User login
› When interpreting hemoglobin A1c (HbA1c) levels, assess for anemia and other comorbidities that can significantly affect the lifespan of red blood cells and skew HbA1c test results. B
› Order nonfasting lipid panels for patients for whom fasting laboratory tests are difficult to obtain, as they have good clinical utility in screening and initial treatment. A
› Avoid routine thyroid-stimulating hormone (TSH) testing in asymptomatic adults; when testing is indicated, start with TSH. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Laboratory mistakes are not defined as diagnostic errors, but they contribute significantly to the thousands of medical errors that occur every year.1 Part of the problem: While accurate interpretation of lab tests often depends on the use of statistical concepts we all learned in medical training, it is difficult to find the time to incorporate these principles into a busy practice.
Overuse of lab tests presents problems, as well. Because “normal ranges” for test results are based on statistical analysis, as many as 5% of patients in a standard distribution fall outside of the range.2 It is important to order only the tests you really need, as extra testing automatically means more false positive results.
This article was written with such pitfalls in mind. In the pages that follow, we focus on 8 types of tests family physicians rely on regularly—all cases in which test results are reliable only if comorbidities, pre- and post-test probabilities, and clinical context are carefully considered. To help you put these lab tests into the proper context, we’ve addressed a key question—and highlighted both pitfalls and pearls—about each.
1. Hemoglobin A1c: How does anemia affect it?
Hemoglobin A1c (HbA1c) can be measured in many ways, including high-performance liquid chromatography, boronate affinity, capillary electrophoresis, and immunoassay, all of which can provide equivalent values without significant variability.3,4 In interpreting these tests, however, it is important to understand the effect that anemia has on HbA1c.
It's important to order only the tests you really need, as extra testing automatically means more false positive results.
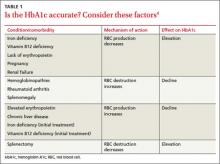
Two primary variables influencing HbA1c are the average glucose level and the average lifespan of red blood cells (RBCs). Normally, there is a direct correlation between average serum glucose and HbA1c.4 In patients with anemia, however, this relationship is less clear, and may be affected by erythropoiesis and RBC destruction.5 In iron deficiency anemia (IDA),6,7 hemoglobin production falls secondary to iron stores, resulting in microcytic cells with a longer lifespan and elevated HbA1c. In at least one study,5,7 HbA1c approached levels associated with diabetes (with increases as high as 1.5%) in nondiabetic patients, but resolved with treatment of IDA.
Increased destruction as well as increased production of RBCs lowers their lifespan, and in turn decreases HbA1c levels (TABLE 1).4 This can be seen in conditions such as splenomegaly and hemoglobinopathies. In patients with hemoglobinopathies, the percentage of hemoglobin A is significantly decreased, often to undetectable levels—thereby making HbA1c tests inaccurate. Hemoglobin electrophoresis and determination of glycation by capillary electrophoresis or high-performance liquid chromatography can be used instead, but neither is practical because of cost and limited availability.4,8,9
THE TAKEAWAY: When you evaluate HbA1c test results, it is crucial to assess the patient for anemia and other conditions or comorbidities that can significantly affect RBC lifespan and skew test results.2,4-6
2. D-dimer: When should you use it?
D-dimer is a fibrin degradation product that is increased when active clotting is present,10 and its assay—which has high sensitivity and low specificity—is widely used to screen for pulmonary embolism (PE) and deep vein thrombosis (DVT). While the minimal number of false negatives makes the D-dimer a good screening test, the higher rate of false positives makes it difficult to arrive at a definitive diagnosis. Appropriate use of the D-dimer assay is crucial to minimize the potential for adverse consequences, such as bleeding in patients who are subjected to unnecessary anticoagulation because of false positive results.
Further testing typically follows. A positive D-dimer test is commonly followed by a computed tomography (CT) scan of the chest or a ventilation/perfusion scan to establish a PE or DVT diagnosis. But this subsequent testing increases both the cost of health care and the patient’s radiation exposure. Use of these subsequent scans can be reduced by first considering the patient’s pretest probability for PE or DVT. The Wells’ Criteria (available at www.mdcalc.com/wells-criteriafor-pulmonary-embolism-pe/) and Geneva Score (Revised) (www.mdcalc.com/genevascore-revised-for-pulmonary-embolism/) can both be used for this purpose.10,11
Patients with high pretest probability should undergo immediate scanning, foregoing the D-dimer—which should be reserved for patients who have a low or moderate pretest probability but sufficient reason to suspect PE or DVT.10-12
The low specificity of the D-dimer assay poses another challenge to its effective use. There are many things that can increase D-dimer levels, such as age, cancer, prolonged immobility, autoimmune disease, inflammation, sickle cell disease, pregnancy, trauma, and surgery.13-15 All these factors must be taken into consideration prior to ordering this test.
In fact, one recent study found that using an age-adjusted D-dimer cutoff (patient’s age in years x 10 mcg/L)—rather than a conventional cutoff of 500 mcg/L—for patients older than 50 years reduces false positives without substantially increasing false negatives.16
Also of note: An anticoagulant can decrease D-dimer levels in plasma, so the test should not be used to rule out PE or DVT in patients who are undergoing anticoagulation.13,15
THE TAKEAWAY: In evaluating patients for PE or DVT, use the Wells’ Criteria or Geneva Score (Revised) to determine a patient’s pretest probability of disease. Use the D-dimer assay to safely rule out these conditions in patients with a low or intermediate pretest probability, but go directly to scans for those with a high pretest probability.
3. Lipid panels: How important is fasting?
Patients are often instructed to report for fasting lab studies, specifically for lipid profiles. Traditionally, this had been defined as an 8- to 12-hour period without food.17 In clinical practice, however, this is often misinterpreted by patients, who may be confused about the duration of the fast or unsure about whether to eat or drink immediately before the test.
Studies investigating the effect of meals on lab values have found that triglycerides are consistently elevated postprandially, to a maximum of 12 hours.18-21 The effect of the fasting state on total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol is more controversial; while some postprandial differences have been detected, the clinical relevance is equivocal.18-21
Nonfasting lipid values can offer useful information, particularly in patients who are unwilling or unable to return for fasting labs. The US Preventive Services Task Force (USPSTF) supports this practice.22 Because guidelines for evaluation and treatment are based on fasting lipids, however, fasting lab work should be used, whenever possible, for initiating treatment and monitoring patients with abnormal values. If nonfasting lipids are used, it is crucial to factor in the postprandial effects on triglycerides and the subsequent difficulty of assessing LDL cholesterol levels.
THE TAKEAWAY: The clinical relevance of postprandial vs fasting lipid levels is equivocal. Nonfasting lipid panels have reasonable clinical utility in screening and initial treatment, particularly in cases in which obtaining fasting lab values may be problematic.18,19
4. Mononucleoosis spot test: When should you use it?
The monospot test is a latex assay that causes hemagglutination of horse RBCs in the presence of heterophile antibodies characteristic of infectious mononucleosis.23 The antibodies develop within the first 7 days of onset of symptoms, but do not peak for 2 to 5 weeks.24 As a result, monospot testing yields a high incidence of false negatives during the first 2 weeks of active infection.25 False negatives are also common in patients younger than 14 years. Heterophile antibodies may be present for up to a year after active infection.24
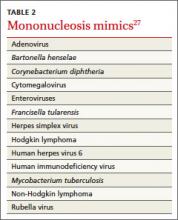
Patients at increased risk for splenic rupture, such as athletes, pose considerable diagnostic difficulty.26 When there is strong clinical suspicion of mononucleosis despite a negative monospot test in such high-risk individuals, follow-up testing is recommended to differentiate it from other mononucleosis-like illnesses (TABLE 2).27 The optimal combination of Epstein-Barr virus (EBV) serologic testing consists of the antibody titration of 4 markers: immunoglobulins M (IgM) and G (IgG) to the viral capsid antigen, IgM to the early antigen, and antibody to Epstein-Barr nuclear antigen (EBNA).28 Acute phase reactants in the setting of an antibody to EBNA could indicate reactivation. A positive test does not exclude other medical causes, however, because up to 20% of patients have acute phase antibodies that persist for years.29
Appropriate diagnosis is important because of the significant morbidity associated with EBV. Risk of splenic injury is greatest between 4 and 21 days after onset of symptoms but persists at 7 weeks,26 so conservative therapy followed by monospot retesting one week later is a reasonable approach.
Mononucleosis or routine tonsillitis? It is important to note that there is no evidence that a positive monospot test will affect the management or outcome of routine tonsillitis, raising questions of the utility of the test in such cases. A better approach: Reserve testing for patients with additional findings—ie, splenomegaly—or whose symptoms have persisted ≥ 2 weeks.
