User login
Sports Injuries of the Hip in Primary Care
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr Paul Nelson Williams. Paul, how are you feeling about sports injuries?
Paul N. Williams, MD: I’m feeling great, Matt.
Watto: You had a sports injury of the hip. Maybe that’s an overshare, Paul, but we talked about it on a podcast with Dr Carlin Senter (part 1 and part 2).
Williams: I think I’ve shared more than my hip injury, for sure.
Watto: Whenever a patient presented with hip pain, I used to pray it was trochanteric bursitis, which now I know is not really the right thing to think about. Intra-articular hip pain presents as anterior hip pain, usually in the crease of the hip. Depending on the patient’s age and history, the differential for that type of pain includes iliopsoas tendonitis, FAI syndrome, a labral tear, a bone stress injury of the femoral neck, or osteoarthritis.
So, what exactly is FAI and how might we diagnose it?
Williams: FAI is what the cool kids call femoral acetabular impingement, and it’s exactly what it sounds like.
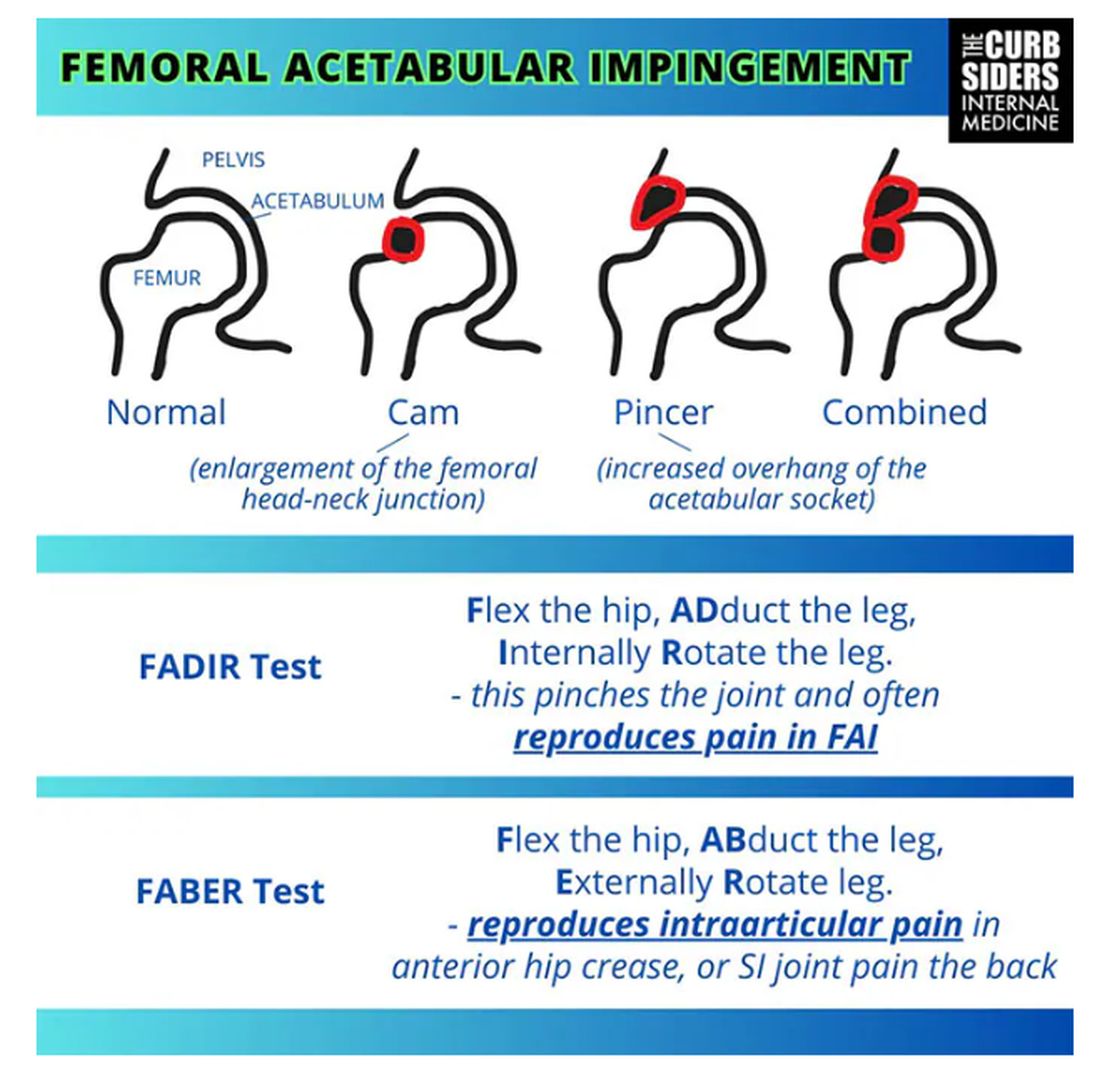
Something is pinching or impinging upon the joint itself and preventing full range of motion. This is a ball-and-socket joint, so it should have tremendous range of motion, able to move in all planes. If it’s impinged, then pain will occur with certain movements. There’s a cam type, which is characterized by enlargement of the femoral head neck junction, or a pincer type, which has more to do with overhang of the acetabulum, and it can also be mixed. In any case, impingement upon the patient’s full range of motion results in pain.
You evaluate this with a couple of tests — the FABER and the FADIR.
The FABER is flexion, abduction, and external rotation, and the FADIR is flexion, adduction, and internal rotation. If you elicit anterior pain with either of those tests, it’s probably one of the intra-articular pathologies, although it is hard to know for sure which one it is because these tests are fairly sensitive but not very specific.
Watto: You can get x-rays to help with the diagnosis. You would order two views of the hip: an AP of the pelvis, which is just a straight-on shot to look for arthritis or fracture. Is there a healthy joint line there? The second is the Dunn view, in which the hip is flexed 90 degrees and abducted about 20 degrees. You are looking for fracture or impingement. You can diagnose FAI based on that view, and you might be able to diagnose a hip stress injury or osteoarthritis.
Unfortunately, you’re not going to see a labral tear, but Dr Senter said that both FAI and labral tears are treated the same way, with physical therapy. Patients with FAI who aren’t getting better might end up going for surgery, so at some point I would refer them to orthopedic surgery. But I feel much more comfortable now diagnosing these conditions with these tests.
Let’s talk a little bit about trochanteric pain syndrome. I used to think it was all bursitis. Why is that not correct?
Williams: It’s nice of you to feign ignorance for the purpose of education. It used to be thought of as bursitis, but these days we know it is probably more likely a tendinopathy.
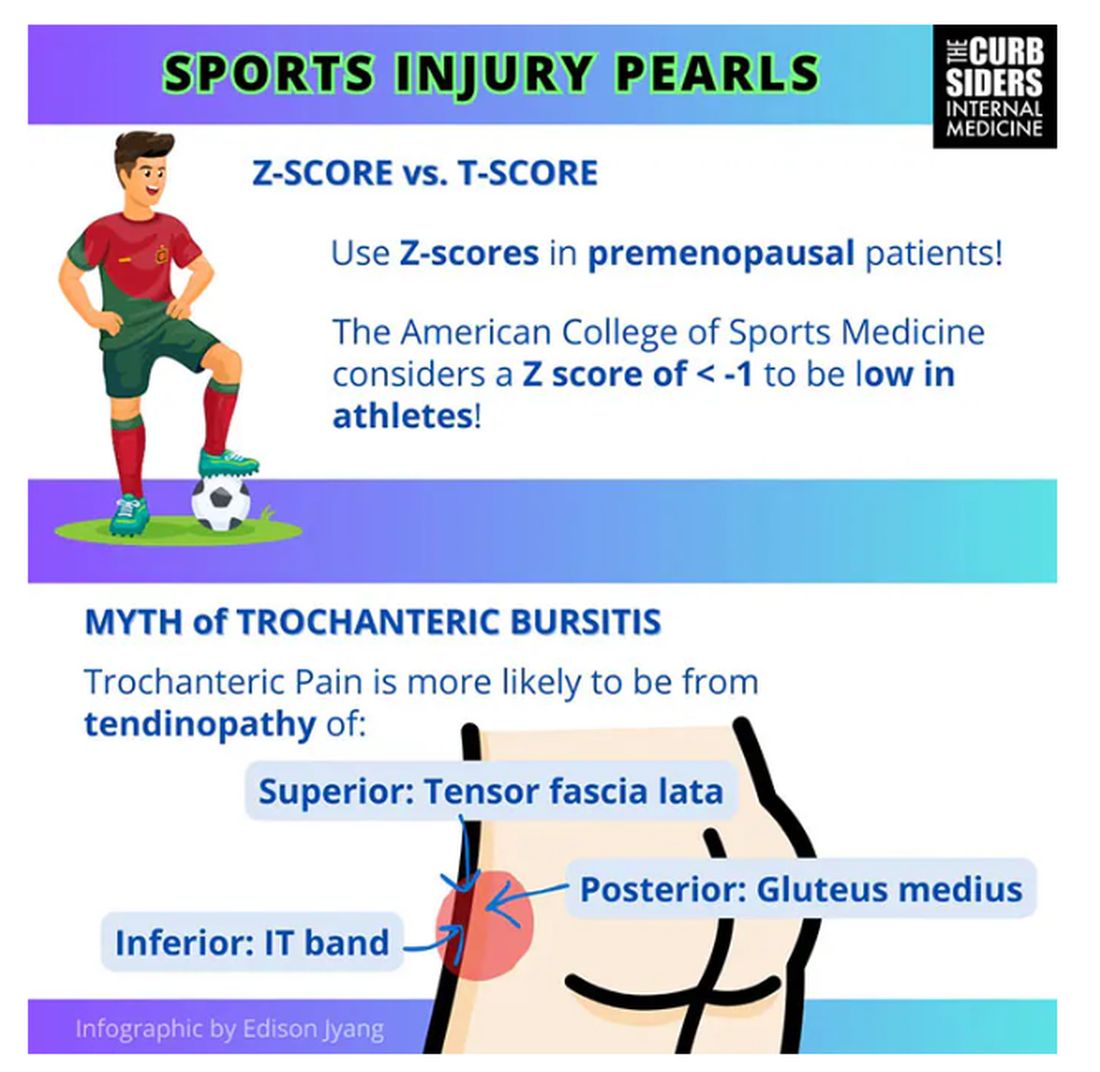
Trochanteric pain syndrome was formerly known as trochanteric bursitis, but the bursa is not typically involved. Trochanteric pain syndrome is a tendinopathy of the surrounding structures: the gluteus medius, the iliotibial band, and the tensor fascia latae. The way these structures relate looks a bit like the face of a clock, as you can see on the infographic. In general, you manage this condition the same way you do with bursitis — physical therapy. You can also give corticosteroid injections. Physical therapy is probably more durable in terms of pain relief and functionality, but in the short term, corticosteroids might provide some degree of analgesia as well.
Watto: The last thing we wanted to mention is bone stress injury, which can occur in high-mileage runners (20 miles or more per week). Patients with bone stress injury need to rest, usually non‒weight bearing, for a period of time.

Treatment of a bone stress fracture depends on which side it’s on (top or bottom). If it’s on the top of the femoral neck (the tension side), it has to be fixed. If it’s on the compression side (the bottom side of the femoral neck), it might be able to be managed conservatively, but many patients are going to need surgery. This is a big deal. But it’s a spectrum; in some cases the bone is merely irritated and unhappy, without a break in the cortex. Those patients might not need surgery.
In patients with a fracture of the femoral neck — especially younger, healthier patients — you should think about getting a bone density test and screening for relative energy deficiency in sport. This used to be called the female athlete triad, which includes disrupted menstrual cycles, being underweight, and fracture. We should be screening patients, asking them in a nonjudgmental way about their relationship with food, to make sure they are getting an appropriate number of calories.
They are actually in an energy deficit. They’re not eating enough to maintain a healthy body with so much activity.
Williams: If you’re interested in this topic, you should refer to the full podcast with Dr Senter which is chock-full of helpful information.
Dr Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, disclosed ties with The Curbsiders.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr Paul Nelson Williams. Paul, how are you feeling about sports injuries?
Paul N. Williams, MD: I’m feeling great, Matt.
Watto: You had a sports injury of the hip. Maybe that’s an overshare, Paul, but we talked about it on a podcast with Dr Carlin Senter (part 1 and part 2).
Williams: I think I’ve shared more than my hip injury, for sure.
Watto: Whenever a patient presented with hip pain, I used to pray it was trochanteric bursitis, which now I know is not really the right thing to think about. Intra-articular hip pain presents as anterior hip pain, usually in the crease of the hip. Depending on the patient’s age and history, the differential for that type of pain includes iliopsoas tendonitis, FAI syndrome, a labral tear, a bone stress injury of the femoral neck, or osteoarthritis.
So, what exactly is FAI and how might we diagnose it?
Williams: FAI is what the cool kids call femoral acetabular impingement, and it’s exactly what it sounds like.

Something is pinching or impinging upon the joint itself and preventing full range of motion. This is a ball-and-socket joint, so it should have tremendous range of motion, able to move in all planes. If it’s impinged, then pain will occur with certain movements. There’s a cam type, which is characterized by enlargement of the femoral head neck junction, or a pincer type, which has more to do with overhang of the acetabulum, and it can also be mixed. In any case, impingement upon the patient’s full range of motion results in pain.
You evaluate this with a couple of tests — the FABER and the FADIR.
The FABER is flexion, abduction, and external rotation, and the FADIR is flexion, adduction, and internal rotation. If you elicit anterior pain with either of those tests, it’s probably one of the intra-articular pathologies, although it is hard to know for sure which one it is because these tests are fairly sensitive but not very specific.
Watto: You can get x-rays to help with the diagnosis. You would order two views of the hip: an AP of the pelvis, which is just a straight-on shot to look for arthritis or fracture. Is there a healthy joint line there? The second is the Dunn view, in which the hip is flexed 90 degrees and abducted about 20 degrees. You are looking for fracture or impingement. You can diagnose FAI based on that view, and you might be able to diagnose a hip stress injury or osteoarthritis.
Unfortunately, you’re not going to see a labral tear, but Dr Senter said that both FAI and labral tears are treated the same way, with physical therapy. Patients with FAI who aren’t getting better might end up going for surgery, so at some point I would refer them to orthopedic surgery. But I feel much more comfortable now diagnosing these conditions with these tests.
Let’s talk a little bit about trochanteric pain syndrome. I used to think it was all bursitis. Why is that not correct?
Williams: It’s nice of you to feign ignorance for the purpose of education. It used to be thought of as bursitis, but these days we know it is probably more likely a tendinopathy.

