User login
SEATTLE – Pre-exposure prophylaxis therapy combined with antiretroviral therapy (ART) appears to be safe in pregnant women, according to an open-label study of high-risk women in Kenya and Uganda who were part of HIV-serodiscordant couples.
The safety profile of the drugs has not been well studied in pregnant women because, in the registration trials of Truvada (emtricitabine and tenofovir disoproxil fumarate; Gilead), women were instructed to stop taking the drugs when they became pregnant. Current guidelines offer counseling and the choice to continue PrEP after a woman becomes pregnant.
“We’ve been trying to gather as much data as we can. This is a small study, but I believe it’s the first study of women who used PrEP throughout their pregnancy,” said Dr Heffron.
The researchers analyzed data among women participating in a PrEP/ART study. Those who became pregnant during the study were counseled and offered the choice to continue PrEP, and the researchers tracked pregnancy and development outcomes in offspring out to 1 year.
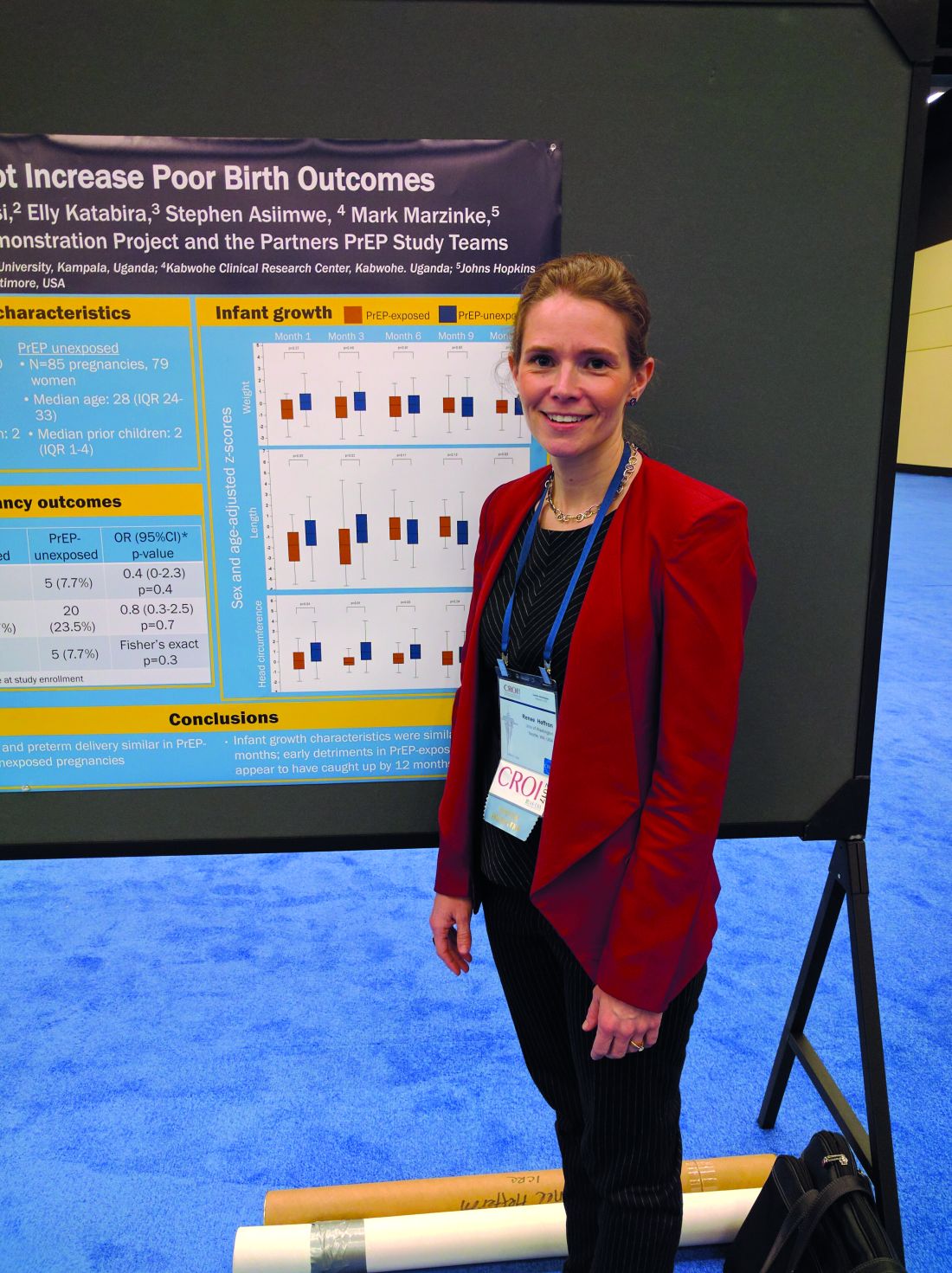
The researchers studied 34 women who became pregnant during the Partners Demonstration Project, which evaluated HIV-prevention preference and adherence among more than 1,000 HIV-serodiscordant couples; 30 of the women (88%) opted to continue PrEP. The researchers compared their outcomes (30 women, 30 pregnancies) to the outcomes of the placebo arm of the Partners PrEP Study (79 women unexposed to PrEP, 88 pregnancies).
The researchers measured medication adherence by recording pill bottle openings via medication event monitoring system caps, which use microcircuits to record the date and time when a bottle is opened. The women opened a pill bottle on a median of 71% of days. A total of 74% of plasma samples showed detectable levels of tenofovir, and 35% had concentrations higher than 40 ng/mL.
The rate of pregnancy loss was similar between the two groups at 16.7% PrEP-exposed patients versus 23.5% PrEP-unexposed patients (adjusted odds ratio, 0.8; P = .7). The frequency of preterm delivery also was similar at 0% PrEP-exposed patients versus 7.7% PrEP unexposed patients (aOR, 0.4; P = .4). There were no congenital anomalies seen among PrEP-exposed babies.