THE TAKEAWAY: Wait at least 2 weeks to conduct monospot testing in patients with routine tonsillitis. If strong clinical suspicion exists, proceed with specific IgM and IgG serologic testing.24,25,27,28
5. Evaluating prescription drug levels: Which factors interfere?
Correct interpretation of lab tests conducted to measure prescription drug levels has major implications with regard to patient safety, particularly for medications with a narrow therapeutic index.
Most drug level tests measure the total concentration, which includes both bound and unbound (free) forms. The unbound forms are the active components of the drug; thus, for an accurate evaluation, it is important to be aware of factors that increase free drug concentration. Chief among them is low protein levels, or hypoalbuminemia.30
Risk factors for hypoalbuminemia include significant burns, advanced age, pregnancy, malnutrition, and human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS).30 HIV/AIDS is a particularly high risk because certain protease inhibitors are highly protein bound.
Drug protein binding is classified as low, moderate, or high. The main proteins involved in the process are albumin, alpha-1-acid glycoprotein, and lipoprotein. Medications that are highly protein bound (>80%) are the most affected by low protein levels: Problems can arise when drugs completely bind to all the available proteins and excess drug availability increases free drug levels.
Medications that are most likely to be affected by a high degree of protein binding include carbamazepine, cyclosporine, mycophenolic acid, phenytoin, protease inhibitors (with the exception of indinavir), tacrolimus, and valproic acid. It is important to consider free levels when you order medication assays for these drugs to avoid misinterpreting the serum levels as being too low-a scenario that raises the risk of drug toxicity and adverse outcomes.30,31
A study of 119 phenytoin samples from 70 patients found significantly higher free phenytoin levels in patients with lower albumin levels.32 Higher free phenytoin levels were also seen in older patients and in those with diminished renal function (creatinine clearance <25 mL/min).32 The degree of protein binding is affected by both the serum drug concentration and the albumin level, with saturable protein binding occurring at higher drug levels.33
Calculate phenytoin levels with this equation. To calculate corrected phenytoin levels in patients with low albumin levels, use the following formula, known as the Sheiner-Tozer equation:34
Concentration adjusted=concentration reported/([adjustment x serum albumin] + 0.1); adjustment=0.2 for creatinine clearance ≥20 or 0.1 for creatinine clearance <20.
Additional causes of misinterpreted drug levels. While hypoalbuminemia plays a major role in the misinterpretation of drug levels, other factors affect serum drug concentration, as well. These include drug-drug interactions, which can significantly increase the concentration of the medications involved, and the timing of the test with regard to medication administration. Digoxin levels, in particular, need to be drawn at least 6 to 8 hours after the last dose is taken to allow for appropriate drug distribution.35
THE TAKEAWAY: It is essential to consider free drug level monitoring in patients who either have hypoalbuminemia or have one or more risk factors for hypoalbuminemia to avoid falsely low estimation of drug levels.36,37
6 Liver function tests: Necessary for patients on statin therapy?
Since statins gained US Food and Drug Administration (FDA) approval, the drugs have been associated with increased liver function tests (LFTs). Indeed, there had been a long-standing belief, based on clinical trials, that by monitoring alanine aminotransferase (ALT) and maintaining it at <3 times the upper limit normal (ULN), hepatotoxicity could be avoided.38 In clinical practice, however, further ALT elevation is frequently allowed based on patient tolerability.
In February 2012, the FDA revised its safety data to reflect this practice.39 The FDA update confirmed that routine LFT monitoring is unnecessary for patients on statins—and that it is not very effective in identifying or preventing liver damage.
Overall, serious hepatotoxicity is very rare, with an incidence ≤2 per 1 million patient-years.39 The National Lipid Association Statin Safety Assessment Task Force recommends repeating LFTs that are 3 to 5 times the ULN within 6 months and continuing with the statin dose if the patient is asymptomatic.38
THE TAKEAWAY: Routine liver function monitoring is not necessary for patients on statins. A better approach: Obtain baseline ALT levels, and repeat the testing only as clinically indicated thereafter.38,39
7. Urine drug screens: Which factors affect their accuracy?
The gold standard for testing for drugs of abuse, urine drug screens (UDS) have good sensitivity and specificity, easy administration, and reasonable cost.40 UDS can detect various narcotics, such as morphine, oxycodone, ,and methadone, and identify other illicit drugs, although which drugs and metabolites are tested for is laboratory- and test-specific.
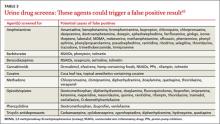
Cross-reactivity. There are 6 currently available immunoassays, all of which use competitive binding between the sample drug and a drug chemically labeled with an enzyme, radioisotope, or fluorophore. The sample drug and labeled drug compete for substrate binding sites on drug-specific antibodies.41,42 Similar to competitive binding for enzymatic reactions in the body, the substrate binding site can experience cross-reactivity—causing substances other than the drug in question to bind to the immunoglobulin, leading to a false positive result (TABLE 3).43 Other factors that can alter the results include the cutoff value of the test and the absorption, distribution, metabolism, and excretion of the drug.42 Thus, a confirmatory test of gas chromatography-mass spectrometry is recommended before making decisions based on the results of UDS.43-45
Routine screens for patients on chronic opioid therapy. Routine use of UDS in emergency departments is no longer recommended, based on evidence that the results are unlikely to have a significant effect on patient management.46 For patients on chronic opioid therapy, however, routine screening has proven helpful in detecting prescription opioid abuse, illicit drug use, and diversion. Up to 34% of patients on prescription opioids have been found to be using illicit drugs, as well.42
THE TAKEAWAY: Use UDS as a tool in managing patients on chronic opioid therapy, but before acting on results, assess for factors, such as the use of oral or topical medications and the cutoff value of the test, that may be associated with false positive or false negative results.43-45
8. Thyroid function testing: When should you test?
Thyroid-stimulating hormone (TSH) is the first-line test when investigating presumed hyper- or hypothyroidism.47,48 Third-generation chemiluminometric assays can reliably measure TSH concentrations <0.01 mU/L by using multiple antibodies to produce a sandwich-type effect on the molecule in question.49
TSH levels exhibit diurnal variation, however, and are affected by other medications, including steroids, opiates, and some antihistamines, among others, as well as comorbidities.47,48 Chronic and acute conditions unrelated to thyroid disease can cause transient changes in TSH concentrations, and have the potential to modify the binding capacity of plasma thyroid hormone binding proteins.48 Thus, TSH should be ordered for hospitalized patients only when clinical suspicion of a thyroid problem exists.48 The USPSTF recommends against routine TSH screening for asymptomatic adults.46
How to respond to abnormal results. For patients found to have abnormal TSH levels, free T4 (fT4) is the next test to order.47,49 An fT4 assay is a superior indicator of thyroid status because it is not affected by changes in iodothyronine-binding proteins, which influence total hormone measurements.49 The results will be elevated in hyperthyroidism and reduced in hypothyroidism.47
Triiodothyronine (T3) measures can be useful in diagnosing Graves’ disease, in which T3 toxicosis may be the initial symptom—or an indication of a relapse. Because T3 is often a peripheral product, however, nonthyroid illnesses and medications can cause artifactually abnormal results.49
Other thyroid-specific labs include thyroid ,antibodies such as antithyroid peroxidase, antithyroglobulin, and TSH receptor, both blocking and stimulating.49 Thyroglobulin is a precursor form of thyroid hormone and should be measured when factitious hyperthyroidism is suspected. Management of hyper- and hypothyroidism often is independent of etiology. Retesting TSH to assess treatment response should be postponed until ≥2 months after any change in medication or dosing.50
Thyroid studies can be very difficult to interpret. TSH should be the first test ordered. However, if TSH values do not match the clinical picture, fT4, T3, and other thyroid tests that are less affected by outside factors can be useful in identifying the cause.
THE TAKEAWAY: Routine TSH testing is not indicated for asymptomatic adults. When evaluating thyroid function is clinically indicated, TSH is the initial test of choice.47,48,51
CORRESPONDENCE
Joshua Tessier, DO, Iowa Lutheran Family Medicine Residency, 840 East University Avenue, Des Moines, IA 50316; [email protected]
1. Ottomano C. Errors in medicine and errors in laboratory medicine: what is the difference? Blood Transfus. 2010;8;79-81.
2. Wallach JB. Introduction to normal values (reference ranges). Interpretation of Diagnostic Tests. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:3-7.
3. Halwachs-Baumann G, Katzensteiner S, Schnedl W, et al. Comparative evaluation of three assay systems for automated determination of hemoglobin A1c. Clin Chem. 1997;43:511-517.
4. Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009;1:9-17.
5. Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109-114.
