User login
The need for prophylaxis of venous thromboembolism (VTE) in hospitalized acutely ill medical patients is well established. Without prophylaxis, hospitalized medical patients develop VTE at a rate of 5% to 15%.1–3 Moreover, pulmonary embolism (PE) occurs more frequently in hospitalized medical patients than in nonmedical patients, and is a leading cause of sudden death in hospitalized medical patients.4,5 Without appropriate prophylaxis, 1 in 20 hospitalized medical patients may suffer a fatal PE.4
PROPHYLAXIS IN MEDICAL PATIENTS: UNDERUSED AND OFTEN INAPPROPRIATE
Despite these risks and the clear indications for VTE prophylaxis in hospitalized medical patients, prophylaxis of VTE is omitted more often in these patients than in hospitalized surgical patients.5 Even when prophylaxis is given, it is often used inappropriately in the medical population. So concludes a recent analysis of data from 196,104 patients with acute medical conditions who were discharged from 227 US hospitals from January 2002 to September 2005.6 Criteria for inclusion in the analysis were patient age of 40 years or older, a hospital stay of 6 days or greater, and an absence of contraindications to anticoagulation. Appropriate prophylaxis was defined in accordance with the Sixth American College of Chest Physicians (ACCP) Consensus Conference on Antithrombotic Therapy.7
The analysis revealed an overall VTE prophylaxis rate of 61.8%, but the rate of appropriate prophylaxis was only 33.9%, meaning that two-thirds of discharged patients did not receive prophylaxis in accordance with ACCP guidelines. When temporal trends were analyzed according to groups based on patients’ diagnosis at admission (acute myocardial infarction, severe lung disease, ischemic stroke, cancer, heart failure, or trauma), the rate of appropriate prophylaxis remained essentially flat from the beginning to the end of the study period for virtually all diagnosis groups.6
Similar findings have emerged from the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE), an ongoing international registry of acutely ill medical patients.8 Data from the first 15,156 patients, enrolled from July 2002 through September 2006, reveal that 50% of patients received pharmacologic and/or mechanical VTE prophylaxis in the hospital, and only 60% of patients who met established criteria for VTE prophylaxis actually received it.
Analysis of the US portion of the IMPROVE data shows that 54% of the US patient sample received some form of VTE prophylaxis; 22% of US patients received intermittent pneumatic compression, 21% received unfractionated heparin (UFH), 14% received low-molecular-weight heparin (LMWH), and 3% wore compression stockings.8 Thus, despite a paucity of data supporting a benefit of intermittent pneumatic compression in this population,9 it was the most frequently used form of prophylaxis in US patients.
CLINICAL TRIALS OF PHARMACOLOGIC PROPHYLAXIS IN MEDICAL PATIENTS
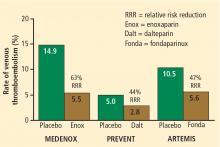
The Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) trial1 randomized 1,102 hospitalized patients to one of two doses of the LMWH enoxaparin (20 mg or 40 mg once daily subcutaneously) or placebo for 6 to 14 days. Compared with placebo, the 40-mg dose of enoxaparin was associated with a 63% reduction in risk of VTE over 3 months of follow-up (P < .001) (Figure 1).
The Prospective Evaluation of Dalteparin Efficacy for Prevention of VTE in Immobilized Patients Trial (PREVENT)2 was a multicenter, randomized, double-blind study comparing the LMWH dalteparin (5,000 IU daily given subcutaneously for 14 days) with placebo in 3,706 acutely ill medical patients. Over 90 days of follow-up, the risk of VTE was reduced by 44% in patients assigned to dalteparin compared with those assigned to placebo (P = .0015) (Figure 1).
The Arixtra for Thromboembolism Prevention in a Medical Indications Study (ARTEMIS)3 randomized 849 medical patients 60 years or older to 6 to 14 days of therapy with the selective factor Xa inhibitor fondaparinux (2.5 mg once daily subcutaneously) or placebo. Compared with the placebo group, fondaparinux recipients had a 47% lower risk of developing VTE by day 15 (P = .029) (Figure 1).
Fewer events and fatal PEs, but no effect on all-cause mortality
A recent meta-analysis by Dentali et al10 further demonstrates the efficacy of anticoagulant therapy for preventing symptomatic VTE in hospitalized medical patients. This analysis included several other trials in addition to the three reviewed above,1–3 for a total of nine randomized studies (seven of which were dou-ble-blind) comprising 19,958 patients. Across the nine studies, anticoagulant prophylaxis was clearly superior to placebo in preventing fatal PE (relative risk, 0.38 [95% CI, 0.21 to 0.69]). There was a strong trend toward a reduction in symptomatic deep vein thrombosis (DVT) with prophylaxis but no effect on all-cause mortality. The meta-analysis also provided reassurance that prophylaxis does not increase the rate of major bleeding.
HOW DO THE PROPHYLAXIS OPTIONS STACK UP?
What the ACCP recommends
Current ACCP guidelines recommend the use of either LMWH or low-dose UFH (5,000 U subcutaneously two or three times daily) as a Grade 1A recommendation for VTE prophylaxis in patients with medical conditions and risk factors for VTE.9 This represents the guidelines’ highest level of recommendation, ie, one that is based on randomized controlled trials (RCTs) without important limitations. In contrast, the 2006 International Consensus Statement, developed as a collaborative effort among expert bodies on VTE, specified a more narrow dosing recommendation for UFH in this patient population (5,000 U three times daily, not twice daily) as well as specifying 40 mg once daily as the recommended dose of enoxaparin and 5,000 IU once daily as the recommended dose of dalteparin.11
For medical patients with risk factors for VTE who have a contraindication to anticoagulant prophylaxis, the ACCP guidelines recommend the use of graduated compression stockings or intermittent pneumatic compression devices as a Grade 1C+ recommendation (“no RCTs but strong RCT results can be unequivocally extrapolated, or overwhelming evidence from observational studies”9).
Current ACCP guidelines do not address the use of fondaparinux in their recommendations for VTE prophylaxis in medical patients.
Getting a handle on bleeding risk
Patient characteristics that exclude pharmacologic thromboprophylaxis due to bleeding risk are generally limited to active bleeding or coagulopathy, as demonstrated by a platelet count less than 50,000 cells/µL or an international normalized ratio greater than 1.5. Additionally, bleeding risk should be carefully assessed if an invasive procedure is planned during a patient’s hospital stay.
It is worth noting that the anticoagulant doses used for VTE prophylaxis are a fraction of those used for treatment of VTE. Thus, if a patient would be treated with full-dose anticoagulation if VTE developed, then that patient should be eligible for VTE prophylaxis.
Because the use of mechanical forms of prophylaxis in medical patients is not truly evidence-based, mechanical prophylaxis should be reserved for medical patients who have a contraindication to anticoagulants, or for use in combination with anticoagulants in patients at very high risk of VTE.
UFH vs LMWH
Two meta-analyses have compared UFH with LMWH for VTE prevention in medical patients.12,13 In a recent analysis that included 10 trials directly comparing the two therapies, 14 trials comparing UFH with control, and 11 trials comparing LMWH with control, Wein et al found a lower risk of DVT with LMWH than with UFH (relative risk, 0.68 [95% CI, 0.52 to 0.88]) but no difference between the therapies in mortality or bleeding risk.12 In an earlier and smaller analysis, Mismetti et al found no significant differences between UFH and LMWH in preventing DVT or death but did find a significant reduction in major bleeding episodes with LMWH versus three-times-daily UFH (52% relative reduction; P = .049).13
Randomized trials also reveal that enoxaparin 40 mg once daily is as efficacious as UFH 5,000 U three times daily for VTE prevention in medical patients.14,15 The above analysis by Wein et al12 and an additional meta-analysis by King and colleagues16 found that three-times-daily dosing of UFH is more efficacious than twice-daily dosing of UFH, but at the expense of more bleeding, including major bleeding.
Economic considerations
Because of differences in drug acquisition costs between UFH and the LMWH agents, several economic evaluations have compared the use of these therapies for prophylaxis in medical patients at risk of VTE.
In an analysis of hospital costs for medical patients receiving VTE prophylaxis from more than 330 US hospitals for the period 2001–2004, Burleigh et al found that mean total hospital costs were higher for patients who received UFH than for those who received LMWH ($7,615 vs $6,866) even though mean drug costs were higher for LMWH ($791 vs $569 for UFH).17 A reduction in hospital length of stay appeared to contribute to the overall savings with LMWH; other contributors may have included costs associated with heparin-induced thrombocytopenia (HIT) in UFH recipients or the extra nursing time required for administering UFH in two or three daily doses.
Leykum et al used a decision analysis model to estimate the economic effect of substituting enoxaparin for UFH in hospitalized medical patients for whom VTE prophylaxis is indicated.18 Cost data were based on Medicare reimbursement rates as well as drug and laboratory costs for a multi-institutional health system. The model assumed HIT incidence rates of 2.7% with UFH and 0.3% with enoxaparin. It also assumed the cost of a daily dose to be $4 for UFH versus $84 for enoxaparin. From the payer perspective, the model showed that substituting enoxaparin for UFH would reduce the overall cost of care by $28.61 per day on a per-patient basis, despite enoxaparin’s higher acquisition cost, and would save $4,550 per quality-adjusted life-year by reducing the incidence of HIT.
Another cost analysis confirms the association between HIT and increased hospital costs. Creekmore et al retrospectively analyzed data from 10,121 adult medical patients who received VTE prophylaxis at the University of Utah Hospital in Salt Lake City from August 2000 to November 2004.19 They found that an admission during which HIT developed incurred a mean cost of $56,364, compared with $15,231 for an admission without HIT. Because LMWH was associated with a lower incidence of HIT compared with UFH (0.084% vs 0.51%, respectively), LMWH reduced the incremental cost of VTE prophylaxis by $13.88 per patient compared with UFH.
THE EXCLAIM TRIAL: IS THERE A ROLE FOR EXTENDED PROPHYLAXIS?