Trochanteric pain syndrome was formerly known as trochanteric bursitis, but the bursa is not typically involved. Trochanteric pain syndrome is a tendinopathy of the surrounding structures: the gluteus medius, the iliotibial band, and the tensor fascia latae. The way these structures relate looks a bit like the face of a clock, as you can see on the infographic. In general, you manage this condition the same way you do with bursitis — physical therapy. You can also give corticosteroid injections. Physical therapy is probably more durable in terms of pain relief and functionality, but in the short term, corticosteroids might provide some degree of analgesia as well.
Watto: The last thing we wanted to mention is bone stress injury, which can occur in high-mileage runners (20 miles or more per week). Patients with bone stress injury need to rest, usually non‒weight bearing, for a period of time.

Treatment of a bone stress fracture depends on which side it’s on (top or bottom). If it’s on the top of the femoral neck (the tension side), it has to be fixed. If it’s on the compression side (the bottom side of the femoral neck), it might be able to be managed conservatively, but many patients are going to need surgery. This is a big deal. But it’s a spectrum; in some cases the bone is merely irritated and unhappy, without a break in the cortex. Those patients might not need surgery.
In patients with a fracture of the femoral neck — especially younger, healthier patients — you should think about getting a bone density test and screening for relative energy deficiency in sport. This used to be called the female athlete triad, which includes disrupted menstrual cycles, being underweight, and fracture. We should be screening patients, asking them in a nonjudgmental way about their relationship with food, to make sure they are getting an appropriate number of calories.
They are actually in an energy deficit. They’re not eating enough to maintain a healthy body with so much activity.
Williams: If you’re interested in this topic, you should refer to the full podcast with Dr Senter which is chock-full of helpful information.
Dr Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, disclosed ties with The Curbsiders.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr Paul Nelson Williams. Paul, how are you feeling about sports injuries?
Paul N. Williams, MD: I’m feeling great, Matt.
Watto: You had a sports injury of the hip. Maybe that’s an overshare, Paul, but we talked about it on a podcast with Dr Carlin Senter (part 1 and part 2).
Williams: I think I’ve shared more than my hip injury, for sure.
Watto: Whenever a patient presented with hip pain, I used to pray it was trochanteric bursitis, which now I know is not really the right thing to think about. Intra-articular hip pain presents as anterior hip pain, usually in the crease of the hip. Depending on the patient’s age and history, the differential for that type of pain includes iliopsoas tendonitis, FAI syndrome, a labral tear, a bone stress injury of the femoral neck, or osteoarthritis.
So, what exactly is FAI and how might we diagnose it?
Williams: FAI is what the cool kids call femoral acetabular impingement, and it’s exactly what it sounds like.

Something is pinching or impinging upon the joint itself and preventing full range of motion. This is a ball-and-socket joint, so it should have tremendous range of motion, able to move in all planes. If it’s impinged, then pain will occur with certain movements. There’s a cam type, which is characterized by enlargement of the femoral head neck junction, or a pincer type, which has more to do with overhang of the acetabulum, and it can also be mixed. In any case, impingement upon the patient’s full range of motion results in pain.
You evaluate this with a couple of tests — the FABER and the FADIR.
The FABER is flexion, abduction, and external rotation, and the FADIR is flexion, adduction, and internal rotation. If you elicit anterior pain with either of those tests, it’s probably one of the intra-articular pathologies, although it is hard to know for sure which one it is because these tests are fairly sensitive but not very specific.
Watto: You can get x-rays to help with the diagnosis. You would order two views of the hip: an AP of the pelvis, which is just a straight-on shot to look for arthritis or fracture. Is there a healthy joint line there? The second is the Dunn view, in which the hip is flexed 90 degrees and abducted about 20 degrees. You are looking for fracture or impingement. You can diagnose FAI based on that view, and you might be able to diagnose a hip stress injury or osteoarthritis.
Unfortunately, you’re not going to see a labral tear, but Dr Senter said that both FAI and labral tears are treated the same way, with physical therapy. Patients with FAI who aren’t getting better might end up going for surgery, so at some point I would refer them to orthopedic surgery. But I feel much more comfortable now diagnosing these conditions with these tests.
Let’s talk a little bit about trochanteric pain syndrome. I used to think it was all bursitis. Why is that not correct?
Williams: It’s nice of you to feign ignorance for the purpose of education. It used to be thought of as bursitis, but these days we know it is probably more likely a tendinopathy.

Trochanteric pain syndrome was formerly known as trochanteric bursitis, but the bursa is not typically involved. Trochanteric pain syndrome is a tendinopathy of the surrounding structures: the gluteus medius, the iliotibial band, and the tensor fascia latae. The way these structures relate looks a bit like the face of a clock, as you can see on the infographic. In general, you manage this condition the same way you do with bursitis — physical therapy. You can also give corticosteroid injections. Physical therapy is probably more durable in terms of pain relief and functionality, but in the short term, corticosteroids might provide some degree of analgesia as well.
Watto: The last thing we wanted to mention is bone stress injury, which can occur in high-mileage runners (20 miles or more per week). Patients with bone stress injury need to rest, usually non‒weight bearing, for a period of time.

Treatment of a bone stress fracture depends on which side it’s on (top or bottom). If it’s on the top of the femoral neck (the tension side), it has to be fixed. If it’s on the compression side (the bottom side of the femoral neck), it might be able to be managed conservatively, but many patients are going to need surgery. This is a big deal. But it’s a spectrum; in some cases the bone is merely irritated and unhappy, without a break in the cortex. Those patients might not need surgery.
In patients with a fracture of the femoral neck — especially younger, healthier patients — you should think about getting a bone density test and screening for relative energy deficiency in sport. This used to be called the female athlete triad, which includes disrupted menstrual cycles, being underweight, and fracture. We should be screening patients, asking them in a nonjudgmental way about their relationship with food, to make sure they are getting an appropriate number of calories.
They are actually in an energy deficit. They’re not eating enough to maintain a healthy body with so much activity.
Williams: If you’re interested in this topic, you should refer to the full podcast with Dr Senter which is chock-full of helpful information.
Dr Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, disclosed ties with The Curbsiders.
A version of this article appeared on Medscape.com.
Using AI to ID Osteoporosis: A Medico-Legal Minefield?
Could an artificial intelligence (AI)–driven tool that mines medical records for suspected cases of osteoporosis be so successful that it becomes a potential liability? Yes, according to Christopher White, PhD, executive director of Maridulu Budyari Gumal, the Sydney Partnership for Health, Education, Research, and Enterprise, a research translation center in Liverpool, Australia.
In a thought-provoking presentation at the Endocrine Society’s AI in Healthcare Virtual Summit, White described the results after his fracture liaison team at Prince of Wales Hospital in Randwick, Australia, tried to plug the “osteoporosis treatment gap” by mining medical records to identify patients with the disorder.
‘Be Careful What You Wish For’
White and colleagues developed a robust standalone database over 20 years that informed fracture risk among patients with osteoporosis in Sydney. The database included all relevant clinical information, as well as bone density measurements, on about 30,000 patients and could be interrogated for randomized controlled trial recruitment.
However, a “crisis” occurred around 2011, when the team received a recruitment request for the first head-to-head comparison of alendronate with romosozumab. “We had numerous postmenopausal women in the age range with the required bone density, but we hadn’t captured the severity of their vertebral fracture or how many they actually had,” White told the this news organization. For recruitment into the study, participants must have had at least two moderate or severe vertebral fractures or a proximal vertebral fracture that was sustained between 3 and 24 months before recruitment.
White turned to his hospital’s mainframe, which had coding data and time intervals for patients who were admitted with vertebral or hip fractures. He calculated how many patients who met the study criteria had been discharged and how many of those he thought he’d be able to capture through the mainframe. He was confident he would have enough, but he was wrong. He underrecruited and could not participate in the trial.
Determined not to wind up in a similar situation in the future, he investigated and found that other centers were struggling with similar problems. This led to a collaboration with four investigators who were using AI and Advanced Encryption Standard (AES) coding to identify patients at risk for osteoporotic fractures. White, meanwhile, had developed a natural language processing tool called XRAIT that also identified patients at fracture risk. A study comparing the two electronic search programs, which screen medical records for fractures, found that both reliably identified patients who had had a fracture. White and his colleagues concluded that hybrid tools combining XRAIT and AES would likely improve the identification of patients with osteoporosis who would require follow-up or might participate in future trials.
Those patients were not being identified sooner for multiple reasons, White explained. Sometimes, the radiologist would report osteoporosis, but it wouldn’t get coded. Or, in the emergency department, a patient with a fracture would be treated and then sent home, and the possibility of osteoporosis wasn’t reported.
“As we went deeper and deeper with our tools into the medical record, we found more and more patients who hadn’t been coded or reported but who actually had osteoporosis,” White said. “It was incredibly prevalent.”
But the number of patients identified was more than the hospital could comfortably handle.
Ironically, he added, “To my relief and probably not to the benefit of the patients, there was a system upgrade of the radiology reporting system, which was incompatible with the natural language processing technology that I had installed. The AI was turned off at that point, but I had a look over the edge and into the mine pit.”
“The lesson learned,” White told this news organization, is “If you mine the medical record for unidentified patients before you know what to do with the output, you create a medico-legal minefield. You need to be careful what you wish for with technology, because it may actually come true.”
Grappling With the Treatment Gap
An (over)abundance of patients is likely contributing to the “osteoporosis treatment gap” that Australia’s fracture liaison services, which handle many of these patients, are grappling with. One recent meta-analysis showed that not all eligible patients are treated and that not all patients who are treated actually start treatment. Another study showed that only a minority of patients — anywhere between 20% and 40% — who start are still persisting at about 3 years, White said.
Various types of fracture liaison services exist, he noted. The model that has been shown to best promote adherence is the one requiring clinicians to “identify, educate [usually, the primary care physician], evaluate, start treatment, continue treatment, and follow-up at 12 months for to confirm that there is adherence.”
What’s happening now, he said, is that the technology is identifying a high number of vertebral crush fractures, and there’s no education or evaluation. “The radiologist just refers the patient to a primary care physician and hopes for the best. AI isn’t contributing to solving the treatment gap problem; it’s amplifying it. It’s ahead of the ability of organizations to accommodate the findings.”
Solutions, he said, would require support at the top of health systems and organizations, and funding to proceed; data surveys concentrating on vertical integration of the medical record to follow patients wherever they are — eg, hospital, primary care — in their health journeys; a workflow with synchronous diagnosis and treatment planning, delivery, monitoring, and payment; and clinical and community champions advocating and “leading the charge in health tech.”
Furthermore, he advised, organizations need to be “very, very careful with safety and security — that is, managing the digital risks.”
“Oscar Wilde said there are two tragedies in life: One is not getting what one wants, and the other is getting it,” White concluded. “In my career, we’ve moved on from not knowing how to treat osteoporosis to knowing how to treat it. And that is both an asset and a liability.”
A version of this article first appeared on Medscape.com.
Could an artificial intelligence (AI)–driven tool that mines medical records for suspected cases of osteoporosis be so successful that it becomes a potential liability? Yes, according to Christopher White, PhD, executive director of Maridulu Budyari Gumal, the Sydney Partnership for Health, Education, Research, and Enterprise, a research translation center in Liverpool, Australia.
In a thought-provoking presentation at the Endocrine Society’s AI in Healthcare Virtual Summit, White described the results after his fracture liaison team at Prince of Wales Hospital in Randwick, Australia, tried to plug the “osteoporosis treatment gap” by mining medical records to identify patients with the disorder.
‘Be Careful What You Wish For’
White and colleagues developed a robust standalone database over 20 years that informed fracture risk among patients with osteoporosis in Sydney. The database included all relevant clinical information, as well as bone density measurements, on about 30,000 patients and could be interrogated for randomized controlled trial recruitment.
However, a “crisis” occurred around 2011, when the team received a recruitment request for the first head-to-head comparison of alendronate with romosozumab. “We had numerous postmenopausal women in the age range with the required bone density, but we hadn’t captured the severity of their vertebral fracture or how many they actually had,” White told the this news organization. For recruitment into the study, participants must have had at least two moderate or severe vertebral fractures or a proximal vertebral fracture that was sustained between 3 and 24 months before recruitment.
White turned to his hospital’s mainframe, which had coding data and time intervals for patients who were admitted with vertebral or hip fractures. He calculated how many patients who met the study criteria had been discharged and how many of those he thought he’d be able to capture through the mainframe. He was confident he would have enough, but he was wrong. He underrecruited and could not participate in the trial.
Determined not to wind up in a similar situation in the future, he investigated and found that other centers were struggling with similar problems. This led to a collaboration with four investigators who were using AI and Advanced Encryption Standard (AES) coding to identify patients at risk for osteoporotic fractures. White, meanwhile, had developed a natural language processing tool called XRAIT that also identified patients at fracture risk. A study comparing the two electronic search programs, which screen medical records for fractures, found that both reliably identified patients who had had a fracture. White and his colleagues concluded that hybrid tools combining XRAIT and AES would likely improve the identification of patients with osteoporosis who would require follow-up or might participate in future trials.
Those patients were not being identified sooner for multiple reasons, White explained. Sometimes, the radiologist would report osteoporosis, but it wouldn’t get coded. Or, in the emergency department, a patient with a fracture would be treated and then sent home, and the possibility of osteoporosis wasn’t reported.
“As we went deeper and deeper with our tools into the medical record, we found more and more patients who hadn’t been coded or reported but who actually had osteoporosis,” White said. “It was incredibly prevalent.”
But the number of patients identified was more than the hospital could comfortably handle.
Ironically, he added, “To my relief and probably not to the benefit of the patients, there was a system upgrade of the radiology reporting system, which was incompatible with the natural language processing technology that I had installed. The AI was turned off at that point, but I had a look over the edge and into the mine pit.”
“The lesson learned,” White told this news organization, is “If you mine the medical record for unidentified patients before you know what to do with the output, you create a medico-legal minefield. You need to be careful what you wish for with technology, because it may actually come true.”
Grappling With the Treatment Gap
An (over)abundance of patients is likely contributing to the “osteoporosis treatment gap” that Australia’s fracture liaison services, which handle many of these patients, are grappling with. One recent meta-analysis showed that not all eligible patients are treated and that not all patients who are treated actually start treatment. Another study showed that only a minority of patients — anywhere between 20% and 40% — who start are still persisting at about 3 years, White said.
Various types of fracture liaison services exist, he noted. The model that has been shown to best promote adherence is the one requiring clinicians to “identify, educate [usually, the primary care physician], evaluate, start treatment, continue treatment, and follow-up at 12 months for to confirm that there is adherence.”