6. Tarim O, Küçükerdog˘an A, Gunay U, et al. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int. 1999;41:357-362.
7. Kim C, Bullard KM, Herman WH, et al. Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care. 2010;33:780-785.
8. Higgins T, Stewart D, Boehr E. Challenges in HbA1c analysis and reporting: an interesting case illustrating the many pitfalls. Clin Biochem. 2008;41:1104-1106.
9. Mongia SK, Little RR, Rohlfing CL, et al. Effects of hemoglobin C and S traits on the results of 14 commercial glycated hemoglobin assays. Am J Clin Pathol. 2008;130:136-140.
10. Brown MD, Rowe BH, Reeves MJ, et al. The accuracy of the enzyme-linked immunosorbent assay D-dimer test in the diagnosis of pulmonary embolism: a meta-analysis. Ann Emerg Med. 2002;40:133-144.
11. Squizzato A, Ageno W. What is the next step in D-dimer research? Education of physicians. Intern Emerg Med. 2006;1:165.
12. Kabrhel C, Mark Courtney D, Camargo CA Jr, et al. Potential impact of adjusting the threshold of the quantitative D-dimer based on pretest probability of acute pulmonary embolism. Acad Emerg Med. 2009;16:325-332.
13. Kabrhel C, Mark Courtney MD, Camargo CA Jr, et al. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med. 2010;17:589-597.
14. Berman AR. Pulmonary embolism in the elderly. Clin Geriatr Med. 2001;17:107-130.
15. Bruinstroop E, van de Ree MA, Huisman MV. The use of Ddimer in specific clinical conditions: a narrative review. Eur J Intern Med. 2009;20:441-446.
16. Schouten HJ, Geersing GI, Koek HL, et al. Diagnostic accuracy ,of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492.
17. Turgeon ML. Linne & Ringsrud’s Clinical Laboratory Science. 5th ed. Saint Louis, MO: Mosby; 2007:50.
18. Cohn JS,McNamara JR, Schaefer EJ. Lipoprotein cholesterol concentrations in the plasma of human subjects as measured in the fed and fasted states. Clin Chem. 1988;34:2456-2459.
19. Watts GF, Cohn JS. Whither the lipid profile: feast, famine, or no free lunch? Clin Chem. 2011;57:363-365.
20. Mora S, Rifai N, Buring JE, et al. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993-1001.
21. Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets. 2009;10:328-335.
22. Screening for lipid disorders in adults: US Preventive Services Task Force Recommendation statement. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/lipid/lipidrs.htm. Accessed March 13, 2014.
23. Wolf DM, Friedrichs I, Toma AG. Lymphocyte-white blood cell count ratio: a quickly available screening tool to differentiate acute purulent tonsillitis from glandular fever. Arch Otolaryngol Head Neck Surg. 2007;133:61-64.
24. McCormack R, O’Shea T. The uptake and use of the Monospot test in patients with tonsillitis. Ir Med J. 2009;102:226-228.
25. Ebell MH. Epstein-Barr virus infectious mononucleosis. Am Fam Physician. 2004;70:1279-1287.
26. Waninger KN, Harcke HT. Determination of safe return to play for athletes recovering from infectious mononucleosis: a review of the literature. Clin J Sport Med. 2005;15:410-416.
27. Hurt C, Tammaro D. Diagnostic evaluation of mononucleosislike illnesses. Am J Med. 2007;120:911.e1-911.e8.
28. Vouloumanou EK, Rafailidis PI, Falagas ME. Current diagnosis and management of infectious mononucleosis. Curr Opin Hematol. 2012;19:14-20.
29. Epstein-Barr virus and infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/laboratory-testing.html. Updated January 7, 2014. Accessed March 12, 2014.
30. Dasgupta A. Clinical utility of free drug monitoring. Clin Chem Lab Med. 2002;40:986-993.
31. Dasgupta A. Usefulness of monitoring free (unbound) concentrations of therapeutic drugs in patient management. Clin Chim Acta. 2007;377:1-13.
32. Iwamoto T, Kagawa Y, Naito Y, et al. Clinical evaluation of plasma free phenytoin measurement and factors influencing its protein binding. Biopharm Drug Dispos. 2006;27:77-84.
33. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97:489-493.
34. Wolf GK, McClain CD, Zurakowski D, et al. Total phenytoin concentrations do not accurately predict free phenytoin concentrations in critically ill children. Pediatr Crit Care Med. 2006;7:434-439; quiz 440.
35. Lanoxin (digoxin) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2011.
36. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
37. De Backer G, Ambrosini E, Borch-Johnsen K, et al; Third Joint Force of European and other Societies on Cardiovascular Disease and Prevention in Clinical Practice. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis. 2003;171:145-155.
38. McKenney JM, Davidson MH, Jacobson TA, et al. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
39. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Updated July 3, 2012. Accessed May 17, 2013.
40. Eskridge KD, Guthrie SK. Clinical issues associated with urine testing of substances of abuse. Pharmacotherapy. 1997;17:497-510.
41. Herring C, Muzyk AJ, Johnston C. Interferences with urine drug screens. J Pharm Pract. 2001;24:102-108.
42. Jaffee WB, Trucco E, Teter C, et al. Focus on alcohol & drug abuse: ensuring validity in urine drug testing. Psychiatr Serv. 2008;59:140-142.
43. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67:1344-1350.
44. Moeller KE, Lee KC, Kissack JC. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc. 2008;83:66-76.
45. Christo PJ, Manchikanti L, Ruan X, et al. Urine drug testing in chronic pain. Pain Physician. 2011;14:123-143.
46. Tenenbein M. Do you really need that emergency drug screen? Clin Toxicol (Phila). 2009;47:286-291.
47. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86:244-251.
48. UK guidelines for the use of thyroid function tests. British Thyroid Association Web site. Available at: http://www.british-thyroid-association.org/info-for-patients/Docs/TFT_guideline_final_version_July_2006.pdf. Accessed March 11, 2014.
49. Volpé, R. Rational use of thyroid function tests. Crit Rev Clin Lab Sci. 1997;34:405-438.
50. Graber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
51. Helfand M; US Preventive Services Task Force. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2004;140:128-141.
› When interpreting hemoglobin A1c (HbA1c) levels, assess for anemia and other comorbidities that can significantly affect the lifespan of red blood cells and skew HbA1c test results. B
› Order nonfasting lipid panels for patients for whom fasting laboratory tests are difficult to obtain, as they have good clinical utility in screening and initial treatment. A
› Avoid routine thyroid-stimulating hormone (TSH) testing in asymptomatic adults; when testing is indicated, start with TSH. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Laboratory mistakes are not defined as diagnostic errors, but they contribute significantly to the thousands of medical errors that occur every year.1 Part of the problem: While accurate interpretation of lab tests often depends on the use of statistical concepts we all learned in medical training, it is difficult to find the time to incorporate these principles into a busy practice.
Overuse of lab tests presents problems, as well. Because “normal ranges” for test results are based on statistical analysis, as many as 5% of patients in a standard distribution fall outside of the range.2 It is important to order only the tests you really need, as extra testing automatically means more false positive results.
This article was written with such pitfalls in mind. In the pages that follow, we focus on 8 types of tests family physicians rely on regularly—all cases in which test results are reliable only if comorbidities, pre- and post-test probabilities, and clinical context are carefully considered. To help you put these lab tests into the proper context, we’ve addressed a key question—and highlighted both pitfalls and pearls—about each.
1. Hemoglobin A1c: How does anemia affect it?
Hemoglobin A1c (HbA1c) can be measured in many ways, including high-performance liquid chromatography, boronate affinity, capillary electrophoresis, and immunoassay, all of which can provide equivalent values without significant variability.3,4 In interpreting these tests, however, it is important to understand the effect that anemia has on HbA1c.
It's important to order only the tests you really need, as extra testing automatically means more false positive results.
Two primary variables influencing HbA1c are the average glucose level and the average lifespan of red blood cells (RBCs). Normally, there is a direct correlation between average serum glucose and HbA1c.4 In patients with anemia, however, this relationship is less clear, and may be affected by erythropoiesis and RBC destruction.5 In iron deficiency anemia (IDA),6,7 hemoglobin production falls secondary to iron stores, resulting in microcytic cells with a longer lifespan and elevated HbA1c. In at least one study,5,7 HbA1c approached levels associated with diabetes (with increases as high as 1.5%) in nondiabetic patients, but resolved with treatment of IDA.
