User login
Nearly half of orthopedic surgery patients do not receive appropriate prophylaxis for venous thromboembolism (VTE), as defined by American College of Chest Physicians (ACCP) consensus guidelines, according to a recent analysis of a nationwide database of hospital admissions.1 Even in teaching hospitals, compliance with consensus guidelines for thromboprophylaxis is suboptimal. In a study of adherence to the ACCP guidelines for VTE prevention among 1,907 surgical patients at 10 teaching hospitals, only 45.2% of hip fracture patients received optimal VTE prophylaxis.2 Rates of optimal prophylaxis were higher among patients undergoing hip arthroplasty and knee arthroplasty—84.3% and 75.9%, respectively—but were still in need of improvement.2
GROWING INTEREST IN POSTOPERATIVE VTE PROPHYLAXIS AS A QUALITY INDICATOR
As noted in the introductory article in this supplement, the Joint Commission on Accreditation of Healthcare Organizations has taken notice of these shortcomings and has proposed national consensus standards for VTE prevention and treatment.3 Among its proposed standards are two related to risk assessment and prophylaxis: whether risk assessment/prophylaxis is ordered within 24 hours of hospital admission and within 24 hours of transfer to the intensive care unit.
Other quality-monitoring initiatives are focused specifically on VTE in the surgical population. The Surgical Care Improvement Project (SCIP) has approved two quality measures with respect to VTE prevention: (1) the proportion of surgical patients for whom recommended VTE prophylaxis is ordered, and (2) the proportion of patients who receive appropriate VTE prophylaxis (based on ACCP guideline recommendations) within 24 hours before or after surgery.4
In the future, two other VTE-related quality measures from SCIP may be implemented by the Centers for Medicare and Medicaid Services: (1) how often intra- or postoperative pulmonary embolism (PE) is diagnosed during the index hospitalization and within 30 days of surgery, and (2) how often intra- or postoperative deep vein thrombosis (DVT) is diagnosed during the index hospitalization and within 30 days of surgery.5
VTE RISK IN ORTHOPEDIC SURGERY
Surgical patients can be stratified into four VTE risk levels—low, moderate, high, and highest—based on age, surgery type, surgery duration, duration of immobilization, and other risk factors.6 For patients undergoing orthopedic surgery, these levels may be defined according to the following patient and surgical characteristics:
- Low risk—surgery duration of less than 30 minutes, age less than 40 years, repair of small fractures
- Moderate risk—age of 40 to 60 years, arthroscopy or repair of lower leg fractures, postoperative plaster cast
- High risk—age greater than 60 years, or age 40 to 60 years with additional VTE risk factors, or immobilization for greater than 4 days
- Highest risk—hip or knee arthroplasty, hip fracture repair, repair of open lower leg fractures, major trauma or spinal cord injury, or multiple risk factors for VTE (age > 40 years, prior VTE, cancer, or hypercoagulable state).
For patients in the low-risk category, no specific prophylaxis is indicated beyond early and aggressive ambulation.6 For those in all other risk categories, prophylaxis with pharmacologic anticoagulant agents and/or mechanical devices is indicated, as reviewed below.
All major orthopedic procedures confer highest risk level
Notably, the “highest risk” category includes any patient undergoing hip or knee arthroplasty or hip fracture repair. Among orthopedic surgery patients in this highest-risk category, rates of VTE events in the absence of prophylaxis are as follows:6
- Calf DVT, 40% to 80%
- Proximal DVT, 10% to 20%
- Clinical PE, 4% to 10%
- Fatal PE, 0.2% to 5%.
Hip replacement poses greater risk than knee replacement
Within this overall highest-risk category, thromboembolic risk in the absence of prophylaxis differs among procedures. Although patients undergoing hip replacement and those undergoing knee replacement have similar rates of DVT of any type,6,7 hip replacement is associated with higher rates of the more clinically important events, specifically proximal DVT and PE. In the absence of prophylaxis, proximal DVT occurs in 23% to 36% of hip replacement patients as opposed to 9% to 20% of knee replacement patients; similarly, PE occurs in 0.7% to 30% of hip replacement patients as compared with 1.8% to 7.0% of knee replacement patients.6,7
What about bleeding risk?
For many orthopedic surgeons, the risk of bleeding as a result of anticoagulant prophylaxis of VTE looms larger than the risk of VTE itself. This is likely because bleeding, when it does occur, is likely to occur more acutely than VTE does and may directly compromise the result of the operation. For this reason, orthopedic surgeons may be more likely to actually witness bleeding events than VTE events (especially fatal PEs) while their patients are still under their care, leading to a misperception of the relative risks of anticoagulation-related bleeding and thromboembolism.
