User login
Using the World Health Organization’s online Fracture Risk Assessment Tool (FRAX) may help you decide when to initiate treatment for patients with osteopenia. C
Consider using intravenous bisphosphonates as first-line therapy for women with postmenopausal osteoporosis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
To prevent bone loss and fractures in postmenopausal osteoporosis, the best choice of medication is one a patient will actually take. Bisphosphonates are the standard of care for maintaining or increasing bone mass and reducing excessive bone turnover,1 and oral bisphosphonates have proven to be safe and effective in reducing osteoporotic fractures. However, numerous studies have shown that the effectiveness of oral bisphosphonates is compromised by poor patient compliance in taking the medication as directed and by poor persistence in continuing the medication over the long term.
Intravenous (IV) bisphosphonates are another option: They bypass the GI tract and thereby avoid the difficult requirements of oral dosing that many patients end up disregarding. And because IV administration occurs under medical supervision, it ensures persistence throughout the entire dosing interval. IV ibandronate 3 mg is administered every 3 months, and IV zoledronic acid 5 mg is administered once a year.
Osteoporosis and osteoporotic fractures are markedly underdiagnosed
Delmas and colleagues evaluated the underdetection of vertebral fractures in an international, multicenter prospective study of 2451 women 65 to 80 years of age who had not received a diagnosis of osteoporosis.2 Expert review of radiographic reports identified at least 1 mild vertebral fracture in 32% of the study population, but one-third of these cases had not been identified before the review.
Determine bone mineral density. Detection of osteoporosis depends in part on measuring bone mineral density (BMD) of the hip and spine by dual-energy x-ray absorptiometry scan. BMD correlates with bone strength and helps predict fracture risk. The World Health Organization has established definitions for bone integrity based on BMD (TABLE).3
One standard deviation below normal equals a loss of 10% to 15% of bone mass. A patient with a T-score of –2.5 has lost >25% of bone mass. Although patients with osteoporosis have the highest probability of fracture, studies have consistently found that patients with osteopenia can also sustain fractures. Therefore, assess other risk factors for bone loss (identified below) when evaluating a patient.4-6
A useful assessment tool. To identify patients at risk for fractures, the World Health Organization offers a Web-based Fracture Risk Assessment Tool (FRAX).7 This tool, which takes into account risk factors and a femoral neck T-score, helps predict the 10-year probability of a hip or other major osteoporotic fracture. FRAX recognizes the following risk factors: age, sex, weight, height, fracture history, parental history of a hip fracture, cigarette use, long-term use of glucocorticoids, rheumatoid arthritis, concomitant disorders known to cause secondary osteoporosis, and daily alcohol consumption. If a T-score is not available, a patient’s body mass index may be used to estimate fracture risk. This free tool is available at www.shef.ac.uk/FRAX. Fracture risk calculation may also help resolve questions about when to initiate treatment for patients with osteopenia.
TABLE
Defining bone integrity by bone mineral density
| Normal | BMD is within 1 SD of that of a young normal adult (T-score ≥–1.0) |
| Low bone mass (osteopenia) | BMD is between 1.0 and 2.5 SD below that of a young normal adult (T-score between –1.0 and –2.5) |
| Osteoporosis | BMD is at least 2.5 SD below that of a young normal adult (T-score ≤–2.5) |
| BMD, bone mineral density; SD, standard deviation. | |
| Source: WHO Study Group on Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis. WHO Technical Report Series, No. 843. 1994.3 | |
Undertreatment of osteoporosis is also a significant problem
A study based on National Health and Nutrition Examination Survey data reported that fewer than 20% of women and men who had sustained an osteoporotic fracture or were at high risk for fracture were being treated with antiresorptive agents.8
National Osteoporosis Foundation (NOF) guidelines9 recommend considering treatment for postmenopausal women 50 years of age or older who exhibit the following:
- A hip or vertebral fracture
- T-score ≤–2.5 at the femoral neck, total hip, or spine after appropriate evaluation to exclude secondary causes
- Low bone mass and a 10-year probability of hip fracture ≥3%, or a 10-year probability of any major osteoporosis-related fracture ≥20%, based on the FRAX calculation.
The NOF Clinician’s Guide to Prevention and Treatment of Osteoporosis is available at http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf.
Also discuss with patients the importance of adequate supplementation with calcium and vitamin D. All patients with osteoporosis should consume at least 1200 mg of calcium and 800 to 1000 IU of vitamin D each day.9
Oral bisphosphonate therapies are effective …
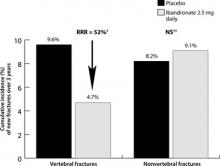
Oral bisphosphonates (alendronate, risedronate, and ibandronate) effectively reduce fracture risk at various skeletal sites, and are generally safe and well tolerated. In a 3-year clinical trial involving postmenopausal women with low femoral neck BMD and at least 1 baseline vertebral fracture, alendronate 10 mg/d, compared with placebo, reduced the risk of vertebral fracture by 47% (8% vs 15%; absolute risk reduction [ARR], 7%) and hip fracture by 51% (1.1% vs 2.2%; ARR, 1.1%).10 Risedronate 5 mg/d has been shown to reduce the risk of vertebral fracture compared with placebo by up to 49% (18% vs 29%; ARR, 11%)11 and nonvertebral fracture by up to 39% (5.2% vs 8.4%; ARR, 3.2%)12 in similar populations. Oral ibandronate reduced the risk of vertebral fracture by 52% (4.7% vs 9.6%; ARR, 4.9%) at doses of 2.5 mg/d compared with placebo; this study was not powered to assess hip fracture reduction, and there was a similar number of nonvertebral osteoporotic fractures in both groups.13
… but patients have trouble with compliance
Rates of compliance (taking medication regularly and properly) at 1 year are poor, ranging from 30% to 55%.14-16 Studies show a strong link between poor compliance with oral bisphosphonates and increased fracture rates, as well as increased health care costs.15-20
Compliance problems associated with oral bisphosphonates may be attributed, in part, to their complex dosing requirements.14 Because these agents are poorly absorbed, patients must fast overnight before ingestion, and then avoid eating, drinking, or taking other medications for 30 to 60 minutes afterward. To reduce the potential for gastrointestinal (GI) irritation, patients must also maintain an upright position for at least 30 to 60 minutes after ingestion. Even when health care providers give complete instructions, between 25% and 50% of patients disregard at least 1 requirement.21,22
The complex dosing regimens with oral bisphosphonates may be particularly challenging for patients with cognitive deficits. Noncompliance also rises with an increase in the number of comorbid conditions, with an increase in the number of medications a patient takes unrelated to osteoporosis, and with older age.23
Studies examining persistence (the length of time a patient continues treatment) with osteoporosis therapy have confirmed that 32% to 55% of patients stop taking their oral medications for osteoporosis within 1 year.14,15,23,24 Several studies have reported that weekly dosing is associated with greater persistence than daily osteoporosis therapy.14 Monthly dosing may yield additional improvements. For example, an open-label, randomized trial found that patients who received once-monthly ibandronate and support measures showed greater persistence at 6 months, compared with those receiving weekly alendronate (56.6% vs 38.6%, P<.0001).24 Nevertheless, weekly and monthly regimens result in only modest improvements in persistence, and approximately 50% of all patients discontinue therapy by 6 months.14,24-26 The recent availability of IV bisphosphonates administered under medical supervision offers a potential advantage in increasing persistence.