Although the previously discussed studies have clearly demonstrated the benefit of in-hospital VTE prophylaxis for acutely ill medical patients, none has rigorously examined extended-duration out-of-hospital prophylaxis in these patients. This represents an important gap in the literature, since a substantial proportion of VTE develops in the outpatient setting within 3 months of a hospitalization, and most outpatient VTE episodes occur within 1 month of a preceding hospitalization.20
To begin to fill this gap, the Extended Clinical Prophylaxis in Acutely Ill Medical Patients (EXCLAIM) trial was conducted to compare extended-duration LMWH prophylaxis with a standard LMWH prophylaxis regimen in acutely ill medical patients using a prospective, multicenter, randomized, double-blind, placebo-controlled design.21
Patients and study design
Patients were eligible for enrollment if they were aged 40 years or older and had recent immobilization (≤ 3 days), a predefined acute medical illness, and either level 1 mobility (total bed rest or sedentary state) or level 2 mobility (level 1 with bathroom privileges). The predefined acute medical illnesses consisted of New York Heart Association class III/IV heart failure, acute respiratory insufficiency, or other acute medical conditions, including post-acute ischemic stroke, acute infection without septic shock, and active cancer.
All patients received open-label enoxaparin 40 mg subcutaneously once daily for 10 ± 4 days, after which they were randomized to either enoxaparin 40 mg subcutaneously once daily or placebo for an additional 28 ± 4 days.
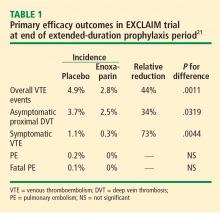
The primary efficacy end point was the incidence of VTE events, defined as asymptomatic DVT documented by mandatory ultrasonography at the end of the double-blind treatment period (28 ± 4 days) or as symptomatic DVT, symptomatic PE, or fatal PE at any time during the double-blind period. Symptomatic DVT was confirmed by objective tests; PE was confirmed by ventilation-perfusion scan, computed tomography, angiography, or autopsy.
Secondary efficacy end points were mortality at the end of the double-blind period, at 3 months, and at 6 months, as well as the incidence of VTE at 3 months.
The primary safety outcome measure was the incidence of major hemorrhage during the double-blind period; secondary safety measures were rates of major and minor hemorrhage, minor hemorrhage, HIT, and serious adverse events.
Population amended at planned interim analysis
After approximately half of the patients were enrolled, a planned and blinded interim analysis for futility concluded that the study was unlikely to show a statistically significant advantage of enoxaparin over placebo. The trial’s steering committee followed the suggestion of its data safety monitoring board to redefine the inclusion criteria to refocus enrollment on patients with a high risk of VTE. A blinded analysis was performed to identify this subgroup.
The resulting amended inclusion criteria were the same as above except that level 2 mobility had to be accompanied by at least one of three additional high-risk criteria: (1) age greater than 75 years, (2) history of prior VTE, or (3) diagnosis of cancer.
The trial’s main exclusion criteria were evidence of active bleeding, a contraindication to anticoagulation, receipt of prophylactic LMWH or UFH more than 72 hours prior to enrollment, treatment with an oral anticoagulant within 72 hours of enrollment, major surgery within the prior 3 months, cerebral stroke with bleeding, and persistent renal failure (creatinine clearance < 30 mL/min).


Results
The amended study population included 5,105 patients, 5,049 of whom received open-label enoxaparin. Of this group, 2,013 were randomized to active extended prophylaxis with enoxparain and 2,027 to placebo. Baseline characteristics, including level of mobility, were similar between the two groups.
The efficacy of extended prophylaxis with enoxaparin was enduring, as the cumulative incidence of VTE events at day 90 was significantly lower in enoxaparin recipients than in placebo recipients (3.0% vs 5.2%; relative reduction of 42%; P = .0115).
There was no difference in all-cause mortality at 6 months between the enoxaparin and placebo groups (10.1% vs 8.9%, respectively; P = .179).
Safety. Major hemorrhage was significantly more frequent in the enoxaparin arm, occurring in 0.60% of enoxaparin recipients compared with 0.15% of placebo recipients (P = .019). Minor bleeding was also more common with enoxaparin (5.20% vs 3.70%; P = .024).
Conclusions
The EXCLAIM trial found that an extended-duration (38-day) enoxaparin regimen significantly reduced the overall incidence of VTE relative to a 10-day enoxaparin regimen in acutely ill medical patients with reduced mobility. At the same time, the extended regimen was associated with a significant increase in the rate of major bleeding, although the incidence of major bleeding was low. The investigators concluded that the net clinical effect of extended-duration prophylaxis with enoxaparin is favorable, as only 46 patients would need to be treated to prevent one VTE event, whereas 224 patients would need to be treated to result in one major bleeding event.21
For this reason, it is reasonable to consider extended prophylaxis for hospitalized medical patients after identifying these patients’ risk factors. In keeping with the trial’s amended inclusion criteria, patients older than age 75 and those with cancer or prior VTE should receive special consideration for extended prophylaxis.
RECOMMENDED APPROACH TO VTE PREVENTION IN HOSPITALIZED MEDICAL PATIENTS
- All hospitalized medical patients should be screened at the time of admission, and patients at risk for VTE should receive prophylaxis.
- All patients with reduced mobility and one or more other risk factors for VTE are candidates for prophylaxis.
- Patients should be reassessed daily for the development of VTE risk factors during their hospitalization if risk factors are absent on admission.
- If screening or reassessment reveals any VTE risk factors, pharmacologic prophylaxis is the mainstay of therapy. If exclusion criteria for pharmacologic prophylaxis are present, mechanical prophylaxis with graduated compression stockings and intermittent compression devices should be used. For very high-risk medical patients without a contraindication to anticoagulants, combination prophylaxis with both an anticoagulant and mechanical devices is preferred.
- In this patient population, LMWH agents are preferred as pharmacologic prophylaxis over UFH and over fondaparinux (which is not currently approved by the US Food and Drug Administration for this population).
- If UFH is to be used in this patient population, 5,000 U three times daily is the preferred dosage.
- Extended pharmacologic prophylaxis should be considered in patients older than age 75 and in patients with a cancer diagnosis or a prior VTE episode.
DISCUSSION: ADDITIONAL PERSPECTIVES FROM THE AUTHORS
Dr. Jaffer: Dr. Spyropoulos, are there any guidelines, other than those from the ACCP, that speak to VTE prophylaxis in hospitalized medical patients? If so, what are their take-home messages and how do they differ from the ACCP guidelines?
Dr. Spyropoulos: I was part of the group that developed the International Consensus Statement (ICS) published in International Angiology in 2006,11 which is more recent than the latest ACCP guidelines, which were published in 2004. The ICS drew on much of the same data that the ACCP did, but we did an updated review of clinical trials.
For VTE prophylaxis in hospitalized medical patients, the ICS recommendations are more specific with regard to the type, dose, and dosing frequency of anticoagulant agents. First, they specify doses for both LMWH agents in this patient setting: 40 mg once daily for enoxaparin, and 5,000 IU once daily for dalteparin.
The ICS document also states that if UFH is the choice for prophylaxis, a regimen of 5,000 U three times daily should be considered. In the past year alone, two analyses suggest that three-times-daily dosing of UFH in medical patients provides superior efficacy to twice-daily dosing, although perhaps at the expense of more bleeding episodes.12,16 It is important to remember that no large placebo-controlled trial supports the efficacy of a UFH regimen of 5,000 U twice daily in this population.
Finally, the ICS document states that fondaparinux 2.5 mg once daily is a viable option for prophylaxis in medical patients, based on the ARTEMIS trial,3 even though this represents an off-label use.
Dr. Jaffer: Real-world use of VTE prophylaxis is far from optimal, especially in medical patients, and this is partly a result of system-of-care issues. I’d like to conclude by asking each of my colleagues to offer your perspectives on how your own institutions have improved their systems of care to promote better use of VTE prophylaxis and what lessons might be shared with others. Dr. McKean, you work at Brigham and Women’s Hospital, which recently reported impressive results with an electronic alert system designed to increase clinicians’ consideration of VTE risk assessment and use of prophylaxis.24 Please tell us about that study and the alert system.
Dr. McKean: Despite many educational initiatives at Brigham and Women’s Hospital, there were still some patients at high risk for VTE who were not receiving appropriate prophylaxis. What Dr. Samuel Goldhaber and his colleagues wanted to determine was whether changing the system of care could result in a reduced incidence of VTE.24 They devised a computer software program linked to the patient database that used eight common risk factors to determine each hospitalized patient’s risk profile for VTE. Each risk factor was weighted according to a point scale, with major risk factors (cancer, prior VTE, or hypercoagulability) assigned 3 points, the intermediate risk factor of surgery assigned 2 points, and minor risk factors (advanced age, obesity, immobility, or use of hormone replacement therapy or oral contraceptives) assigned 1 point. For patients with a total risk score of 4 or greater, the computer screen generates a color-coded VTE risk alert that requires the physician to acknowledge the alert and choose one of three options: order prophylaxis as appropriate, review a 60-page document on the computer to learn more about prophylaxis, or do nothing.
The study found that hospitalized patients who were randomized to treatment under the computer alert system were significantly more likely to receive VTE prophylaxis and significantly less likely to develop VTE than were patients randomized to a control group. The alert system reduced the risk of DVT or PE at 90 days by 41% in patients considered to be at high risk. It was particularly interesting that the incidence of VTE was lower in the intervention group even when physicians chose not to use prophylaxis, which suggests that simply having this alert system in place improved outcomes, perhaps by raising awareness of the risk of VTE.24
Additional studies are needed to better understand physicians’ behavior and determine why they seem to have a disproportionate fear of the risk of bleeding relative to the risk of clotting, including fatal PE, because that is really the heart of the matter. When patients are not given prophylaxis, often it is because of fear of bleeding. It is not clear, however, why some of these patients did not receive mechanical devices as an alternative form of prophylaxis, but this seems to be the case worldwide, as shown recently by the multinational ENDORSE study.22 Meanwhile, as we await studies to better understand physician perceptions and behaviors regarding prophylaxis, we need to work hard to change the system of care.