What’s happening now, he said, is that the technology is identifying a high number of vertebral crush fractures, and there’s no education or evaluation. “The radiologist just refers the patient to a primary care physician and hopes for the best. AI isn’t contributing to solving the treatment gap problem; it’s amplifying it. It’s ahead of the ability of organizations to accommodate the findings.”
Solutions, he said, would require support at the top of health systems and organizations, and funding to proceed; data surveys concentrating on vertical integration of the medical record to follow patients wherever they are — eg, hospital, primary care — in their health journeys; a workflow with synchronous diagnosis and treatment planning, delivery, monitoring, and payment; and clinical and community champions advocating and “leading the charge in health tech.”
Furthermore, he advised, organizations need to be “very, very careful with safety and security — that is, managing the digital risks.”
“Oscar Wilde said there are two tragedies in life: One is not getting what one wants, and the other is getting it,” White concluded. “In my career, we’ve moved on from not knowing how to treat osteoporosis to knowing how to treat it. And that is both an asset and a liability.”
A version of this article first appeared on Medscape.com.
Could an artificial intelligence (AI)–driven tool that mines medical records for suspected cases of osteoporosis be so successful that it becomes a potential liability? Yes, according to Christopher White, PhD, executive director of Maridulu Budyari Gumal, the Sydney Partnership for Health, Education, Research, and Enterprise, a research translation center in Liverpool, Australia.
In a thought-provoking presentation at the Endocrine Society’s AI in Healthcare Virtual Summit, White described the results after his fracture liaison team at Prince of Wales Hospital in Randwick, Australia, tried to plug the “osteoporosis treatment gap” by mining medical records to identify patients with the disorder.
‘Be Careful What You Wish For’
White and colleagues developed a robust standalone database over 20 years that informed fracture risk among patients with osteoporosis in Sydney. The database included all relevant clinical information, as well as bone density measurements, on about 30,000 patients and could be interrogated for randomized controlled trial recruitment.
However, a “crisis” occurred around 2011, when the team received a recruitment request for the first head-to-head comparison of alendronate with romosozumab. “We had numerous postmenopausal women in the age range with the required bone density, but we hadn’t captured the severity of their vertebral fracture or how many they actually had,” White told the this news organization. For recruitment into the study, participants must have had at least two moderate or severe vertebral fractures or a proximal vertebral fracture that was sustained between 3 and 24 months before recruitment.
White turned to his hospital’s mainframe, which had coding data and time intervals for patients who were admitted with vertebral or hip fractures. He calculated how many patients who met the study criteria had been discharged and how many of those he thought he’d be able to capture through the mainframe. He was confident he would have enough, but he was wrong. He underrecruited and could not participate in the trial.
Determined not to wind up in a similar situation in the future, he investigated and found that other centers were struggling with similar problems. This led to a collaboration with four investigators who were using AI and Advanced Encryption Standard (AES) coding to identify patients at risk for osteoporotic fractures. White, meanwhile, had developed a natural language processing tool called XRAIT that also identified patients at fracture risk. A study comparing the two electronic search programs, which screen medical records for fractures, found that both reliably identified patients who had had a fracture. White and his colleagues concluded that hybrid tools combining XRAIT and AES would likely improve the identification of patients with osteoporosis who would require follow-up or might participate in future trials.
Those patients were not being identified sooner for multiple reasons, White explained. Sometimes, the radiologist would report osteoporosis, but it wouldn’t get coded. Or, in the emergency department, a patient with a fracture would be treated and then sent home, and the possibility of osteoporosis wasn’t reported.
“As we went deeper and deeper with our tools into the medical record, we found more and more patients who hadn’t been coded or reported but who actually had osteoporosis,” White said. “It was incredibly prevalent.”
But the number of patients identified was more than the hospital could comfortably handle.
Ironically, he added, “To my relief and probably not to the benefit of the patients, there was a system upgrade of the radiology reporting system, which was incompatible with the natural language processing technology that I had installed. The AI was turned off at that point, but I had a look over the edge and into the mine pit.”
“The lesson learned,” White told this news organization, is “If you mine the medical record for unidentified patients before you know what to do with the output, you create a medico-legal minefield. You need to be careful what you wish for with technology, because it may actually come true.”
Grappling With the Treatment Gap
An (over)abundance of patients is likely contributing to the “osteoporosis treatment gap” that Australia’s fracture liaison services, which handle many of these patients, are grappling with. One recent meta-analysis showed that not all eligible patients are treated and that not all patients who are treated actually start treatment. Another study showed that only a minority of patients — anywhere between 20% and 40% — who start are still persisting at about 3 years, White said.
Various types of fracture liaison services exist, he noted. The model that has been shown to best promote adherence is the one requiring clinicians to “identify, educate [usually, the primary care physician], evaluate, start treatment, continue treatment, and follow-up at 12 months for to confirm that there is adherence.”
What’s happening now, he said, is that the technology is identifying a high number of vertebral crush fractures, and there’s no education or evaluation. “The radiologist just refers the patient to a primary care physician and hopes for the best. AI isn’t contributing to solving the treatment gap problem; it’s amplifying it. It’s ahead of the ability of organizations to accommodate the findings.”
Solutions, he said, would require support at the top of health systems and organizations, and funding to proceed; data surveys concentrating on vertical integration of the medical record to follow patients wherever they are — eg, hospital, primary care — in their health journeys; a workflow with synchronous diagnosis and treatment planning, delivery, monitoring, and payment; and clinical and community champions advocating and “leading the charge in health tech.”
Furthermore, he advised, organizations need to be “very, very careful with safety and security — that is, managing the digital risks.”
“Oscar Wilde said there are two tragedies in life: One is not getting what one wants, and the other is getting it,” White concluded. “In my career, we’ve moved on from not knowing how to treat osteoporosis to knowing how to treat it. And that is both an asset and a liability.”
A version of this article first appeared on Medscape.com.
Exercising Longer May Boost Weight Loss, Meta-Analysis Shows
TOPLINE:
Aerobic exercise shows a linear relationship with weight loss, with 30 minutes of weekly exercise linked to reduced body weight, waist circumference, and body fat in adults who were overweight or had obesity.
METHODOLOGY:
- Researchers conducted a meta-analysis of randomized clinical trials to investigate the association of varying intensities and durations of aerobic exercise with adiposity measures in adults with obesity or who were overweight.
- Overall, 116 randomized clinical trials that spanned across North America, Asia, Europe, Australia, South America, and Africa and involved 6880 adults (mean age, 46 years; 61% women) were included.
- The trials were required to have intervention durations of at least 8 weeks; all trials used supervised aerobic exercise, such as walking or running, while the control groups remained sedentary or continued usual activities.
- The intensity of exercise was defined as: Light (40%-55% maximum heart rate), moderate (55%-70% maximum heart rate), and vigorous (70%-90% maximum heart rate).
- The primary outcomes were body weight changes and adverse events; the secondary outcomes included changes in waist circumference, quality-of-life scores, and reduction in medications like antihypertensives.
TAKEAWAY:
- Every 30 minutes of aerobic exercise per week was also associated with lower waist circumference (mean difference, −0.56 cm; 95% CI, –0.67 to –0.45), body fat percentage (mean difference, –0.37%; 95% CI, –0.43 to –0.31), and body fat mass (mean difference, –0.20 kg; 95% CI, –0.32 to –0.08), along with reduced visceral and subcutaneous adipose tissue.
- A dose-response meta-analysis revealed that body fat percentage improved most significantly with 150 minutes of aerobic exercise per week, while body weight and waist circumference decreased linearly with increasing duration of aerobic exercise at 300 min/wk at different intensities.
- Adverse events with aerobic exercise were mostly mild or moderate musculoskeletal symptoms.
IN PRACTICE:
“Point-specific estimates for different aerobic exercise duration and intensity can help patients and healthcare professionals select the optimal aerobic exercise duration and intensity according to their weight loss goals,” the authors wrote.
SOURCE:
The study was led by Ahmad Jayedi, PhD, of the Department of Epidemiology and Biostatistics in the School of Public Health at the Imperial College London in England. It was published online on December 26, 2024, in JAMA Network Open.
LIMITATIONS:
High heterogeneity was present in the data. Only one trial included measures of health-related quality of life, and two studies included measures of medication use. Dietary habits and smoking status of participants were not included in studies, so any potential effects were not risk adjusted for.
DISCLOSURES:
No funding sources were reported. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Aerobic exercise shows a linear relationship with weight loss, with 30 minutes of weekly exercise linked to reduced body weight, waist circumference, and body fat in adults who were overweight or had obesity.
METHODOLOGY:
- Researchers conducted a meta-analysis of randomized clinical trials to investigate the association of varying intensities and durations of aerobic exercise with adiposity measures in adults with obesity or who were overweight.
- Overall, 116 randomized clinical trials that spanned across North America, Asia, Europe, Australia, South America, and Africa and involved 6880 adults (mean age, 46 years; 61% women) were included.
- The trials were required to have intervention durations of at least 8 weeks; all trials used supervised aerobic exercise, such as walking or running, while the control groups remained sedentary or continued usual activities.
- The intensity of exercise was defined as: Light (40%-55% maximum heart rate), moderate (55%-70% maximum heart rate), and vigorous (70%-90% maximum heart rate).
- The primary outcomes were body weight changes and adverse events; the secondary outcomes included changes in waist circumference, quality-of-life scores, and reduction in medications like antihypertensives.
TAKEAWAY:
- Every 30 minutes of aerobic exercise per week was also associated with lower waist circumference (mean difference, −0.56 cm; 95% CI, –0.67 to –0.45), body fat percentage (mean difference, –0.37%; 95% CI, –0.43 to –0.31), and body fat mass (mean difference, –0.20 kg; 95% CI, –0.32 to –0.08), along with reduced visceral and subcutaneous adipose tissue.
- A dose-response meta-analysis revealed that body fat percentage improved most significantly with 150 minutes of aerobic exercise per week, while body weight and waist circumference decreased linearly with increasing duration of aerobic exercise at 300 min/wk at different intensities.
- Adverse events with aerobic exercise were mostly mild or moderate musculoskeletal symptoms.
IN PRACTICE:
“Point-specific estimates for different aerobic exercise duration and intensity can help patients and healthcare professionals select the optimal aerobic exercise duration and intensity according to their weight loss goals,” the authors wrote.
SOURCE:
The study was led by Ahmad Jayedi, PhD, of the Department of Epidemiology and Biostatistics in the School of Public Health at the Imperial College London in England. It was published online on December 26, 2024, in JAMA Network Open.
LIMITATIONS:
High heterogeneity was present in the data. Only one trial included measures of health-related quality of life, and two studies included measures of medication use. Dietary habits and smoking status of participants were not included in studies, so any potential effects were not risk adjusted for.
DISCLOSURES:
No funding sources were reported. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Aerobic exercise shows a linear relationship with weight loss, with 30 minutes of weekly exercise linked to reduced body weight, waist circumference, and body fat in adults who were overweight or had obesity.
METHODOLOGY:
- Researchers conducted a meta-analysis of randomized clinical trials to investigate the association of varying intensities and durations of aerobic exercise with adiposity measures in adults with obesity or who were overweight.
- Overall, 116 randomized clinical trials that spanned across North America, Asia, Europe, Australia, South America, and Africa and involved 6880 adults (mean age, 46 years; 61% women) were included.
- The trials were required to have intervention durations of at least 8 weeks; all trials used supervised aerobic exercise, such as walking or running, while the control groups remained sedentary or continued usual activities.
- The intensity of exercise was defined as: Light (40%-55% maximum heart rate), moderate (55%-70% maximum heart rate), and vigorous (70%-90% maximum heart rate).
- The primary outcomes were body weight changes and adverse events; the secondary outcomes included changes in waist circumference, quality-of-life scores, and reduction in medications like antihypertensives.
TAKEAWAY:
- Every 30 minutes of aerobic exercise per week was also associated with lower waist circumference (mean difference, −0.56 cm; 95% CI, –0.67 to –0.45), body fat percentage (mean difference, –0.37%; 95% CI, –0.43 to –0.31), and body fat mass (mean difference, –0.20 kg; 95% CI, –0.32 to –0.08), along with reduced visceral and subcutaneous adipose tissue.
- A dose-response meta-analysis revealed that body fat percentage improved most significantly with 150 minutes of aerobic exercise per week, while body weight and waist circumference decreased linearly with increasing duration of aerobic exercise at 300 min/wk at different intensities.
- Adverse events with aerobic exercise were mostly mild or moderate musculoskeletal symptoms.
IN PRACTICE:
“Point-specific estimates for different aerobic exercise duration and intensity can help patients and healthcare professionals select the optimal aerobic exercise duration and intensity according to their weight loss goals,” the authors wrote.
SOURCE:
The study was led by Ahmad Jayedi, PhD, of the Department of Epidemiology and Biostatistics in the School of Public Health at the Imperial College London in England. It was published online on December 26, 2024, in JAMA Network Open.
LIMITATIONS:
High heterogeneity was present in the data. Only one trial included measures of health-related quality of life, and two studies included measures of medication use. Dietary habits and smoking status of participants were not included in studies, so any potential effects were not risk adjusted for.
DISCLOSURES:
No funding sources were reported. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Stem Cell Transplant Effective for Children With Arthritis
TOPLINE:
METHODOLOGY:
- Retrospective cohort study of 13 children with refractory systemic juvenile idiopathic arthritis–related lung disease (sJIA-LD) who had allogeneic hematopoietic stem cell transplantation (HSCT).
- Children whose median age was 9 years at transplantation underwent HSCT at nine hospitals in the United States and Europe between January 2018 and October 2022, with a median follow-up of 16 months.
- Outcomes included transplant-related complications, pulmonary outcomes (eg, oxygen dependence and chest CT findings), and overall outcomes (eg, complete response, partial response, and death).
TAKEAWAY:
- Five patients developed acute graft vs host disease of varying grades, but none experienced chronic disease.
- All nine surviving patients achieved a complete response at the last follow-up, with no sJIA characteristics or need for immunosuppressive therapy or supplemental oxygen.
- Four patients died from complications including cytomegalovirus pneumonitis (n = 2), intracranial hemorrhage (n = 1), and progressive sJIA-LD (n = 1).
- Of six patients who underwent posttransplant chest CT, three had improved lung health, two had stable lung disease, and one experienced worsening lung disease, ultimately resulting in death.
IN PRACTICE:
“Allogeneic HSCT should be considered for treatment-refractory sJIA-LD,” the authors wrote.
“Efforts are being pursued for earlier recognition of patients with sJIA-LD at risk of adverse reactions to biologics. Early detection should help to avoid repeated treatments that are less effective and possibly deleterious and consider therapeutic approaches (eg, anti–[interleukin]-18 or [interferon]-delta–targeted treatments) that might act as a bridge therapy to control disease activity before HSCT,” wrote the author of an accompanying editorial.
