User login
A number of new therapies for cutaneous diseases are helping dermatologists improve patient care.
One such agent, ingenol mebutate, is a plant extract being used to treat actinic keratoses, Dr. J. Mark Jackson explained at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation.
An active component of radium weed, the extract is believed to induce primary necrosis by a neutrophil-mediated, antibody-dependent cellular toxicity. Ingenol mebutate is widely used in Australia to treat actinic keratoses, said Dr. Jackson, a clinical professor of medicine and dermatology at the University of Louisville in Kentucky.
Phase III trial results were released at press time by LEO Pharma, the manufacturer of ingenol mebutate. A 0.05% concentration of ingenol mebutate was used for 2 consecutive days in 250 patients with actinic keratoses on non-head locations. According to the company's press release, the gel met its primary clinical endpoint of complete lesion clearance. The study's full findings are expected to be presented at the upcoming meeting of the American Academy of Dermatology.
In the phase II, randomized, double-blind, placebo-controlled trial, 58 patients with 5 biopsy-proven actinic keratoses were treated with ingenol mebutate gel or placebo on days 1 and 2 or days 1 and 8 (Australasian J. Dermatol. 2009;50:16-22).
Complete clearance was achieved in 71% of actinic keratoses treated with the 0.05% formulation of ingenol mebutate, compared with 25% of those treated with the 0.01% formulation, 40% of those treated with the 0.0025% formulation, and 32% of those treated with the placebo gel.
Side effects were mild to moderate, including four cases of hypopigmentation at the treatment sites and one case of persistent scabbing and pain that extended beyond the study period in three of five actinic keratoses.
The use of itraconazole for treating palmoplantar pustulosis is another advance, he said. In a recent open-label study, six patients with palmoplantar pustulosis and no other evidence of psoriasis received 100 mg itraconazole daily for 1 month, followed by every other day for 1 month (Dermatol. Ther. 2009;22:85-9).
Three patients achieved complete response, with improvement after 2 weeks of treatment. The remaining three patients developed no new pustules but continued to have some disease activity. All patients experienced relapse, but retreatment controlled disease in two patients.
During a discussion of retapamulin, which is approved for the topical treatment of impetigo due to Staphylococcus aureus (methicillin-susceptible S. aureus only) and S. pyogenes in patients older than 9 months of age, Dr. Jackson noted that the agent can cause irritation to the nasal mucosa "and is, therefore, not a good treatment for many patients with nasal carriage of MRSA. If nasal carriage is resistant to mupirocin, you may try retapamulin, but you should warn the patient in advance to try a test area first."
Cutaneous reactions can occur in response to epidermal growth factor receptors (EGFRs), Dr. Jackson warned. A prospective study of 30 patients taking either cetuximab or erlotinib for cancer noted acneiform pustule and follicular eruptions in a seborrheic distribution 7-10 days after therapy (J. Am. Acad. Dermatol. 2006;55:429-37).
"Palmoplantar eruptions have also been reported," he said. "So have xerosis, pyogenic granulomas, and paronychia." Cutaneous reactions to EGFRs are dose dependent and recur with subsequent treatment.
Be aware of the procoagulant effects of thalidomide, which is approved for the treatment of chronic recurrent and severe erythema nodosum leprosum and is used for many other inflammatory dermatologic conditions, he said. A study of 25 patients found that deep vein thrombosis occurred in 20% of patients taking thalidomide for various inflammatory conditions (J. Dermatolog. Treat. 2007;18:335-40).
Patients at particular risk include those with systemic lupus erythematosus, a malignancy, antiphospholipid syndrome, smokers, women on oral contraceptives, patients with hyperhomocysteinemia, and patients withdrawing from antimalarials, he added.
Dr. Jackson advised that dermatologists should have a basic understanding of interstitial granulomatous dermatitis, first described in 1993. Also known as granulomatous dermatitis with associated arthritis, the condition "generally presents as erythematous, violaceous, hyperpigmented, annular plaques on the trunk and thighs," he said. "Sometimes there is an acral distribution."
Histopathology reveals dense inflammatory mixed cell infiltrate, necrobiosis, sparse mucin deposition, and no vasculitis.
Associated diseases include rheumatoid arthritis, systemic lupus erythematosus, and other autoimmune conditions. The disease can also be triggered by infections and certain medications, including ACE inhibitors, calcium channel blockers, beta blockers, and lipid lowering agents.
Treatment options include glucocorticoids, NSAIDs, and TNF-alpha inhibitors.
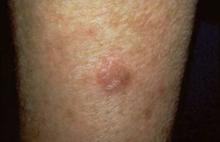
Several new therapies are under study to help physicians treat cutaneous diseases such as actinic keratoses (shown here on an elbow). Photo courtesy Dr. Roger I. Ceilley.
Dr. Jackson disclosed that he has received research, honoraria, consulting or other support from Abbott, Amgen, Biogen Idec, Centocor Ortho Biotech, Ferndale Laboratories, Galderma, Genentech, Medicis, Novartis, Promius Pharma, Quinnova Pharmaceuticals, Roche, and Stiefel.