Dr. Deitelzweig: Over the past couple of years, the Ochsner Clinic has grown from a one-hospital teaching organization to a seven-hospital system with a mix of closed and open medical staff. The challenge is how to take a process that worked well in the one center, where appropriate prophylaxis was used about 90% of the time, and transfer it to the other centers in the larger system. We have endorsed several types of performance tools, such as the change-acceleration processes used by General Electric. The aim is to share a vision of heightening awareness. To do that, we have worked to mobilize the key stakeholders, at least half of them, to build algorithms that they all will endorse. It is easier said than done, however, and we have found it essential to involve both physicians and non-physician colleagues from pharmacy and nursing who have political and organizational clout.
Dr. Brotman: At Johns Hopkins, I took a bit more draconian approach to this issue because I thought that hospitalists often knew that they should be using VTE prophylaxis but sometimes weren’t, and I am not convinced that clinicians always look at prompts. So we came up with a system that incorporates both billing and documentation simultaneously. We put a hard stop on users’ documentation so that they could not sign off on a note or bill for their care until they checked off the kind of VTE prophylaxis they were using. Since hospitalists ultimately care about billing for their work, this system has at least ensured that everybody has considered and documented VTE prophylaxis on a daily basis. There are other hard stops that can be implemented in computer order-entry systems as well, and we are considering ways to roll them out on a broader scale.
However, all of these systems can have problems because patient situations change from day to day. For instance, VTE prophylaxis is not necessarily indicated in a 38-year-old ambulatory patient who comes in with a sickle cell crisis, but you will need to reconsider if the patient ends up in acute chest syndrome in the intensive care unit. I do not yet have a good way to ensure that this is being done on a daily basis with all patients.
Dr. Amin: At the University of California, Irvine, we implemented an electronic alert system, but we locked users in so that they could not complete their admission orders until they answered questions about VTE prevention. This practice increased our VTE prophylaxis rates tremendously. Because we are a level I trauma center, we allow users to bypass the screens one time, but the next time they log in, even to get a simple lab result, they have to answer the questions about VTE prevention.
With any system you develop, you also have to continue with the education process, because clinicians sometimes get into bad habits or simply forget things.
Dr. Spyropolous: At Lovelace Medical Center, we didn’t have the sophistication of an electronic order-entry system, but we had an experienced clinical pharmacist (the director of inpatient pharmacy) who helped to develop and champion VTE prevention guidelines that have then been used throughout the system in close conjunction with our hospitalists’ rounds. This system has been used successfully for the past 7 years.
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thrombo-embolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med 1999; 341:793–800.
- Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation 2004; 110:874–879.
- Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ 2006; 332:325–329.
- Baglin TP, White K, Charles A. Fatal pulmonary embolism in hos-pitalised medical patients. J Clin Pathol 1997; 50:609–610.
- Piazza G, Seddighzadeh A, Goldhaber SZ. Double trouble for 2,609 hospitalized medical patients who developed deep vein thrombosis: prophylaxis omitted more often and pulmonary embolism more frequent. Chest 2007; 132:554–561.
- Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost 2007; 5:1610–1616.
- Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest 2001; 119(1 Suppl):132S–175S.
- Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest 2007; 132:936–945.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thrombo-embolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med 2007; 146:278–288.
- Cardiovascular Disease Educational and Research Trust; Cyprus Cardiovascular Disease Educational and Research Trust; European Venous Forum; International Surgical Thrombosis Forum; International Union of Angiology; Union Internationale de Phlébologie. Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence). Int Angiol 2006; 25:101–161.
- Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta-analysis of randomized controlled trials. Arch Intern Med 2007; 167:1476–1486.
- Mismetti P, Laporte-Simitsidis S, Tardy B, et al. Prevention of venous thromboembolism in internal medicine with unfractionated or low-molecular-weight heparins: a meta-analysis of randomised clinical trials. Thromb Haemost 2000; 83:14–19.
- Lechler E, Schramm W, Flosbach CW. The venous thrombotic risk in non-surgical patients: epidemiological data and efficacy/safety profile of a low-molecular-weight heparin (enoxaparin). The Prime Study Group. Haemostasis 1996; 26(Suppl 2):49–56.
- Kleber FX, Witt C, Vogel G, et al; the PRINCE Study Group. Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease. Am Heart J 2003; 145:614–621.
- King CS, Holley AB, Jackson JL, Shorr AF, Moores LK. Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a meta-analysis. Chest 2007; 131:507–516.
- Burleigh E, Wang C, Foster D, et al. Thromboprophylaxis in medically ill patients at risk for venous thromboembolism. Am J Health Syst Pharm 2006; 63(20 Suppl 6):S23–S29.
- Leykum L, Pugh J, Diuguid D, Papadopoulos K. Cost utility of substituting enoxaparin for unfractionated heparin for prophylaxis of venous thrombosis in the hospitalized medical patient. J Hosp Med 2006; 1:168–176.
- Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy 2006; 26:1438–1445.
- Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med 2007; 167:1471–1475.
- Hull RD, Schellong SM Tapson VF, et al. Extended-duration venous thromboembolism (VTE) prophylaxis in acutely ill medical patients with recent reduced mobility: the EXCLAIM study. Presentation at: International Society on Thrombosis and Haemo-stasis XXIst Congress; July 6–12, 2007; Geneva, Switzerland.
- Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008; 371:387–394.
- Kahn SR, Panju A, Geerts W, et al; CURVE study investigators. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res 2007; 119:145–155.
- Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent thromboembolism among hospitalized patients. N Engl J Med 2005; 352:969–977.
- Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 1991; 151:933–938.
- Howell MD, Geraci JM, Knowlton AA. Congestive heart failure and outpatient risk of venous thromboembolism: a retrospective, case-control study. J Clin Epidemiol 2001; 54:810–816.
- Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet 2006; 367:1075–1079.
- Alikhan R, Cohen AT, Combe S, et al. Prevention of venous thromboembolism in medical patients with enoxaparin: a subgroup analysis of the MEDENOX study. Blood Coagul Firbinolysis 2003; 14:341–346.
- Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107(23 Suppl 1):I9–I16.
- Greinacher A, Warkentin TE. Recognition, treatment, and prevention of heparin-induced thrombocytopenia: review and update. Thromb Res 2006; 118:165–176.
- Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 2005; 106:2710–2715.
- Sanderink GJ, Guimart CG, Ozoux ML, Jariwala NU, Shukla UA, Boutouyrie BX. Pharmacokinetics and pharmacodynamics of the prophylactic dose of enoxaparin once daily over 4 days in patients with renal impairment. Thromb Res 2002; 105:225–231.
- Douketis J, Cook D, Zytaruk N, et al. Dalteparin thromboprophylaxis in critically ill patients with severe renal insufficiency: the DIRECT study [abstract]. J Thromb Haemost 2007; 5(Suppl 2): P-S-680.
- Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg 2002; 12:19–24.
The need for prophylaxis of venous thromboembolism (VTE) in hospitalized acutely ill medical patients is well established. Without prophylaxis, hospitalized medical patients develop VTE at a rate of 5% to 15%.1–3 Moreover, pulmonary embolism (PE) occurs more frequently in hospitalized medical patients than in nonmedical patients, and is a leading cause of sudden death in hospitalized medical patients.4,5 Without appropriate prophylaxis, 1 in 20 hospitalized medical patients may suffer a fatal PE.4
PROPHYLAXIS IN MEDICAL PATIENTS: UNDERUSED AND OFTEN INAPPROPRIATE
Despite these risks and the clear indications for VTE prophylaxis in hospitalized medical patients, prophylaxis of VTE is omitted more often in these patients than in hospitalized surgical patients.5 Even when prophylaxis is given, it is often used inappropriately in the medical population. So concludes a recent analysis of data from 196,104 patients with acute medical conditions who were discharged from 227 US hospitals from January 2002 to September 2005.6 Criteria for inclusion in the analysis were patient age of 40 years or older, a hospital stay of 6 days or greater, and an absence of contraindications to anticoagulation. Appropriate prophylaxis was defined in accordance with the Sixth American College of Chest Physicians (ACCP) Consensus Conference on Antithrombotic Therapy.7
The analysis revealed an overall VTE prophylaxis rate of 61.8%, but the rate of appropriate prophylaxis was only 33.9%, meaning that two-thirds of discharged patients did not receive prophylaxis in accordance with ACCP guidelines. When temporal trends were analyzed according to groups based on patients’ diagnosis at admission (acute myocardial infarction, severe lung disease, ischemic stroke, cancer, heart failure, or trauma), the rate of appropriate prophylaxis remained essentially flat from the beginning to the end of the study period for virtually all diagnosis groups.6
Similar findings have emerged from the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE), an ongoing international registry of acutely ill medical patients.8 Data from the first 15,156 patients, enrolled from July 2002 through September 2006, reveal that 50% of patients received pharmacologic and/or mechanical VTE prophylaxis in the hospital, and only 60% of patients who met established criteria for VTE prophylaxis actually received it.
Analysis of the US portion of the IMPROVE data shows that 54% of the US patient sample received some form of VTE prophylaxis; 22% of US patients received intermittent pneumatic compression, 21% received unfractionated heparin (UFH), 14% received low-molecular-weight heparin (LMWH), and 3% wore compression stockings.8 Thus, despite a paucity of data supporting a benefit of intermittent pneumatic compression in this population,9 it was the most frequently used form of prophylaxis in US patients.
CLINICAL TRIALS OF PHARMACOLOGIC PROPHYLAXIS IN MEDICAL PATIENTS
The Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) trial1 randomized 1,102 hospitalized patients to one of two doses of the LMWH enoxaparin (20 mg or 40 mg once daily subcutaneously) or placebo for 6 to 14 days. Compared with placebo, the 40-mg dose of enoxaparin was associated with a 63% reduction in risk of VTE over 3 months of follow-up (P < .001) (Figure 1).