SOURCE:
Michael G. Matt, MD, and Daniel Drozdov, MD, led the study, which was published online on December 20, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Limitations included sampling bias and heterogeneity in clinical follow-up. The small sample size made it difficult to identify variables affecting survival and the achievement of a complete response. Additionally, many patients had relatively short follow-up periods.
DISCLOSURES:
This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health. Several authors reported receiving advisory board fees, consulting fees, honoraria, grant funds, and stocks and shares from various research institutes and pharmaceutical organizations.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Retrospective cohort study of 13 children with refractory systemic juvenile idiopathic arthritis–related lung disease (sJIA-LD) who had allogeneic hematopoietic stem cell transplantation (HSCT).
- Children whose median age was 9 years at transplantation underwent HSCT at nine hospitals in the United States and Europe between January 2018 and October 2022, with a median follow-up of 16 months.
- Outcomes included transplant-related complications, pulmonary outcomes (eg, oxygen dependence and chest CT findings), and overall outcomes (eg, complete response, partial response, and death).
TAKEAWAY:
- Five patients developed acute graft vs host disease of varying grades, but none experienced chronic disease.
- All nine surviving patients achieved a complete response at the last follow-up, with no sJIA characteristics or need for immunosuppressive therapy or supplemental oxygen.
- Four patients died from complications including cytomegalovirus pneumonitis (n = 2), intracranial hemorrhage (n = 1), and progressive sJIA-LD (n = 1).
- Of six patients who underwent posttransplant chest CT, three had improved lung health, two had stable lung disease, and one experienced worsening lung disease, ultimately resulting in death.
IN PRACTICE:
“Allogeneic HSCT should be considered for treatment-refractory sJIA-LD,” the authors wrote.
“Efforts are being pursued for earlier recognition of patients with sJIA-LD at risk of adverse reactions to biologics. Early detection should help to avoid repeated treatments that are less effective and possibly deleterious and consider therapeutic approaches (eg, anti–[interleukin]-18 or [interferon]-delta–targeted treatments) that might act as a bridge therapy to control disease activity before HSCT,” wrote the author of an accompanying editorial.
SOURCE:
Michael G. Matt, MD, and Daniel Drozdov, MD, led the study, which was published online on December 20, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Limitations included sampling bias and heterogeneity in clinical follow-up. The small sample size made it difficult to identify variables affecting survival and the achievement of a complete response. Additionally, many patients had relatively short follow-up periods.
DISCLOSURES:
This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health. Several authors reported receiving advisory board fees, consulting fees, honoraria, grant funds, and stocks and shares from various research institutes and pharmaceutical organizations.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Retrospective cohort study of 13 children with refractory systemic juvenile idiopathic arthritis–related lung disease (sJIA-LD) who had allogeneic hematopoietic stem cell transplantation (HSCT).
- Children whose median age was 9 years at transplantation underwent HSCT at nine hospitals in the United States and Europe between January 2018 and October 2022, with a median follow-up of 16 months.
- Outcomes included transplant-related complications, pulmonary outcomes (eg, oxygen dependence and chest CT findings), and overall outcomes (eg, complete response, partial response, and death).
TAKEAWAY:
- Five patients developed acute graft vs host disease of varying grades, but none experienced chronic disease.
- All nine surviving patients achieved a complete response at the last follow-up, with no sJIA characteristics or need for immunosuppressive therapy or supplemental oxygen.
- Four patients died from complications including cytomegalovirus pneumonitis (n = 2), intracranial hemorrhage (n = 1), and progressive sJIA-LD (n = 1).
- Of six patients who underwent posttransplant chest CT, three had improved lung health, two had stable lung disease, and one experienced worsening lung disease, ultimately resulting in death.
IN PRACTICE:
“Allogeneic HSCT should be considered for treatment-refractory sJIA-LD,” the authors wrote.
“Efforts are being pursued for earlier recognition of patients with sJIA-LD at risk of adverse reactions to biologics. Early detection should help to avoid repeated treatments that are less effective and possibly deleterious and consider therapeutic approaches (eg, anti–[interleukin]-18 or [interferon]-delta–targeted treatments) that might act as a bridge therapy to control disease activity before HSCT,” wrote the author of an accompanying editorial.
SOURCE:
Michael G. Matt, MD, and Daniel Drozdov, MD, led the study, which was published online on December 20, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Limitations included sampling bias and heterogeneity in clinical follow-up. The small sample size made it difficult to identify variables affecting survival and the achievement of a complete response. Additionally, many patients had relatively short follow-up periods.
DISCLOSURES:
This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health. Several authors reported receiving advisory board fees, consulting fees, honoraria, grant funds, and stocks and shares from various research institutes and pharmaceutical organizations.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
FDA Approves Ustekinumab Biosimilar Steqeyma, the Seventh of Its Kind
The Food and Drug Administration (FDA) has approved ustekinumab-stba (Steqeyma) as a biosimilar to the interleukin-12 and -23 inhibitor ustekinumab (Stelara) for the treatment of adults with active Crohn’s disease or ulcerative colitis and for both children aged ≥ 6 years and adults with moderate to severe plaque psoriasis or active psoriatic arthritis.
This is the seventh ustekinumab biosimilar approved by the FDA. The biosimilar, developed by Celltrion, has a license entry date in February 2025 as part of the settlement and license agreement with the manufacturer of the reference biologic, Johnson & Johnson.
Ustekinumab-stba will be available in two formulations: A subcutaneous injection in two strengths — a 45 mg/0.5 mL or 90 mg/1 mL solution in a single-dose, prefilled syringe — and an intravenous infusion of a 130 mg/26 mL (5 mg/mL) solution in a single-dose vial.
“The approval of Steqeyma reflects Celltrion’s continued investment in providing treatment options to patients diagnosed with ulcerative colitis, Crohn’s disease, psoriasis, and psoriatic arthritis,” said Thomas Nusbickel, Chief Commercial Officer at Celltrion USA, Jersey City, New Jersey, in a press release.
The FDA has previously approved the company’s adalimumab biosimilar Yuflyma and its infliximab biosimilar Zymfentra.
The full prescribing information for ustekinumab-stba is available here.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved ustekinumab-stba (Steqeyma) as a biosimilar to the interleukin-12 and -23 inhibitor ustekinumab (Stelara) for the treatment of adults with active Crohn’s disease or ulcerative colitis and for both children aged ≥ 6 years and adults with moderate to severe plaque psoriasis or active psoriatic arthritis.
This is the seventh ustekinumab biosimilar approved by the FDA. The biosimilar, developed by Celltrion, has a license entry date in February 2025 as part of the settlement and license agreement with the manufacturer of the reference biologic, Johnson & Johnson.
Ustekinumab-stba will be available in two formulations: A subcutaneous injection in two strengths — a 45 mg/0.5 mL or 90 mg/1 mL solution in a single-dose, prefilled syringe — and an intravenous infusion of a 130 mg/26 mL (5 mg/mL) solution in a single-dose vial.
“The approval of Steqeyma reflects Celltrion’s continued investment in providing treatment options to patients diagnosed with ulcerative colitis, Crohn’s disease, psoriasis, and psoriatic arthritis,” said Thomas Nusbickel, Chief Commercial Officer at Celltrion USA, Jersey City, New Jersey, in a press release.
The FDA has previously approved the company’s adalimumab biosimilar Yuflyma and its infliximab biosimilar Zymfentra.
The full prescribing information for ustekinumab-stba is available here.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved ustekinumab-stba (Steqeyma) as a biosimilar to the interleukin-12 and -23 inhibitor ustekinumab (Stelara) for the treatment of adults with active Crohn’s disease or ulcerative colitis and for both children aged ≥ 6 years and adults with moderate to severe plaque psoriasis or active psoriatic arthritis.
This is the seventh ustekinumab biosimilar approved by the FDA. The biosimilar, developed by Celltrion, has a license entry date in February 2025 as part of the settlement and license agreement with the manufacturer of the reference biologic, Johnson & Johnson.
Ustekinumab-stba will be available in two formulations: A subcutaneous injection in two strengths — a 45 mg/0.5 mL or 90 mg/1 mL solution in a single-dose, prefilled syringe — and an intravenous infusion of a 130 mg/26 mL (5 mg/mL) solution in a single-dose vial.
“The approval of Steqeyma reflects Celltrion’s continued investment in providing treatment options to patients diagnosed with ulcerative colitis, Crohn’s disease, psoriasis, and psoriatic arthritis,” said Thomas Nusbickel, Chief Commercial Officer at Celltrion USA, Jersey City, New Jersey, in a press release.
The FDA has previously approved the company’s adalimumab biosimilar Yuflyma and its infliximab biosimilar Zymfentra.
The full prescribing information for ustekinumab-stba is available here.
A version of this article first appeared on Medscape.com.
Project’s Improvement in JIA Outcome Disparities Sets Stage for Further Interventions
WASHINGTON — A quality improvement project aimed at reducing racial disparities in juvenile idiopathic arthritis (JIA) led to a modest reduction in the overall clinical Juvenile Arthritis Disease Activity Score (cJADAS) and a 17% reduction in the disparity gap between Black and White patients, according to a study presented at the annual meeting of the American College of Rheumatology.
“Our work has led to initial progress in all groups, but we did not fully close the gap in outcomes,” Dori Abel, MD, MSHP, an attending rheumatologist at Children’s Hospital of Philadelphia in Pennsylvania, told attendees. But the project still revealed that it’s feasible to improve outcomes and reduce disparities with a “multipronged, equity-driven approach,” she said. “Stratifying data by demographic variables can reveal important differences in health care delivery and outcomes, catalyzing improvement efforts.”
Giya Harry, MD, MPH, MSc, an associate professor of pediatric rheumatology at Wake Forest University School of Medicine in Winston-Salem, North Carolina, was not involved in the study but praised both the effort and the progress made.
“The results are promising and suggest that with additional interventions targeting other key drivers, the team may be successful in completely eliminating the disparity in outcomes,” Harry said in an interview. “I applaud the hard work of Dr Abel and the other members of the team for doing the important work of characterizing the very complex issue of disparities in JIA outcomes across different race groups.”
It will now be important to build upon what the physicians learned during this process, said Harry, also the chair of the Diversity, Equity, Inclusion, and Accessibility committee of the Childhood Arthritis and Rheumatology Research Alliance.
“Patience is needed as they cycle through interventions with an emphasis on other key drivers” of disparities, Harry said.
Targeting Factors That Clinicians Can Potentially Influence
In her presentation, Abel discussed the various barriers that interfere with patients’ ability to move up the “JIA escalator” of getting referred and diagnosed, starting treatment and getting control of the disease, and monitoring and managing the disease and flares. These barriers include difficulties with access, trust, finances, insurance, caregivers’ missed work, medication burden, side effects, system barriers, and exhaustion and depression among caregivers and patients.
These barriers then contribute to disparities in JIA outcomes. In the STOP-JIA study, for example, Black children had greater polyarthritis disease activity in the first year and greater odds of radiographic damage, Abel noted. At her own institution, despite a mean cJADAS of 2.9 for the whole population of patients with JIA, the average was 5.0 for non-Hispanic Black patients, compared with 2.6 for non-Hispanic White patients.
The team therefore developed and implemented a quality improvement initiative aimed at improving the overall mean cJADAS and narrowing the gap between Black and White patients. The goal was to reduce the mean cJADAS to 2.7 by July 2024 and simultaneously reduce the cJADAS in Black patients by 1.2 units, or 50% of the baseline disparity gap, without increasing the existing gap.
The team first explored the many overlapping and interacting drivers of disparities within the realms of community characteristics, JIA treatment course, patient/family characteristics, organizational infrastructure, divisional infrastructure, and provider characteristics. While many of the individual factors driving disparities are outside clinicians’ control, “there are some domains clinicians may be able to directly influence, such as provider characteristics, JIA treatment course, and possibly divisional infrastructure,” Harry noted, and the team appeared to choose goals that fell under domains within clinicians’ potential influence.
The research team focused their efforts on four areas: Consistent outcome documentation, application of JIA best practices, providing access to at-risk patients, and team awareness and agency.
As part of improving consistent outcome documentation, they integrated outcome metrics into data visualization tools so that gaps were more evident. Applying JIA best practices included standardizing their approach to assessing medication adherence and barriers, with changes to the JIA note templates in the electronic health record and updates to medication adherence handouts.
Providing access to at-risk patients included several components:
- Creating a population management team
- Defining a target population to engage with for earlier follow-up
- Using a monthly batch outreach to defined patients
- Having a coordinator or social worker reach out to those who don’t make appointments
- Using a new JIA/high disease activity video follow-up program.
Finally, team awareness and agency involved giving physicians monthly access to mean cJADAS values for their own patients and at the division level. They also held quarterly disparity mitigation workshops.
Although the institution’s JIA population grew 13%, from 776 to 878 patients, over the course of the study, from January 2023 to May 2024, there was minimal change in the characteristics of the patient population. By May 2024, two thirds of patients (68%) were women, and 23% had public insurance. The population included 67% non-Hispanic White, 9% Hispanic/Latino, 7% non-Hispanic Black, and 4% Asian patients.
One third of the patients (32%) had the oligoarticular subtype, and other subtypes included enthesitis-related at 16%, polyarticular rheumatoid factor (RF)–negative at 15%, systemic at 7%, psoriatic at 6%, undifferentiated at 5%, and polyarticular RF-positive at 4%; data on subtype were unavailable for 14%. Most of their patients (71%) were in a high or very high quintile of the Childhood Opportunity Index, and 12% were in a low or very low quintile.
Results of the Quality Improvement Project
As of May 2024, the team had reached most of the goals they had set in terms of individual metrics. They met their goal of having a complete cJADAS calculated in more than 80% of JIA visits each month. With a goal of having over 90% of JIA monthly visits include disease activity target attestations, they reached 95% by May.
They aimed to have over half of JIA monthly visits include documentation of medication adherence/barrier assessment, and 75% of monthly visits had one. For their monthly outreach goal for overdue visits, they aimed to contact more than 75% of patients within 30 days if they were newly overdue for a follow-up visit but had only reached 47% by May 2024. The team had also completed 154 Maintenance of Certification assessments by May.
From initiation of project planning in January 2023 through May 2024, the overall JIA patient population experienced an improvement in cJADAS from 2.9 to 2.54. In individual cJADAS components, the mean patient global score improved from 1.71 to 1.47, the physician global score improved from 0.81 to 0.75, and the joint count score improved from 0.71 to 0.68.