Increased destruction as well as increased production of RBCs lowers their lifespan, and in turn decreases HbA1c levels (TABLE 1).4 This can be seen in conditions such as splenomegaly and hemoglobinopathies. In patients with hemoglobinopathies, the percentage of hemoglobin A is significantly decreased, often to undetectable levels—thereby making HbA1c tests inaccurate. Hemoglobin electrophoresis and determination of glycation by capillary electrophoresis or high-performance liquid chromatography can be used instead, but neither is practical because of cost and limited availability.4,8,9
THE TAKEAWAY: When you evaluate HbA1c test results, it is crucial to assess the patient for anemia and other conditions or comorbidities that can significantly affect RBC lifespan and skew test results.2,4-6
2. D-dimer: When should you use it?
D-dimer is a fibrin degradation product that is increased when active clotting is present,10 and its assay—which has high sensitivity and low specificity—is widely used to screen for pulmonary embolism (PE) and deep vein thrombosis (DVT). While the minimal number of false negatives makes the D-dimer a good screening test, the higher rate of false positives makes it difficult to arrive at a definitive diagnosis. Appropriate use of the D-dimer assay is crucial to minimize the potential for adverse consequences, such as bleeding in patients who are subjected to unnecessary anticoagulation because of false positive results.
Further testing typically follows. A positive D-dimer test is commonly followed by a computed tomography (CT) scan of the chest or a ventilation/perfusion scan to establish a PE or DVT diagnosis. But this subsequent testing increases both the cost of health care and the patient’s radiation exposure. Use of these subsequent scans can be reduced by first considering the patient’s pretest probability for PE or DVT. The Wells’ Criteria (available at www.mdcalc.com/wells-criteriafor-pulmonary-embolism-pe/) and Geneva Score (Revised) (www.mdcalc.com/genevascore-revised-for-pulmonary-embolism/) can both be used for this purpose.10,11
Patients with high pretest probability should undergo immediate scanning, foregoing the D-dimer—which should be reserved for patients who have a low or moderate pretest probability but sufficient reason to suspect PE or DVT.10-12
The low specificity of the D-dimer assay poses another challenge to its effective use. There are many things that can increase D-dimer levels, such as age, cancer, prolonged immobility, autoimmune disease, inflammation, sickle cell disease, pregnancy, trauma, and surgery.13-15 All these factors must be taken into consideration prior to ordering this test.
In fact, one recent study found that using an age-adjusted D-dimer cutoff (patient’s age in years x 10 mcg/L)—rather than a conventional cutoff of 500 mcg/L—for patients older than 50 years reduces false positives without substantially increasing false negatives.16
Also of note: An anticoagulant can decrease D-dimer levels in plasma, so the test should not be used to rule out PE or DVT in patients who are undergoing anticoagulation.13,15
THE TAKEAWAY: In evaluating patients for PE or DVT, use the Wells’ Criteria or Geneva Score (Revised) to determine a patient’s pretest probability of disease. Use the D-dimer assay to safely rule out these conditions in patients with a low or intermediate pretest probability, but go directly to scans for those with a high pretest probability.
3. Lipid panels: How important is fasting?
Patients are often instructed to report for fasting lab studies, specifically for lipid profiles. Traditionally, this had been defined as an 8- to 12-hour period without food.17 In clinical practice, however, this is often misinterpreted by patients, who may be confused about the duration of the fast or unsure about whether to eat or drink immediately before the test.
Studies investigating the effect of meals on lab values have found that triglycerides are consistently elevated postprandially, to a maximum of 12 hours.18-21 The effect of the fasting state on total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol is more controversial; while some postprandial differences have been detected, the clinical relevance is equivocal.18-21
Nonfasting lipid values can offer useful information, particularly in patients who are unwilling or unable to return for fasting labs. The US Preventive Services Task Force (USPSTF) supports this practice.22 Because guidelines for evaluation and treatment are based on fasting lipids, however, fasting lab work should be used, whenever possible, for initiating treatment and monitoring patients with abnormal values. If nonfasting lipids are used, it is crucial to factor in the postprandial effects on triglycerides and the subsequent difficulty of assessing LDL cholesterol levels.
THE TAKEAWAY: The clinical relevance of postprandial vs fasting lipid levels is equivocal. Nonfasting lipid panels have reasonable clinical utility in screening and initial treatment, particularly in cases in which obtaining fasting lab values may be problematic.18,19
4. Mononucleoosis spot test: When should you use it?
The monospot test is a latex assay that causes hemagglutination of horse RBCs in the presence of heterophile antibodies characteristic of infectious mononucleosis.23 The antibodies develop within the first 7 days of onset of symptoms, but do not peak for 2 to 5 weeks.24 As a result, monospot testing yields a high incidence of false negatives during the first 2 weeks of active infection.25 False negatives are also common in patients younger than 14 years. Heterophile antibodies may be present for up to a year after active infection.24
Patients at increased risk for splenic rupture, such as athletes, pose considerable diagnostic difficulty.26 When there is strong clinical suspicion of mononucleosis despite a negative monospot test in such high-risk individuals, follow-up testing is recommended to differentiate it from other mononucleosis-like illnesses (TABLE 2).27 The optimal combination of Epstein-Barr virus (EBV) serologic testing consists of the antibody titration of 4 markers: immunoglobulins M (IgM) and G (IgG) to the viral capsid antigen, IgM to the early antigen, and antibody to Epstein-Barr nuclear antigen (EBNA).28 Acute phase reactants in the setting of an antibody to EBNA could indicate reactivation. A positive test does not exclude other medical causes, however, because up to 20% of patients have acute phase antibodies that persist for years.29
Appropriate diagnosis is important because of the significant morbidity associated with EBV. Risk of splenic injury is greatest between 4 and 21 days after onset of symptoms but persists at 7 weeks,26 so conservative therapy followed by monospot retesting one week later is a reasonable approach.
Mononucleosis or routine tonsillitis? It is important to note that there is no evidence that a positive monospot test will affect the management or outcome of routine tonsillitis, raising questions of the utility of the test in such cases. A better approach: Reserve testing for patients with additional findings—ie, splenomegaly—or whose symptoms have persisted ≥ 2 weeks.
THE TAKEAWAY: Wait at least 2 weeks to conduct monospot testing in patients with routine tonsillitis. If strong clinical suspicion exists, proceed with specific IgM and IgG serologic testing.24,25,27,28
5. Evaluating prescription drug levels: Which factors interfere?
Correct interpretation of lab tests conducted to measure prescription drug levels has major implications with regard to patient safety, particularly for medications with a narrow therapeutic index.
Most drug level tests measure the total concentration, which includes both bound and unbound (free) forms. The unbound forms are the active components of the drug; thus, for an accurate evaluation, it is important to be aware of factors that increase free drug concentration. Chief among them is low protein levels, or hypoalbuminemia.30
Risk factors for hypoalbuminemia include significant burns, advanced age, pregnancy, malnutrition, and human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS).30 HIV/AIDS is a particularly high risk because certain protease inhibitors are highly protein bound.
Drug protein binding is classified as low, moderate, or high. The main proteins involved in the process are albumin, alpha-1-acid glycoprotein, and lipoprotein. Medications that are highly protein bound (>80%) are the most affected by low protein levels: Problems can arise when drugs completely bind to all the available proteins and excess drug availability increases free drug levels.
Medications that are most likely to be affected by a high degree of protein binding include carbamazepine, cyclosporine, mycophenolic acid, phenytoin, protease inhibitors (with the exception of indinavir), tacrolimus, and valproic acid. It is important to consider free levels when you order medication assays for these drugs to avoid misinterpreting the serum levels as being too low-a scenario that raises the risk of drug toxicity and adverse outcomes.30,31
A study of 119 phenytoin samples from 70 patients found significantly higher free phenytoin levels in patients with lower albumin levels.32 Higher free phenytoin levels were also seen in older patients and in those with diminished renal function (creatinine clearance <25 mL/min).32 The degree of protein binding is affected by both the serum drug concentration and the albumin level, with saturable protein binding occurring at higher drug levels.33
Calculate phenytoin levels with this equation. To calculate corrected phenytoin levels in patients with low albumin levels, use the following formula, known as the Sheiner-Tozer equation:34
Concentration adjusted=concentration reported/([adjustment x serum albumin] + 0.1); adjustment=0.2 for creatinine clearance ≥20 or 0.1 for creatinine clearance <20.
Additional causes of misinterpreted drug levels. While hypoalbuminemia plays a major role in the misinterpretation of drug levels, other factors affect serum drug concentration, as well. These include drug-drug interactions, which can significantly increase the concentration of the medications involved, and the timing of the test with regard to medication administration. Digoxin levels, in particular, need to be drawn at least 6 to 8 hours after the last dose is taken to allow for appropriate drug distribution.35
THE TAKEAWAY: It is essential to consider free drug level monitoring in patients who either have hypoalbuminemia or have one or more risk factors for hypoalbuminemia to avoid falsely low estimation of drug levels.36,37
6 Liver function tests: Necessary for patients on statin therapy?
Since statins gained US Food and Drug Administration (FDA) approval, the drugs have been associated with increased liver function tests (LFTs). Indeed, there had been a long-standing belief, based on clinical trials, that by monitoring alanine aminotransferase (ALT) and maintaining it at <3 times the upper limit normal (ULN), hepatotoxicity could be avoided.38 In clinical practice, however, further ALT elevation is frequently allowed based on patient tolerability.
