, such as PTLD. However, pulmonary PTLD is seen more often in lung transplant recipients than in patients who have undergone transplantation of other solid organs. In kidney transplant recipients, PTLD most commonly manifests in the allograft itself, gastrointestinal tract, central nervous system, or lymph nodes; lung involvement is less common. Chest imaging in affected patients may reveal nodular or reticulonodular infiltrates of basilar predominance, solitary or multiple masses, cavitating or necrotic lesions, and/or lymphadenopathy. In this patient who has undergone renal transplantation, late-onset PTLD with isolated pulmonary involvement, with only ground-glass opacities on lung imaging, would be an atypical presentation of an uncommon syndrome.
Despite empiric treatment with antibiotics and antiviral agents, the patient’s fever persisted. His respiratory rate increased to 30 breaths per minute. His hypoxia worsened, and he required nasal cannula high-flow oxygen supplementation at 30 L/min with a fraction of inspired oxygen (FiO2) of 40%. On the fifth hospital day, contrast CT scan of the chest and abdomen showed new infiltrates in the bilateral upper lung fields as well as an area of low density in the tail of the pancreas without a focal mass (Figure 1). At this point, BAL was performed, and fluid PCR analysis returned positive for Pneumocystis jirovecii. CMV direct immunoperoxidase staining of leukocytes with peroxidase-labeled monoclonal antibody (C7-HRP test) was positive at five cells per 7.35 × 104 peripheral blood leukocytes. The serum Epstein-Barr virus (EBV) viral capsid antigen (VCA) IgG was positive, while VCA IgM and EBV nuclear antigen IgG were negative. A bone marrow biopsy revealed mild hemophagocytosis. His serum soluble interleukin-2 (sIL2R) level was elevated at 5,254 U/mL (normal range, 122-496 U/mL). Given the BAL Pneumocystis PCR result, the dose of prednisolone was increased to 30 mg/day, and the patient’s fever subsided. Supplemental oxygen was weaned to an FiO2 of 35%.
These studies should be interpreted carefully considering the biphasic clinical course. After two months of exertional dyspnea, the patient acutely developed persistent fever and progressive lung infiltrates. His clinical course, the positive PCR assay for Pneumocystis jirovecii in BAL fluid, and the compatible lung imaging findings make Pneumocystis jirovecii a likely pathogen. But PCP may only explain the second phase of this patient’s illness, considering its often-fulminant course in HIV-negative patients. To explain the two months of exertional dyspnea, marrow hemophagocytosis, pancreatic abnormality, and perhaps even the patient’s heightened susceptibility to PCP infection, an index of suspicion should be maintained for a separate, antecedent process. This could be either an indolent infection (eg, CMV or Aspergillus pneumonia) or a malignancy (eg, lymphoma or PTLD). Completion of serum serologic testing for viruses, bacteria, and fungi and comprehensive BAL fluid analysis (culture, viral PCR, and cytology) is recommended.
A CMV antigenemia assay returned positive, suggesting prior CMV infection. However, to diagnose CMV pneumonia, the virus must be detected in BAL fluid by PCR or cytologic analysis. CMV infection has been associated with cytopenias, HLH, pancreatic infiltration, and an increased risk for fungal infections and EBV-related PTLD. CMV infection could explain the first phase of this patient’s illness. Serum and BAL PCR for CMV are advised. Meanwhile, EBV testing indicates prior infection but does not distinguish between recent or more distant infection. EBV has been implicated in the pathophysiology of PTLD, as EBV-infected lymphoid tissue may proliferate in a variety of organs under reduced T-cell surveillance. EBV infection or PTLD with resulting immunomodulation may pose other explanations for this patient’s development of PCP infection. Cytologic analysis of the BAL fluid and marrow aspirate for evidence of PTLD is warranted. Finally, CMV, EBV, and PTLD have each been reported to trigger HLH. Though this patient has fevers, mild marrow hemophagocytosis, elevated serum ferritin, and elevated serum IL-2 receptor levels, he does not meet other diagnostic criteria for HLH (such as more pronounced cytopenias, splenomegaly, hypertriglyceridemia, hypofibrinogenemia, and low or absent natural killer T-cell activity). However, HLH may be muted in this patient because he was prescribed cyclosporine, which has been used in HLH treatment protocols.