In the non-Hispanic Black population, the mean cJADAS improved from 5.06 in January 2023 to 4.31 in May 2024. Mean cJADAS in the non-Hispanic White population fell from 2.63 to 2.29. With a difference of 0.4 points fewer between the Black and White populations, the disparity gap closed by 17%.
One of the team’s next steps will be to focus on the Hispanic population in 2024-2025 by optimizing language services, working toward greater family involvement to better understand barriers to care, and ongoing population management.
Abel and Harry had no disclosures. No external funding was noted.
A version of this article appeared on Medscape.com.
WASHINGTON — A quality improvement project aimed at reducing racial disparities in juvenile idiopathic arthritis (JIA) led to a modest reduction in the overall clinical Juvenile Arthritis Disease Activity Score (cJADAS) and a 17% reduction in the disparity gap between Black and White patients, according to a study presented at the annual meeting of the American College of Rheumatology.
“Our work has led to initial progress in all groups, but we did not fully close the gap in outcomes,” Dori Abel, MD, MSHP, an attending rheumatologist at Children’s Hospital of Philadelphia in Pennsylvania, told attendees. But the project still revealed that it’s feasible to improve outcomes and reduce disparities with a “multipronged, equity-driven approach,” she said. “Stratifying data by demographic variables can reveal important differences in health care delivery and outcomes, catalyzing improvement efforts.”
Giya Harry, MD, MPH, MSc, an associate professor of pediatric rheumatology at Wake Forest University School of Medicine in Winston-Salem, North Carolina, was not involved in the study but praised both the effort and the progress made.
“The results are promising and suggest that with additional interventions targeting other key drivers, the team may be successful in completely eliminating the disparity in outcomes,” Harry said in an interview. “I applaud the hard work of Dr Abel and the other members of the team for doing the important work of characterizing the very complex issue of disparities in JIA outcomes across different race groups.”
It will now be important to build upon what the physicians learned during this process, said Harry, also the chair of the Diversity, Equity, Inclusion, and Accessibility committee of the Childhood Arthritis and Rheumatology Research Alliance.
“Patience is needed as they cycle through interventions with an emphasis on other key drivers” of disparities, Harry said.
Targeting Factors That Clinicians Can Potentially Influence
In her presentation, Abel discussed the various barriers that interfere with patients’ ability to move up the “JIA escalator” of getting referred and diagnosed, starting treatment and getting control of the disease, and monitoring and managing the disease and flares. These barriers include difficulties with access, trust, finances, insurance, caregivers’ missed work, medication burden, side effects, system barriers, and exhaustion and depression among caregivers and patients.
These barriers then contribute to disparities in JIA outcomes. In the STOP-JIA study, for example, Black children had greater polyarthritis disease activity in the first year and greater odds of radiographic damage, Abel noted. At her own institution, despite a mean cJADAS of 2.9 for the whole population of patients with JIA, the average was 5.0 for non-Hispanic Black patients, compared with 2.6 for non-Hispanic White patients.
The team therefore developed and implemented a quality improvement initiative aimed at improving the overall mean cJADAS and narrowing the gap between Black and White patients. The goal was to reduce the mean cJADAS to 2.7 by July 2024 and simultaneously reduce the cJADAS in Black patients by 1.2 units, or 50% of the baseline disparity gap, without increasing the existing gap.
The team first explored the many overlapping and interacting drivers of disparities within the realms of community characteristics, JIA treatment course, patient/family characteristics, organizational infrastructure, divisional infrastructure, and provider characteristics. While many of the individual factors driving disparities are outside clinicians’ control, “there are some domains clinicians may be able to directly influence, such as provider characteristics, JIA treatment course, and possibly divisional infrastructure,” Harry noted, and the team appeared to choose goals that fell under domains within clinicians’ potential influence.
The research team focused their efforts on four areas: Consistent outcome documentation, application of JIA best practices, providing access to at-risk patients, and team awareness and agency.
As part of improving consistent outcome documentation, they integrated outcome metrics into data visualization tools so that gaps were more evident. Applying JIA best practices included standardizing their approach to assessing medication adherence and barriers, with changes to the JIA note templates in the electronic health record and updates to medication adherence handouts.
Providing access to at-risk patients included several components:
- Creating a population management team
- Defining a target population to engage with for earlier follow-up
- Using a monthly batch outreach to defined patients
- Having a coordinator or social worker reach out to those who don’t make appointments
- Using a new JIA/high disease activity video follow-up program.
Finally, team awareness and agency involved giving physicians monthly access to mean cJADAS values for their own patients and at the division level. They also held quarterly disparity mitigation workshops.
Although the institution’s JIA population grew 13%, from 776 to 878 patients, over the course of the study, from January 2023 to May 2024, there was minimal change in the characteristics of the patient population. By May 2024, two thirds of patients (68%) were women, and 23% had public insurance. The population included 67% non-Hispanic White, 9% Hispanic/Latino, 7% non-Hispanic Black, and 4% Asian patients.
One third of the patients (32%) had the oligoarticular subtype, and other subtypes included enthesitis-related at 16%, polyarticular rheumatoid factor (RF)–negative at 15%, systemic at 7%, psoriatic at 6%, undifferentiated at 5%, and polyarticular RF-positive at 4%; data on subtype were unavailable for 14%. Most of their patients (71%) were in a high or very high quintile of the Childhood Opportunity Index, and 12% were in a low or very low quintile.
Results of the Quality Improvement Project
As of May 2024, the team had reached most of the goals they had set in terms of individual metrics. They met their goal of having a complete cJADAS calculated in more than 80% of JIA visits each month. With a goal of having over 90% of JIA monthly visits include disease activity target attestations, they reached 95% by May.
They aimed to have over half of JIA monthly visits include documentation of medication adherence/barrier assessment, and 75% of monthly visits had one. For their monthly outreach goal for overdue visits, they aimed to contact more than 75% of patients within 30 days if they were newly overdue for a follow-up visit but had only reached 47% by May 2024. The team had also completed 154 Maintenance of Certification assessments by May.
From initiation of project planning in January 2023 through May 2024, the overall JIA patient population experienced an improvement in cJADAS from 2.9 to 2.54. In individual cJADAS components, the mean patient global score improved from 1.71 to 1.47, the physician global score improved from 0.81 to 0.75, and the joint count score improved from 0.71 to 0.68.
In the non-Hispanic Black population, the mean cJADAS improved from 5.06 in January 2023 to 4.31 in May 2024. Mean cJADAS in the non-Hispanic White population fell from 2.63 to 2.29. With a difference of 0.4 points fewer between the Black and White populations, the disparity gap closed by 17%.
One of the team’s next steps will be to focus on the Hispanic population in 2024-2025 by optimizing language services, working toward greater family involvement to better understand barriers to care, and ongoing population management.
Abel and Harry had no disclosures. No external funding was noted.
A version of this article appeared on Medscape.com.
WASHINGTON — A quality improvement project aimed at reducing racial disparities in juvenile idiopathic arthritis (JIA) led to a modest reduction in the overall clinical Juvenile Arthritis Disease Activity Score (cJADAS) and a 17% reduction in the disparity gap between Black and White patients, according to a study presented at the annual meeting of the American College of Rheumatology.
“Our work has led to initial progress in all groups, but we did not fully close the gap in outcomes,” Dori Abel, MD, MSHP, an attending rheumatologist at Children’s Hospital of Philadelphia in Pennsylvania, told attendees. But the project still revealed that it’s feasible to improve outcomes and reduce disparities with a “multipronged, equity-driven approach,” she said. “Stratifying data by demographic variables can reveal important differences in health care delivery and outcomes, catalyzing improvement efforts.”
Giya Harry, MD, MPH, MSc, an associate professor of pediatric rheumatology at Wake Forest University School of Medicine in Winston-Salem, North Carolina, was not involved in the study but praised both the effort and the progress made.
“The results are promising and suggest that with additional interventions targeting other key drivers, the team may be successful in completely eliminating the disparity in outcomes,” Harry said in an interview. “I applaud the hard work of Dr Abel and the other members of the team for doing the important work of characterizing the very complex issue of disparities in JIA outcomes across different race groups.”
It will now be important to build upon what the physicians learned during this process, said Harry, also the chair of the Diversity, Equity, Inclusion, and Accessibility committee of the Childhood Arthritis and Rheumatology Research Alliance.
“Patience is needed as they cycle through interventions with an emphasis on other key drivers” of disparities, Harry said.
Targeting Factors That Clinicians Can Potentially Influence
In her presentation, Abel discussed the various barriers that interfere with patients’ ability to move up the “JIA escalator” of getting referred and diagnosed, starting treatment and getting control of the disease, and monitoring and managing the disease and flares. These barriers include difficulties with access, trust, finances, insurance, caregivers’ missed work, medication burden, side effects, system barriers, and exhaustion and depression among caregivers and patients.
These barriers then contribute to disparities in JIA outcomes. In the STOP-JIA study, for example, Black children had greater polyarthritis disease activity in the first year and greater odds of radiographic damage, Abel noted. At her own institution, despite a mean cJADAS of 2.9 for the whole population of patients with JIA, the average was 5.0 for non-Hispanic Black patients, compared with 2.6 for non-Hispanic White patients.
The team therefore developed and implemented a quality improvement initiative aimed at improving the overall mean cJADAS and narrowing the gap between Black and White patients. The goal was to reduce the mean cJADAS to 2.7 by July 2024 and simultaneously reduce the cJADAS in Black patients by 1.2 units, or 50% of the baseline disparity gap, without increasing the existing gap.
The team first explored the many overlapping and interacting drivers of disparities within the realms of community characteristics, JIA treatment course, patient/family characteristics, organizational infrastructure, divisional infrastructure, and provider characteristics. While many of the individual factors driving disparities are outside clinicians’ control, “there are some domains clinicians may be able to directly influence, such as provider characteristics, JIA treatment course, and possibly divisional infrastructure,” Harry noted, and the team appeared to choose goals that fell under domains within clinicians’ potential influence.
The research team focused their efforts on four areas: Consistent outcome documentation, application of JIA best practices, providing access to at-risk patients, and team awareness and agency.
As part of improving consistent outcome documentation, they integrated outcome metrics into data visualization tools so that gaps were more evident. Applying JIA best practices included standardizing their approach to assessing medication adherence and barriers, with changes to the JIA note templates in the electronic health record and updates to medication adherence handouts.
Providing access to at-risk patients included several components:
- Creating a population management team
- Defining a target population to engage with for earlier follow-up
- Using a monthly batch outreach to defined patients
- Having a coordinator or social worker reach out to those who don’t make appointments
- Using a new JIA/high disease activity video follow-up program.
Finally, team awareness and agency involved giving physicians monthly access to mean cJADAS values for their own patients and at the division level. They also held quarterly disparity mitigation workshops.
Although the institution’s JIA population grew 13%, from 776 to 878 patients, over the course of the study, from January 2023 to May 2024, there was minimal change in the characteristics of the patient population. By May 2024, two thirds of patients (68%) were women, and 23% had public insurance. The population included 67% non-Hispanic White, 9% Hispanic/Latino, 7% non-Hispanic Black, and 4% Asian patients.
One third of the patients (32%) had the oligoarticular subtype, and other subtypes included enthesitis-related at 16%, polyarticular rheumatoid factor (RF)–negative at 15%, systemic at 7%, psoriatic at 6%, undifferentiated at 5%, and polyarticular RF-positive at 4%; data on subtype were unavailable for 14%. Most of their patients (71%) were in a high or very high quintile of the Childhood Opportunity Index, and 12% were in a low or very low quintile.
Results of the Quality Improvement Project
As of May 2024, the team had reached most of the goals they had set in terms of individual metrics. They met their goal of having a complete cJADAS calculated in more than 80% of JIA visits each month. With a goal of having over 90% of JIA monthly visits include disease activity target attestations, they reached 95% by May.
They aimed to have over half of JIA monthly visits include documentation of medication adherence/barrier assessment, and 75% of monthly visits had one. For their monthly outreach goal for overdue visits, they aimed to contact more than 75% of patients within 30 days if they were newly overdue for a follow-up visit but had only reached 47% by May 2024. The team had also completed 154 Maintenance of Certification assessments by May.
From initiation of project planning in January 2023 through May 2024, the overall JIA patient population experienced an improvement in cJADAS from 2.9 to 2.54. In individual cJADAS components, the mean patient global score improved from 1.71 to 1.47, the physician global score improved from 0.81 to 0.75, and the joint count score improved from 0.71 to 0.68.
In the non-Hispanic Black population, the mean cJADAS improved from 5.06 in January 2023 to 4.31 in May 2024. Mean cJADAS in the non-Hispanic White population fell from 2.63 to 2.29. With a difference of 0.4 points fewer between the Black and White populations, the disparity gap closed by 17%.
One of the team’s next steps will be to focus on the Hispanic population in 2024-2025 by optimizing language services, working toward greater family involvement to better understand barriers to care, and ongoing population management.
Abel and Harry had no disclosures. No external funding was noted.
A version of this article appeared on Medscape.com.
FROM ACR 2024
Could Biomarkers Help to Detect Lung Disease Earlier in Systemic JIA?
WASHINGTON — Children who have systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) have distinct biomarker profiles that may hold potential in eventually detecting LD earlier and identifying personalized treatment, according to research presented at the American College of Rheumatology (ACR) 2024 Annual Meeting.
Established risk factors for LD, which affects up to 1 in every 20 patients with sJIA, include being of a younger age, having more macrophage activation syndrome (MAS) episodes, and having more adverse reactions to biologics, Esraa Eloseily, MD, MS, an assistant professor of pediatrics at UT Southwestern Children’s Medical Center, Dallas, told attendees.
“The pathophysiology remains unclear and debate centers around elevated IL-18 [interleukin 18] and T-cell activation in association with HLA-DRB1*15/DRESS [drug reaction with eosinophilia and systemic symptoms] reactions to biologics, and thus, we have urgent unmet needs to understand the prevalence, the pathogenesis, disease biomarkers, and influence of biologics,” Eloseily said.
Their study confirmed that patients with LD tended to be younger and have more MAS. The researchers also found lower hemoglobin and higher white blood cell counts and platelets in patients with sJIA-LD, as well as a higher medication burden, particularly with steroids, biologics, and Janus kinase (JAK) inhibitors.
Randy Cron, MD, PhD, director of the Division of Pediatric Rheumatology at the University of Alabama at Birmingham, attended the presentation and noted that every additional piece of information is helpful in filling out the picture of what we know and can predict about sJIA-LD development.
“We’re all learning as we go, so the more people that study this, the better,” Cron told Medscape Medical News. “Even if it’s just seeing things that other groups have seen or really solidifying the risk factors for the development of lung disease, I think, at this point, that’s one of the most clinically relevant things: Do we screen? Who do we screen? Certainly, kids who have very young age of onset, children who develop macrophage activation syndrome, children who have high IL-18 levels.”