SDEF and this news organization are owned by Elsevier.
A number of new therapies for cutaneous diseases are helping dermatologists improve patient care.
One such agent, ingenol mebutate, is a plant extract being used to treat actinic keratoses, Dr. J. Mark Jackson explained at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation.
An active component of radium weed, the extract is believed to induce primary necrosis by a neutrophil-mediated, antibody-dependent cellular toxicity. Ingenol mebutate is widely used in Australia to treat actinic keratoses, said Dr. Jackson, a clinical professor of medicine and dermatology at the University of Louisville in Kentucky.
Phase III trial results were released at press time by LEO Pharma, the manufacturer of ingenol mebutate. A 0.05% concentration of ingenol mebutate was used for 2 consecutive days in 250 patients with actinic keratoses on non-head locations. According to the company's press release, the gel met its primary clinical endpoint of complete lesion clearance. The study's full findings are expected to be presented at the upcoming meeting of the American Academy of Dermatology.
In the phase II, randomized, double-blind, placebo-controlled trial, 58 patients with 5 biopsy-proven actinic keratoses were treated with ingenol mebutate gel or placebo on days 1 and 2 or days 1 and 8 (Australasian J. Dermatol. 2009;50:16-22).
Complete clearance was achieved in 71% of actinic keratoses treated with the 0.05% formulation of ingenol mebutate, compared with 25% of those treated with the 0.01% formulation, 40% of those treated with the 0.0025% formulation, and 32% of those treated with the placebo gel.
Side effects were mild to moderate, including four cases of hypopigmentation at the treatment sites and one case of persistent scabbing and pain that extended beyond the study period in three of five actinic keratoses.
The use of itraconazole for treating palmoplantar pustulosis is another advance, he said. In a recent open-label study, six patients with palmoplantar pustulosis and no other evidence of psoriasis received 100 mg itraconazole daily for 1 month, followed by every other day for 1 month (Dermatol. Ther. 2009;22:85-9).
Three patients achieved complete response, with improvement after 2 weeks of treatment. The remaining three patients developed no new pustules but continued to have some disease activity. All patients experienced relapse, but retreatment controlled disease in two patients.
During a discussion of retapamulin, which is approved for the topical treatment of impetigo due to Staphylococcus aureus (methicillin-susceptible S. aureus only) and S. pyogenes in patients older than 9 months of age, Dr. Jackson noted that the agent can cause irritation to the nasal mucosa "and is, therefore, not a good treatment for many patients with nasal carriage of MRSA. If nasal carriage is resistant to mupirocin, you may try retapamulin, but you should warn the patient in advance to try a test area first."
Cutaneous reactions can occur in response to epidermal growth factor receptors (EGFRs), Dr. Jackson warned. A prospective study of 30 patients taking either cetuximab or erlotinib for cancer noted acneiform pustule and follicular eruptions in a seborrheic distribution 7-10 days after therapy (J. Am. Acad. Dermatol. 2006;55:429-37).
"Palmoplantar eruptions have also been reported," he said. "So have xerosis, pyogenic granulomas, and paronychia." Cutaneous reactions to EGFRs are dose dependent and recur with subsequent treatment.
Be aware of the procoagulant effects of thalidomide, which is approved for the treatment of chronic recurrent and severe erythema nodosum leprosum and is used for many other inflammatory dermatologic conditions, he said. A study of 25 patients found that deep vein thrombosis occurred in 20% of patients taking thalidomide for various inflammatory conditions (J. Dermatolog. Treat. 2007;18:335-40).
Patients at particular risk include those with systemic lupus erythematosus, a malignancy, antiphospholipid syndrome, smokers, women on oral contraceptives, patients with hyperhomocysteinemia, and patients withdrawing from antimalarials, he added.
Dr. Jackson advised that dermatologists should have a basic understanding of interstitial granulomatous dermatitis, first described in 1993. Also known as granulomatous dermatitis with associated arthritis, the condition "generally presents as erythematous, violaceous, hyperpigmented, annular plaques on the trunk and thighs," he said. "Sometimes there is an acral distribution."
Histopathology reveals dense inflammatory mixed cell infiltrate, necrobiosis, sparse mucin deposition, and no vasculitis.
Associated diseases include rheumatoid arthritis, systemic lupus erythematosus, and other autoimmune conditions. The disease can also be triggered by infections and certain medications, including ACE inhibitors, calcium channel blockers, beta blockers, and lipid lowering agents.
Treatment options include glucocorticoids, NSAIDs, and TNF-alpha inhibitors.
Several new therapies are under study to help physicians treat cutaneous diseases such as actinic keratoses (shown here on an elbow). Photo courtesy Dr. Roger I. Ceilley.
Dr. Jackson disclosed that he has received research, honoraria, consulting or other support from Abbott, Amgen, Biogen Idec, Centocor Ortho Biotech, Ferndale Laboratories, Galderma, Genentech, Medicis, Novartis, Promius Pharma, Quinnova Pharmaceuticals, Roche, and Stiefel.