What we know about the effectiveness of IV bisphosphonates
IV ibandronate 3 mg every 3 months and IV zoledronic acid 5 mg every 12 months are approved for the treatment of postmenopausal osteoporosis. Health care professionals can provide the ibandronate IV injection or zoledronic acid infusion in their office or refer patients to a local provider or infusion center.
Ibandronate. The effectiveness of IV ibandronate is based on clinical trials with daily oral ibandronate, such as the BONE (oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe) study (FIGURE 1).
The DIVA (Dosing IntraVenous Administration) study compared IV ibandronate 3 mg every 3 months (n=471) with daily oral dosing with ibandronate 2.5 mg (n=470) in women with postmenopausal osteoporosis.27 The IV regimen was significantly superior to daily oral dosing, with increases in mean lumbar spine BMD of 4.8% for the IV formulation and 3.8% for daily oral therapy (P<.001). Similar improvements in BMD occurred at other bone sites.
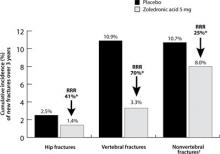
Zoledronic acid. The effectiveness of IV zoledronic acid, administered over a period lasting at least 15 minutes, was shown in the 3-year Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly-Pivotal Fracture Trial (HORIZON-PFT).28 An annual infusion of zoledronic acid 5 mg in postmenopausal women with osteoporosis (n=3875) compared with placebo (n=3861) reduced the risk of hip fractures, vertebral fractures, and nonvertebral fractures by 41% (1.4% vs 2.5%; ARR, 1.1%), 70% (3.3% vs 10.9%; ARR, 7.6%), and 25% (8% vs 10.7%; ARR, 2.7%), respectively (P≤.002) (FIGURE 2). The numbers of patients needed to treat (NNT) to prevent 1 fracture at 3 years were 91, 13, and 37 for hip, vertebral, and nonvertebral fractures, respectively. IV zoledronic acid also significantly increased BMD and reduced bone turnover markers.
Therapy underused after hip fracture. Most patients are not properly evaluated or treated for osteoporosis after a hip fracture. Zoledronic acid is the only bisphosphonate that has been specifically studied in this population to evaluate its effectiveness in preventing additional fractures. In the HORIZON-Recurrent Fracture Trial, patients were randomized to receive IV zoledronic acid 5 mg (n=1065) or placebo (n=1062) within 90 days after repair of a hip fracture and yearly thereafter.29 Compared with placebo, an annual infusion of IV zoledronic acid resulted in a 35% reduction in the rate of any new clinical fracture (13.9% vs 8.6%; P=.001; NNT to prevent 1 fracture at 3 years=19). BMD of the contralateral hip increased in the zoledronic acid group by 5.5% after 3 years compared with a 0.9% decrease in the placebo group (P<.001). In addition, there was a 28% reduction in all-cause mortality (9.6% for zoledronic acid vs 13.3% for placebo; P=.01).
This study demonstrated that a yearly infusion of zoledronic acid is effective in preventing subsequent clinical fractures in patients who have recently suffered a hip fracture and that it may reduce all-cause mortality. These results support the need for careful discharge planning after a hip fracture to include appropriate treatment to prevent subsequent fractures.
FIGURE 1
Daily oral ibandronate (2.5 mg*) reduced risk of vertebral fractures in the BONE study (n=982)
*Ibandronate 2.5 mg tablets are not available on the market.
†P<.001 vs placebo.
‡No significant difference vs placebo.
BONE, oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe; RR, relative risk reduction.
Data from Chesnut CH II, et al. J Bone Miner Res. 2004.13
FIGURE 2
Intravenous zoledronic acid (5 mg, once yearly) reduced risk of fractures in the HORIZON-Pivotal Fracture Trial (n=7736)
*P≤.002 vs placebo.
†Hip fracture was not excluded from analysis of nonvertebral fracture.
HORIZON, Health Outcomes and Reduced Incidence with Zoledronic acid Once Yearly; RR, relative risk reduction.
Data from Black DM et al. N Engl J Med. 2007.28
GI issues and flu-like symptoms are among the side effects
Side effects of oral bisphosphonates include such GI problems as dyspepsia, nausea, and reflux. There is also a small risk of developing inflammation of the esophagus and gastric ulcers.
In some patients, IV bisphosphonates can cause transient flu-like symptoms (eg, myalgia, headache, pyrexia) within 3 days of administration.27-30 These symptoms are generally mild and last a few days, and they can be reduced by taking acetaminophen or ibuprofen for several days after the injection or infusion. Symptoms are uncommon with subsequent injections or infusions. Counsel patients about the possibility of these symptoms and how to manage them.
The safety of switching directly from weekly oral alendronate 70 mg to IV zoledronic acid 5 mg was evaluated in a 12-month randomized, double-blind study. The results showed that switching to zoledronic acid was well tolerated.31 The overall cost of IV annual zoledronic acid is similar to the annual cost of a branded oral bisphosphonate.
When to avoid bisphosphonates
Give no bisphosphonate, oral or IV, to a patient with significant renal impairment because the drugs are excreted renally. Be sure a patient’s calcium level is normal and that she is not vitamin D deficient. Due to the use of sunscreens and sun avoidance, vitamin D deficiency is common, even in sunny climates. Consider using vitamin D supplementation, as needed, before infusion of an IV bisphosphonate. And emphasize the importance of lifelong calcium and vitamin D supplementation to all patients with osteoporosis.
Osteonecrosis of the jaw (ONJ) in bisphosphonate-treated patients with osteoporosis is rare (<1 in 100,000 patient-years in non-cancer patients).32,33 Most cases of ONJ in this setting have occurred “in cancer patients treated with intravenous bisphosphonates undergoing dental procedures. Some cases have occurred in patients with postmenopausal osteoporosis treated with either oral or intravenous bisphosphonates.”34 A prescriber should perform a routine oral examination before initiating bisphosphonate treatment. Consider referring patients who have a history of concomitant risk factors (eg, cancer, chemotherapy, radiotherapy, corticosteroids, poor oral hygiene, pre-existing dental disease or infection, anemia, coagulopathy) for a dental examination and appropriate preventive dentistry.
While on treatment, patients with concomitant risk factors should avoid invasive dental procedures, if possible. If ONJ develops during bisphosphonate therapy, dental surgery may exacerbate the condition. If a dental procedure is unavoidable, no data are available to suggest whether discontinuation of bisphosphonate treatment reduces the risk of ONJ. The treating physician must rely on clinical judgment regarding the benefits/risks for an individual.34
Possible downside to long-term use. Two studies presented at the 2010 annual meeting of the American Academy of Orthopaedic Surgeons showed that long-term use of oral bisphosphonates may diminish bone quality while increasing bone quantity, perhaps increasing the risk of atypical femoral fractures. In one study of 111 postmenopausal women—61 who received bisphosphonate therapy and 50 non-bisphosphonate controls—the bisphosphonate group exhibited improved structural integrity early in the course of treatment, but those gains were diminished at 4 years.35
In the other study, bone biopsy samples taken from the lateral femur in 21 postmenopausal women with femoral fractures (12 who received bisphosphonate therapy for an average of 8.5 years and 9 non-bisphosphonate controls) indicated that bisphosphonate therapy reduced the heterogeneity of bone tissue properties.36
The Food and Drug Administration has said it is looking closely at all evidence, but that its review of data to date has not shown an unequivocal association between bisphosphonate use and increased risk of atypical femoral fractures.37
A new approach to the management of osteoporosis
To avoid noncompliance problems and the associated increase in fracture risk, consider IV bisphosphonates for first-line therapy in women with postmenopausal osteoporosis. The intermittent dosing regimens of IV bisphosphonates ensure 100% persistence throughout the dosing interval.