Study Results
The study compared 37 patients with sJIA-LD from 16 Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry sites with 141 patients with sJIA but without LD who had similar follow-up durations in the CARRA Registry.
Disease duration for patients with sJIA-LD was defined as the time from their initial sJIA diagnosis to their baseline sJIA-LD cohort visit, which was considered their index visit. In patients without LD, duration was from their enrollment in the CARRA Registry to their last CARRA Registry visit, which was considered their index visit.
The patients with sJIA-LD were significantly younger — a median age of 1 year — at onset of sJIA than those without LD, who had a median age of 5 years (P < .0019). The patients with sJIA-LD were also younger (median age, 7 years) at their index visit than those without LD (median age, 10 years) (P < .0001).
There were also significant differences in medication usage between those with and without LD. While 40.5% of patients with sJIA-LD were using steroids at their index visit, only 11.4% of those without LD were using steroids (P < .0001). Yet the mean dose of steroids was significantly lower in those with LD (5.45 mg/d) than in those without (20.7 mg/d). In addition, nearly half the patients with sJIA-LD had ever used cyclosporin A (45.7%) compared with 2.8% of those without LD (P < .0001), and 17.1% of patients with sJIA-LD had used mycophenolate mofetil compared with 0.7% of those without LD (P = .0002).
Similar disparities were seen for usage of biologics and JAK inhibitors: Anakinra (82.9% vs 56.7%; P = .0036), abatacept (8.6% vs 1.4%; P = .053), tofacitinib (57.1% vs 5.7%; P < .0001), ruxolitinib (25.7% vs 0%; P < .0001), baricitinib (8.6% vs 0%; P = .007), and emapalumab (23% vs 0.7%; P < .0001). Further, 5.7% of those with sJIA-LD had taken etoposide and 22.9% had received intravenous immunoglobulin compared with 0% and 4.3%, respectively, in those without LD (P = .04 and P = .001).
Laboratory parameters of patients with sJIA-LD were also significantly different from those of patients without LD, including a higher white blood cell count (8.8 × 109/L vs 8.1 × 109/L; P = .01), higher platelets (316.5 × 109/L vs 311.2 × 109/L; P = .03), and lower hemoglobin (11.5 g/dL vs 12.6 g/dL; P = .007). Ferritin levels trended nonsignificantly higher in patients with sJIA-LD (506 ng/mL vs 173.2 ng/mL; P = .09), and aspartate aminotransferase levels were significantly higher (37 U/L vs 28.72 U/L; P < .0001).
Patients’ overall well-being was “unexpectedly” higher in patients with sJIA-LD (P = .007), Eloseily noted, including the parent/patient rating (P = .027). However, more of the patients without LD had an excellent (61%) or very good (20.4%) health-related quality of life compared with those with LD (13% and 39%), and nearly one third of patients with sJIA-LD (30.4%) had only fair health-related quality of life compared with 5.5% without LD (P = .0002).
In line with known risk factors, most of the patients with sJIA-LD had a prior MAS episode (67.6%) compared with 10.6% of those without LD (P < .0001). Mortality was also higher in those with LD, two of whom died, whereas none without LD died (P = .04).
While existing biomarkers have been reported, they lack independent validation, Eloseily told attendees. Among the known key biomarkers are IL-18/interferon (IFN)-gamma axis, which are known to drive MAS and pulmonary inflammation, especially in those with MAS and LD; ICAM-5 and MMP-7, which is linked to fibrosis and tissue remodeling; and CCL11, CCL17, and GDF-15, which is linked to fibrosis and inflammation.
Because the CARRA Registry has limited availability of biosamples for most patients, the researchers used plasma samples from the FROST study for 27 patients with sJIA-LD and 46 patients without LD. When they compared 23 biomarkers between the groups, most of the known key biomarkers, as well as several other biomarkers, were significantly elevated in those with LD compared with in those without:
- MMP-7 (P = .001)
- IFN gamma (P = .008)
- CCL11 (P < .0001)
- CCL17 (P = .002)
- CCL15 (P < .0001)
- MCP-1 (P = .0003)
- MCP-3 (P = .02)
- CCL25 (P < .0001)
- CD25 (P < .0001)
- GDF-15 (P < .0001)
- TRAIL (P < .0001)
- IL-23 (P = .002)
They found that IL-18 only trended higher (P = .07), and there were not adequate data for ICAM-5.
The study was limited by the differences in disease duration between the compared groups and the limited availability of biosamples, which they only had from patients enrolled in the FROST study.
The research was funded by CARRA and the Arthritis Foundation. Eloseily reported no disclosures. Cron reported serving as an adviser for AbbVie/Abbott and Sobi, receiving grant funding and speaking and consulting fees from Pfizer, and receiving royalties from Springer.
A version of this article appeared on Medscape.com.
WASHINGTON — Children who have systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) have distinct biomarker profiles that may hold potential in eventually detecting LD earlier and identifying personalized treatment, according to research presented at the American College of Rheumatology (ACR) 2024 Annual Meeting.
Established risk factors for LD, which affects up to 1 in every 20 patients with sJIA, include being of a younger age, having more macrophage activation syndrome (MAS) episodes, and having more adverse reactions to biologics, Esraa Eloseily, MD, MS, an assistant professor of pediatrics at UT Southwestern Children’s Medical Center, Dallas, told attendees.
“The pathophysiology remains unclear and debate centers around elevated IL-18 [interleukin 18] and T-cell activation in association with HLA-DRB1*15/DRESS [drug reaction with eosinophilia and systemic symptoms] reactions to biologics, and thus, we have urgent unmet needs to understand the prevalence, the pathogenesis, disease biomarkers, and influence of biologics,” Eloseily said.
Their study confirmed that patients with LD tended to be younger and have more MAS. The researchers also found lower hemoglobin and higher white blood cell counts and platelets in patients with sJIA-LD, as well as a higher medication burden, particularly with steroids, biologics, and Janus kinase (JAK) inhibitors.
Randy Cron, MD, PhD, director of the Division of Pediatric Rheumatology at the University of Alabama at Birmingham, attended the presentation and noted that every additional piece of information is helpful in filling out the picture of what we know and can predict about sJIA-LD development.
“We’re all learning as we go, so the more people that study this, the better,” Cron told Medscape Medical News. “Even if it’s just seeing things that other groups have seen or really solidifying the risk factors for the development of lung disease, I think, at this point, that’s one of the most clinically relevant things: Do we screen? Who do we screen? Certainly, kids who have very young age of onset, children who develop macrophage activation syndrome, children who have high IL-18 levels.”
Study Results
The study compared 37 patients with sJIA-LD from 16 Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry sites with 141 patients with sJIA but without LD who had similar follow-up durations in the CARRA Registry.
Disease duration for patients with sJIA-LD was defined as the time from their initial sJIA diagnosis to their baseline sJIA-LD cohort visit, which was considered their index visit. In patients without LD, duration was from their enrollment in the CARRA Registry to their last CARRA Registry visit, which was considered their index visit.
The patients with sJIA-LD were significantly younger — a median age of 1 year — at onset of sJIA than those without LD, who had a median age of 5 years (P < .0019). The patients with sJIA-LD were also younger (median age, 7 years) at their index visit than those without LD (median age, 10 years) (P < .0001).
There were also significant differences in medication usage between those with and without LD. While 40.5% of patients with sJIA-LD were using steroids at their index visit, only 11.4% of those without LD were using steroids (P < .0001). Yet the mean dose of steroids was significantly lower in those with LD (5.45 mg/d) than in those without (20.7 mg/d). In addition, nearly half the patients with sJIA-LD had ever used cyclosporin A (45.7%) compared with 2.8% of those without LD (P < .0001), and 17.1% of patients with sJIA-LD had used mycophenolate mofetil compared with 0.7% of those without LD (P = .0002).
Similar disparities were seen for usage of biologics and JAK inhibitors: Anakinra (82.9% vs 56.7%; P = .0036), abatacept (8.6% vs 1.4%; P = .053), tofacitinib (57.1% vs 5.7%; P < .0001), ruxolitinib (25.7% vs 0%; P < .0001), baricitinib (8.6% vs 0%; P = .007), and emapalumab (23% vs 0.7%; P < .0001). Further, 5.7% of those with sJIA-LD had taken etoposide and 22.9% had received intravenous immunoglobulin compared with 0% and 4.3%, respectively, in those without LD (P = .04 and P = .001).
Laboratory parameters of patients with sJIA-LD were also significantly different from those of patients without LD, including a higher white blood cell count (8.8 × 109/L vs 8.1 × 109/L; P = .01), higher platelets (316.5 × 109/L vs 311.2 × 109/L; P = .03), and lower hemoglobin (11.5 g/dL vs 12.6 g/dL; P = .007). Ferritin levels trended nonsignificantly higher in patients with sJIA-LD (506 ng/mL vs 173.2 ng/mL; P = .09), and aspartate aminotransferase levels were significantly higher (37 U/L vs 28.72 U/L; P < .0001).
Patients’ overall well-being was “unexpectedly” higher in patients with sJIA-LD (P = .007), Eloseily noted, including the parent/patient rating (P = .027). However, more of the patients without LD had an excellent (61%) or very good (20.4%) health-related quality of life compared with those with LD (13% and 39%), and nearly one third of patients with sJIA-LD (30.4%) had only fair health-related quality of life compared with 5.5% without LD (P = .0002).
In line with known risk factors, most of the patients with sJIA-LD had a prior MAS episode (67.6%) compared with 10.6% of those without LD (P < .0001). Mortality was also higher in those with LD, two of whom died, whereas none without LD died (P = .04).
While existing biomarkers have been reported, they lack independent validation, Eloseily told attendees. Among the known key biomarkers are IL-18/interferon (IFN)-gamma axis, which are known to drive MAS and pulmonary inflammation, especially in those with MAS and LD; ICAM-5 and MMP-7, which is linked to fibrosis and tissue remodeling; and CCL11, CCL17, and GDF-15, which is linked to fibrosis and inflammation.
Because the CARRA Registry has limited availability of biosamples for most patients, the researchers used plasma samples from the FROST study for 27 patients with sJIA-LD and 46 patients without LD. When they compared 23 biomarkers between the groups, most of the known key biomarkers, as well as several other biomarkers, were significantly elevated in those with LD compared with in those without:
- MMP-7 (P = .001)
- IFN gamma (P = .008)
- CCL11 (P < .0001)
- CCL17 (P = .002)
- CCL15 (P < .0001)
- MCP-1 (P = .0003)
- MCP-3 (P = .02)
- CCL25 (P < .0001)
- CD25 (P < .0001)
- GDF-15 (P < .0001)
- TRAIL (P < .0001)
- IL-23 (P = .002)
They found that IL-18 only trended higher (P = .07), and there were not adequate data for ICAM-5.
The study was limited by the differences in disease duration between the compared groups and the limited availability of biosamples, which they only had from patients enrolled in the FROST study.
The research was funded by CARRA and the Arthritis Foundation. Eloseily reported no disclosures. Cron reported serving as an adviser for AbbVie/Abbott and Sobi, receiving grant funding and speaking and consulting fees from Pfizer, and receiving royalties from Springer.
A version of this article appeared on Medscape.com.
WASHINGTON — Children who have systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) have distinct biomarker profiles that may hold potential in eventually detecting LD earlier and identifying personalized treatment, according to research presented at the American College of Rheumatology (ACR) 2024 Annual Meeting.
Established risk factors for LD, which affects up to 1 in every 20 patients with sJIA, include being of a younger age, having more macrophage activation syndrome (MAS) episodes, and having more adverse reactions to biologics, Esraa Eloseily, MD, MS, an assistant professor of pediatrics at UT Southwestern Children’s Medical Center, Dallas, told attendees.
“The pathophysiology remains unclear and debate centers around elevated IL-18 [interleukin 18] and T-cell activation in association with HLA-DRB1*15/DRESS [drug reaction with eosinophilia and systemic symptoms] reactions to biologics, and thus, we have urgent unmet needs to understand the prevalence, the pathogenesis, disease biomarkers, and influence of biologics,” Eloseily said.
Their study confirmed that patients with LD tended to be younger and have more MAS. The researchers also found lower hemoglobin and higher white blood cell counts and platelets in patients with sJIA-LD, as well as a higher medication burden, particularly with steroids, biologics, and Janus kinase (JAK) inhibitors.
Randy Cron, MD, PhD, director of the Division of Pediatric Rheumatology at the University of Alabama at Birmingham, attended the presentation and noted that every additional piece of information is helpful in filling out the picture of what we know and can predict about sJIA-LD development.
“We’re all learning as we go, so the more people that study this, the better,” Cron told Medscape Medical News. “Even if it’s just seeing things that other groups have seen or really solidifying the risk factors for the development of lung disease, I think, at this point, that’s one of the most clinically relevant things: Do we screen? Who do we screen? Certainly, kids who have very young age of onset, children who develop macrophage activation syndrome, children who have high IL-18 levels.”
Study Results
The study compared 37 patients with sJIA-LD from 16 Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry sites with 141 patients with sJIA but without LD who had similar follow-up durations in the CARRA Registry.
Disease duration for patients with sJIA-LD was defined as the time from their initial sJIA diagnosis to their baseline sJIA-LD cohort visit, which was considered their index visit. In patients without LD, duration was from their enrollment in the CARRA Registry to their last CARRA Registry visit, which was considered their index visit.
The patients with sJIA-LD were significantly younger — a median age of 1 year — at onset of sJIA than those without LD, who had a median age of 5 years (P < .0019). The patients with sJIA-LD were also younger (median age, 7 years) at their index visit than those without LD (median age, 10 years) (P < .0001).
There were also significant differences in medication usage between those with and without LD. While 40.5% of patients with sJIA-LD were using steroids at their index visit, only 11.4% of those without LD were using steroids (P < .0001). Yet the mean dose of steroids was significantly lower in those with LD (5.45 mg/d) than in those without (20.7 mg/d). In addition, nearly half the patients with sJIA-LD had ever used cyclosporin A (45.7%) compared with 2.8% of those without LD (P < .0001), and 17.1% of patients with sJIA-LD had used mycophenolate mofetil compared with 0.7% of those without LD (P = .0002).
Similar disparities were seen for usage of biologics and JAK inhibitors: Anakinra (82.9% vs 56.7%; P = .0036), abatacept (8.6% vs 1.4%; P = .053), tofacitinib (57.1% vs 5.7%; P < .0001), ruxolitinib (25.7% vs 0%; P < .0001), baricitinib (8.6% vs 0%; P = .007), and emapalumab (23% vs 0.7%; P < .0001). Further, 5.7% of those with sJIA-LD had taken etoposide and 22.9% had received intravenous immunoglobulin compared with 0% and 4.3%, respectively, in those without LD (P = .04 and P = .001).