The Prospective Evaluation of Dalteparin Efficacy for Prevention of VTE in Immobilized Patients Trial (PREVENT)2 was a multicenter, randomized, double-blind study comparing the LMWH dalteparin (5,000 IU daily given subcutaneously for 14 days) with placebo in 3,706 acutely ill medical patients. Over 90 days of follow-up, the risk of VTE was reduced by 44% in patients assigned to dalteparin compared with those assigned to placebo (P = .0015) (Figure 1).
The Arixtra for Thromboembolism Prevention in a Medical Indications Study (ARTEMIS)3 randomized 849 medical patients 60 years or older to 6 to 14 days of therapy with the selective factor Xa inhibitor fondaparinux (2.5 mg once daily subcutaneously) or placebo. Compared with the placebo group, fondaparinux recipients had a 47% lower risk of developing VTE by day 15 (P = .029) (Figure 1).
Fewer events and fatal PEs, but no effect on all-cause mortality
A recent meta-analysis by Dentali et al10 further demonstrates the efficacy of anticoagulant therapy for preventing symptomatic VTE in hospitalized medical patients. This analysis included several other trials in addition to the three reviewed above,1–3 for a total of nine randomized studies (seven of which were dou-ble-blind) comprising 19,958 patients. Across the nine studies, anticoagulant prophylaxis was clearly superior to placebo in preventing fatal PE (relative risk, 0.38 [95% CI, 0.21 to 0.69]). There was a strong trend toward a reduction in symptomatic deep vein thrombosis (DVT) with prophylaxis but no effect on all-cause mortality. The meta-analysis also provided reassurance that prophylaxis does not increase the rate of major bleeding.
HOW DO THE PROPHYLAXIS OPTIONS STACK UP?
What the ACCP recommends
Current ACCP guidelines recommend the use of either LMWH or low-dose UFH (5,000 U subcutaneously two or three times daily) as a Grade 1A recommendation for VTE prophylaxis in patients with medical conditions and risk factors for VTE.9 This represents the guidelines’ highest level of recommendation, ie, one that is based on randomized controlled trials (RCTs) without important limitations. In contrast, the 2006 International Consensus Statement, developed as a collaborative effort among expert bodies on VTE, specified a more narrow dosing recommendation for UFH in this patient population (5,000 U three times daily, not twice daily) as well as specifying 40 mg once daily as the recommended dose of enoxaparin and 5,000 IU once daily as the recommended dose of dalteparin.11
For medical patients with risk factors for VTE who have a contraindication to anticoagulant prophylaxis, the ACCP guidelines recommend the use of graduated compression stockings or intermittent pneumatic compression devices as a Grade 1C+ recommendation (“no RCTs but strong RCT results can be unequivocally extrapolated, or overwhelming evidence from observational studies”9).
Current ACCP guidelines do not address the use of fondaparinux in their recommendations for VTE prophylaxis in medical patients.
Getting a handle on bleeding risk
Patient characteristics that exclude pharmacologic thromboprophylaxis due to bleeding risk are generally limited to active bleeding or coagulopathy, as demonstrated by a platelet count less than 50,000 cells/µL or an international normalized ratio greater than 1.5. Additionally, bleeding risk should be carefully assessed if an invasive procedure is planned during a patient’s hospital stay.
It is worth noting that the anticoagulant doses used for VTE prophylaxis are a fraction of those used for treatment of VTE. Thus, if a patient would be treated with full-dose anticoagulation if VTE developed, then that patient should be eligible for VTE prophylaxis.
Because the use of mechanical forms of prophylaxis in medical patients is not truly evidence-based, mechanical prophylaxis should be reserved for medical patients who have a contraindication to anticoagulants, or for use in combination with anticoagulants in patients at very high risk of VTE.
UFH vs LMWH
Two meta-analyses have compared UFH with LMWH for VTE prevention in medical patients.12,13 In a recent analysis that included 10 trials directly comparing the two therapies, 14 trials comparing UFH with control, and 11 trials comparing LMWH with control, Wein et al found a lower risk of DVT with LMWH than with UFH (relative risk, 0.68 [95% CI, 0.52 to 0.88]) but no difference between the therapies in mortality or bleeding risk.12 In an earlier and smaller analysis, Mismetti et al found no significant differences between UFH and LMWH in preventing DVT or death but did find a significant reduction in major bleeding episodes with LMWH versus three-times-daily UFH (52% relative reduction; P = .049).13
Randomized trials also reveal that enoxaparin 40 mg once daily is as efficacious as UFH 5,000 U three times daily for VTE prevention in medical patients.14,15 The above analysis by Wein et al12 and an additional meta-analysis by King and colleagues16 found that three-times-daily dosing of UFH is more efficacious than twice-daily dosing of UFH, but at the expense of more bleeding, including major bleeding.
Economic considerations
Because of differences in drug acquisition costs between UFH and the LMWH agents, several economic evaluations have compared the use of these therapies for prophylaxis in medical patients at risk of VTE.
In an analysis of hospital costs for medical patients receiving VTE prophylaxis from more than 330 US hospitals for the period 2001–2004, Burleigh et al found that mean total hospital costs were higher for patients who received UFH than for those who received LMWH ($7,615 vs $6,866) even though mean drug costs were higher for LMWH ($791 vs $569 for UFH).17 A reduction in hospital length of stay appeared to contribute to the overall savings with LMWH; other contributors may have included costs associated with heparin-induced thrombocytopenia (HIT) in UFH recipients or the extra nursing time required for administering UFH in two or three daily doses.
Leykum et al used a decision analysis model to estimate the economic effect of substituting enoxaparin for UFH in hospitalized medical patients for whom VTE prophylaxis is indicated.18 Cost data were based on Medicare reimbursement rates as well as drug and laboratory costs for a multi-institutional health system. The model assumed HIT incidence rates of 2.7% with UFH and 0.3% with enoxaparin. It also assumed the cost of a daily dose to be $4 for UFH versus $84 for enoxaparin. From the payer perspective, the model showed that substituting enoxaparin for UFH would reduce the overall cost of care by $28.61 per day on a per-patient basis, despite enoxaparin’s higher acquisition cost, and would save $4,550 per quality-adjusted life-year by reducing the incidence of HIT.
Another cost analysis confirms the association between HIT and increased hospital costs. Creekmore et al retrospectively analyzed data from 10,121 adult medical patients who received VTE prophylaxis at the University of Utah Hospital in Salt Lake City from August 2000 to November 2004.19 They found that an admission during which HIT developed incurred a mean cost of $56,364, compared with $15,231 for an admission without HIT. Because LMWH was associated with a lower incidence of HIT compared with UFH (0.084% vs 0.51%, respectively), LMWH reduced the incremental cost of VTE prophylaxis by $13.88 per patient compared with UFH.
THE EXCLAIM TRIAL: IS THERE A ROLE FOR EXTENDED PROPHYLAXIS?
Although the previously discussed studies have clearly demonstrated the benefit of in-hospital VTE prophylaxis for acutely ill medical patients, none has rigorously examined extended-duration out-of-hospital prophylaxis in these patients. This represents an important gap in the literature, since a substantial proportion of VTE develops in the outpatient setting within 3 months of a hospitalization, and most outpatient VTE episodes occur within 1 month of a preceding hospitalization.20
To begin to fill this gap, the Extended Clinical Prophylaxis in Acutely Ill Medical Patients (EXCLAIM) trial was conducted to compare extended-duration LMWH prophylaxis with a standard LMWH prophylaxis regimen in acutely ill medical patients using a prospective, multicenter, randomized, double-blind, placebo-controlled design.21
Patients and study design
Patients were eligible for enrollment if they were aged 40 years or older and had recent immobilization (≤ 3 days), a predefined acute medical illness, and either level 1 mobility (total bed rest or sedentary state) or level 2 mobility (level 1 with bathroom privileges). The predefined acute medical illnesses consisted of New York Heart Association class III/IV heart failure, acute respiratory insufficiency, or other acute medical conditions, including post-acute ischemic stroke, acute infection without septic shock, and active cancer.
All patients received open-label enoxaparin 40 mg subcutaneously once daily for 10 ± 4 days, after which they were randomized to either enoxaparin 40 mg subcutaneously once daily or placebo for an additional 28 ± 4 days.
The primary efficacy end point was the incidence of VTE events, defined as asymptomatic DVT documented by mandatory ultrasonography at the end of the double-blind treatment period (28 ± 4 days) or as symptomatic DVT, symptomatic PE, or fatal PE at any time during the double-blind period. Symptomatic DVT was confirmed by objective tests; PE was confirmed by ventilation-perfusion scan, computed tomography, angiography, or autopsy.
Secondary efficacy end points were mortality at the end of the double-blind period, at 3 months, and at 6 months, as well as the incidence of VTE at 3 months.
The primary safety outcome measure was the incidence of major hemorrhage during the double-blind period; secondary safety measures were rates of major and minor hemorrhage, minor hemorrhage, HIT, and serious adverse events.
Population amended at planned interim analysis
After approximately half of the patients were enrolled, a planned and blinded interim analysis for futility concluded that the study was unlikely to show a statistically significant advantage of enoxaparin over placebo. The trial’s steering committee followed the suggestion of its data safety monitoring board to redefine the inclusion criteria to refocus enrollment on patients with a high risk of VTE. A blinded analysis was performed to identify this subgroup.
The resulting amended inclusion criteria were the same as above except that level 2 mobility had to be accompanied by at least one of three additional high-risk criteria: (1) age greater than 75 years, (2) history of prior VTE, or (3) diagnosis of cancer.
The trial’s main exclusion criteria were evidence of active bleeding, a contraindication to anticoagulation, receipt of prophylactic LMWH or UFH more than 72 hours prior to enrollment, treatment with an oral anticoagulant within 72 hours of enrollment, major surgery within the prior 3 months, cerebral stroke with bleeding, and persistent renal failure (creatinine clearance < 30 mL/min).


Results
The amended study population included 5,105 patients, 5,049 of whom received open-label enoxaparin. Of this group, 2,013 were randomized to active extended prophylaxis with enoxparain and 2,027 to placebo. Baseline characteristics, including level of mobility, were similar between the two groups.