CORRESPONDENCE Raymond Cole, DO, Michigan State University College of Osteopathic Medicine, Osteoporosis Testing Center of Michigan, 107 Chicago Street, Brooklyn, MI 49230; [email protected]
1. Chapurlat RD, Delmas PD. Drug insight: bisphosphonates for postmenopausal osteoporosis. Nat Clin Pract Endocrinol Metab. 2006;2:211-219.
2. Delmas PD, van de Langerijt L, Watts NB, et al. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT Study. J Bone Miner Res. 2005;20:557-563.
3. WHO Study Group on Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO Study Group. Geneva, Switzerland: World Health Organization; 1994. WHO Technical Report Series, No. 843.
4. Siris ES, Chen Y-T, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108-1112.
5. Pasco JA, Seeman E, Henry MJ, et al. The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos Int. 2006;17:1404-1409.
6. Cranney A, Jamal SA, Tsang JF, et al. Low bone mineral density and fracture burden in postmenopausal women. CMAJ. 2007;177:575-580.
7. Kanis JA, Burlet N, Cooper C, et al. European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19:399-428.
8. Gehlbach SH, Avrunin JS, Puleo E, et al. Fracture risk and antiresorptive medication use in older women in the USA. Osteoporos Int. 2007;18:805-810.
9. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Available at: http://www.nof.org/professionals/NOF_Clinicians_Guide.pdf. Accessed March 25, 2008.
10. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535-1541.
11. Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83-91.
12. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282:1344-1352.
13. Chesnut CH, III, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249.
14. Cramer JA, Gold DT, Silverman SL, et al. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023-1031.
15. Weycker D, Macarios D, Edelsberg J, et al. Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int. 2007;18:271-277.
16. Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81:1013-1022.
17. Caro JJ, Ishak KJ, Huybrechts KF, et al. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004;15:1003-1008.
18. Briesacher BA, Andrade SE, Yood RA, et al. Consequences of poor compliance with bisphosphonates. Bone. 2007;41:882-887.
19. Rabenda V, Mertens R, Fabri V, et al. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. 2008;19:811-818.
20. Earnshaw SR, Graham CN, Ettinger B, et al. Cost-effectiveness of bisphosphonate therapies for women with postmenopausal osteoporosis: implications of improved persistence with less frequently administered oral bisphosphonates. Curr Med Res Opin. 2007;23:2517-2529.
21. Hamilton B, McCoy K, Taggart H. Tolerability and compliance with risedronate in clinical practice. Osteoporos Int. 2003;14:259-262.
22. Ettinger MP. Aging bone and osteoporosis: strategies for preventing fractures in the elderly. Arch Intern Med. 2003;163:2237-2246.
23. Solomon DH, Avorn J, Katz JN, et al. Compliance with osteoporosis medications. Arch Intern Med. 2005;165:2414-2419.
24. Cooper A, Drake J, Brankin E. The PERSIST Investigators. Treatment persistence with once-monthly ibandronate and patient support vs once-weekly alendronate: results from the PERSIST study. Int J Clin Pract. 2006;60:896-905.
25. Weiss TW, Henderson SC, McHorney CA, et al. Persistence across weekly and monthly bisphosphonates: analysis of US retail pharmacy prescription refills. Curr Res Med Opin. 2007;23:2193-2203.
26. Penning-van Beest FJA, Goettsch WG, Erkens JA, et al. Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther. 2006;28:236-242.
27. Delmas PD, Adami S, Strugala C, et al. Intravenous ibandronate injections in postmenopausal women with osteoporosis. Arthritis Rheum. 2006;54:1838-1846.
28. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809-1822.
29. Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
30. Adami S, Felsenberg D, Christiansen C, et al. Efficacy and safety of ibandronate given by intravenous injection once every 3 months. Bone. 2004;34:881-889.
31. McClung M, Recker R, Miller P, et al. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone. 2007;41:122-128.
32. Felsenberg D, Hoffmeister B, Amling M, et al. Kiefernekrosen nach hoch dosierter Bisphosphonattherapie. Dtsch Arztebl. 2006;103:3078-3081.
33. American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc. 2006;137:1144-1150.
34. Reclast [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; October 2009. Available at: http://www.pharma.us.novartis.com:80/product/pi/pdf/reclast.pdf. Accessed May 12, 2010.
35. Ding A. The structural effects of long-term bisphosphonate treatment leading to atypical hip fractures. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 10, 2010; New Orleans, La.
36. Gladnick B. The effects of long-term bisphosphonate use on bone quality. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11, 2010; New Orleans, La.
37. FDA Drug Safety Communication: Ongoing safety review of oral bisphosphonates and atypical subtrochanteric femur fractures. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203891.htm. Accessed April 20, 2010.
Using the World Health Organization’s online Fracture Risk Assessment Tool (FRAX) may help you decide when to initiate treatment for patients with osteopenia. C
Consider using intravenous bisphosphonates as first-line therapy for women with postmenopausal osteoporosis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
To prevent bone loss and fractures in postmenopausal osteoporosis, the best choice of medication is one a patient will actually take. Bisphosphonates are the standard of care for maintaining or increasing bone mass and reducing excessive bone turnover,1 and oral bisphosphonates have proven to be safe and effective in reducing osteoporotic fractures. However, numerous studies have shown that the effectiveness of oral bisphosphonates is compromised by poor patient compliance in taking the medication as directed and by poor persistence in continuing the medication over the long term.
Intravenous (IV) bisphosphonates are another option: They bypass the GI tract and thereby avoid the difficult requirements of oral dosing that many patients end up disregarding. And because IV administration occurs under medical supervision, it ensures persistence throughout the entire dosing interval. IV ibandronate 3 mg is administered every 3 months, and IV zoledronic acid 5 mg is administered once a year.
Osteoporosis and osteoporotic fractures are markedly underdiagnosed
Delmas and colleagues evaluated the underdetection of vertebral fractures in an international, multicenter prospective study of 2451 women 65 to 80 years of age who had not received a diagnosis of osteoporosis.2 Expert review of radiographic reports identified at least 1 mild vertebral fracture in 32% of the study population, but one-third of these cases had not been identified before the review.
Determine bone mineral density. Detection of osteoporosis depends in part on measuring bone mineral density (BMD) of the hip and spine by dual-energy x-ray absorptiometry scan. BMD correlates with bone strength and helps predict fracture risk. The World Health Organization has established definitions for bone integrity based on BMD (TABLE).3
One standard deviation below normal equals a loss of 10% to 15% of bone mass. A patient with a T-score of –2.5 has lost >25% of bone mass. Although patients with osteoporosis have the highest probability of fracture, studies have consistently found that patients with osteopenia can also sustain fractures. Therefore, assess other risk factors for bone loss (identified below) when evaluating a patient.4-6
A useful assessment tool. To identify patients at risk for fractures, the World Health Organization offers a Web-based Fracture Risk Assessment Tool (FRAX).7 This tool, which takes into account risk factors and a femoral neck T-score, helps predict the 10-year probability of a hip or other major osteoporotic fracture. FRAX recognizes the following risk factors: age, sex, weight, height, fracture history, parental history of a hip fracture, cigarette use, long-term use of glucocorticoids, rheumatoid arthritis, concomitant disorders known to cause secondary osteoporosis, and daily alcohol consumption. If a T-score is not available, a patient’s body mass index may be used to estimate fracture risk. This free tool is available at www.shef.ac.uk/FRAX. Fracture risk calculation may also help resolve questions about when to initiate treatment for patients with osteopenia.