Laboratory parameters of patients with sJIA-LD were also significantly different from those of patients without LD, including a higher white blood cell count (8.8 × 109/L vs 8.1 × 109/L; P = .01), higher platelets (316.5 × 109/L vs 311.2 × 109/L; P = .03), and lower hemoglobin (11.5 g/dL vs 12.6 g/dL; P = .007). Ferritin levels trended nonsignificantly higher in patients with sJIA-LD (506 ng/mL vs 173.2 ng/mL; P = .09), and aspartate aminotransferase levels were significantly higher (37 U/L vs 28.72 U/L; P < .0001).
Patients’ overall well-being was “unexpectedly” higher in patients with sJIA-LD (P = .007), Eloseily noted, including the parent/patient rating (P = .027). However, more of the patients without LD had an excellent (61%) or very good (20.4%) health-related quality of life compared with those with LD (13% and 39%), and nearly one third of patients with sJIA-LD (30.4%) had only fair health-related quality of life compared with 5.5% without LD (P = .0002).
In line with known risk factors, most of the patients with sJIA-LD had a prior MAS episode (67.6%) compared with 10.6% of those without LD (P < .0001). Mortality was also higher in those with LD, two of whom died, whereas none without LD died (P = .04).
While existing biomarkers have been reported, they lack independent validation, Eloseily told attendees. Among the known key biomarkers are IL-18/interferon (IFN)-gamma axis, which are known to drive MAS and pulmonary inflammation, especially in those with MAS and LD; ICAM-5 and MMP-7, which is linked to fibrosis and tissue remodeling; and CCL11, CCL17, and GDF-15, which is linked to fibrosis and inflammation.
Because the CARRA Registry has limited availability of biosamples for most patients, the researchers used plasma samples from the FROST study for 27 patients with sJIA-LD and 46 patients without LD. When they compared 23 biomarkers between the groups, most of the known key biomarkers, as well as several other biomarkers, were significantly elevated in those with LD compared with in those without:
- MMP-7 (P = .001)
- IFN gamma (P = .008)
- CCL11 (P < .0001)
- CCL17 (P = .002)
- CCL15 (P < .0001)
- MCP-1 (P = .0003)
- MCP-3 (P = .02)
- CCL25 (P < .0001)
- CD25 (P < .0001)
- GDF-15 (P < .0001)
- TRAIL (P < .0001)
- IL-23 (P = .002)
They found that IL-18 only trended higher (P = .07), and there were not adequate data for ICAM-5.
The study was limited by the differences in disease duration between the compared groups and the limited availability of biosamples, which they only had from patients enrolled in the FROST study.
The research was funded by CARRA and the Arthritis Foundation. Eloseily reported no disclosures. Cron reported serving as an adviser for AbbVie/Abbott and Sobi, receiving grant funding and speaking and consulting fees from Pfizer, and receiving royalties from Springer.
A version of this article appeared on Medscape.com.
FROM ACR 2024
New Approaches to Research Beyond Massive Clinical Trials
This transcript has been edited for clarity.
I want to briefly present a fascinating effort, one that needs to be applauded and applauded again, and then we need to scratch our collective heads and ask, why did we do it and what did we learn?
I’m referring to a report recently published in Annals of Internal Medicine, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women: Postintervention Follow-up of a Randomized Clinical Trial.” The title of this report does not do it justice. This was a massive effort — one could, I believe, even use the term Herculean — to ask an important question that was asked more than 20 years ago.
This was a national women’s health initiative to answer these questions. The study looked at 36,282 postmenopausal women who, at the time of agreeing to be randomized in this trial, had no history of breast or colorectal cancer. This was a 7-year randomized intervention effort, and 40 centers across the United States participated, obviously funded by the government. Randomization was one-to-one to placebo or 1000 mg calcium and 400 international units of vitamin D3 daily.
They looked at the incidence of colorectal cancer, breast cancer, and total cancer, and importantly as an endpoint, total cardiovascular disease and hip fractures. They didn’t comment on hip fractures in this particular analysis. Obviously, hip fractures relate to this question of osteoporosis in postmenopausal women.
Here’s the bottom line: With a median follow-up now of 22.3 years — that’s not 2 years, but 22.3 years — there was a 7% decrease in cancer mortality in the population that received the calcium and vitamin D3. This is nothing to snicker at, and nothing at which to say, “Wow. That’s not important.”
However, in this analysis involving several tens of thousands of women, there was a 6% increase in cardiovascular disease mortality noted and reported. Overall, there was no effect on all-cause mortality of this intervention, with a hazard ratio — you rarely see this — of 1.00.
There is much that can be said, but I will summarize my comments very briefly. Criticize this if you want. It’s not inappropriate to criticize, but what was the individual impact of the calcium vs vitamin D? If they had only used one vs the other, or used both but in separate arms of the trial, and you could have separated what might have caused the decrease in cancer mortality and not the increased cardiovascular disease… This was designed more than 20 years ago. That’s one point.
The second is, how many more tens of thousands of patients would they have had to add to do this, and at what cost? This was a massive study, a national study, and a simple study in terms of the intervention. It was low risk except if you look at the long-term outcome. You can only imagine how much it would cost to do that study today — not the cost of the calcium, the vitamin D3, but the cost of doing the trial that was concluded to have no impact.
From a societal perspective, this was an important question to answer, certainly then. What did we learn and at what cost? The bottom line is that we have to figure out a way of answering these kinds of questions.
Perhaps now they should be from real-world data, looking at electronic medical records or at a variety of other population-based data so that we can get the answer — not in 20 years but in perhaps 2 months, because we’ve looked at the data using artificial intelligence to help us to answer these questions; and maybe not 36,000 patients but 360,000 individuals looked at over this period of time.
Again, I’m proposing an alternative solution because the questions that were asked 20 years ago remain important today. This cannot be the way that we, in the future, try to answer them, certainly from the perspective of cost and also the perspective of time to get the answers.
Let me conclude by, again, applauding these researchers because of the quality of the work they started out doing and ended up doing and reporting. Also, I think we’ve learned that we have to come up with alternative ways to answer what were important questions then and are important questions today.
Dr. Markman, Professor of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center; President, Medicine & Science, City of Hope Atlanta, Chicago, Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I want to briefly present a fascinating effort, one that needs to be applauded and applauded again, and then we need to scratch our collective heads and ask, why did we do it and what did we learn?
I’m referring to a report recently published in Annals of Internal Medicine, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women: Postintervention Follow-up of a Randomized Clinical Trial.” The title of this report does not do it justice. This was a massive effort — one could, I believe, even use the term Herculean — to ask an important question that was asked more than 20 years ago.
This was a national women’s health initiative to answer these questions. The study looked at 36,282 postmenopausal women who, at the time of agreeing to be randomized in this trial, had no history of breast or colorectal cancer. This was a 7-year randomized intervention effort, and 40 centers across the United States participated, obviously funded by the government. Randomization was one-to-one to placebo or 1000 mg calcium and 400 international units of vitamin D3 daily.
They looked at the incidence of colorectal cancer, breast cancer, and total cancer, and importantly as an endpoint, total cardiovascular disease and hip fractures. They didn’t comment on hip fractures in this particular analysis. Obviously, hip fractures relate to this question of osteoporosis in postmenopausal women.
Here’s the bottom line: With a median follow-up now of 22.3 years — that’s not 2 years, but 22.3 years — there was a 7% decrease in cancer mortality in the population that received the calcium and vitamin D3. This is nothing to snicker at, and nothing at which to say, “Wow. That’s not important.”
However, in this analysis involving several tens of thousands of women, there was a 6% increase in cardiovascular disease mortality noted and reported. Overall, there was no effect on all-cause mortality of this intervention, with a hazard ratio — you rarely see this — of 1.00.
There is much that can be said, but I will summarize my comments very briefly. Criticize this if you want. It’s not inappropriate to criticize, but what was the individual impact of the calcium vs vitamin D? If they had only used one vs the other, or used both but in separate arms of the trial, and you could have separated what might have caused the decrease in cancer mortality and not the increased cardiovascular disease… This was designed more than 20 years ago. That’s one point.
The second is, how many more tens of thousands of patients would they have had to add to do this, and at what cost? This was a massive study, a national study, and a simple study in terms of the intervention. It was low risk except if you look at the long-term outcome. You can only imagine how much it would cost to do that study today — not the cost of the calcium, the vitamin D3, but the cost of doing the trial that was concluded to have no impact.
From a societal perspective, this was an important question to answer, certainly then. What did we learn and at what cost? The bottom line is that we have to figure out a way of answering these kinds of questions.
Perhaps now they should be from real-world data, looking at electronic medical records or at a variety of other population-based data so that we can get the answer — not in 20 years but in perhaps 2 months, because we’ve looked at the data using artificial intelligence to help us to answer these questions; and maybe not 36,000 patients but 360,000 individuals looked at over this period of time.
Again, I’m proposing an alternative solution because the questions that were asked 20 years ago remain important today. This cannot be the way that we, in the future, try to answer them, certainly from the perspective of cost and also the perspective of time to get the answers.
Let me conclude by, again, applauding these researchers because of the quality of the work they started out doing and ended up doing and reporting. Also, I think we’ve learned that we have to come up with alternative ways to answer what were important questions then and are important questions today.
Dr. Markman, Professor of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center; President, Medicine & Science, City of Hope Atlanta, Chicago, Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I want to briefly present a fascinating effort, one that needs to be applauded and applauded again, and then we need to scratch our collective heads and ask, why did we do it and what did we learn?
I’m referring to a report recently published in Annals of Internal Medicine, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women: Postintervention Follow-up of a Randomized Clinical Trial.” The title of this report does not do it justice. This was a massive effort — one could, I believe, even use the term Herculean — to ask an important question that was asked more than 20 years ago.
This was a national women’s health initiative to answer these questions. The study looked at 36,282 postmenopausal women who, at the time of agreeing to be randomized in this trial, had no history of breast or colorectal cancer. This was a 7-year randomized intervention effort, and 40 centers across the United States participated, obviously funded by the government. Randomization was one-to-one to placebo or 1000 mg calcium and 400 international units of vitamin D3 daily.
They looked at the incidence of colorectal cancer, breast cancer, and total cancer, and importantly as an endpoint, total cardiovascular disease and hip fractures. They didn’t comment on hip fractures in this particular analysis. Obviously, hip fractures relate to this question of osteoporosis in postmenopausal women.
Here’s the bottom line: With a median follow-up now of 22.3 years — that’s not 2 years, but 22.3 years — there was a 7% decrease in cancer mortality in the population that received the calcium and vitamin D3. This is nothing to snicker at, and nothing at which to say, “Wow. That’s not important.”
However, in this analysis involving several tens of thousands of women, there was a 6% increase in cardiovascular disease mortality noted and reported. Overall, there was no effect on all-cause mortality of this intervention, with a hazard ratio — you rarely see this — of 1.00.
There is much that can be said, but I will summarize my comments very briefly. Criticize this if you want. It’s not inappropriate to criticize, but what was the individual impact of the calcium vs vitamin D? If they had only used one vs the other, or used both but in separate arms of the trial, and you could have separated what might have caused the decrease in cancer mortality and not the increased cardiovascular disease… This was designed more than 20 years ago. That’s one point.
The second is, how many more tens of thousands of patients would they have had to add to do this, and at what cost? This was a massive study, a national study, and a simple study in terms of the intervention. It was low risk except if you look at the long-term outcome. You can only imagine how much it would cost to do that study today — not the cost of the calcium, the vitamin D3, but the cost of doing the trial that was concluded to have no impact.
From a societal perspective, this was an important question to answer, certainly then. What did we learn and at what cost? The bottom line is that we have to figure out a way of answering these kinds of questions.
Perhaps now they should be from real-world data, looking at electronic medical records or at a variety of other population-based data so that we can get the answer — not in 20 years but in perhaps 2 months, because we’ve looked at the data using artificial intelligence to help us to answer these questions; and maybe not 36,000 patients but 360,000 individuals looked at over this period of time.
Again, I’m proposing an alternative solution because the questions that were asked 20 years ago remain important today. This cannot be the way that we, in the future, try to answer them, certainly from the perspective of cost and also the perspective of time to get the answers.
Let me conclude by, again, applauding these researchers because of the quality of the work they started out doing and ended up doing and reporting. Also, I think we’ve learned that we have to come up with alternative ways to answer what were important questions then and are important questions today.
Dr. Markman, Professor of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center; President, Medicine & Science, City of Hope Atlanta, Chicago, Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
Spinal Cord Stimulation Promising for Chronic Back, Leg Pain
TOPLINE:
Spinal cord stimulation (SCS) therapies for chronic back and/or leg pain is superior to conventional medical management (CMM) for reduced pain intensity and functional disability, new research suggests.
METHODOLOGY:
- Researchers performed a systematic review and network meta-analysis of 13 randomized clinical trials that compared conventional and novel SCS therapies with CMM.
- More than 1500 adults with chronic back and/or leg pain and no past history of receiving SCS therapies were included.
- Novel therapies included high frequency, burst, differential target multiplexed, and closed-loop SCS; conventional therapies included tonic SCS wave forms.
- Study outcomes included pain intensity in the back and in the leg, proportion of patients achieving at least 50% pain reduction in the back and in the leg, quality of life as measured by the EuroQol-5 Dimensions (EQ-5D) index, and functional disability on the Oswestry Disability Index.
- The analysis included data from multiple follow-up points at 3, 6, 12, and 24 months, with 6-month data being those from the longest mutually reported timepoint across all outcomes.
TAKEAWAY:
- Both conventional and novel SCS therapies demonstrated superior efficacy vs CMM in pain reduction, but the novel SCS therapies were more likely to provide ≥ 50% reduction in back pain (odds ratio, 8.76; 95% credible interval [CrI], 3.84-22.31).
- Both SCS therapies showed a significant reduction in pain intensity, with novel SCS providing the greatest mean difference (MD) for back pain (–2.34; 95% CrI, –2.96 to –1.73) and lower leg pain (MD, –4.01; 95% CrI, –5.31 to –2.75).
- Quality of life improved with both types of SCS therapies, with novel SCS therapies yielding the highest MD (0.17; 95% CrI, 0.13-0.21) in EQ-5D index score.
- Conventional SCS showed greater improvement in functionality vs CMM, yielding the lowest MD (–7.10; 95% CrI, –10.91 to –3.36) in Oswestry Disability Index score.
IN PRACTICE:
“We found that SCS was associated with improved pain and QOL [quality of life] and reduced disability, compared with CMM, after 6 months of follow-up. These findings highlight the potential of SCS therapies as an effective and valuable option in chronic pain management,” the investigators wrote.