In February 2012, the FDA revised its safety data to reflect this practice.39 The FDA update confirmed that routine LFT monitoring is unnecessary for patients on statins—and that it is not very effective in identifying or preventing liver damage.
Overall, serious hepatotoxicity is very rare, with an incidence ≤2 per 1 million patient-years.39 The National Lipid Association Statin Safety Assessment Task Force recommends repeating LFTs that are 3 to 5 times the ULN within 6 months and continuing with the statin dose if the patient is asymptomatic.38
THE TAKEAWAY: Routine liver function monitoring is not necessary for patients on statins. A better approach: Obtain baseline ALT levels, and repeat the testing only as clinically indicated thereafter.38,39
7. Urine drug screens: Which factors affect their accuracy?
The gold standard for testing for drugs of abuse, urine drug screens (UDS) have good sensitivity and specificity, easy administration, and reasonable cost.40 UDS can detect various narcotics, such as morphine, oxycodone, ,and methadone, and identify other illicit drugs, although which drugs and metabolites are tested for is laboratory- and test-specific.
Cross-reactivity. There are 6 currently available immunoassays, all of which use competitive binding between the sample drug and a drug chemically labeled with an enzyme, radioisotope, or fluorophore. The sample drug and labeled drug compete for substrate binding sites on drug-specific antibodies.41,42 Similar to competitive binding for enzymatic reactions in the body, the substrate binding site can experience cross-reactivity—causing substances other than the drug in question to bind to the immunoglobulin, leading to a false positive result (TABLE 3).43 Other factors that can alter the results include the cutoff value of the test and the absorption, distribution, metabolism, and excretion of the drug.42 Thus, a confirmatory test of gas chromatography-mass spectrometry is recommended before making decisions based on the results of UDS.43-45
Routine screens for patients on chronic opioid therapy. Routine use of UDS in emergency departments is no longer recommended, based on evidence that the results are unlikely to have a significant effect on patient management.46 For patients on chronic opioid therapy, however, routine screening has proven helpful in detecting prescription opioid abuse, illicit drug use, and diversion. Up to 34% of patients on prescription opioids have been found to be using illicit drugs, as well.42
THE TAKEAWAY: Use UDS as a tool in managing patients on chronic opioid therapy, but before acting on results, assess for factors, such as the use of oral or topical medications and the cutoff value of the test, that may be associated with false positive or false negative results.43-45
8. Thyroid function testing: When should you test?
Thyroid-stimulating hormone (TSH) is the first-line test when investigating presumed hyper- or hypothyroidism.47,48 Third-generation chemiluminometric assays can reliably measure TSH concentrations <0.01 mU/L by using multiple antibodies to produce a sandwich-type effect on the molecule in question.49
TSH levels exhibit diurnal variation, however, and are affected by other medications, including steroids, opiates, and some antihistamines, among others, as well as comorbidities.47,48 Chronic and acute conditions unrelated to thyroid disease can cause transient changes in TSH concentrations, and have the potential to modify the binding capacity of plasma thyroid hormone binding proteins.48 Thus, TSH should be ordered for hospitalized patients only when clinical suspicion of a thyroid problem exists.48 The USPSTF recommends against routine TSH screening for asymptomatic adults.46
How to respond to abnormal results. For patients found to have abnormal TSH levels, free T4 (fT4) is the next test to order.47,49 An fT4 assay is a superior indicator of thyroid status because it is not affected by changes in iodothyronine-binding proteins, which influence total hormone measurements.49 The results will be elevated in hyperthyroidism and reduced in hypothyroidism.47
Triiodothyronine (T3) measures can be useful in diagnosing Graves’ disease, in which T3 toxicosis may be the initial symptom—or an indication of a relapse. Because T3 is often a peripheral product, however, nonthyroid illnesses and medications can cause artifactually abnormal results.49
Other thyroid-specific labs include thyroid ,antibodies such as antithyroid peroxidase, antithyroglobulin, and TSH receptor, both blocking and stimulating.49 Thyroglobulin is a precursor form of thyroid hormone and should be measured when factitious hyperthyroidism is suspected. Management of hyper- and hypothyroidism often is independent of etiology. Retesting TSH to assess treatment response should be postponed until ≥2 months after any change in medication or dosing.50
Thyroid studies can be very difficult to interpret. TSH should be the first test ordered. However, if TSH values do not match the clinical picture, fT4, T3, and other thyroid tests that are less affected by outside factors can be useful in identifying the cause.
THE TAKEAWAY: Routine TSH testing is not indicated for asymptomatic adults. When evaluating thyroid function is clinically indicated, TSH is the initial test of choice.47,48,51
CORRESPONDENCE
Joshua Tessier, DO, Iowa Lutheran Family Medicine Residency, 840 East University Avenue, Des Moines, IA 50316; [email protected]
› When interpreting hemoglobin A1c (HbA1c) levels, assess for anemia and other comorbidities that can significantly affect the lifespan of red blood cells and skew HbA1c test results. B
› Order nonfasting lipid panels for patients for whom fasting laboratory tests are difficult to obtain, as they have good clinical utility in screening and initial treatment. A
› Avoid routine thyroid-stimulating hormone (TSH) testing in asymptomatic adults; when testing is indicated, start with TSH. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Laboratory mistakes are not defined as diagnostic errors, but they contribute significantly to the thousands of medical errors that occur every year.1 Part of the problem: While accurate interpretation of lab tests often depends on the use of statistical concepts we all learned in medical training, it is difficult to find the time to incorporate these principles into a busy practice.
Overuse of lab tests presents problems, as well. Because “normal ranges” for test results are based on statistical analysis, as many as 5% of patients in a standard distribution fall outside of the range.2 It is important to order only the tests you really need, as extra testing automatically means more false positive results.
This article was written with such pitfalls in mind. In the pages that follow, we focus on 8 types of tests family physicians rely on regularly—all cases in which test results are reliable only if comorbidities, pre- and post-test probabilities, and clinical context are carefully considered. To help you put these lab tests into the proper context, we’ve addressed a key question—and highlighted both pitfalls and pearls—about each.
1. Hemoglobin A1c: How does anemia affect it?
Hemoglobin A1c (HbA1c) can be measured in many ways, including high-performance liquid chromatography, boronate affinity, capillary electrophoresis, and immunoassay, all of which can provide equivalent values without significant variability.3,4 In interpreting these tests, however, it is important to understand the effect that anemia has on HbA1c.
It's important to order only the tests you really need, as extra testing automatically means more false positive results.
Two primary variables influencing HbA1c are the average glucose level and the average lifespan of red blood cells (RBCs). Normally, there is a direct correlation between average serum glucose and HbA1c.4 In patients with anemia, however, this relationship is less clear, and may be affected by erythropoiesis and RBC destruction.5 In iron deficiency anemia (IDA),6,7 hemoglobin production falls secondary to iron stores, resulting in microcytic cells with a longer lifespan and elevated HbA1c. In at least one study,5,7 HbA1c approached levels associated with diabetes (with increases as high as 1.5%) in nondiabetic patients, but resolved with treatment of IDA.
Increased destruction as well as increased production of RBCs lowers their lifespan, and in turn decreases HbA1c levels (TABLE 1).4 This can be seen in conditions such as splenomegaly and hemoglobinopathies. In patients with hemoglobinopathies, the percentage of hemoglobin A is significantly decreased, often to undetectable levels—thereby making HbA1c tests inaccurate. Hemoglobin electrophoresis and determination of glycation by capillary electrophoresis or high-performance liquid chromatography can be used instead, but neither is practical because of cost and limited availability.4,8,9
THE TAKEAWAY: When you evaluate HbA1c test results, it is crucial to assess the patient for anemia and other conditions or comorbidities that can significantly affect RBC lifespan and skew test results.2,4-6
2. D-dimer: When should you use it?
D-dimer is a fibrin degradation product that is increased when active clotting is present,10 and its assay—which has high sensitivity and low specificity—is widely used to screen for pulmonary embolism (PE) and deep vein thrombosis (DVT). While the minimal number of false negatives makes the D-dimer a good screening test, the higher rate of false positives makes it difficult to arrive at a definitive diagnosis. Appropriate use of the D-dimer assay is crucial to minimize the potential for adverse consequences, such as bleeding in patients who are subjected to unnecessary anticoagulation because of false positive results.