TABLE
Defining bone integrity by bone mineral density
| Normal | BMD is within 1 SD of that of a young normal adult (T-score ≥–1.0) |
| Low bone mass (osteopenia) | BMD is between 1.0 and 2.5 SD below that of a young normal adult (T-score between –1.0 and –2.5) |
| Osteoporosis | BMD is at least 2.5 SD below that of a young normal adult (T-score ≤–2.5) |
| BMD, bone mineral density; SD, standard deviation. | |
| Source: WHO Study Group on Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis. WHO Technical Report Series, No. 843. 1994.3 | |
Undertreatment of osteoporosis is also a significant problem
A study based on National Health and Nutrition Examination Survey data reported that fewer than 20% of women and men who had sustained an osteoporotic fracture or were at high risk for fracture were being treated with antiresorptive agents.8
National Osteoporosis Foundation (NOF) guidelines9 recommend considering treatment for postmenopausal women 50 years of age or older who exhibit the following:
- A hip or vertebral fracture
- T-score ≤–2.5 at the femoral neck, total hip, or spine after appropriate evaluation to exclude secondary causes
- Low bone mass and a 10-year probability of hip fracture ≥3%, or a 10-year probability of any major osteoporosis-related fracture ≥20%, based on the FRAX calculation.
The NOF Clinician’s Guide to Prevention and Treatment of Osteoporosis is available at http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf.
Also discuss with patients the importance of adequate supplementation with calcium and vitamin D. All patients with osteoporosis should consume at least 1200 mg of calcium and 800 to 1000 IU of vitamin D each day.9
Oral bisphosphonate therapies are effective …
Oral bisphosphonates (alendronate, risedronate, and ibandronate) effectively reduce fracture risk at various skeletal sites, and are generally safe and well tolerated. In a 3-year clinical trial involving postmenopausal women with low femoral neck BMD and at least 1 baseline vertebral fracture, alendronate 10 mg/d, compared with placebo, reduced the risk of vertebral fracture by 47% (8% vs 15%; absolute risk reduction [ARR], 7%) and hip fracture by 51% (1.1% vs 2.2%; ARR, 1.1%).10 Risedronate 5 mg/d has been shown to reduce the risk of vertebral fracture compared with placebo by up to 49% (18% vs 29%; ARR, 11%)11 and nonvertebral fracture by up to 39% (5.2% vs 8.4%; ARR, 3.2%)12 in similar populations. Oral ibandronate reduced the risk of vertebral fracture by 52% (4.7% vs 9.6%; ARR, 4.9%) at doses of 2.5 mg/d compared with placebo; this study was not powered to assess hip fracture reduction, and there was a similar number of nonvertebral osteoporotic fractures in both groups.13
… but patients have trouble with compliance
Rates of compliance (taking medication regularly and properly) at 1 year are poor, ranging from 30% to 55%.14-16 Studies show a strong link between poor compliance with oral bisphosphonates and increased fracture rates, as well as increased health care costs.15-20
Compliance problems associated with oral bisphosphonates may be attributed, in part, to their complex dosing requirements.14 Because these agents are poorly absorbed, patients must fast overnight before ingestion, and then avoid eating, drinking, or taking other medications for 30 to 60 minutes afterward. To reduce the potential for gastrointestinal (GI) irritation, patients must also maintain an upright position for at least 30 to 60 minutes after ingestion. Even when health care providers give complete instructions, between 25% and 50% of patients disregard at least 1 requirement.21,22
The complex dosing regimens with oral bisphosphonates may be particularly challenging for patients with cognitive deficits. Noncompliance also rises with an increase in the number of comorbid conditions, with an increase in the number of medications a patient takes unrelated to osteoporosis, and with older age.23
Studies examining persistence (the length of time a patient continues treatment) with osteoporosis therapy have confirmed that 32% to 55% of patients stop taking their oral medications for osteoporosis within 1 year.14,15,23,24 Several studies have reported that weekly dosing is associated with greater persistence than daily osteoporosis therapy.14 Monthly dosing may yield additional improvements. For example, an open-label, randomized trial found that patients who received once-monthly ibandronate and support measures showed greater persistence at 6 months, compared with those receiving weekly alendronate (56.6% vs 38.6%, P<.0001).24 Nevertheless, weekly and monthly regimens result in only modest improvements in persistence, and approximately 50% of all patients discontinue therapy by 6 months.14,24-26 The recent availability of IV bisphosphonates administered under medical supervision offers a potential advantage in increasing persistence.
What we know about the effectiveness of IV bisphosphonates
IV ibandronate 3 mg every 3 months and IV zoledronic acid 5 mg every 12 months are approved for the treatment of postmenopausal osteoporosis. Health care professionals can provide the ibandronate IV injection or zoledronic acid infusion in their office or refer patients to a local provider or infusion center.
Ibandronate. The effectiveness of IV ibandronate is based on clinical trials with daily oral ibandronate, such as the BONE (oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe) study (FIGURE 1).
The DIVA (Dosing IntraVenous Administration) study compared IV ibandronate 3 mg every 3 months (n=471) with daily oral dosing with ibandronate 2.5 mg (n=470) in women with postmenopausal osteoporosis.27 The IV regimen was significantly superior to daily oral dosing, with increases in mean lumbar spine BMD of 4.8% for the IV formulation and 3.8% for daily oral therapy (P<.001). Similar improvements in BMD occurred at other bone sites.
Zoledronic acid. The effectiveness of IV zoledronic acid, administered over a period lasting at least 15 minutes, was shown in the 3-year Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly-Pivotal Fracture Trial (HORIZON-PFT).28 An annual infusion of zoledronic acid 5 mg in postmenopausal women with osteoporosis (n=3875) compared with placebo (n=3861) reduced the risk of hip fractures, vertebral fractures, and nonvertebral fractures by 41% (1.4% vs 2.5%; ARR, 1.1%), 70% (3.3% vs 10.9%; ARR, 7.6%), and 25% (8% vs 10.7%; ARR, 2.7%), respectively (P≤.002) (FIGURE 2). The numbers of patients needed to treat (NNT) to prevent 1 fracture at 3 years were 91, 13, and 37 for hip, vertebral, and nonvertebral fractures, respectively. IV zoledronic acid also significantly increased BMD and reduced bone turnover markers.
Therapy underused after hip fracture. Most patients are not properly evaluated or treated for osteoporosis after a hip fracture. Zoledronic acid is the only bisphosphonate that has been specifically studied in this population to evaluate its effectiveness in preventing additional fractures. In the HORIZON-Recurrent Fracture Trial, patients were randomized to receive IV zoledronic acid 5 mg (n=1065) or placebo (n=1062) within 90 days after repair of a hip fracture and yearly thereafter.29 Compared with placebo, an annual infusion of IV zoledronic acid resulted in a 35% reduction in the rate of any new clinical fracture (13.9% vs 8.6%; P=.001; NNT to prevent 1 fracture at 3 years=19). BMD of the contralateral hip increased in the zoledronic acid group by 5.5% after 3 years compared with a 0.9% decrease in the placebo group (P<.001). In addition, there was a 28% reduction in all-cause mortality (9.6% for zoledronic acid vs 13.3% for placebo; P=.01).