SOURCE:
The study was led by Frank J.P.M. Huygen, PhD, MD, Erasmus Medical Center, Rotterdam, the Netherlands. It was published online in JAMA Network Open.
LIMITATIONS:
The lack of randomized clinical trials with long-term follow-up data restricted the inclusion of extended outcome assessments. Most included studies showed a high risk for bias. Safety estimates could not be evaluated as adverse events were only reported as procedure-related outcomes, which are not applicable for CMM. Additionally, the network meta-analytical approach, which combined evidence from studies with varying patient eligibility criteria, may have introduced bias because of between-study heterogeneity.
DISCLOSURES:
This study was funded by Medtronic. Huygen reported receiving personal fees from Abbott, Saluda, and Grunenthal outside the submitted work. The four other authors reported receiving funding from Medtronic.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Spinal cord stimulation (SCS) therapies for chronic back and/or leg pain is superior to conventional medical management (CMM) for reduced pain intensity and functional disability, new research suggests.
METHODOLOGY:
- Researchers performed a systematic review and network meta-analysis of 13 randomized clinical trials that compared conventional and novel SCS therapies with CMM.
- More than 1500 adults with chronic back and/or leg pain and no past history of receiving SCS therapies were included.
- Novel therapies included high frequency, burst, differential target multiplexed, and closed-loop SCS; conventional therapies included tonic SCS wave forms.
- Study outcomes included pain intensity in the back and in the leg, proportion of patients achieving at least 50% pain reduction in the back and in the leg, quality of life as measured by the EuroQol-5 Dimensions (EQ-5D) index, and functional disability on the Oswestry Disability Index.
- The analysis included data from multiple follow-up points at 3, 6, 12, and 24 months, with 6-month data being those from the longest mutually reported timepoint across all outcomes.
TAKEAWAY:
- Both conventional and novel SCS therapies demonstrated superior efficacy vs CMM in pain reduction, but the novel SCS therapies were more likely to provide ≥ 50% reduction in back pain (odds ratio, 8.76; 95% credible interval [CrI], 3.84-22.31).
- Both SCS therapies showed a significant reduction in pain intensity, with novel SCS providing the greatest mean difference (MD) for back pain (–2.34; 95% CrI, –2.96 to –1.73) and lower leg pain (MD, –4.01; 95% CrI, –5.31 to –2.75).
- Quality of life improved with both types of SCS therapies, with novel SCS therapies yielding the highest MD (0.17; 95% CrI, 0.13-0.21) in EQ-5D index score.
- Conventional SCS showed greater improvement in functionality vs CMM, yielding the lowest MD (–7.10; 95% CrI, –10.91 to –3.36) in Oswestry Disability Index score.
IN PRACTICE:
“We found that SCS was associated with improved pain and QOL [quality of life] and reduced disability, compared with CMM, after 6 months of follow-up. These findings highlight the potential of SCS therapies as an effective and valuable option in chronic pain management,” the investigators wrote.
SOURCE:
The study was led by Frank J.P.M. Huygen, PhD, MD, Erasmus Medical Center, Rotterdam, the Netherlands. It was published online in JAMA Network Open.
LIMITATIONS:
The lack of randomized clinical trials with long-term follow-up data restricted the inclusion of extended outcome assessments. Most included studies showed a high risk for bias. Safety estimates could not be evaluated as adverse events were only reported as procedure-related outcomes, which are not applicable for CMM. Additionally, the network meta-analytical approach, which combined evidence from studies with varying patient eligibility criteria, may have introduced bias because of between-study heterogeneity.
DISCLOSURES:
This study was funded by Medtronic. Huygen reported receiving personal fees from Abbott, Saluda, and Grunenthal outside the submitted work. The four other authors reported receiving funding from Medtronic.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Spinal cord stimulation (SCS) therapies for chronic back and/or leg pain is superior to conventional medical management (CMM) for reduced pain intensity and functional disability, new research suggests.
METHODOLOGY:
- Researchers performed a systematic review and network meta-analysis of 13 randomized clinical trials that compared conventional and novel SCS therapies with CMM.
- More than 1500 adults with chronic back and/or leg pain and no past history of receiving SCS therapies were included.
- Novel therapies included high frequency, burst, differential target multiplexed, and closed-loop SCS; conventional therapies included tonic SCS wave forms.
- Study outcomes included pain intensity in the back and in the leg, proportion of patients achieving at least 50% pain reduction in the back and in the leg, quality of life as measured by the EuroQol-5 Dimensions (EQ-5D) index, and functional disability on the Oswestry Disability Index.
- The analysis included data from multiple follow-up points at 3, 6, 12, and 24 months, with 6-month data being those from the longest mutually reported timepoint across all outcomes.
TAKEAWAY:
- Both conventional and novel SCS therapies demonstrated superior efficacy vs CMM in pain reduction, but the novel SCS therapies were more likely to provide ≥ 50% reduction in back pain (odds ratio, 8.76; 95% credible interval [CrI], 3.84-22.31).
- Both SCS therapies showed a significant reduction in pain intensity, with novel SCS providing the greatest mean difference (MD) for back pain (–2.34; 95% CrI, –2.96 to –1.73) and lower leg pain (MD, –4.01; 95% CrI, –5.31 to –2.75).
- Quality of life improved with both types of SCS therapies, with novel SCS therapies yielding the highest MD (0.17; 95% CrI, 0.13-0.21) in EQ-5D index score.
- Conventional SCS showed greater improvement in functionality vs CMM, yielding the lowest MD (–7.10; 95% CrI, –10.91 to –3.36) in Oswestry Disability Index score.
IN PRACTICE:
“We found that SCS was associated with improved pain and QOL [quality of life] and reduced disability, compared with CMM, after 6 months of follow-up. These findings highlight the potential of SCS therapies as an effective and valuable option in chronic pain management,” the investigators wrote.
SOURCE:
The study was led by Frank J.P.M. Huygen, PhD, MD, Erasmus Medical Center, Rotterdam, the Netherlands. It was published online in JAMA Network Open.
LIMITATIONS:
The lack of randomized clinical trials with long-term follow-up data restricted the inclusion of extended outcome assessments. Most included studies showed a high risk for bias. Safety estimates could not be evaluated as adverse events were only reported as procedure-related outcomes, which are not applicable for CMM. Additionally, the network meta-analytical approach, which combined evidence from studies with varying patient eligibility criteria, may have introduced bias because of between-study heterogeneity.
DISCLOSURES:
This study was funded by Medtronic. Huygen reported receiving personal fees from Abbott, Saluda, and Grunenthal outside the submitted work. The four other authors reported receiving funding from Medtronic.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Oxidative Stress Marker May Signal Fracture Risk in T2D
TOPLINE:
Elevated levels of plasma F2-isoprostanes, a reliable marker of oxidative stress, are associated with an increased risk for fractures in older ambulatory patients with type 2 diabetes (T2D) independently of bone density.
METHODOLOGY:
- Patients with T2D face an increased risk for fractures at any given bone mineral density; oxidative stress levels (reflected in circulating F2-isoprostanes), which are elevated in T2D, are associated with other T2D complications, and may weaken bone integrity.
- Researchers analyzed data from an observational cohort study to investigate the association between the levels of circulating F2-isoprostanes and the risk for clinical fractures in older patients with T2D.
- The data included 703 older ambulatory adults (baseline age, 70-79 years; about half White individuals and half Black individuals ; about half men and half women) from the Health, Aging and Body Composition Study, of whom 132 had T2D.
- Plasma F2-isoprostane levels were measured using baseline serum samples; bone turnover markers were also measured including procollagen type 1 N-terminal propeptide, osteocalcin, and C-terminal telopeptide of type 1 collagen.
- Incident clinical fractures were tracked over a follow-up period of up to 17.3 years, with fractures verified through radiology reports.
TAKEAWAY:
- Overall, 25.8% patients in the T2D group and 23.5% adults in the non-diabetes group reported an incident clinical fracture during a mean follow-up period of 6.2 and 8.0 years, respectively.
- In patients with T2D, the risk for incident clinical fracture increased by 93% for every standard deviation increase in the log F2-isoprostane serum levels (hazard ratio [HR], 1.93; 95% CI, 1.26-2.95; P = .002) independently of baseline bone density, medication use, and other risk factors, with no such association reported in individuals without T2D (HR, 0.98; 95% CI, 0.81-1.18; P = .79).
- In the T2D group, elevated plasma F2-isoprostane levels were also associated with a decrease in total hip bone mineral density over 4 years (r = −0.28; P = .008), but not in the non-diabetes group.
- No correlation was found between plasma F2-isoprostane levels and circulating advanced glycoxidation end-products, bone turnover markers, or A1c levels in either group.
IN PRACTICE:
“Oxidative stress in T2D may play an important role in the decline of bone quality and not just bone quantity,” the authors wrote.
SOURCE:
This study was led by Bowen Wang, PhD, Rensselaer Polytechnic Institute, Troy, New York. It was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
This study was conducted in a well-functioning elderly population with only White and Black participants, which may limit the generalizability of the findings to other age groups or less healthy populations. Additionally, the study did not assess prevalent vertebral fracture risk due to the small sample size.
DISCLOSURES:
This study was supported by the US National Institute on Aging and the Intramural Research Program of the US National Institutes of Health and the Dr and Ms Sands and Sands Family for Orthopaedic Research. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Elevated levels of plasma F2-isoprostanes, a reliable marker of oxidative stress, are associated with an increased risk for fractures in older ambulatory patients with type 2 diabetes (T2D) independently of bone density.
METHODOLOGY:
- Patients with T2D face an increased risk for fractures at any given bone mineral density; oxidative stress levels (reflected in circulating F2-isoprostanes), which are elevated in T2D, are associated with other T2D complications, and may weaken bone integrity.
- Researchers analyzed data from an observational cohort study to investigate the association between the levels of circulating F2-isoprostanes and the risk for clinical fractures in older patients with T2D.
- The data included 703 older ambulatory adults (baseline age, 70-79 years; about half White individuals and half Black individuals ; about half men and half women) from the Health, Aging and Body Composition Study, of whom 132 had T2D.
- Plasma F2-isoprostane levels were measured using baseline serum samples; bone turnover markers were also measured including procollagen type 1 N-terminal propeptide, osteocalcin, and C-terminal telopeptide of type 1 collagen.
- Incident clinical fractures were tracked over a follow-up period of up to 17.3 years, with fractures verified through radiology reports.
TAKEAWAY:
- Overall, 25.8% patients in the T2D group and 23.5% adults in the non-diabetes group reported an incident clinical fracture during a mean follow-up period of 6.2 and 8.0 years, respectively.
- In patients with T2D, the risk for incident clinical fracture increased by 93% for every standard deviation increase in the log F2-isoprostane serum levels (hazard ratio [HR], 1.93; 95% CI, 1.26-2.95; P = .002) independently of baseline bone density, medication use, and other risk factors, with no such association reported in individuals without T2D (HR, 0.98; 95% CI, 0.81-1.18; P = .79).
- In the T2D group, elevated plasma F2-isoprostane levels were also associated with a decrease in total hip bone mineral density over 4 years (r = −0.28; P = .008), but not in the non-diabetes group.
- No correlation was found between plasma F2-isoprostane levels and circulating advanced glycoxidation end-products, bone turnover markers, or A1c levels in either group.
IN PRACTICE:
“Oxidative stress in T2D may play an important role in the decline of bone quality and not just bone quantity,” the authors wrote.
SOURCE:
This study was led by Bowen Wang, PhD, Rensselaer Polytechnic Institute, Troy, New York. It was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
This study was conducted in a well-functioning elderly population with only White and Black participants, which may limit the generalizability of the findings to other age groups or less healthy populations. Additionally, the study did not assess prevalent vertebral fracture risk due to the small sample size.
DISCLOSURES:
This study was supported by the US National Institute on Aging and the Intramural Research Program of the US National Institutes of Health and the Dr and Ms Sands and Sands Family for Orthopaedic Research. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Elevated levels of plasma F2-isoprostanes, a reliable marker of oxidative stress, are associated with an increased risk for fractures in older ambulatory patients with type 2 diabetes (T2D) independently of bone density.
METHODOLOGY:
- Patients with T2D face an increased risk for fractures at any given bone mineral density; oxidative stress levels (reflected in circulating F2-isoprostanes), which are elevated in T2D, are associated with other T2D complications, and may weaken bone integrity.
- Researchers analyzed data from an observational cohort study to investigate the association between the levels of circulating F2-isoprostanes and the risk for clinical fractures in older patients with T2D.
- The data included 703 older ambulatory adults (baseline age, 70-79 years; about half White individuals and half Black individuals ; about half men and half women) from the Health, Aging and Body Composition Study, of whom 132 had T2D.
- Plasma F2-isoprostane levels were measured using baseline serum samples; bone turnover markers were also measured including procollagen type 1 N-terminal propeptide, osteocalcin, and C-terminal telopeptide of type 1 collagen.
- Incident clinical fractures were tracked over a follow-up period of up to 17.3 years, with fractures verified through radiology reports.
TAKEAWAY:
- Overall, 25.8% patients in the T2D group and 23.5% adults in the non-diabetes group reported an incident clinical fracture during a mean follow-up period of 6.2 and 8.0 years, respectively.
- In patients with T2D, the risk for incident clinical fracture increased by 93% for every standard deviation increase in the log F2-isoprostane serum levels (hazard ratio [HR], 1.93; 95% CI, 1.26-2.95; P = .002) independently of baseline bone density, medication use, and other risk factors, with no such association reported in individuals without T2D (HR, 0.98; 95% CI, 0.81-1.18; P = .79).
- In the T2D group, elevated plasma F2-isoprostane levels were also associated with a decrease in total hip bone mineral density over 4 years (r = −0.28; P = .008), but not in the non-diabetes group.
- No correlation was found between plasma F2-isoprostane levels and circulating advanced glycoxidation end-products, bone turnover markers, or A1c levels in either group.
IN PRACTICE:
“Oxidative stress in T2D may play an important role in the decline of bone quality and not just bone quantity,” the authors wrote.
SOURCE:
This study was led by Bowen Wang, PhD, Rensselaer Polytechnic Institute, Troy, New York. It was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
This study was conducted in a well-functioning elderly population with only White and Black participants, which may limit the generalizability of the findings to other age groups or less healthy populations. Additionally, the study did not assess prevalent vertebral fracture risk due to the small sample size.
DISCLOSURES:
This study was supported by the US National Institute on Aging and the Intramural Research Program of the US National Institutes of Health and the Dr and Ms Sands and Sands Family for Orthopaedic Research. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.