Further testing typically follows. A positive D-dimer test is commonly followed by a computed tomography (CT) scan of the chest or a ventilation/perfusion scan to establish a PE or DVT diagnosis. But this subsequent testing increases both the cost of health care and the patient’s radiation exposure. Use of these subsequent scans can be reduced by first considering the patient’s pretest probability for PE or DVT. The Wells’ Criteria (available at www.mdcalc.com/wells-criteriafor-pulmonary-embolism-pe/) and Geneva Score (Revised) (www.mdcalc.com/genevascore-revised-for-pulmonary-embolism/) can both be used for this purpose.10,11
Patients with high pretest probability should undergo immediate scanning, foregoing the D-dimer—which should be reserved for patients who have a low or moderate pretest probability but sufficient reason to suspect PE or DVT.10-12
The low specificity of the D-dimer assay poses another challenge to its effective use. There are many things that can increase D-dimer levels, such as age, cancer, prolonged immobility, autoimmune disease, inflammation, sickle cell disease, pregnancy, trauma, and surgery.13-15 All these factors must be taken into consideration prior to ordering this test.
In fact, one recent study found that using an age-adjusted D-dimer cutoff (patient’s age in years x 10 mcg/L)—rather than a conventional cutoff of 500 mcg/L—for patients older than 50 years reduces false positives without substantially increasing false negatives.16
Also of note: An anticoagulant can decrease D-dimer levels in plasma, so the test should not be used to rule out PE or DVT in patients who are undergoing anticoagulation.13,15
THE TAKEAWAY: In evaluating patients for PE or DVT, use the Wells’ Criteria or Geneva Score (Revised) to determine a patient’s pretest probability of disease. Use the D-dimer assay to safely rule out these conditions in patients with a low or intermediate pretest probability, but go directly to scans for those with a high pretest probability.
3. Lipid panels: How important is fasting?
Patients are often instructed to report for fasting lab studies, specifically for lipid profiles. Traditionally, this had been defined as an 8- to 12-hour period without food.17 In clinical practice, however, this is often misinterpreted by patients, who may be confused about the duration of the fast or unsure about whether to eat or drink immediately before the test.
Studies investigating the effect of meals on lab values have found that triglycerides are consistently elevated postprandially, to a maximum of 12 hours.18-21 The effect of the fasting state on total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol is more controversial; while some postprandial differences have been detected, the clinical relevance is equivocal.18-21
Nonfasting lipid values can offer useful information, particularly in patients who are unwilling or unable to return for fasting labs. The US Preventive Services Task Force (USPSTF) supports this practice.22 Because guidelines for evaluation and treatment are based on fasting lipids, however, fasting lab work should be used, whenever possible, for initiating treatment and monitoring patients with abnormal values. If nonfasting lipids are used, it is crucial to factor in the postprandial effects on triglycerides and the subsequent difficulty of assessing LDL cholesterol levels.
THE TAKEAWAY: The clinical relevance of postprandial vs fasting lipid levels is equivocal. Nonfasting lipid panels have reasonable clinical utility in screening and initial treatment, particularly in cases in which obtaining fasting lab values may be problematic.18,19
4. Mononucleoosis spot test: When should you use it?
The monospot test is a latex assay that causes hemagglutination of horse RBCs in the presence of heterophile antibodies characteristic of infectious mononucleosis.23 The antibodies develop within the first 7 days of onset of symptoms, but do not peak for 2 to 5 weeks.24 As a result, monospot testing yields a high incidence of false negatives during the first 2 weeks of active infection.25 False negatives are also common in patients younger than 14 years. Heterophile antibodies may be present for up to a year after active infection.24
Patients at increased risk for splenic rupture, such as athletes, pose considerable diagnostic difficulty.26 When there is strong clinical suspicion of mononucleosis despite a negative monospot test in such high-risk individuals, follow-up testing is recommended to differentiate it from other mononucleosis-like illnesses (TABLE 2).27 The optimal combination of Epstein-Barr virus (EBV) serologic testing consists of the antibody titration of 4 markers: immunoglobulins M (IgM) and G (IgG) to the viral capsid antigen, IgM to the early antigen, and antibody to Epstein-Barr nuclear antigen (EBNA).28 Acute phase reactants in the setting of an antibody to EBNA could indicate reactivation. A positive test does not exclude other medical causes, however, because up to 20% of patients have acute phase antibodies that persist for years.29
Appropriate diagnosis is important because of the significant morbidity associated with EBV. Risk of splenic injury is greatest between 4 and 21 days after onset of symptoms but persists at 7 weeks,26 so conservative therapy followed by monospot retesting one week later is a reasonable approach.
Mononucleosis or routine tonsillitis? It is important to note that there is no evidence that a positive monospot test will affect the management or outcome of routine tonsillitis, raising questions of the utility of the test in such cases. A better approach: Reserve testing for patients with additional findings—ie, splenomegaly—or whose symptoms have persisted ≥ 2 weeks.
THE TAKEAWAY: Wait at least 2 weeks to conduct monospot testing in patients with routine tonsillitis. If strong clinical suspicion exists, proceed with specific IgM and IgG serologic testing.24,25,27,28
5. Evaluating prescription drug levels: Which factors interfere?
Correct interpretation of lab tests conducted to measure prescription drug levels has major implications with regard to patient safety, particularly for medications with a narrow therapeutic index.
Most drug level tests measure the total concentration, which includes both bound and unbound (free) forms. The unbound forms are the active components of the drug; thus, for an accurate evaluation, it is important to be aware of factors that increase free drug concentration. Chief among them is low protein levels, or hypoalbuminemia.30
Risk factors for hypoalbuminemia include significant burns, advanced age, pregnancy, malnutrition, and human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS).30 HIV/AIDS is a particularly high risk because certain protease inhibitors are highly protein bound.
Drug protein binding is classified as low, moderate, or high. The main proteins involved in the process are albumin, alpha-1-acid glycoprotein, and lipoprotein. Medications that are highly protein bound (>80%) are the most affected by low protein levels: Problems can arise when drugs completely bind to all the available proteins and excess drug availability increases free drug levels.
Medications that are most likely to be affected by a high degree of protein binding include carbamazepine, cyclosporine, mycophenolic acid, phenytoin, protease inhibitors (with the exception of indinavir), tacrolimus, and valproic acid. It is important to consider free levels when you order medication assays for these drugs to avoid misinterpreting the serum levels as being too low-a scenario that raises the risk of drug toxicity and adverse outcomes.30,31
A study of 119 phenytoin samples from 70 patients found significantly higher free phenytoin levels in patients with lower albumin levels.32 Higher free phenytoin levels were also seen in older patients and in those with diminished renal function (creatinine clearance <25 mL/min).32 The degree of protein binding is affected by both the serum drug concentration and the albumin level, with saturable protein binding occurring at higher drug levels.33
Calculate phenytoin levels with this equation. To calculate corrected phenytoin levels in patients with low albumin levels, use the following formula, known as the Sheiner-Tozer equation:34
Concentration adjusted=concentration reported/([adjustment x serum albumin] + 0.1); adjustment=0.2 for creatinine clearance ≥20 or 0.1 for creatinine clearance <20.
Additional causes of misinterpreted drug levels. While hypoalbuminemia plays a major role in the misinterpretation of drug levels, other factors affect serum drug concentration, as well. These include drug-drug interactions, which can significantly increase the concentration of the medications involved, and the timing of the test with regard to medication administration. Digoxin levels, in particular, need to be drawn at least 6 to 8 hours after the last dose is taken to allow for appropriate drug distribution.35
THE TAKEAWAY: It is essential to consider free drug level monitoring in patients who either have hypoalbuminemia or have one or more risk factors for hypoalbuminemia to avoid falsely low estimation of drug levels.36,37
6 Liver function tests: Necessary for patients on statin therapy?
Since statins gained US Food and Drug Administration (FDA) approval, the drugs have been associated with increased liver function tests (LFTs). Indeed, there had been a long-standing belief, based on clinical trials, that by monitoring alanine aminotransferase (ALT) and maintaining it at <3 times the upper limit normal (ULN), hepatotoxicity could be avoided.38 In clinical practice, however, further ALT elevation is frequently allowed based on patient tolerability.
In February 2012, the FDA revised its safety data to reflect this practice.39 The FDA update confirmed that routine LFT monitoring is unnecessary for patients on statins—and that it is not very effective in identifying or preventing liver damage.