This study demonstrated that a yearly infusion of zoledronic acid is effective in preventing subsequent clinical fractures in patients who have recently suffered a hip fracture and that it may reduce all-cause mortality. These results support the need for careful discharge planning after a hip fracture to include appropriate treatment to prevent subsequent fractures.
FIGURE 1
Daily oral ibandronate (2.5 mg*) reduced risk of vertebral fractures in the BONE study (n=982)
*Ibandronate 2.5 mg tablets are not available on the market.
†P<.001 vs placebo.
‡No significant difference vs placebo.
BONE, oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe; RR, relative risk reduction.
Data from Chesnut CH II, et al. J Bone Miner Res. 2004.13
FIGURE 2
Intravenous zoledronic acid (5 mg, once yearly) reduced risk of fractures in the HORIZON-Pivotal Fracture Trial (n=7736)
*P≤.002 vs placebo.
†Hip fracture was not excluded from analysis of nonvertebral fracture.
HORIZON, Health Outcomes and Reduced Incidence with Zoledronic acid Once Yearly; RR, relative risk reduction.
Data from Black DM et al. N Engl J Med. 2007.28
GI issues and flu-like symptoms are among the side effects
Side effects of oral bisphosphonates include such GI problems as dyspepsia, nausea, and reflux. There is also a small risk of developing inflammation of the esophagus and gastric ulcers.
In some patients, IV bisphosphonates can cause transient flu-like symptoms (eg, myalgia, headache, pyrexia) within 3 days of administration.27-30 These symptoms are generally mild and last a few days, and they can be reduced by taking acetaminophen or ibuprofen for several days after the injection or infusion. Symptoms are uncommon with subsequent injections or infusions. Counsel patients about the possibility of these symptoms and how to manage them.
The safety of switching directly from weekly oral alendronate 70 mg to IV zoledronic acid 5 mg was evaluated in a 12-month randomized, double-blind study. The results showed that switching to zoledronic acid was well tolerated.31 The overall cost of IV annual zoledronic acid is similar to the annual cost of a branded oral bisphosphonate.
When to avoid bisphosphonates
Give no bisphosphonate, oral or IV, to a patient with significant renal impairment because the drugs are excreted renally. Be sure a patient’s calcium level is normal and that she is not vitamin D deficient. Due to the use of sunscreens and sun avoidance, vitamin D deficiency is common, even in sunny climates. Consider using vitamin D supplementation, as needed, before infusion of an IV bisphosphonate. And emphasize the importance of lifelong calcium and vitamin D supplementation to all patients with osteoporosis.
Osteonecrosis of the jaw (ONJ) in bisphosphonate-treated patients with osteoporosis is rare (<1 in 100,000 patient-years in non-cancer patients).32,33 Most cases of ONJ in this setting have occurred “in cancer patients treated with intravenous bisphosphonates undergoing dental procedures. Some cases have occurred in patients with postmenopausal osteoporosis treated with either oral or intravenous bisphosphonates.”34 A prescriber should perform a routine oral examination before initiating bisphosphonate treatment. Consider referring patients who have a history of concomitant risk factors (eg, cancer, chemotherapy, radiotherapy, corticosteroids, poor oral hygiene, pre-existing dental disease or infection, anemia, coagulopathy) for a dental examination and appropriate preventive dentistry.
While on treatment, patients with concomitant risk factors should avoid invasive dental procedures, if possible. If ONJ develops during bisphosphonate therapy, dental surgery may exacerbate the condition. If a dental procedure is unavoidable, no data are available to suggest whether discontinuation of bisphosphonate treatment reduces the risk of ONJ. The treating physician must rely on clinical judgment regarding the benefits/risks for an individual.34
Possible downside to long-term use. Two studies presented at the 2010 annual meeting of the American Academy of Orthopaedic Surgeons showed that long-term use of oral bisphosphonates may diminish bone quality while increasing bone quantity, perhaps increasing the risk of atypical femoral fractures. In one study of 111 postmenopausal women—61 who received bisphosphonate therapy and 50 non-bisphosphonate controls—the bisphosphonate group exhibited improved structural integrity early in the course of treatment, but those gains were diminished at 4 years.35
In the other study, bone biopsy samples taken from the lateral femur in 21 postmenopausal women with femoral fractures (12 who received bisphosphonate therapy for an average of 8.5 years and 9 non-bisphosphonate controls) indicated that bisphosphonate therapy reduced the heterogeneity of bone tissue properties.36
The Food and Drug Administration has said it is looking closely at all evidence, but that its review of data to date has not shown an unequivocal association between bisphosphonate use and increased risk of atypical femoral fractures.37
A new approach to the management of osteoporosis
To avoid noncompliance problems and the associated increase in fracture risk, consider IV bisphosphonates for first-line therapy in women with postmenopausal osteoporosis. The intermittent dosing regimens of IV bisphosphonates ensure 100% persistence throughout the dosing interval.
CORRESPONDENCE Raymond Cole, DO, Michigan State University College of Osteopathic Medicine, Osteoporosis Testing Center of Michigan, 107 Chicago Street, Brooklyn, MI 49230; [email protected]
Using the World Health Organization’s online Fracture Risk Assessment Tool (FRAX) may help you decide when to initiate treatment for patients with osteopenia. C
Consider using intravenous bisphosphonates as first-line therapy for women with postmenopausal osteoporosis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
To prevent bone loss and fractures in postmenopausal osteoporosis, the best choice of medication is one a patient will actually take. Bisphosphonates are the standard of care for maintaining or increasing bone mass and reducing excessive bone turnover,1 and oral bisphosphonates have proven to be safe and effective in reducing osteoporotic fractures. However, numerous studies have shown that the effectiveness of oral bisphosphonates is compromised by poor patient compliance in taking the medication as directed and by poor persistence in continuing the medication over the long term.
Intravenous (IV) bisphosphonates are another option: They bypass the GI tract and thereby avoid the difficult requirements of oral dosing that many patients end up disregarding. And because IV administration occurs under medical supervision, it ensures persistence throughout the entire dosing interval. IV ibandronate 3 mg is administered every 3 months, and IV zoledronic acid 5 mg is administered once a year.
Osteoporosis and osteoporotic fractures are markedly underdiagnosed
Delmas and colleagues evaluated the underdetection of vertebral fractures in an international, multicenter prospective study of 2451 women 65 to 80 years of age who had not received a diagnosis of osteoporosis.2 Expert review of radiographic reports identified at least 1 mild vertebral fracture in 32% of the study population, but one-third of these cases had not been identified before the review.