The efficacy of extended prophylaxis with enoxaparin was enduring, as the cumulative incidence of VTE events at day 90 was significantly lower in enoxaparin recipients than in placebo recipients (3.0% vs 5.2%; relative reduction of 42%; P = .0115).
There was no difference in all-cause mortality at 6 months between the enoxaparin and placebo groups (10.1% vs 8.9%, respectively; P = .179).
Safety. Major hemorrhage was significantly more frequent in the enoxaparin arm, occurring in 0.60% of enoxaparin recipients compared with 0.15% of placebo recipients (P = .019). Minor bleeding was also more common with enoxaparin (5.20% vs 3.70%; P = .024).
Conclusions
The EXCLAIM trial found that an extended-duration (38-day) enoxaparin regimen significantly reduced the overall incidence of VTE relative to a 10-day enoxaparin regimen in acutely ill medical patients with reduced mobility. At the same time, the extended regimen was associated with a significant increase in the rate of major bleeding, although the incidence of major bleeding was low. The investigators concluded that the net clinical effect of extended-duration prophylaxis with enoxaparin is favorable, as only 46 patients would need to be treated to prevent one VTE event, whereas 224 patients would need to be treated to result in one major bleeding event.21
For this reason, it is reasonable to consider extended prophylaxis for hospitalized medical patients after identifying these patients’ risk factors. In keeping with the trial’s amended inclusion criteria, patients older than age 75 and those with cancer or prior VTE should receive special consideration for extended prophylaxis.
RECOMMENDED APPROACH TO VTE PREVENTION IN HOSPITALIZED MEDICAL PATIENTS
- All hospitalized medical patients should be screened at the time of admission, and patients at risk for VTE should receive prophylaxis.
- All patients with reduced mobility and one or more other risk factors for VTE are candidates for prophylaxis.
- Patients should be reassessed daily for the development of VTE risk factors during their hospitalization if risk factors are absent on admission.
- If screening or reassessment reveals any VTE risk factors, pharmacologic prophylaxis is the mainstay of therapy. If exclusion criteria for pharmacologic prophylaxis are present, mechanical prophylaxis with graduated compression stockings and intermittent compression devices should be used. For very high-risk medical patients without a contraindication to anticoagulants, combination prophylaxis with both an anticoagulant and mechanical devices is preferred.
- In this patient population, LMWH agents are preferred as pharmacologic prophylaxis over UFH and over fondaparinux (which is not currently approved by the US Food and Drug Administration for this population).
- If UFH is to be used in this patient population, 5,000 U three times daily is the preferred dosage.
- Extended pharmacologic prophylaxis should be considered in patients older than age 75 and in patients with a cancer diagnosis or a prior VTE episode.
DISCUSSION: ADDITIONAL PERSPECTIVES FROM THE AUTHORS
Dr. Jaffer: Dr. Spyropoulos, are there any guidelines, other than those from the ACCP, that speak to VTE prophylaxis in hospitalized medical patients? If so, what are their take-home messages and how do they differ from the ACCP guidelines?
Dr. Spyropoulos: I was part of the group that developed the International Consensus Statement (ICS) published in International Angiology in 2006,11 which is more recent than the latest ACCP guidelines, which were published in 2004. The ICS drew on much of the same data that the ACCP did, but we did an updated review of clinical trials.
For VTE prophylaxis in hospitalized medical patients, the ICS recommendations are more specific with regard to the type, dose, and dosing frequency of anticoagulant agents. First, they specify doses for both LMWH agents in this patient setting: 40 mg once daily for enoxaparin, and 5,000 IU once daily for dalteparin.
The ICS document also states that if UFH is the choice for prophylaxis, a regimen of 5,000 U three times daily should be considered. In the past year alone, two analyses suggest that three-times-daily dosing of UFH in medical patients provides superior efficacy to twice-daily dosing, although perhaps at the expense of more bleeding episodes.12,16 It is important to remember that no large placebo-controlled trial supports the efficacy of a UFH regimen of 5,000 U twice daily in this population.
Finally, the ICS document states that fondaparinux 2.5 mg once daily is a viable option for prophylaxis in medical patients, based on the ARTEMIS trial,3 even though this represents an off-label use.
Dr. Jaffer: Real-world use of VTE prophylaxis is far from optimal, especially in medical patients, and this is partly a result of system-of-care issues. I’d like to conclude by asking each of my colleagues to offer your perspectives on how your own institutions have improved their systems of care to promote better use of VTE prophylaxis and what lessons might be shared with others. Dr. McKean, you work at Brigham and Women’s Hospital, which recently reported impressive results with an electronic alert system designed to increase clinicians’ consideration of VTE risk assessment and use of prophylaxis.24 Please tell us about that study and the alert system.
Dr. McKean: Despite many educational initiatives at Brigham and Women’s Hospital, there were still some patients at high risk for VTE who were not receiving appropriate prophylaxis. What Dr. Samuel Goldhaber and his colleagues wanted to determine was whether changing the system of care could result in a reduced incidence of VTE.24 They devised a computer software program linked to the patient database that used eight common risk factors to determine each hospitalized patient’s risk profile for VTE. Each risk factor was weighted according to a point scale, with major risk factors (cancer, prior VTE, or hypercoagulability) assigned 3 points, the intermediate risk factor of surgery assigned 2 points, and minor risk factors (advanced age, obesity, immobility, or use of hormone replacement therapy or oral contraceptives) assigned 1 point. For patients with a total risk score of 4 or greater, the computer screen generates a color-coded VTE risk alert that requires the physician to acknowledge the alert and choose one of three options: order prophylaxis as appropriate, review a 60-page document on the computer to learn more about prophylaxis, or do nothing.
The study found that hospitalized patients who were randomized to treatment under the computer alert system were significantly more likely to receive VTE prophylaxis and significantly less likely to develop VTE than were patients randomized to a control group. The alert system reduced the risk of DVT or PE at 90 days by 41% in patients considered to be at high risk. It was particularly interesting that the incidence of VTE was lower in the intervention group even when physicians chose not to use prophylaxis, which suggests that simply having this alert system in place improved outcomes, perhaps by raising awareness of the risk of VTE.24
Additional studies are needed to better understand physicians’ behavior and determine why they seem to have a disproportionate fear of the risk of bleeding relative to the risk of clotting, including fatal PE, because that is really the heart of the matter. When patients are not given prophylaxis, often it is because of fear of bleeding. It is not clear, however, why some of these patients did not receive mechanical devices as an alternative form of prophylaxis, but this seems to be the case worldwide, as shown recently by the multinational ENDORSE study.22 Meanwhile, as we await studies to better understand physician perceptions and behaviors regarding prophylaxis, we need to work hard to change the system of care.
Dr. Deitelzweig: Over the past couple of years, the Ochsner Clinic has grown from a one-hospital teaching organization to a seven-hospital system with a mix of closed and open medical staff. The challenge is how to take a process that worked well in the one center, where appropriate prophylaxis was used about 90% of the time, and transfer it to the other centers in the larger system. We have endorsed several types of performance tools, such as the change-acceleration processes used by General Electric. The aim is to share a vision of heightening awareness. To do that, we have worked to mobilize the key stakeholders, at least half of them, to build algorithms that they all will endorse. It is easier said than done, however, and we have found it essential to involve both physicians and non-physician colleagues from pharmacy and nursing who have political and organizational clout.
Dr. Brotman: At Johns Hopkins, I took a bit more draconian approach to this issue because I thought that hospitalists often knew that they should be using VTE prophylaxis but sometimes weren’t, and I am not convinced that clinicians always look at prompts. So we came up with a system that incorporates both billing and documentation simultaneously. We put a hard stop on users’ documentation so that they could not sign off on a note or bill for their care until they checked off the kind of VTE prophylaxis they were using. Since hospitalists ultimately care about billing for their work, this system has at least ensured that everybody has considered and documented VTE prophylaxis on a daily basis. There are other hard stops that can be implemented in computer order-entry systems as well, and we are considering ways to roll them out on a broader scale.
However, all of these systems can have problems because patient situations change from day to day. For instance, VTE prophylaxis is not necessarily indicated in a 38-year-old ambulatory patient who comes in with a sickle cell crisis, but you will need to reconsider if the patient ends up in acute chest syndrome in the intensive care unit. I do not yet have a good way to ensure that this is being done on a daily basis with all patients.
Dr. Amin: At the University of California, Irvine, we implemented an electronic alert system, but we locked users in so that they could not complete their admission orders until they answered questions about VTE prevention. This practice increased our VTE prophylaxis rates tremendously. Because we are a level I trauma center, we allow users to bypass the screens one time, but the next time they log in, even to get a simple lab result, they have to answer the questions about VTE prevention.
With any system you develop, you also have to continue with the education process, because clinicians sometimes get into bad habits or simply forget things.
Dr. Spyropolous: At Lovelace Medical Center, we didn’t have the sophistication of an electronic order-entry system, but we had an experienced clinical pharmacist (the director of inpatient pharmacy) who helped to develop and champion VTE prevention guidelines that have then been used throughout the system in close conjunction with our hospitalists’ rounds. This system has been used successfully for the past 7 years.