Overall, serious hepatotoxicity is very rare, with an incidence ≤2 per 1 million patient-years.39 The National Lipid Association Statin Safety Assessment Task Force recommends repeating LFTs that are 3 to 5 times the ULN within 6 months and continuing with the statin dose if the patient is asymptomatic.38
THE TAKEAWAY: Routine liver function monitoring is not necessary for patients on statins. A better approach: Obtain baseline ALT levels, and repeat the testing only as clinically indicated thereafter.38,39
7. Urine drug screens: Which factors affect their accuracy?
The gold standard for testing for drugs of abuse, urine drug screens (UDS) have good sensitivity and specificity, easy administration, and reasonable cost.40 UDS can detect various narcotics, such as morphine, oxycodone, ,and methadone, and identify other illicit drugs, although which drugs and metabolites are tested for is laboratory- and test-specific.
Cross-reactivity. There are 6 currently available immunoassays, all of which use competitive binding between the sample drug and a drug chemically labeled with an enzyme, radioisotope, or fluorophore. The sample drug and labeled drug compete for substrate binding sites on drug-specific antibodies.41,42 Similar to competitive binding for enzymatic reactions in the body, the substrate binding site can experience cross-reactivity—causing substances other than the drug in question to bind to the immunoglobulin, leading to a false positive result (TABLE 3).43 Other factors that can alter the results include the cutoff value of the test and the absorption, distribution, metabolism, and excretion of the drug.42 Thus, a confirmatory test of gas chromatography-mass spectrometry is recommended before making decisions based on the results of UDS.43-45
Routine screens for patients on chronic opioid therapy. Routine use of UDS in emergency departments is no longer recommended, based on evidence that the results are unlikely to have a significant effect on patient management.46 For patients on chronic opioid therapy, however, routine screening has proven helpful in detecting prescription opioid abuse, illicit drug use, and diversion. Up to 34% of patients on prescription opioids have been found to be using illicit drugs, as well.42
THE TAKEAWAY: Use UDS as a tool in managing patients on chronic opioid therapy, but before acting on results, assess for factors, such as the use of oral or topical medications and the cutoff value of the test, that may be associated with false positive or false negative results.43-45
8. Thyroid function testing: When should you test?
Thyroid-stimulating hormone (TSH) is the first-line test when investigating presumed hyper- or hypothyroidism.47,48 Third-generation chemiluminometric assays can reliably measure TSH concentrations <0.01 mU/L by using multiple antibodies to produce a sandwich-type effect on the molecule in question.49
TSH levels exhibit diurnal variation, however, and are affected by other medications, including steroids, opiates, and some antihistamines, among others, as well as comorbidities.47,48 Chronic and acute conditions unrelated to thyroid disease can cause transient changes in TSH concentrations, and have the potential to modify the binding capacity of plasma thyroid hormone binding proteins.48 Thus, TSH should be ordered for hospitalized patients only when clinical suspicion of a thyroid problem exists.48 The USPSTF recommends against routine TSH screening for asymptomatic adults.46
How to respond to abnormal results. For patients found to have abnormal TSH levels, free T4 (fT4) is the next test to order.47,49 An fT4 assay is a superior indicator of thyroid status because it is not affected by changes in iodothyronine-binding proteins, which influence total hormone measurements.49 The results will be elevated in hyperthyroidism and reduced in hypothyroidism.47
Triiodothyronine (T3) measures can be useful in diagnosing Graves’ disease, in which T3 toxicosis may be the initial symptom—or an indication of a relapse. Because T3 is often a peripheral product, however, nonthyroid illnesses and medications can cause artifactually abnormal results.49
Other thyroid-specific labs include thyroid ,antibodies such as antithyroid peroxidase, antithyroglobulin, and TSH receptor, both blocking and stimulating.49 Thyroglobulin is a precursor form of thyroid hormone and should be measured when factitious hyperthyroidism is suspected. Management of hyper- and hypothyroidism often is independent of etiology. Retesting TSH to assess treatment response should be postponed until ≥2 months after any change in medication or dosing.50
Thyroid studies can be very difficult to interpret. TSH should be the first test ordered. However, if TSH values do not match the clinical picture, fT4, T3, and other thyroid tests that are less affected by outside factors can be useful in identifying the cause.
THE TAKEAWAY: Routine TSH testing is not indicated for asymptomatic adults. When evaluating thyroid function is clinically indicated, TSH is the initial test of choice.47,48,51
CORRESPONDENCE
Joshua Tessier, DO, Iowa Lutheran Family Medicine Residency, 840 East University Avenue, Des Moines, IA 50316; [email protected]
1. Ottomano C. Errors in medicine and errors in laboratory medicine: what is the difference? Blood Transfus. 2010;8;79-81.
2. Wallach JB. Introduction to normal values (reference ranges). Interpretation of Diagnostic Tests. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:3-7.
3. Halwachs-Baumann G, Katzensteiner S, Schnedl W, et al. Comparative evaluation of three assay systems for automated determination of hemoglobin A1c. Clin Chem. 1997;43:511-517.
4. Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009;1:9-17.
5. Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109-114.
6. Tarim O, Küçükerdog˘an A, Gunay U, et al. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int. 1999;41:357-362.
7. Kim C, Bullard KM, Herman WH, et al. Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care. 2010;33:780-785.
8. Higgins T, Stewart D, Boehr E. Challenges in HbA1c analysis and reporting: an interesting case illustrating the many pitfalls. Clin Biochem. 2008;41:1104-1106.
9. Mongia SK, Little RR, Rohlfing CL, et al. Effects of hemoglobin C and S traits on the results of 14 commercial glycated hemoglobin assays. Am J Clin Pathol. 2008;130:136-140.
10. Brown MD, Rowe BH, Reeves MJ, et al. The accuracy of the enzyme-linked immunosorbent assay D-dimer test in the diagnosis of pulmonary embolism: a meta-analysis. Ann Emerg Med. 2002;40:133-144.
11. Squizzato A, Ageno W. What is the next step in D-dimer research? Education of physicians. Intern Emerg Med. 2006;1:165.
12. Kabrhel C, Mark Courtney D, Camargo CA Jr, et al. Potential impact of adjusting the threshold of the quantitative D-dimer based on pretest probability of acute pulmonary embolism. Acad Emerg Med. 2009;16:325-332.
13. Kabrhel C, Mark Courtney MD, Camargo CA Jr, et al. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med. 2010;17:589-597.
14. Berman AR. Pulmonary embolism in the elderly. Clin Geriatr Med. 2001;17:107-130.
15. Bruinstroop E, van de Ree MA, Huisman MV. The use of Ddimer in specific clinical conditions: a narrative review. Eur J Intern Med. 2009;20:441-446.
16. Schouten HJ, Geersing GI, Koek HL, et al. Diagnostic accuracy ,of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492.
17. Turgeon ML. Linne & Ringsrud’s Clinical Laboratory Science. 5th ed. Saint Louis, MO: Mosby; 2007:50.
18. Cohn JS,McNamara JR, Schaefer EJ. Lipoprotein cholesterol concentrations in the plasma of human subjects as measured in the fed and fasted states. Clin Chem. 1988;34:2456-2459.
19. Watts GF, Cohn JS. Whither the lipid profile: feast, famine, or no free lunch? Clin Chem. 2011;57:363-365.
20. Mora S, Rifai N, Buring JE, et al. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993-1001.
21. Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets. 2009;10:328-335.
22. Screening for lipid disorders in adults: US Preventive Services Task Force Recommendation statement. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/lipid/lipidrs.htm. Accessed March 13, 2014.
23. Wolf DM, Friedrichs I, Toma AG. Lymphocyte-white blood cell count ratio: a quickly available screening tool to differentiate acute purulent tonsillitis from glandular fever. Arch Otolaryngol Head Neck Surg. 2007;133:61-64.
24. McCormack R, O’Shea T. The uptake and use of the Monospot test in patients with tonsillitis. Ir Med J. 2009;102:226-228.
25. Ebell MH. Epstein-Barr virus infectious mononucleosis. Am Fam Physician. 2004;70:1279-1287.
26. Waninger KN, Harcke HT. Determination of safe return to play for athletes recovering from infectious mononucleosis: a review of the literature. Clin J Sport Med. 2005;15:410-416.
27. Hurt C, Tammaro D. Diagnostic evaluation of mononucleosislike illnesses. Am J Med. 2007;120:911.e1-911.e8.
28. Vouloumanou EK, Rafailidis PI, Falagas ME. Current diagnosis and management of infectious mononucleosis. Curr Opin Hematol. 2012;19:14-20.
29. Epstein-Barr virus and infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/laboratory-testing.html. Updated January 7, 2014. Accessed March 12, 2014.
30. Dasgupta A. Clinical utility of free drug monitoring. Clin Chem Lab Med. 2002;40:986-993.
31. Dasgupta A. Usefulness of monitoring free (unbound) concentrations of therapeutic drugs in patient management. Clin Chim Acta. 2007;377:1-13.