Determine bone mineral density. Detection of osteoporosis depends in part on measuring bone mineral density (BMD) of the hip and spine by dual-energy x-ray absorptiometry scan. BMD correlates with bone strength and helps predict fracture risk. The World Health Organization has established definitions for bone integrity based on BMD (TABLE).3
One standard deviation below normal equals a loss of 10% to 15% of bone mass. A patient with a T-score of –2.5 has lost >25% of bone mass. Although patients with osteoporosis have the highest probability of fracture, studies have consistently found that patients with osteopenia can also sustain fractures. Therefore, assess other risk factors for bone loss (identified below) when evaluating a patient.4-6
A useful assessment tool. To identify patients at risk for fractures, the World Health Organization offers a Web-based Fracture Risk Assessment Tool (FRAX).7 This tool, which takes into account risk factors and a femoral neck T-score, helps predict the 10-year probability of a hip or other major osteoporotic fracture. FRAX recognizes the following risk factors: age, sex, weight, height, fracture history, parental history of a hip fracture, cigarette use, long-term use of glucocorticoids, rheumatoid arthritis, concomitant disorders known to cause secondary osteoporosis, and daily alcohol consumption. If a T-score is not available, a patient’s body mass index may be used to estimate fracture risk. This free tool is available at www.shef.ac.uk/FRAX. Fracture risk calculation may also help resolve questions about when to initiate treatment for patients with osteopenia.
TABLE
Defining bone integrity by bone mineral density
| Normal | BMD is within 1 SD of that of a young normal adult (T-score ≥–1.0) |
| Low bone mass (osteopenia) | BMD is between 1.0 and 2.5 SD below that of a young normal adult (T-score between –1.0 and –2.5) |
| Osteoporosis | BMD is at least 2.5 SD below that of a young normal adult (T-score ≤–2.5) |
| BMD, bone mineral density; SD, standard deviation. | |
| Source: WHO Study Group on Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis. WHO Technical Report Series, No. 843. 1994.3 | |
Undertreatment of osteoporosis is also a significant problem
A study based on National Health and Nutrition Examination Survey data reported that fewer than 20% of women and men who had sustained an osteoporotic fracture or were at high risk for fracture were being treated with antiresorptive agents.8
National Osteoporosis Foundation (NOF) guidelines9 recommend considering treatment for postmenopausal women 50 years of age or older who exhibit the following:
- A hip or vertebral fracture
- T-score ≤–2.5 at the femoral neck, total hip, or spine after appropriate evaluation to exclude secondary causes
- Low bone mass and a 10-year probability of hip fracture ≥3%, or a 10-year probability of any major osteoporosis-related fracture ≥20%, based on the FRAX calculation.
The NOF Clinician’s Guide to Prevention and Treatment of Osteoporosis is available at http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf.
Also discuss with patients the importance of adequate supplementation with calcium and vitamin D. All patients with osteoporosis should consume at least 1200 mg of calcium and 800 to 1000 IU of vitamin D each day.9
Oral bisphosphonate therapies are effective …
Oral bisphosphonates (alendronate, risedronate, and ibandronate) effectively reduce fracture risk at various skeletal sites, and are generally safe and well tolerated. In a 3-year clinical trial involving postmenopausal women with low femoral neck BMD and at least 1 baseline vertebral fracture, alendronate 10 mg/d, compared with placebo, reduced the risk of vertebral fracture by 47% (8% vs 15%; absolute risk reduction [ARR], 7%) and hip fracture by 51% (1.1% vs 2.2%; ARR, 1.1%).10 Risedronate 5 mg/d has been shown to reduce the risk of vertebral fracture compared with placebo by up to 49% (18% vs 29%; ARR, 11%)11 and nonvertebral fracture by up to 39% (5.2% vs 8.4%; ARR, 3.2%)12 in similar populations. Oral ibandronate reduced the risk of vertebral fracture by 52% (4.7% vs 9.6%; ARR, 4.9%) at doses of 2.5 mg/d compared with placebo; this study was not powered to assess hip fracture reduction, and there was a similar number of nonvertebral osteoporotic fractures in both groups.13
… but patients have trouble with compliance
Rates of compliance (taking medication regularly and properly) at 1 year are poor, ranging from 30% to 55%.14-16 Studies show a strong link between poor compliance with oral bisphosphonates and increased fracture rates, as well as increased health care costs.15-20
Compliance problems associated with oral bisphosphonates may be attributed, in part, to their complex dosing requirements.14 Because these agents are poorly absorbed, patients must fast overnight before ingestion, and then avoid eating, drinking, or taking other medications for 30 to 60 minutes afterward. To reduce the potential for gastrointestinal (GI) irritation, patients must also maintain an upright position for at least 30 to 60 minutes after ingestion. Even when health care providers give complete instructions, between 25% and 50% of patients disregard at least 1 requirement.21,22
The complex dosing regimens with oral bisphosphonates may be particularly challenging for patients with cognitive deficits. Noncompliance also rises with an increase in the number of comorbid conditions, with an increase in the number of medications a patient takes unrelated to osteoporosis, and with older age.23
Studies examining persistence (the length of time a patient continues treatment) with osteoporosis therapy have confirmed that 32% to 55% of patients stop taking their oral medications for osteoporosis within 1 year.14,15,23,24 Several studies have reported that weekly dosing is associated with greater persistence than daily osteoporosis therapy.14 Monthly dosing may yield additional improvements. For example, an open-label, randomized trial found that patients who received once-monthly ibandronate and support measures showed greater persistence at 6 months, compared with those receiving weekly alendronate (56.6% vs 38.6%, P<.0001).24 Nevertheless, weekly and monthly regimens result in only modest improvements in persistence, and approximately 50% of all patients discontinue therapy by 6 months.14,24-26 The recent availability of IV bisphosphonates administered under medical supervision offers a potential advantage in increasing persistence.
What we know about the effectiveness of IV bisphosphonates
IV ibandronate 3 mg every 3 months and IV zoledronic acid 5 mg every 12 months are approved for the treatment of postmenopausal osteoporosis. Health care professionals can provide the ibandronate IV injection or zoledronic acid infusion in their office or refer patients to a local provider or infusion center.
Ibandronate. The effectiveness of IV ibandronate is based on clinical trials with daily oral ibandronate, such as the BONE (oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe) study (FIGURE 1).
The DIVA (Dosing IntraVenous Administration) study compared IV ibandronate 3 mg every 3 months (n=471) with daily oral dosing with ibandronate 2.5 mg (n=470) in women with postmenopausal osteoporosis.27 The IV regimen was significantly superior to daily oral dosing, with increases in mean lumbar spine BMD of 4.8% for the IV formulation and 3.8% for daily oral therapy (P<.001). Similar improvements in BMD occurred at other bone sites.
Zoledronic acid. The effectiveness of IV zoledronic acid, administered over a period lasting at least 15 minutes, was shown in the 3-year Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly-Pivotal Fracture Trial (HORIZON-PFT).28 An annual infusion of zoledronic acid 5 mg in postmenopausal women with osteoporosis (n=3875) compared with placebo (n=3861) reduced the risk of hip fractures, vertebral fractures, and nonvertebral fractures by 41% (1.4% vs 2.5%; ARR, 1.1%), 70% (3.3% vs 10.9%; ARR, 7.6%), and 25% (8% vs 10.7%; ARR, 2.7%), respectively (P≤.002) (FIGURE 2). The numbers of patients needed to treat (NNT) to prevent 1 fracture at 3 years were 91, 13, and 37 for hip, vertebral, and nonvertebral fractures, respectively. IV zoledronic acid also significantly increased BMD and reduced bone turnover markers.