The researchers also looked at growth outcomes out to 1 year, including standardized measures of head circumferences, height, and weight. In early measurements, PrEP-exposed babies were slightly smaller on average than were unexposed babies, but by 12 months, the two groups were indistinguishable. Dr. Heffron suspects the unexposed population may have been slightly larger than average.
The study was funded by the Bill & Melinda Gates Foundation, the National Institute of Mental Health, and the United States Agency for International Development. Dr Heffron reported having no financial disclosures.
SEATTLE – Pre-exposure prophylaxis therapy combined with antiretroviral therapy (ART) appears to be safe in pregnant women, according to an open-label study of high-risk women in Kenya and Uganda who were part of HIV-serodiscordant couples.
The safety profile of the drugs has not been well studied in pregnant women because, in the registration trials of Truvada (emtricitabine and tenofovir disoproxil fumarate; Gilead), women were instructed to stop taking the drugs when they became pregnant. Current guidelines offer counseling and the choice to continue PrEP after a woman becomes pregnant.
“We’ve been trying to gather as much data as we can. This is a small study, but I believe it’s the first study of women who used PrEP throughout their pregnancy,” said Dr Heffron.
The researchers analyzed data among women participating in a PrEP/ART study. Those who became pregnant during the study were counseled and offered the choice to continue PrEP, and the researchers tracked pregnancy and development outcomes in offspring out to 1 year.
The researchers studied 34 women who became pregnant during the Partners Demonstration Project, which evaluated HIV-prevention preference and adherence among more than 1,000 HIV-serodiscordant couples; 30 of the women (88%) opted to continue PrEP. The researchers compared their outcomes (30 women, 30 pregnancies) to the outcomes of the placebo arm of the Partners PrEP Study (79 women unexposed to PrEP, 88 pregnancies).
The researchers measured medication adherence by recording pill bottle openings via medication event monitoring system caps, which use microcircuits to record the date and time when a bottle is opened. The women opened a pill bottle on a median of 71% of days. A total of 74% of plasma samples showed detectable levels of tenofovir, and 35% had concentrations higher than 40 ng/mL.