The need for prophylaxis of venous thromboembolism (VTE) in hospitalized acutely ill medical patients is well established. Without prophylaxis, hospitalized medical patients develop VTE at a rate of 5% to 15%.1–3 Moreover, pulmonary embolism (PE) occurs more frequently in hospitalized medical patients than in nonmedical patients, and is a leading cause of sudden death in hospitalized medical patients.4,5 Without appropriate prophylaxis, 1 in 20 hospitalized medical patients may suffer a fatal PE.4
PROPHYLAXIS IN MEDICAL PATIENTS: UNDERUSED AND OFTEN INAPPROPRIATE
Despite these risks and the clear indications for VTE prophylaxis in hospitalized medical patients, prophylaxis of VTE is omitted more often in these patients than in hospitalized surgical patients.5 Even when prophylaxis is given, it is often used inappropriately in the medical population. So concludes a recent analysis of data from 196,104 patients with acute medical conditions who were discharged from 227 US hospitals from January 2002 to September 2005.6 Criteria for inclusion in the analysis were patient age of 40 years or older, a hospital stay of 6 days or greater, and an absence of contraindications to anticoagulation. Appropriate prophylaxis was defined in accordance with the Sixth American College of Chest Physicians (ACCP) Consensus Conference on Antithrombotic Therapy.7
The analysis revealed an overall VTE prophylaxis rate of 61.8%, but the rate of appropriate prophylaxis was only 33.9%, meaning that two-thirds of discharged patients did not receive prophylaxis in accordance with ACCP guidelines. When temporal trends were analyzed according to groups based on patients’ diagnosis at admission (acute myocardial infarction, severe lung disease, ischemic stroke, cancer, heart failure, or trauma), the rate of appropriate prophylaxis remained essentially flat from the beginning to the end of the study period for virtually all diagnosis groups.6
Similar findings have emerged from the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE), an ongoing international registry of acutely ill medical patients.8 Data from the first 15,156 patients, enrolled from July 2002 through September 2006, reveal that 50% of patients received pharmacologic and/or mechanical VTE prophylaxis in the hospital, and only 60% of patients who met established criteria for VTE prophylaxis actually received it.
Analysis of the US portion of the IMPROVE data shows that 54% of the US patient sample received some form of VTE prophylaxis; 22% of US patients received intermittent pneumatic compression, 21% received unfractionated heparin (UFH), 14% received low-molecular-weight heparin (LMWH), and 3% wore compression stockings.8 Thus, despite a paucity of data supporting a benefit of intermittent pneumatic compression in this population,9 it was the most frequently used form of prophylaxis in US patients.
CLINICAL TRIALS OF PHARMACOLOGIC PROPHYLAXIS IN MEDICAL PATIENTS
The Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) trial1 randomized 1,102 hospitalized patients to one of two doses of the LMWH enoxaparin (20 mg or 40 mg once daily subcutaneously) or placebo for 6 to 14 days. Compared with placebo, the 40-mg dose of enoxaparin was associated with a 63% reduction in risk of VTE over 3 months of follow-up (P < .001) (Figure 1).
The Prospective Evaluation of Dalteparin Efficacy for Prevention of VTE in Immobilized Patients Trial (PREVENT)2 was a multicenter, randomized, double-blind study comparing the LMWH dalteparin (5,000 IU daily given subcutaneously for 14 days) with placebo in 3,706 acutely ill medical patients. Over 90 days of follow-up, the risk of VTE was reduced by 44% in patients assigned to dalteparin compared with those assigned to placebo (P = .0015) (Figure 1).
The Arixtra for Thromboembolism Prevention in a Medical Indications Study (ARTEMIS)3 randomized 849 medical patients 60 years or older to 6 to 14 days of therapy with the selective factor Xa inhibitor fondaparinux (2.5 mg once daily subcutaneously) or placebo. Compared with the placebo group, fondaparinux recipients had a 47% lower risk of developing VTE by day 15 (P = .029) (Figure 1).
Fewer events and fatal PEs, but no effect on all-cause mortality
A recent meta-analysis by Dentali et al10 further demonstrates the efficacy of anticoagulant therapy for preventing symptomatic VTE in hospitalized medical patients. This analysis included several other trials in addition to the three reviewed above,1–3 for a total of nine randomized studies (seven of which were dou-ble-blind) comprising 19,958 patients. Across the nine studies, anticoagulant prophylaxis was clearly superior to placebo in preventing fatal PE (relative risk, 0.38 [95% CI, 0.21 to 0.69]). There was a strong trend toward a reduction in symptomatic deep vein thrombosis (DVT) with prophylaxis but no effect on all-cause mortality. The meta-analysis also provided reassurance that prophylaxis does not increase the rate of major bleeding.
HOW DO THE PROPHYLAXIS OPTIONS STACK UP?
What the ACCP recommends
Current ACCP guidelines recommend the use of either LMWH or low-dose UFH (5,000 U subcutaneously two or three times daily) as a Grade 1A recommendation for VTE prophylaxis in patients with medical conditions and risk factors for VTE.9 This represents the guidelines’ highest level of recommendation, ie, one that is based on randomized controlled trials (RCTs) without important limitations. In contrast, the 2006 International Consensus Statement, developed as a collaborative effort among expert bodies on VTE, specified a more narrow dosing recommendation for UFH in this patient population (5,000 U three times daily, not twice daily) as well as specifying 40 mg once daily as the recommended dose of enoxaparin and 5,000 IU once daily as the recommended dose of dalteparin.11
For medical patients with risk factors for VTE who have a contraindication to anticoagulant prophylaxis, the ACCP guidelines recommend the use of graduated compression stockings or intermittent pneumatic compression devices as a Grade 1C+ recommendation (“no RCTs but strong RCT results can be unequivocally extrapolated, or overwhelming evidence from observational studies”9).
Current ACCP guidelines do not address the use of fondaparinux in their recommendations for VTE prophylaxis in medical patients.
Getting a handle on bleeding risk
Patient characteristics that exclude pharmacologic thromboprophylaxis due to bleeding risk are generally limited to active bleeding or coagulopathy, as demonstrated by a platelet count less than 50,000 cells/µL or an international normalized ratio greater than 1.5. Additionally, bleeding risk should be carefully assessed if an invasive procedure is planned during a patient’s hospital stay.
It is worth noting that the anticoagulant doses used for VTE prophylaxis are a fraction of those used for treatment of VTE. Thus, if a patient would be treated with full-dose anticoagulation if VTE developed, then that patient should be eligible for VTE prophylaxis.
Because the use of mechanical forms of prophylaxis in medical patients is not truly evidence-based, mechanical prophylaxis should be reserved for medical patients who have a contraindication to anticoagulants, or for use in combination with anticoagulants in patients at very high risk of VTE.
UFH vs LMWH
Two meta-analyses have compared UFH with LMWH for VTE prevention in medical patients.12,13 In a recent analysis that included 10 trials directly comparing the two therapies, 14 trials comparing UFH with control, and 11 trials comparing LMWH with control, Wein et al found a lower risk of DVT with LMWH than with UFH (relative risk, 0.68 [95% CI, 0.52 to 0.88]) but no difference between the therapies in mortality or bleeding risk.12 In an earlier and smaller analysis, Mismetti et al found no significant differences between UFH and LMWH in preventing DVT or death but did find a significant reduction in major bleeding episodes with LMWH versus three-times-daily UFH (52% relative reduction; P = .049).13
Randomized trials also reveal that enoxaparin 40 mg once daily is as efficacious as UFH 5,000 U three times daily for VTE prevention in medical patients.14,15 The above analysis by Wein et al12 and an additional meta-analysis by King and colleagues16 found that three-times-daily dosing of UFH is more efficacious than twice-daily dosing of UFH, but at the expense of more bleeding, including major bleeding.
Economic considerations
Because of differences in drug acquisition costs between UFH and the LMWH agents, several economic evaluations have compared the use of these therapies for prophylaxis in medical patients at risk of VTE.
In an analysis of hospital costs for medical patients receiving VTE prophylaxis from more than 330 US hospitals for the period 2001–2004, Burleigh et al found that mean total hospital costs were higher for patients who received UFH than for those who received LMWH ($7,615 vs $6,866) even though mean drug costs were higher for LMWH ($791 vs $569 for UFH).17 A reduction in hospital length of stay appeared to contribute to the overall savings with LMWH; other contributors may have included costs associated with heparin-induced thrombocytopenia (HIT) in UFH recipients or the extra nursing time required for administering UFH in two or three daily doses.
Leykum et al used a decision analysis model to estimate the economic effect of substituting enoxaparin for UFH in hospitalized medical patients for whom VTE prophylaxis is indicated.18 Cost data were based on Medicare reimbursement rates as well as drug and laboratory costs for a multi-institutional health system. The model assumed HIT incidence rates of 2.7% with UFH and 0.3% with enoxaparin. It also assumed the cost of a daily dose to be $4 for UFH versus $84 for enoxaparin. From the payer perspective, the model showed that substituting enoxaparin for UFH would reduce the overall cost of care by $28.61 per day on a per-patient basis, despite enoxaparin’s higher acquisition cost, and would save $4,550 per quality-adjusted life-year by reducing the incidence of HIT.
Another cost analysis confirms the association between HIT and increased hospital costs. Creekmore et al retrospectively analyzed data from 10,121 adult medical patients who received VTE prophylaxis at the University of Utah Hospital in Salt Lake City from August 2000 to November 2004.19 They found that an admission during which HIT developed incurred a mean cost of $56,364, compared with $15,231 for an admission without HIT. Because LMWH was associated with a lower incidence of HIT compared with UFH (0.084% vs 0.51%, respectively), LMWH reduced the incremental cost of VTE prophylaxis by $13.88 per patient compared with UFH.
THE EXCLAIM TRIAL: IS THERE A ROLE FOR EXTENDED PROPHYLAXIS?
Although the previously discussed studies have clearly demonstrated the benefit of in-hospital VTE prophylaxis for acutely ill medical patients, none has rigorously examined extended-duration out-of-hospital prophylaxis in these patients. This represents an important gap in the literature, since a substantial proportion of VTE develops in the outpatient setting within 3 months of a hospitalization, and most outpatient VTE episodes occur within 1 month of a preceding hospitalization.20
To begin to fill this gap, the Extended Clinical Prophylaxis in Acutely Ill Medical Patients (EXCLAIM) trial was conducted to compare extended-duration LMWH prophylaxis with a standard LMWH prophylaxis regimen in acutely ill medical patients using a prospective, multicenter, randomized, double-blind, placebo-controlled design.21
Patients and study design
Patients were eligible for enrollment if they were aged 40 years or older and had recent immobilization (≤ 3 days), a predefined acute medical illness, and either level 1 mobility (total bed rest or sedentary state) or level 2 mobility (level 1 with bathroom privileges). The predefined acute medical illnesses consisted of New York Heart Association class III/IV heart failure, acute respiratory insufficiency, or other acute medical conditions, including post-acute ischemic stroke, acute infection without septic shock, and active cancer.