Therapy underused after hip fracture. Most patients are not properly evaluated or treated for osteoporosis after a hip fracture. Zoledronic acid is the only bisphosphonate that has been specifically studied in this population to evaluate its effectiveness in preventing additional fractures. In the HORIZON-Recurrent Fracture Trial, patients were randomized to receive IV zoledronic acid 5 mg (n=1065) or placebo (n=1062) within 90 days after repair of a hip fracture and yearly thereafter.29 Compared with placebo, an annual infusion of IV zoledronic acid resulted in a 35% reduction in the rate of any new clinical fracture (13.9% vs 8.6%; P=.001; NNT to prevent 1 fracture at 3 years=19). BMD of the contralateral hip increased in the zoledronic acid group by 5.5% after 3 years compared with a 0.9% decrease in the placebo group (P<.001). In addition, there was a 28% reduction in all-cause mortality (9.6% for zoledronic acid vs 13.3% for placebo; P=.01).
This study demonstrated that a yearly infusion of zoledronic acid is effective in preventing subsequent clinical fractures in patients who have recently suffered a hip fracture and that it may reduce all-cause mortality. These results support the need for careful discharge planning after a hip fracture to include appropriate treatment to prevent subsequent fractures.
FIGURE 1
Daily oral ibandronate (2.5 mg*) reduced risk of vertebral fractures in the BONE study (n=982)
*Ibandronate 2.5 mg tablets are not available on the market.
†P<.001 vs placebo.
‡No significant difference vs placebo.
BONE, oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe; RR, relative risk reduction.
Data from Chesnut CH II, et al. J Bone Miner Res. 2004.13
FIGURE 2
Intravenous zoledronic acid (5 mg, once yearly) reduced risk of fractures in the HORIZON-Pivotal Fracture Trial (n=7736)
*P≤.002 vs placebo.
†Hip fracture was not excluded from analysis of nonvertebral fracture.
HORIZON, Health Outcomes and Reduced Incidence with Zoledronic acid Once Yearly; RR, relative risk reduction.
Data from Black DM et al. N Engl J Med. 2007.28
GI issues and flu-like symptoms are among the side effects
Side effects of oral bisphosphonates include such GI problems as dyspepsia, nausea, and reflux. There is also a small risk of developing inflammation of the esophagus and gastric ulcers.
In some patients, IV bisphosphonates can cause transient flu-like symptoms (eg, myalgia, headache, pyrexia) within 3 days of administration.27-30 These symptoms are generally mild and last a few days, and they can be reduced by taking acetaminophen or ibuprofen for several days after the injection or infusion. Symptoms are uncommon with subsequent injections or infusions. Counsel patients about the possibility of these symptoms and how to manage them.
The safety of switching directly from weekly oral alendronate 70 mg to IV zoledronic acid 5 mg was evaluated in a 12-month randomized, double-blind study. The results showed that switching to zoledronic acid was well tolerated.31 The overall cost of IV annual zoledronic acid is similar to the annual cost of a branded oral bisphosphonate.
When to avoid bisphosphonates
Give no bisphosphonate, oral or IV, to a patient with significant renal impairment because the drugs are excreted renally. Be sure a patient’s calcium level is normal and that she is not vitamin D deficient. Due to the use of sunscreens and sun avoidance, vitamin D deficiency is common, even in sunny climates. Consider using vitamin D supplementation, as needed, before infusion of an IV bisphosphonate. And emphasize the importance of lifelong calcium and vitamin D supplementation to all patients with osteoporosis.
Osteonecrosis of the jaw (ONJ) in bisphosphonate-treated patients with osteoporosis is rare (<1 in 100,000 patient-years in non-cancer patients).32,33 Most cases of ONJ in this setting have occurred “in cancer patients treated with intravenous bisphosphonates undergoing dental procedures. Some cases have occurred in patients with postmenopausal osteoporosis treated with either oral or intravenous bisphosphonates.”34 A prescriber should perform a routine oral examination before initiating bisphosphonate treatment. Consider referring patients who have a history of concomitant risk factors (eg, cancer, chemotherapy, radiotherapy, corticosteroids, poor oral hygiene, pre-existing dental disease or infection, anemia, coagulopathy) for a dental examination and appropriate preventive dentistry.
While on treatment, patients with concomitant risk factors should avoid invasive dental procedures, if possible. If ONJ develops during bisphosphonate therapy, dental surgery may exacerbate the condition. If a dental procedure is unavoidable, no data are available to suggest whether discontinuation of bisphosphonate treatment reduces the risk of ONJ. The treating physician must rely on clinical judgment regarding the benefits/risks for an individual.34
Possible downside to long-term use. Two studies presented at the 2010 annual meeting of the American Academy of Orthopaedic Surgeons showed that long-term use of oral bisphosphonates may diminish bone quality while increasing bone quantity, perhaps increasing the risk of atypical femoral fractures. In one study of 111 postmenopausal women—61 who received bisphosphonate therapy and 50 non-bisphosphonate controls—the bisphosphonate group exhibited improved structural integrity early in the course of treatment, but those gains were diminished at 4 years.35
In the other study, bone biopsy samples taken from the lateral femur in 21 postmenopausal women with femoral fractures (12 who received bisphosphonate therapy for an average of 8.5 years and 9 non-bisphosphonate controls) indicated that bisphosphonate therapy reduced the heterogeneity of bone tissue properties.36
The Food and Drug Administration has said it is looking closely at all evidence, but that its review of data to date has not shown an unequivocal association between bisphosphonate use and increased risk of atypical femoral fractures.37
A new approach to the management of osteoporosis
To avoid noncompliance problems and the associated increase in fracture risk, consider IV bisphosphonates for first-line therapy in women with postmenopausal osteoporosis. The intermittent dosing regimens of IV bisphosphonates ensure 100% persistence throughout the dosing interval.
CORRESPONDENCE Raymond Cole, DO, Michigan State University College of Osteopathic Medicine, Osteoporosis Testing Center of Michigan, 107 Chicago Street, Brooklyn, MI 49230; [email protected]
1. Chapurlat RD, Delmas PD. Drug insight: bisphosphonates for postmenopausal osteoporosis. Nat Clin Pract Endocrinol Metab. 2006;2:211-219.
2. Delmas PD, van de Langerijt L, Watts NB, et al. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT Study. J Bone Miner Res. 2005;20:557-563.
3. WHO Study Group on Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO Study Group. Geneva, Switzerland: World Health Organization; 1994. WHO Technical Report Series, No. 843.
4. Siris ES, Chen Y-T, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108-1112.
5. Pasco JA, Seeman E, Henry MJ, et al. The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos Int. 2006;17:1404-1409.
6. Cranney A, Jamal SA, Tsang JF, et al. Low bone mineral density and fracture burden in postmenopausal women. CMAJ. 2007;177:575-580.
7. Kanis JA, Burlet N, Cooper C, et al. European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19:399-428.
8. Gehlbach SH, Avrunin JS, Puleo E, et al. Fracture risk and antiresorptive medication use in older women in the USA. Osteoporos Int. 2007;18:805-810.
9. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Available at: http://www.nof.org/professionals/NOF_Clinicians_Guide.pdf. Accessed March 25, 2008.
10. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535-1541.
11. Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83-91.
12. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282:1344-1352.
13. Chesnut CH, III, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249.
14. Cramer JA, Gold DT, Silverman SL, et al. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023-1031.
15. Weycker D, Macarios D, Edelsberg J, et al. Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int. 2007;18:271-277.
16. Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81:1013-1022.