32. Iwamoto T, Kagawa Y, Naito Y, et al. Clinical evaluation of plasma free phenytoin measurement and factors influencing its protein binding. Biopharm Drug Dispos. 2006;27:77-84.
33. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97:489-493.
34. Wolf GK, McClain CD, Zurakowski D, et al. Total phenytoin concentrations do not accurately predict free phenytoin concentrations in critically ill children. Pediatr Crit Care Med. 2006;7:434-439; quiz 440.
35. Lanoxin (digoxin) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2011.
36. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
37. De Backer G, Ambrosini E, Borch-Johnsen K, et al; Third Joint Force of European and other Societies on Cardiovascular Disease and Prevention in Clinical Practice. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis. 2003;171:145-155.
38. McKenney JM, Davidson MH, Jacobson TA, et al. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
39. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Updated July 3, 2012. Accessed May 17, 2013.
40. Eskridge KD, Guthrie SK. Clinical issues associated with urine testing of substances of abuse. Pharmacotherapy. 1997;17:497-510.
41. Herring C, Muzyk AJ, Johnston C. Interferences with urine drug screens. J Pharm Pract. 2001;24:102-108.
42. Jaffee WB, Trucco E, Teter C, et al. Focus on alcohol & drug abuse: ensuring validity in urine drug testing. Psychiatr Serv. 2008;59:140-142.
43. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67:1344-1350.
44. Moeller KE, Lee KC, Kissack JC. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc. 2008;83:66-76.
45. Christo PJ, Manchikanti L, Ruan X, et al. Urine drug testing in chronic pain. Pain Physician. 2011;14:123-143.
46. Tenenbein M. Do you really need that emergency drug screen? Clin Toxicol (Phila). 2009;47:286-291.
47. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86:244-251.
48. UK guidelines for the use of thyroid function tests. British Thyroid Association Web site. Available at: http://www.british-thyroid-association.org/info-for-patients/Docs/TFT_guideline_final_version_July_2006.pdf. Accessed March 11, 2014.
49. Volpé, R. Rational use of thyroid function tests. Crit Rev Clin Lab Sci. 1997;34:405-438.
50. Graber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
51. Helfand M; US Preventive Services Task Force. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2004;140:128-141.
1. Ottomano C. Errors in medicine and errors in laboratory medicine: what is the difference? Blood Transfus. 2010;8;79-81.
2. Wallach JB. Introduction to normal values (reference ranges). Interpretation of Diagnostic Tests. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:3-7.
3. Halwachs-Baumann G, Katzensteiner S, Schnedl W, et al. Comparative evaluation of three assay systems for automated determination of hemoglobin A1c. Clin Chem. 1997;43:511-517.
4. Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009;1:9-17.
5. Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109-114.
6. Tarim O, Küçükerdog˘an A, Gunay U, et al. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int. 1999;41:357-362.
7. Kim C, Bullard KM, Herman WH, et al. Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care. 2010;33:780-785.
8. Higgins T, Stewart D, Boehr E. Challenges in HbA1c analysis and reporting: an interesting case illustrating the many pitfalls. Clin Biochem. 2008;41:1104-1106.
9. Mongia SK, Little RR, Rohlfing CL, et al. Effects of hemoglobin C and S traits on the results of 14 commercial glycated hemoglobin assays. Am J Clin Pathol. 2008;130:136-140.
10. Brown MD, Rowe BH, Reeves MJ, et al. The accuracy of the enzyme-linked immunosorbent assay D-dimer test in the diagnosis of pulmonary embolism: a meta-analysis. Ann Emerg Med. 2002;40:133-144.
11. Squizzato A, Ageno W. What is the next step in D-dimer research? Education of physicians. Intern Emerg Med. 2006;1:165.
12. Kabrhel C, Mark Courtney D, Camargo CA Jr, et al. Potential impact of adjusting the threshold of the quantitative D-dimer based on pretest probability of acute pulmonary embolism. Acad Emerg Med. 2009;16:325-332.
13. Kabrhel C, Mark Courtney MD, Camargo CA Jr, et al. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med. 2010;17:589-597.
14. Berman AR. Pulmonary embolism in the elderly. Clin Geriatr Med. 2001;17:107-130.
15. Bruinstroop E, van de Ree MA, Huisman MV. The use of Ddimer in specific clinical conditions: a narrative review. Eur J Intern Med. 2009;20:441-446.
16. Schouten HJ, Geersing GI, Koek HL, et al. Diagnostic accuracy ,of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492.
17. Turgeon ML. Linne & Ringsrud’s Clinical Laboratory Science. 5th ed. Saint Louis, MO: Mosby; 2007:50.
18. Cohn JS,McNamara JR, Schaefer EJ. Lipoprotein cholesterol concentrations in the plasma of human subjects as measured in the fed and fasted states. Clin Chem. 1988;34:2456-2459.
19. Watts GF, Cohn JS. Whither the lipid profile: feast, famine, or no free lunch? Clin Chem. 2011;57:363-365.
20. Mora S, Rifai N, Buring JE, et al. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993-1001.
21. Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets. 2009;10:328-335.
22. Screening for lipid disorders in adults: US Preventive Services Task Force Recommendation statement. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/lipid/lipidrs.htm. Accessed March 13, 2014.
23. Wolf DM, Friedrichs I, Toma AG. Lymphocyte-white blood cell count ratio: a quickly available screening tool to differentiate acute purulent tonsillitis from glandular fever. Arch Otolaryngol Head Neck Surg. 2007;133:61-64.
24. McCormack R, O’Shea T. The uptake and use of the Monospot test in patients with tonsillitis. Ir Med J. 2009;102:226-228.
25. Ebell MH. Epstein-Barr virus infectious mononucleosis. Am Fam Physician. 2004;70:1279-1287.
26. Waninger KN, Harcke HT. Determination of safe return to play for athletes recovering from infectious mononucleosis: a review of the literature. Clin J Sport Med. 2005;15:410-416.
27. Hurt C, Tammaro D. Diagnostic evaluation of mononucleosislike illnesses. Am J Med. 2007;120:911.e1-911.e8.
28. Vouloumanou EK, Rafailidis PI, Falagas ME. Current diagnosis and management of infectious mononucleosis. Curr Opin Hematol. 2012;19:14-20.
29. Epstein-Barr virus and infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/laboratory-testing.html. Updated January 7, 2014. Accessed March 12, 2014.
30. Dasgupta A. Clinical utility of free drug monitoring. Clin Chem Lab Med. 2002;40:986-993.
31. Dasgupta A. Usefulness of monitoring free (unbound) concentrations of therapeutic drugs in patient management. Clin Chim Acta. 2007;377:1-13.
32. Iwamoto T, Kagawa Y, Naito Y, et al. Clinical evaluation of plasma free phenytoin measurement and factors influencing its protein binding. Biopharm Drug Dispos. 2006;27:77-84.
33. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97:489-493.
34. Wolf GK, McClain CD, Zurakowski D, et al. Total phenytoin concentrations do not accurately predict free phenytoin concentrations in critically ill children. Pediatr Crit Care Med. 2006;7:434-439; quiz 440.
35. Lanoxin (digoxin) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2011.
36. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
37. De Backer G, Ambrosini E, Borch-Johnsen K, et al; Third Joint Force of European and other Societies on Cardiovascular Disease and Prevention in Clinical Practice. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis. 2003;171:145-155.
38. McKenney JM, Davidson MH, Jacobson TA, et al. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
39. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Updated July 3, 2012. Accessed May 17, 2013.
40. Eskridge KD, Guthrie SK. Clinical issues associated with urine testing of substances of abuse. Pharmacotherapy. 1997;17:497-510.
41. Herring C, Muzyk AJ, Johnston C. Interferences with urine drug screens. J Pharm Pract. 2001;24:102-108.
42. Jaffee WB, Trucco E, Teter C, et al. Focus on alcohol & drug abuse: ensuring validity in urine drug testing. Psychiatr Serv. 2008;59:140-142.
43. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67:1344-1350.
44. Moeller KE, Lee KC, Kissack JC. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc. 2008;83:66-76.
45. Christo PJ, Manchikanti L, Ruan X, et al. Urine drug testing in chronic pain. Pain Physician. 2011;14:123-143.
46. Tenenbein M. Do you really need that emergency drug screen? Clin Toxicol (Phila). 2009;47:286-291.
47. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86:244-251.
48. UK guidelines for the use of thyroid function tests. British Thyroid Association Web site. Available at: http://www.british-thyroid-association.org/info-for-patients/Docs/TFT_guideline_final_version_July_2006.pdf. Accessed March 11, 2014.
49. Volpé, R. Rational use of thyroid function tests. Crit Rev Clin Lab Sci. 1997;34:405-438.
50. Graber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
51. Helfand M; US Preventive Services Task Force. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2004;140:128-141.