All patients received open-label enoxaparin 40 mg subcutaneously once daily for 10 ± 4 days, after which they were randomized to either enoxaparin 40 mg subcutaneously once daily or placebo for an additional 28 ± 4 days.
The primary efficacy end point was the incidence of VTE events, defined as asymptomatic DVT documented by mandatory ultrasonography at the end of the double-blind treatment period (28 ± 4 days) or as symptomatic DVT, symptomatic PE, or fatal PE at any time during the double-blind period. Symptomatic DVT was confirmed by objective tests; PE was confirmed by ventilation-perfusion scan, computed tomography, angiography, or autopsy.
Secondary efficacy end points were mortality at the end of the double-blind period, at 3 months, and at 6 months, as well as the incidence of VTE at 3 months.
The primary safety outcome measure was the incidence of major hemorrhage during the double-blind period; secondary safety measures were rates of major and minor hemorrhage, minor hemorrhage, HIT, and serious adverse events.
Population amended at planned interim analysis
After approximately half of the patients were enrolled, a planned and blinded interim analysis for futility concluded that the study was unlikely to show a statistically significant advantage of enoxaparin over placebo. The trial’s steering committee followed the suggestion of its data safety monitoring board to redefine the inclusion criteria to refocus enrollment on patients with a high risk of VTE. A blinded analysis was performed to identify this subgroup.
The resulting amended inclusion criteria were the same as above except that level 2 mobility had to be accompanied by at least one of three additional high-risk criteria: (1) age greater than 75 years, (2) history of prior VTE, or (3) diagnosis of cancer.
The trial’s main exclusion criteria were evidence of active bleeding, a contraindication to anticoagulation, receipt of prophylactic LMWH or UFH more than 72 hours prior to enrollment, treatment with an oral anticoagulant within 72 hours of enrollment, major surgery within the prior 3 months, cerebral stroke with bleeding, and persistent renal failure (creatinine clearance < 30 mL/min).


Results
The amended study population included 5,105 patients, 5,049 of whom received open-label enoxaparin. Of this group, 2,013 were randomized to active extended prophylaxis with enoxparain and 2,027 to placebo. Baseline characteristics, including level of mobility, were similar between the two groups.
The efficacy of extended prophylaxis with enoxaparin was enduring, as the cumulative incidence of VTE events at day 90 was significantly lower in enoxaparin recipients than in placebo recipients (3.0% vs 5.2%; relative reduction of 42%; P = .0115).
There was no difference in all-cause mortality at 6 months between the enoxaparin and placebo groups (10.1% vs 8.9%, respectively; P = .179).
Safety. Major hemorrhage was significantly more frequent in the enoxaparin arm, occurring in 0.60% of enoxaparin recipients compared with 0.15% of placebo recipients (P = .019). Minor bleeding was also more common with enoxaparin (5.20% vs 3.70%; P = .024).
Conclusions
The EXCLAIM trial found that an extended-duration (38-day) enoxaparin regimen significantly reduced the overall incidence of VTE relative to a 10-day enoxaparin regimen in acutely ill medical patients with reduced mobility. At the same time, the extended regimen was associated with a significant increase in the rate of major bleeding, although the incidence of major bleeding was low. The investigators concluded that the net clinical effect of extended-duration prophylaxis with enoxaparin is favorable, as only 46 patients would need to be treated to prevent one VTE event, whereas 224 patients would need to be treated to result in one major bleeding event.21
For this reason, it is reasonable to consider extended prophylaxis for hospitalized medical patients after identifying these patients’ risk factors. In keeping with the trial’s amended inclusion criteria, patients older than age 75 and those with cancer or prior VTE should receive special consideration for extended prophylaxis.
RECOMMENDED APPROACH TO VTE PREVENTION IN HOSPITALIZED MEDICAL PATIENTS
- All hospitalized medical patients should be screened at the time of admission, and patients at risk for VTE should receive prophylaxis.
- All patients with reduced mobility and one or more other risk factors for VTE are candidates for prophylaxis.
- Patients should be reassessed daily for the development of VTE risk factors during their hospitalization if risk factors are absent on admission.
- If screening or reassessment reveals any VTE risk factors, pharmacologic prophylaxis is the mainstay of therapy. If exclusion criteria for pharmacologic prophylaxis are present, mechanical prophylaxis with graduated compression stockings and intermittent compression devices should be used. For very high-risk medical patients without a contraindication to anticoagulants, combination prophylaxis with both an anticoagulant and mechanical devices is preferred.
- In this patient population, LMWH agents are preferred as pharmacologic prophylaxis over UFH and over fondaparinux (which is not currently approved by the US Food and Drug Administration for this population).
- If UFH is to be used in this patient population, 5,000 U three times daily is the preferred dosage.
- Extended pharmacologic prophylaxis should be considered in patients older than age 75 and in patients with a cancer diagnosis or a prior VTE episode.
DISCUSSION: ADDITIONAL PERSPECTIVES FROM THE AUTHORS
Dr. Jaffer: Dr. Spyropoulos, are there any guidelines, other than those from the ACCP, that speak to VTE prophylaxis in hospitalized medical patients? If so, what are their take-home messages and how do they differ from the ACCP guidelines?
Dr. Spyropoulos: I was part of the group that developed the International Consensus Statement (ICS) published in International Angiology in 2006,11 which is more recent than the latest ACCP guidelines, which were published in 2004. The ICS drew on much of the same data that the ACCP did, but we did an updated review of clinical trials.
For VTE prophylaxis in hospitalized medical patients, the ICS recommendations are more specific with regard to the type, dose, and dosing frequency of anticoagulant agents. First, they specify doses for both LMWH agents in this patient setting: 40 mg once daily for enoxaparin, and 5,000 IU once daily for dalteparin.
The ICS document also states that if UFH is the choice for prophylaxis, a regimen of 5,000 U three times daily should be considered. In the past year alone, two analyses suggest that three-times-daily dosing of UFH in medical patients provides superior efficacy to twice-daily dosing, although perhaps at the expense of more bleeding episodes.12,16 It is important to remember that no large placebo-controlled trial supports the efficacy of a UFH regimen of 5,000 U twice daily in this population.
Finally, the ICS document states that fondaparinux 2.5 mg once daily is a viable option for prophylaxis in medical patients, based on the ARTEMIS trial,3 even though this represents an off-label use.
Dr. Jaffer: Real-world use of VTE prophylaxis is far from optimal, especially in medical patients, and this is partly a result of system-of-care issues. I’d like to conclude by asking each of my colleagues to offer your perspectives on how your own institutions have improved their systems of care to promote better use of VTE prophylaxis and what lessons might be shared with others. Dr. McKean, you work at Brigham and Women’s Hospital, which recently reported impressive results with an electronic alert system designed to increase clinicians’ consideration of VTE risk assessment and use of prophylaxis.24 Please tell us about that study and the alert system.
Dr. McKean: Despite many educational initiatives at Brigham and Women’s Hospital, there were still some patients at high risk for VTE who were not receiving appropriate prophylaxis. What Dr. Samuel Goldhaber and his colleagues wanted to determine was whether changing the system of care could result in a reduced incidence of VTE.24 They devised a computer software program linked to the patient database that used eight common risk factors to determine each hospitalized patient’s risk profile for VTE. Each risk factor was weighted according to a point scale, with major risk factors (cancer, prior VTE, or hypercoagulability) assigned 3 points, the intermediate risk factor of surgery assigned 2 points, and minor risk factors (advanced age, obesity, immobility, or use of hormone replacement therapy or oral contraceptives) assigned 1 point. For patients with a total risk score of 4 or greater, the computer screen generates a color-coded VTE risk alert that requires the physician to acknowledge the alert and choose one of three options: order prophylaxis as appropriate, review a 60-page document on the computer to learn more about prophylaxis, or do nothing.
The study found that hospitalized patients who were randomized to treatment under the computer alert system were significantly more likely to receive VTE prophylaxis and significantly less likely to develop VTE than were patients randomized to a control group. The alert system reduced the risk of DVT or PE at 90 days by 41% in patients considered to be at high risk. It was particularly interesting that the incidence of VTE was lower in the intervention group even when physicians chose not to use prophylaxis, which suggests that simply having this alert system in place improved outcomes, perhaps by raising awareness of the risk of VTE.24
Additional studies are needed to better understand physicians’ behavior and determine why they seem to have a disproportionate fear of the risk of bleeding relative to the risk of clotting, including fatal PE, because that is really the heart of the matter. When patients are not given prophylaxis, often it is because of fear of bleeding. It is not clear, however, why some of these patients did not receive mechanical devices as an alternative form of prophylaxis, but this seems to be the case worldwide, as shown recently by the multinational ENDORSE study.22 Meanwhile, as we await studies to better understand physician perceptions and behaviors regarding prophylaxis, we need to work hard to change the system of care.
Dr. Deitelzweig: Over the past couple of years, the Ochsner Clinic has grown from a one-hospital teaching organization to a seven-hospital system with a mix of closed and open medical staff. The challenge is how to take a process that worked well in the one center, where appropriate prophylaxis was used about 90% of the time, and transfer it to the other centers in the larger system. We have endorsed several types of performance tools, such as the change-acceleration processes used by General Electric. The aim is to share a vision of heightening awareness. To do that, we have worked to mobilize the key stakeholders, at least half of them, to build algorithms that they all will endorse. It is easier said than done, however, and we have found it essential to involve both physicians and non-physician colleagues from pharmacy and nursing who have political and organizational clout.
Dr. Brotman: At Johns Hopkins, I took a bit more draconian approach to this issue because I thought that hospitalists often knew that they should be using VTE prophylaxis but sometimes weren’t, and I am not convinced that clinicians always look at prompts. So we came up with a system that incorporates both billing and documentation simultaneously. We put a hard stop on users’ documentation so that they could not sign off on a note or bill for their care until they checked off the kind of VTE prophylaxis they were using. Since hospitalists ultimately care about billing for their work, this system has at least ensured that everybody has considered and documented VTE prophylaxis on a daily basis. There are other hard stops that can be implemented in computer order-entry systems as well, and we are considering ways to roll them out on a broader scale.