17. Caro JJ, Ishak KJ, Huybrechts KF, et al. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004;15:1003-1008.
18. Briesacher BA, Andrade SE, Yood RA, et al. Consequences of poor compliance with bisphosphonates. Bone. 2007;41:882-887.
19. Rabenda V, Mertens R, Fabri V, et al. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. 2008;19:811-818.
20. Earnshaw SR, Graham CN, Ettinger B, et al. Cost-effectiveness of bisphosphonate therapies for women with postmenopausal osteoporosis: implications of improved persistence with less frequently administered oral bisphosphonates. Curr Med Res Opin. 2007;23:2517-2529.
21. Hamilton B, McCoy K, Taggart H. Tolerability and compliance with risedronate in clinical practice. Osteoporos Int. 2003;14:259-262.
22. Ettinger MP. Aging bone and osteoporosis: strategies for preventing fractures in the elderly. Arch Intern Med. 2003;163:2237-2246.
23. Solomon DH, Avorn J, Katz JN, et al. Compliance with osteoporosis medications. Arch Intern Med. 2005;165:2414-2419.
24. Cooper A, Drake J, Brankin E. The PERSIST Investigators. Treatment persistence with once-monthly ibandronate and patient support vs once-weekly alendronate: results from the PERSIST study. Int J Clin Pract. 2006;60:896-905.
25. Weiss TW, Henderson SC, McHorney CA, et al. Persistence across weekly and monthly bisphosphonates: analysis of US retail pharmacy prescription refills. Curr Res Med Opin. 2007;23:2193-2203.
26. Penning-van Beest FJA, Goettsch WG, Erkens JA, et al. Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther. 2006;28:236-242.
27. Delmas PD, Adami S, Strugala C, et al. Intravenous ibandronate injections in postmenopausal women with osteoporosis. Arthritis Rheum. 2006;54:1838-1846.
28. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809-1822.
29. Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
30. Adami S, Felsenberg D, Christiansen C, et al. Efficacy and safety of ibandronate given by intravenous injection once every 3 months. Bone. 2004;34:881-889.
31. McClung M, Recker R, Miller P, et al. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone. 2007;41:122-128.
32. Felsenberg D, Hoffmeister B, Amling M, et al. Kiefernekrosen nach hoch dosierter Bisphosphonattherapie. Dtsch Arztebl. 2006;103:3078-3081.
33. American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc. 2006;137:1144-1150.
34. Reclast [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; October 2009. Available at: http://www.pharma.us.novartis.com:80/product/pi/pdf/reclast.pdf. Accessed May 12, 2010.
35. Ding A. The structural effects of long-term bisphosphonate treatment leading to atypical hip fractures. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 10, 2010; New Orleans, La.
36. Gladnick B. The effects of long-term bisphosphonate use on bone quality. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11, 2010; New Orleans, La.
37. FDA Drug Safety Communication: Ongoing safety review of oral bisphosphonates and atypical subtrochanteric femur fractures. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203891.htm. Accessed April 20, 2010.
1. Chapurlat RD, Delmas PD. Drug insight: bisphosphonates for postmenopausal osteoporosis. Nat Clin Pract Endocrinol Metab. 2006;2:211-219.
2. Delmas PD, van de Langerijt L, Watts NB, et al. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT Study. J Bone Miner Res. 2005;20:557-563.
3. WHO Study Group on Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO Study Group. Geneva, Switzerland: World Health Organization; 1994. WHO Technical Report Series, No. 843.
4. Siris ES, Chen Y-T, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108-1112.
5. Pasco JA, Seeman E, Henry MJ, et al. The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos Int. 2006;17:1404-1409.
6. Cranney A, Jamal SA, Tsang JF, et al. Low bone mineral density and fracture burden in postmenopausal women. CMAJ. 2007;177:575-580.
7. Kanis JA, Burlet N, Cooper C, et al. European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19:399-428.
8. Gehlbach SH, Avrunin JS, Puleo E, et al. Fracture risk and antiresorptive medication use in older women in the USA. Osteoporos Int. 2007;18:805-810.
9. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Available at: http://www.nof.org/professionals/NOF_Clinicians_Guide.pdf. Accessed March 25, 2008.
10. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535-1541.
11. Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83-91.
12. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282:1344-1352.
13. Chesnut CH, III, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249.
14. Cramer JA, Gold DT, Silverman SL, et al. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023-1031.
15. Weycker D, Macarios D, Edelsberg J, et al. Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int. 2007;18:271-277.
16. Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81:1013-1022.
17. Caro JJ, Ishak KJ, Huybrechts KF, et al. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004;15:1003-1008.
18. Briesacher BA, Andrade SE, Yood RA, et al. Consequences of poor compliance with bisphosphonates. Bone. 2007;41:882-887.
19. Rabenda V, Mertens R, Fabri V, et al. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. 2008;19:811-818.
20. Earnshaw SR, Graham CN, Ettinger B, et al. Cost-effectiveness of bisphosphonate therapies for women with postmenopausal osteoporosis: implications of improved persistence with less frequently administered oral bisphosphonates. Curr Med Res Opin. 2007;23:2517-2529.
21. Hamilton B, McCoy K, Taggart H. Tolerability and compliance with risedronate in clinical practice. Osteoporos Int. 2003;14:259-262.
22. Ettinger MP. Aging bone and osteoporosis: strategies for preventing fractures in the elderly. Arch Intern Med. 2003;163:2237-2246.
23. Solomon DH, Avorn J, Katz JN, et al. Compliance with osteoporosis medications. Arch Intern Med. 2005;165:2414-2419.
24. Cooper A, Drake J, Brankin E. The PERSIST Investigators. Treatment persistence with once-monthly ibandronate and patient support vs once-weekly alendronate: results from the PERSIST study. Int J Clin Pract. 2006;60:896-905.
25. Weiss TW, Henderson SC, McHorney CA, et al. Persistence across weekly and monthly bisphosphonates: analysis of US retail pharmacy prescription refills. Curr Res Med Opin. 2007;23:2193-2203.
26. Penning-van Beest FJA, Goettsch WG, Erkens JA, et al. Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther. 2006;28:236-242.
27. Delmas PD, Adami S, Strugala C, et al. Intravenous ibandronate injections in postmenopausal women with osteoporosis. Arthritis Rheum. 2006;54:1838-1846.
28. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809-1822.
29. Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
30. Adami S, Felsenberg D, Christiansen C, et al. Efficacy and safety of ibandronate given by intravenous injection once every 3 months. Bone. 2004;34:881-889.
31. McClung M, Recker R, Miller P, et al. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone. 2007;41:122-128.
32. Felsenberg D, Hoffmeister B, Amling M, et al. Kiefernekrosen nach hoch dosierter Bisphosphonattherapie. Dtsch Arztebl. 2006;103:3078-3081.
33. American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc. 2006;137:1144-1150.
34. Reclast [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; October 2009. Available at: http://www.pharma.us.novartis.com:80/product/pi/pdf/reclast.pdf. Accessed May 12, 2010.
35. Ding A. The structural effects of long-term bisphosphonate treatment leading to atypical hip fractures. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 10, 2010; New Orleans, La.
36. Gladnick B. The effects of long-term bisphosphonate use on bone quality. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11, 2010; New Orleans, La.
37. FDA Drug Safety Communication: Ongoing safety review of oral bisphosphonates and atypical subtrochanteric femur fractures. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203891.htm. Accessed April 20, 2010.