SDEF and this news organization are owned by Elsevier.
A number of new therapies for cutaneous diseases are helping dermatologists improve patient care.
One such agent, ingenol mebutate, is a plant extract being used to treat actinic keratoses, Dr. J. Mark Jackson explained at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation.
An active component of radium weed, the extract is believed to induce primary necrosis by a neutrophil-mediated, antibody-dependent cellular toxicity. Ingenol mebutate is widely used in Australia to treat actinic keratoses, said Dr. Jackson, a clinical professor of medicine and dermatology at the University of Louisville in Kentucky.
Phase III trial results were released at press time by LEO Pharma, the manufacturer of ingenol mebutate. A 0.05% concentration of ingenol mebutate was used for 2 consecutive days in 250 patients with actinic keratoses on non-head locations. According to the company's press release, the gel met its primary clinical endpoint of complete lesion clearance. The study's full findings are expected to be presented at the upcoming meeting of the American Academy of Dermatology.
In the phase II, randomized, double-blind, placebo-controlled trial, 58 patients with 5 biopsy-proven actinic keratoses were treated with ingenol mebutate gel or placebo on days 1 and 2 or days 1 and 8 (Australasian J. Dermatol. 2009;50:16-22).
Complete clearance was achieved in 71% of actinic keratoses treated with the 0.05% formulation of ingenol mebutate, compared with 25% of those treated with the 0.01% formulation, 40% of those treated with the 0.0025% formulation, and 32% of those treated with the placebo gel.
Side effects were mild to moderate, including four cases of hypopigmentation at the treatment sites and one case of persistent scabbing and pain that extended beyond the study period in three of five actinic keratoses.
The use of itraconazole for treating palmoplantar pustulosis is another advance, he said. In a recent open-label study, six patients with palmoplantar pustulosis and no other evidence of psoriasis received 100 mg itraconazole daily for 1 month, followed by every other day for 1 month (Dermatol. Ther. 2009;22:85-9).
Three patients achieved complete response, with improvement after 2 weeks of treatment. The remaining three patients developed no new pustules but continued to have some disease activity. All patients experienced relapse, but retreatment controlled disease in two patients.
During a discussion of retapamulin, which is approved for the topical treatment of impetigo due to Staphylococcus aureus (methicillin-susceptible S. aureus only) and S. pyogenes in patients older than 9 months of age, Dr. Jackson noted that the agent can cause irritation to the nasal mucosa "and is, therefore, not a good treatment for many patients with nasal carriage of MRSA. If nasal carriage is resistant to mupirocin, you may try retapamulin, but you should warn the patient in advance to try a test area first."
Cutaneous reactions can occur in response to epidermal growth factor receptors (EGFRs), Dr. Jackson warned. A prospective study of 30 patients taking either cetuximab or erlotinib for cancer noted acneiform pustule and follicular eruptions in a seborrheic distribution 7-10 days after therapy (J. Am. Acad. Dermatol. 2006;55:429-37).
"Palmoplantar eruptions have also been reported," he said. "So have xerosis, pyogenic granulomas, and paronychia." Cutaneous reactions to EGFRs are dose dependent and recur with subsequent treatment.
Be aware of the procoagulant effects of thalidomide, which is approved for the treatment of chronic recurrent and severe erythema nodosum leprosum and is used for many other inflammatory dermatologic conditions, he said. A study of 25 patients found that deep vein thrombosis occurred in 20% of patients taking thalidomide for various inflammatory conditions (J. Dermatolog. Treat. 2007;18:335-40).
Patients at particular risk include those with systemic lupus erythematosus, a malignancy, antiphospholipid syndrome, smokers, women on oral contraceptives, patients with hyperhomocysteinemia, and patients withdrawing from antimalarials, he added.
Dr. Jackson advised that dermatologists should have a basic understanding of interstitial granulomatous dermatitis, first described in 1993. Also known as granulomatous dermatitis with associated arthritis, the condition "generally presents as erythematous, violaceous, hyperpigmented, annular plaques on the trunk and thighs," he said. "Sometimes there is an acral distribution."
Histopathology reveals dense inflammatory mixed cell infiltrate, necrobiosis, sparse mucin deposition, and no vasculitis.
Associated diseases include rheumatoid arthritis, systemic lupus erythematosus, and other autoimmune conditions. The disease can also be triggered by infections and certain medications, including ACE inhibitors, calcium channel blockers, beta blockers, and lipid lowering agents.
Treatment options include glucocorticoids, NSAIDs, and TNF-alpha inhibitors.
Several new therapies are under study to help physicians treat cutaneous diseases such as actinic keratoses (shown here on an elbow). Photo courtesy Dr. Roger I. Ceilley.
Dr. Jackson disclosed that he has received research, honoraria, consulting or other support from Abbott, Amgen, Biogen Idec, Centocor Ortho Biotech, Ferndale Laboratories, Galderma, Genentech, Medicis, Novartis, Promius Pharma, Quinnova Pharmaceuticals, Roche, and Stiefel.
SDEF and this news organization are owned by Elsevier.