However, all of these systems can have problems because patient situations change from day to day. For instance, VTE prophylaxis is not necessarily indicated in a 38-year-old ambulatory patient who comes in with a sickle cell crisis, but you will need to reconsider if the patient ends up in acute chest syndrome in the intensive care unit. I do not yet have a good way to ensure that this is being done on a daily basis with all patients.
Dr. Amin: At the University of California, Irvine, we implemented an electronic alert system, but we locked users in so that they could not complete their admission orders until they answered questions about VTE prevention. This practice increased our VTE prophylaxis rates tremendously. Because we are a level I trauma center, we allow users to bypass the screens one time, but the next time they log in, even to get a simple lab result, they have to answer the questions about VTE prevention.
With any system you develop, you also have to continue with the education process, because clinicians sometimes get into bad habits or simply forget things.
Dr. Spyropolous: At Lovelace Medical Center, we didn’t have the sophistication of an electronic order-entry system, but we had an experienced clinical pharmacist (the director of inpatient pharmacy) who helped to develop and champion VTE prevention guidelines that have then been used throughout the system in close conjunction with our hospitalists’ rounds. This system has been used successfully for the past 7 years.
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thrombo-embolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med 1999; 341:793–800.
- Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation 2004; 110:874–879.
- Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ 2006; 332:325–329.
- Baglin TP, White K, Charles A. Fatal pulmonary embolism in hos-pitalised medical patients. J Clin Pathol 1997; 50:609–610.
- Piazza G, Seddighzadeh A, Goldhaber SZ. Double trouble for 2,609 hospitalized medical patients who developed deep vein thrombosis: prophylaxis omitted more often and pulmonary embolism more frequent. Chest 2007; 132:554–561.
- Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost 2007; 5:1610–1616.
- Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest 2001; 119(1 Suppl):132S–175S.
- Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest 2007; 132:936–945.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thrombo-embolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med 2007; 146:278–288.
- Cardiovascular Disease Educational and Research Trust; Cyprus Cardiovascular Disease Educational and Research Trust; European Venous Forum; International Surgical Thrombosis Forum; International Union of Angiology; Union Internationale de Phlébologie. Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence). Int Angiol 2006; 25:101–161.
- Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta-analysis of randomized controlled trials. Arch Intern Med 2007; 167:1476–1486.
- Mismetti P, Laporte-Simitsidis S, Tardy B, et al. Prevention of venous thromboembolism in internal medicine with unfractionated or low-molecular-weight heparins: a meta-analysis of randomised clinical trials. Thromb Haemost 2000; 83:14–19.
- Lechler E, Schramm W, Flosbach CW. The venous thrombotic risk in non-surgical patients: epidemiological data and efficacy/safety profile of a low-molecular-weight heparin (enoxaparin). The Prime Study Group. Haemostasis 1996; 26(Suppl 2):49–56.
- Kleber FX, Witt C, Vogel G, et al; the PRINCE Study Group. Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease. Am Heart J 2003; 145:614–621.
- King CS, Holley AB, Jackson JL, Shorr AF, Moores LK. Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a meta-analysis. Chest 2007; 131:507–516.
- Burleigh E, Wang C, Foster D, et al. Thromboprophylaxis in medically ill patients at risk for venous thromboembolism. Am J Health Syst Pharm 2006; 63(20 Suppl 6):S23–S29.
- Leykum L, Pugh J, Diuguid D, Papadopoulos K. Cost utility of substituting enoxaparin for unfractionated heparin for prophylaxis of venous thrombosis in the hospitalized medical patient. J Hosp Med 2006; 1:168–176.
- Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy 2006; 26:1438–1445.
- Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med 2007; 167:1471–1475.
- Hull RD, Schellong SM Tapson VF, et al. Extended-duration venous thromboembolism (VTE) prophylaxis in acutely ill medical patients with recent reduced mobility: the EXCLAIM study. Presentation at: International Society on Thrombosis and Haemo-stasis XXIst Congress; July 6–12, 2007; Geneva, Switzerland.
- Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008; 371:387–394.
- Kahn SR, Panju A, Geerts W, et al; CURVE study investigators. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res 2007; 119:145–155.
- Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent thromboembolism among hospitalized patients. N Engl J Med 2005; 352:969–977.
- Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 1991; 151:933–938.
- Howell MD, Geraci JM, Knowlton AA. Congestive heart failure and outpatient risk of venous thromboembolism: a retrospective, case-control study. J Clin Epidemiol 2001; 54:810–816.
- Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet 2006; 367:1075–1079.
- Alikhan R, Cohen AT, Combe S, et al. Prevention of venous thromboembolism in medical patients with enoxaparin: a subgroup analysis of the MEDENOX study. Blood Coagul Firbinolysis 2003; 14:341–346.
- Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107(23 Suppl 1):I9–I16.
- Greinacher A, Warkentin TE. Recognition, treatment, and prevention of heparin-induced thrombocytopenia: review and update. Thromb Res 2006; 118:165–176.
- Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 2005; 106:2710–2715.
- Sanderink GJ, Guimart CG, Ozoux ML, Jariwala NU, Shukla UA, Boutouyrie BX. Pharmacokinetics and pharmacodynamics of the prophylactic dose of enoxaparin once daily over 4 days in patients with renal impairment. Thromb Res 2002; 105:225–231.
- Douketis J, Cook D, Zytaruk N, et al. Dalteparin thromboprophylaxis in critically ill patients with severe renal insufficiency: the DIRECT study [abstract]. J Thromb Haemost 2007; 5(Suppl 2): P-S-680.
- Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg 2002; 12:19–24.
- Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thrombo-embolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med 1999; 341:793–800.
- Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation 2004; 110:874–879.
- Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ 2006; 332:325–329.
- Baglin TP, White K, Charles A. Fatal pulmonary embolism in hos-pitalised medical patients. J Clin Pathol 1997; 50:609–610.
- Piazza G, Seddighzadeh A, Goldhaber SZ. Double trouble for 2,609 hospitalized medical patients who developed deep vein thrombosis: prophylaxis omitted more often and pulmonary embolism more frequent. Chest 2007; 132:554–561.
- Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost 2007; 5:1610–1616.
- Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest 2001; 119(1 Suppl):132S–175S.
- Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest 2007; 132:936–945.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thrombo-embolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med 2007; 146:278–288.
- Cardiovascular Disease Educational and Research Trust; Cyprus Cardiovascular Disease Educational and Research Trust; European Venous Forum; International Surgical Thrombosis Forum; International Union of Angiology; Union Internationale de Phlébologie. Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence). Int Angiol 2006; 25:101–161.
- Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta-analysis of randomized controlled trials. Arch Intern Med 2007; 167:1476–1486.
- Mismetti P, Laporte-Simitsidis S, Tardy B, et al. Prevention of venous thromboembolism in internal medicine with unfractionated or low-molecular-weight heparins: a meta-analysis of randomised clinical trials. Thromb Haemost 2000; 83:14–19.
- Lechler E, Schramm W, Flosbach CW. The venous thrombotic risk in non-surgical patients: epidemiological data and efficacy/safety profile of a low-molecular-weight heparin (enoxaparin). The Prime Study Group. Haemostasis 1996; 26(Suppl 2):49–56.
- Kleber FX, Witt C, Vogel G, et al; the PRINCE Study Group. Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease. Am Heart J 2003; 145:614–621.
- King CS, Holley AB, Jackson JL, Shorr AF, Moores LK. Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a meta-analysis. Chest 2007; 131:507–516.
- Burleigh E, Wang C, Foster D, et al. Thromboprophylaxis in medically ill patients at risk for venous thromboembolism. Am J Health Syst Pharm 2006; 63(20 Suppl 6):S23–S29.
- Leykum L, Pugh J, Diuguid D, Papadopoulos K. Cost utility of substituting enoxaparin for unfractionated heparin for prophylaxis of venous thrombosis in the hospitalized medical patient. J Hosp Med 2006; 1:168–176.
- Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy 2006; 26:1438–1445.
- Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med 2007; 167:1471–1475.
- Hull RD, Schellong SM Tapson VF, et al. Extended-duration venous thromboembolism (VTE) prophylaxis in acutely ill medical patients with recent reduced mobility: the EXCLAIM study. Presentation at: International Society on Thrombosis and Haemo-stasis XXIst Congress; July 6–12, 2007; Geneva, Switzerland.
- Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008; 371:387–394.
- Kahn SR, Panju A, Geerts W, et al; CURVE study investigators. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res 2007; 119:145–155.
- Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent thromboembolism among hospitalized patients. N Engl J Med 2005; 352:969–977.
- Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 1991; 151:933–938.
- Howell MD, Geraci JM, Knowlton AA. Congestive heart failure and outpatient risk of venous thromboembolism: a retrospective, case-control study. J Clin Epidemiol 2001; 54:810–816.
- Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet 2006; 367:1075–1079.
- Alikhan R, Cohen AT, Combe S, et al. Prevention of venous thromboembolism in medical patients with enoxaparin: a subgroup analysis of the MEDENOX study. Blood Coagul Firbinolysis 2003; 14:341–346.
- Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107(23 Suppl 1):I9–I16.
- Greinacher A, Warkentin TE. Recognition, treatment, and prevention of heparin-induced thrombocytopenia: review and update. Thromb Res 2006; 118:165–176.
- Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 2005; 106:2710–2715.
- Sanderink GJ, Guimart CG, Ozoux ML, Jariwala NU, Shukla UA, Boutouyrie BX. Pharmacokinetics and pharmacodynamics of the prophylactic dose of enoxaparin once daily over 4 days in patients with renal impairment. Thromb Res 2002; 105:225–231.
- Douketis J, Cook D, Zytaruk N, et al. Dalteparin thromboprophylaxis in critically ill patients with severe renal insufficiency: the DIRECT study [abstract]. J Thromb Haemost 2007; 5(Suppl 2): P-S-680.
- Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg 2002; 12:19–24.