On the 11th hospital day, the patient developed hemorrhagic shock due to massive hematemesis and was transferred to the intensive care unit. His hemoglobin level was 5.9 g/dL. A total of 18 units of packed red blood cells were transfused over the following week for ongoing gastrointestinal bleeding. The serum LDH level increased to 4,139 IU/L, and the ferritin level rose to 7,855 ng/mL. The EBV copy level by serum PCR returned at 1 × 106 copies/mL (normal range, less than 2 x 102 copies/mL). The patient was started on methylprednisolone (1 g/day for three days) and transitioned to dexamethasone and cyclosporine for possible EBV-related HLH. Ceftazidime, vancomycin, trimethoprim-sulfamethoxazole, and ciprofloxacin were administered. Amphotericin-B was initiated empirically for potential fungal pneumonia. Ganciclovir was continued. However, the patient remained in shock despite vasopressors and transfusions and died on the 22nd hospital day.
The patient deteriorated despite broad antimicrobial therapy. Laboratory studies revealed EBV viremia and rising serum LDH. Recent EBV infection may have induced PTLD in the gastrointestinal tract, which is a commonly involved site among affected renal transplant patients. Corticosteroids and stress from critical illness can contribute to intestinal mucosal erosion and bleeding from a luminal PTLD lesion. Overall, the patient’s condition was likely explained by EBV infection, which triggered HLH and gastrointestinal PTLD. The resulting immunomodulation increased his risk for PCP infection beyond that conferred by chronic immunosuppression. It is still possible that he had concomitant CMV pneumonia, Aspergillus pneumonia, or even pulmonary PTLD, in addition to the proven PCP diagnosis.
An autopsy was performed. Atypical lymphocytic infiltration and diffuse alveolar damage were shown in the right upper lobe (Figure 2). EBV RNA-positive atypical lymphocytes coexpressing CD20 were demonstrated in multiple organs including the bone marrow, lungs, heart, stomach, adrenal glands, duodenum, ileum, and mesentery (Figure 3). This confirmed the diagnosis of an underlying EBV-positive posttransplant lymphoproliferative disorder. Serum and BAL CMV PCR assays returned negative. Neither CMV nor Aspergillus was identified in autopsy specimens.
COMMENTARY
A broad differential diagnosis should be considered when acute fever develops in a patient who has undergone solid organ transplantation. Causes may include opportunistic and nonopportunistic infections as well as noninfectious etiologies such as malignancy, organ rejection, inflammatory conditions, and medication toxicity.1,2 As the discussant noted, more than one infection, or both infection and malignancy, can coexist in immunocompromised patients. For example, while viral pathogens such as EBV, CMV, and respiratory syncytial virus can cause illness due to direct tissue infection, they can also exert indirect effects in transplant recipients: acting as cofactors for and enabling other infections by causing immunosuppression (eg, Aspergillus or PCP developing after CMV infection), triggering graft rejection by upregulating proinflammatory cytokines, and inducing oncogenesis (eg, EBV-related PTLD).1,3-5
PTLD is a rare but serious complication of solid organ transplantation and immunosuppression. Most cases are driven by EBV infection and subsequent transformation of infected lymphoid tissue in a variety of organs in the context of reduced T-cell surveillance.6 The incidence of PTLD varies based on the organ transplanted, ranging from 0.8%-2.5% in those who have undergone renal transplantation to 1.0%-5.5% in liver transplant recipients and 3.0%-10% in lung transplant recipients.3 The incidence has increased over the past decade. This may be due to a greater number of solid organ transplantations being performed, aging of the transplant donor/recipient population, new immunosuppressive regimens, and improved PTLD diagnosis due to superior diagnostic tools and clinician awareness.3 However, the mortality rate among solid organ transplant recipients with PTLD remains high, ranging from 40% to 70%.6
Risk factors for PTLD include a greater intensity of T-cell immunosuppression,7 history of pretransplant malignancy, recipient EBV seronegativity and donor seropositivity, and younger age at the time of transplantation.8-10 EBV-related PTLD incidence in solid organ transplant recipients is greatest in the early posttransplantation course (the period of most intense immunosuppression) with over 80% of cases occurring in the first posttransplant year.11
A high index of suspicion for PTLD is warranted in any solid organ transplant recipient who presents with constitutional symptoms, adenopathy, or cytopenias. Clinical suspicion of PTLD can be informed by risk factors, constitutional symptoms, elevated serum LDH, a detectable or rising serum EBV viral load, and radiologic adenopathy or visceral tissue infiltration.12 The clinical presentation of PTLD is heterogeneous and varies in accordance with the organs affected. Extranodal involvement, such as pulmonary, gastrointestinal, and bone marrow involvement, is more common in PTLD than in other types of lymphoma.13 In this patient, the cytopenias, elevated serum LDH level, lung infiltrates, and radiologic pancreatic tail abnormality served as early clues to the presence of underlying PTLD.