The rate of pregnancy loss was similar between the two groups at 16.7% PrEP-exposed patients versus 23.5% PrEP-unexposed patients (adjusted odds ratio, 0.8; P = .7). The frequency of preterm delivery also was similar at 0% PrEP-exposed patients versus 7.7% PrEP unexposed patients (aOR, 0.4; P = .4). There were no congenital anomalies seen among PrEP-exposed babies.
The researchers also looked at growth outcomes out to 1 year, including standardized measures of head circumferences, height, and weight. In early measurements, PrEP-exposed babies were slightly smaller on average than were unexposed babies, but by 12 months, the two groups were indistinguishable. Dr. Heffron suspects the unexposed population may have been slightly larger than average.
The study was funded by the Bill & Melinda Gates Foundation, the National Institute of Mental Health, and the United States Agency for International Development. Dr Heffron reported having no financial disclosures.
SEATTLE – Pre-exposure prophylaxis therapy combined with antiretroviral therapy (ART) appears to be safe in pregnant women, according to an open-label study of high-risk women in Kenya and Uganda who were part of HIV-serodiscordant couples.
The safety profile of the drugs has not been well studied in pregnant women because, in the registration trials of Truvada (emtricitabine and tenofovir disoproxil fumarate; Gilead), women were instructed to stop taking the drugs when they became pregnant. Current guidelines offer counseling and the choice to continue PrEP after a woman becomes pregnant.
“We’ve been trying to gather as much data as we can. This is a small study, but I believe it’s the first study of women who used PrEP throughout their pregnancy,” said Dr Heffron.
The researchers analyzed data among women participating in a PrEP/ART study. Those who became pregnant during the study were counseled and offered the choice to continue PrEP, and the researchers tracked pregnancy and development outcomes in offspring out to 1 year.
The researchers studied 34 women who became pregnant during the Partners Demonstration Project, which evaluated HIV-prevention preference and adherence among more than 1,000 HIV-serodiscordant couples; 30 of the women (88%) opted to continue PrEP. The researchers compared their outcomes (30 women, 30 pregnancies) to the outcomes of the placebo arm of the Partners PrEP Study (79 women unexposed to PrEP, 88 pregnancies).
The researchers measured medication adherence by recording pill bottle openings via medication event monitoring system caps, which use microcircuits to record the date and time when a bottle is opened. The women opened a pill bottle on a median of 71% of days. A total of 74% of plasma samples showed detectable levels of tenofovir, and 35% had concentrations higher than 40 ng/mL.
The rate of pregnancy loss was similar between the two groups at 16.7% PrEP-exposed patients versus 23.5% PrEP-unexposed patients (adjusted odds ratio, 0.8; P = .7). The frequency of preterm delivery also was similar at 0% PrEP-exposed patients versus 7.7% PrEP unexposed patients (aOR, 0.4; P = .4). There were no congenital anomalies seen among PrEP-exposed babies.
The researchers also looked at growth outcomes out to 1 year, including standardized measures of head circumferences, height, and weight. In early measurements, PrEP-exposed babies were slightly smaller on average than were unexposed babies, but by 12 months, the two groups were indistinguishable. Dr. Heffron suspects the unexposed population may have been slightly larger than average.
The study was funded by the Bill & Melinda Gates Foundation, the National Institute of Mental Health, and the United States Agency for International Development. Dr Heffron reported having no financial disclosures.
Key clinical point: The study is the first to confirm safety of PrEP in pregnancy.
Major finding: In this study, 16.7% of PrEP-exposed women experienced pregnancy loss versus 23.5% of unexposed.
Data source: Open-label, case-controlled study of 30 PrEP-exposed women and 79 controls.
Disclosures: The study was funded by the Bill & Melinda Gates Foundation, the National Institute of Mental Health, and the United States Agency for International Development. Dr Heffron reported having no financial disclosures.