The standard approach to diagnosing PTLD is biopsy of a suspicious lesion (adenopathy or an infiltrated visceral organ) with histopathological examination. Pathology may demonstrate distorted tissue architecture, clonal lymphocytes, or EBV-positive lymphocytes.14 Conventional CT is the most commonly used imaging modality to detect adenopathy or tissue infiltration related to PTLD,3 though 18F-fluorodeoxyglucose position-emission tomography (FDG-PET) is also used. Although FDG-PET has high diagnostic accuracy, with an overall sensitivity of 89% and specificity of 89%, false-negative results have been reported, particularly in cases of early PTLD lesions and diffuse large B-cell lymphoma.15 The majority of patients with EBV-associated PTLD demonstrate significant elevations in the serum EBV viral load compared with immunosuppressed controls without PTLD.16 An elevated EBV viral load can support a diagnosis of PTLD, though the absence of EBV viremia does not rule it out.17 Some transplant centers perform posttransplantation monitoring of the serum EBV viral load to aid in PTLD risk assessment and early diagnosis.
Management of PTLD is patient-specific and may involve reduction of immunosuppressive therapy, rituximab, chemotherapy, surgical excision, and/or radiation.13 Reduction of immunosuppression is the cornerstone of treatment.18 In patients who do not respond to the reduction of immunosuppression, rituximab and immunochemotherapy are second-line treatment options. A prospective, multicenter phase 2 trial (the PTLD-1 trial) demonstrated a complete response rate of 40% among patients with PTLD managed with rituximab.19
In summary, this case illustrates the importance of maintaining a broad differential diagnosis when acute fever develops in a patient who has undergone solid organ transplantation. The presence of more than one condition should be considered when the clinical presentation cannot be explained by a single diagnosis, as infections and malignancies can coexist in immunocompromised hosts. This case also highlights an unusual clinical presentation of PTLD, which was heralded mainly by its immunomodulatory effects rather than by compatible symptoms or obvious mass lesions.
Carefully reviewing the patient’s medical history and understanding how it sets the stage for the present illness is an essential step in clinical problem solving, because what is past is prologue.
TEACHING POINTS
- Fever in solid organ transplant recipients should prompt consideration of a broad differential diagnosis, including infection, malignancy, organ graft rejection, autoimmune disease, and medication toxicity.
- PTLD is a rare but serious complication of organ transplantation. Most cases are driven by EBV infection and transformation of infected lymphocytes in a variety of organs in the context of reduced T-cell surveillance. The clinical presentation can be heterogeneous and varies depending on the organs and tissues involved.
- More than one infection, or both infection and malignancy, can coexist in organ transplant recipients. Viral pathogens can exert direct pathologic effects on tissue but can also exert indirect effects, such as contributing to opportunistic infection susceptibility, graft rejection, and oncogenesis.
Disclosures
The authors have nothing to disclose.
Previous Publication
This case was originally reported in the 121st Okinawa Association of Medical Sciences in 2015 in Okinawa, Japan, and the conference abstracts were covered in The Okinawa Medical Journal. The publication did not provide any detailed, step-by-step analysis of clinical decision-making.
1. Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601-2614. https://doi.org/10.1056/NEJMra064928.
2. Bouza E, Loeches B, Muñoz P. Fever of unknown origin in solid organ transplant recipients. Infect Dis Clin North Am. 2007;21(4):1033-1054, ix-x. https://doi.org/10.1016/j.idc.2007.09.001,
3. Kotton CN, Fishman JA. Viral infection in the renal transplant recipient. J Am Soc Nephrol. 2005;16(6):1758-1774. https://doi.org/10.1681/ASN.2004121113.
4. Arend SM, Westendorp RG, Kroon FP, et al. Rejection treatment and cytomegalovirus infection as risk factors for Pneumocystis carinii pneumonia in renal transplant recipients. Clin Infect Dis. 1996;22(6):920-925. https://doi.org/10.1093/clinids/22.6.920.
5. Reinke P, Fietze E, Ode-Hakim S, et al. Late-acute renal allograft rejection and symptomless cytomegalovirus infection. Lancet. 1994;344(8939-8940):1737-1738. https://doi.org/10.1016/S0140-6736(94)92887-8.
6. Tsai DE, Douglas L, Andreadis C, et al. EBV PCR in the diagnosis and monitoring of posttransplant lymphoproliferative disorder: results of a two-arm prospective trial. Am J Transplant. 2008;8(5):1016-1024. https://doi.org/10.1111/j.1600-6143.2008.02183.x.
7. Penn I. Cancers complicating organ transplantation. N Engl J Med. 1990;323(25):1767-1769. https://doi.org/10.1056/NEJM199012203232510
8. Walker RC, Marshall WF, Strickler JG, et al. Pretransplantation assessment of the risk of lymphoproliferative disorder. Clin Infect Dis. 1995;20(5):1346-1353. https://doi.org/10.1093/clinids/20.5.1346.
9. Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4(2):222-230. https://doi.org/10.1046/j.1600-6143.2003.00325.x.
10. Caillard S, Dharnidharka V, Agodoa L, Bohen E, Abbott K. Posttransplant lymphoproliferative disorders after renal transplantation in the United States in era of modern immunosuppression. Transplantation. 2005;80(9):1233-1243. doi: 10.1097/01.tp.0000179639.98338.39.
11. Opelz G, Henderson R. Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients. Lancet. 1993;342(8886-8887):1514-1516. https://doi.org/10.1016/S0140-6736(05)80084-4.
12. Samant H, Kothadia JP. Transplantation Posttransplantation Lymphoproliferative Disorders. Treasure Island, FL: StatPearls Publishing; 2018. PubMed
13. Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med. 2018;378(6):549-562. https://doi.org/10.1056/NEJMra1702693.
14. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. https://doi.org/10.1182/blood-2016-01-643569.
15. Dierickx D, Tousseyn T, Requilé A, et al. The accuracy of positron emission tomography in the detection of posttransplant lymphoproliferative disorder. Haematologica. 2013;98(5):771-775. https://doi.org/10.3324/haematol.2012.074500.
16. Wagner HJ, Wessel M, Jabs W, et al. Patients at risk for development of posttransplant lymphoproliferative disorder: plasma versus peripheral blood mononuclear cells as material for quantification of Epstein-Barr viral load by using real-time quantitative polymerase chain reaction. Transplantation. 2001;72(6):1012-1019. PubMed
17. Baldanti F, Rognoni V, Cascina A, Oggionni T, Tinelli C, Meloni F. Post-transplant lymphoproliferative disorders and Epstein-Barr virus DNAemia in a cohort of lung transplant recipients. Virol J. 2011;8:421. https://doi.org/10.1186/1743-422X-8-421.
18. Parker A, Bowles K, Bradley JA, et al. Management of post-transplant lymphoproliferative disorder in adult solid organ transplant recipients - BCSH and BTS Guidelines. Br J Haematol. 2010;149(5):693-705. https://doi.org/10.1111/j.1365-2141.2010.08160.x.
19. Trappe R, Oertel S, Leblond V, et al. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012;13(2):196-206. https://doi.org/10.1016/S1470-2045(11)70300-X.
1. Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601-2614. https://doi.org/10.1056/NEJMra064928.
2. Bouza E, Loeches B, Muñoz P. Fever of unknown origin in solid organ transplant recipients. Infect Dis Clin North Am. 2007;21(4):1033-1054, ix-x. https://doi.org/10.1016/j.idc.2007.09.001,
3. Kotton CN, Fishman JA. Viral infection in the renal transplant recipient. J Am Soc Nephrol. 2005;16(6):1758-1774. https://doi.org/10.1681/ASN.2004121113.
4. Arend SM, Westendorp RG, Kroon FP, et al. Rejection treatment and cytomegalovirus infection as risk factors for Pneumocystis carinii pneumonia in renal transplant recipients. Clin Infect Dis. 1996;22(6):920-925. https://doi.org/10.1093/clinids/22.6.920.
5. Reinke P, Fietze E, Ode-Hakim S, et al. Late-acute renal allograft rejection and symptomless cytomegalovirus infection. Lancet. 1994;344(8939-8940):1737-1738. https://doi.org/10.1016/S0140-6736(94)92887-8.
6. Tsai DE, Douglas L, Andreadis C, et al. EBV PCR in the diagnosis and monitoring of posttransplant lymphoproliferative disorder: results of a two-arm prospective trial. Am J Transplant. 2008;8(5):1016-1024. https://doi.org/10.1111/j.1600-6143.2008.02183.x.
7. Penn I. Cancers complicating organ transplantation. N Engl J Med. 1990;323(25):1767-1769. https://doi.org/10.1056/NEJM199012203232510
8. Walker RC, Marshall WF, Strickler JG, et al. Pretransplantation assessment of the risk of lymphoproliferative disorder. Clin Infect Dis. 1995;20(5):1346-1353. https://doi.org/10.1093/clinids/20.5.1346.
9. Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4(2):222-230. https://doi.org/10.1046/j.1600-6143.2003.00325.x.
10. Caillard S, Dharnidharka V, Agodoa L, Bohen E, Abbott K. Posttransplant lymphoproliferative disorders after renal transplantation in the United States in era of modern immunosuppression. Transplantation. 2005;80(9):1233-1243. doi: 10.1097/01.tp.0000179639.98338.39.
11. Opelz G, Henderson R. Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients. Lancet. 1993;342(8886-8887):1514-1516. https://doi.org/10.1016/S0140-6736(05)80084-4.
12. Samant H, Kothadia JP. Transplantation Posttransplantation Lymphoproliferative Disorders. Treasure Island, FL: StatPearls Publishing; 2018. PubMed
13. Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med. 2018;378(6):549-562. https://doi.org/10.1056/NEJMra1702693.
14. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. https://doi.org/10.1182/blood-2016-01-643569.
15. Dierickx D, Tousseyn T, Requilé A, et al. The accuracy of positron emission tomography in the detection of posttransplant lymphoproliferative disorder. Haematologica. 2013;98(5):771-775. https://doi.org/10.3324/haematol.2012.074500.
16. Wagner HJ, Wessel M, Jabs W, et al. Patients at risk for development of posttransplant lymphoproliferative disorder: plasma versus peripheral blood mononuclear cells as material for quantification of Epstein-Barr viral load by using real-time quantitative polymerase chain reaction. Transplantation. 2001;72(6):1012-1019. PubMed
17. Baldanti F, Rognoni V, Cascina A, Oggionni T, Tinelli C, Meloni F. Post-transplant lymphoproliferative disorders and Epstein-Barr virus DNAemia in a cohort of lung transplant recipients. Virol J. 2011;8:421. https://doi.org/10.1186/1743-422X-8-421.
18. Parker A, Bowles K, Bradley JA, et al. Management of post-transplant lymphoproliferative disorder in adult solid organ transplant recipients - BCSH and BTS Guidelines. Br J Haematol. 2010;149(5):693-705. https://doi.org/10.1111/j.1365-2141.2010.08160.x.
19. Trappe R, Oertel S, Leblond V, et al. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012;13(2):196-206. https://doi.org/10.1016/S1470-2045(11)70300-X.
© 2019 Society of Hospital Medicine