In reality, rates of major bleeding with pharmacologic prophylaxis of VTE are a tiny fraction of the above-listed rates of VTE events in the absence of prophylaxis in patients undergoing major orthopedic surgery. Reported 30-day rates of major bleeding in patients receiving VTE prophylaxis with heparins range from 0.2% to 1.7%; these rates barely differ from the rates among placebo recipients in the same VTE prophylaxis trials, which range from 0.2% to 1.5%.8,9 Additionally, within the continuum of risk of major bleeding from various medical interventions, VTE prophylaxis with heparins is one of the lowest-risk interventions, posing far less risk than, for example, the use of warfarin in ischemic stroke patients or in patients older than 75 years.
PHARMACOLOGIC OPTIONS FOR VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
As reviewed in the introductory article of this supplement, the arsenal of anticoagulants for use in VTE prophylaxis includes low-dose unfractionated heparin (UFH), low-molecular-weight heparin (LMWH) agents such as dalteparin and enoxaparin, and the factor Xa inhibitor fondaparinux. A few additional comments about these and other anticoagulant options is warranted in the specific context of orthopedic surgery.
Fondaparinux. Because most of its formal US indications are for use as VTE prophylaxis in major orthopedic surgery—including hip replacement, knee replacement, and hip fracture repair—fondaparinux has been studied more widely in orthopedic surgery patients than in the other populations reviewed earlier in this supplement. Nevertheless, its use even in these settings has remained somewhat limited. This may be because of concerns over possible increased bleeding risk relative to some other anticoagulants. Because of bleeding risk, fondaparinux is contraindicated in patients who weigh less than 50 kg, and its package insert recommends caution when it is used in the elderly due to an increased risk of bleeding in patients aged 65 or older. Additionally, the Pentasaccharide in Major Knee Surgery (PENTAMAKS) study found fondaparinux to be associated with a significantly higher incidence of major bleeding compared with enoxaparin (2.1% vs 0.2%; P = .006) in major knee surgery, although it was superior to enoxaparin in preventing VTE.10 Other possible reasons for slow adoption of fondaparinux include its long half-life, which results in a sustained antithrombotic effect, its lack of easy reversibility, and a contraindication in patients with renal insufficiency.11
Limited role for UFH. Low-dose UFH has a more limited role in orthopedic surgery than in other settings requiring VTE prophylaxis, as current ACCP guidelines for VTE prevention recognize it only as a possible option in hip fracture surgery and state that it is not to be considered as sole prophylaxis in patients undergoing hip or knee replacement.6
Warfarin. Although not indicated for use in other VTE prophylaxis settings, the vitamin K antagonist warfarin is recommended as an option for all three major orthopedic surgery indications—knee replacement, hip replacement, and hip fracture repair.6
The key to effective prophylaxis with warfarin is achieving the appropriate intensity of anticoagulation. In two separate analyses, Hylek et al demonstrated a balance between safety and efficacy with warfarin therapy targeted to an international normalized ratio (INR) of 2.0 to 3.0.12,13 An INR greater than 4.0 greatly increased the risk of intracranial hemorrhage, whereas thrombosis was not effectively prevented with an INR less than 2.0.12,13 This latter point should be stressed to orthopedic surgeons, who sometimes aim for INR values below 2.0.
Although anticoagulation clinics are superior to usual care at maintaining the INR within the window of 2.0 to 3.0, only about one-third of patients nationally who take warfarin receive care in such clinics.14 Even with optimal care in anticoagulation clinics, some patients will still receive subtherapeutic or supertherapeutic levels of warfarin, which is one of this agent’s limitations.
Aspirin not recommended as sole agent. Although aspirin is still used as thromboprophylaxis in orthopedic surgery patients, current ACCP guidelines recommend against its use as the sole means of VTE prophylaxis in any patient group.6 The limitations of the evidence for aspirin in this setting are illustrated by the Pulmonary Embolism Prevention study, a multicenter randomized trial in patients undergoing hip fracture (n = 13,356) or hip/knee replacement (n = 4,088).15 Patients received aspirin 160 mg/day or placebo for 5 weeks, starting preoperatively, and were evaluated for outcomes at day 35. Among the hip fracture patients, the rate of symptomatic DVT was lower in the aspirin group than in the placebo group (1.0% vs 1.5%; P = .03), as was the rate of PE (0.7% vs 1.2%, respectively; P = .002), but there was no significant difference in outcomes between the groups among the patients undergoing hip or knee replacement. Notably, 40% of patients in the study also received UFH or LMWH. Further confounding the results, some patients received nonpharmacologic VTE prophylaxis modalities, and others received nonsteroidal anti-inflammatory drugs other than aspirin.
Heparin-induced thrombocytopenia. As noted earlier in this supplement, the incidence of heparin-induced thrombocytopenia (HIT) is markedly higher in patients who receive UFH than in those who receive LMWH. This difference in frequency, which constitutes about a sixfold to eightfold differential, is due to the relationship between standard heparin and platelet factor IV, which can induce formation of IgG antibodies.16 A 50% or greater reduction in platelet count in heparin recipients should prompt consideration of HIT.
Oral direct thrombin inhibitors. Although the oral direct thrombin inhibitor ximelagatran was rejected for approval by the US Food and Drug Administration (FDA) and recently withdrawn from the market worldwide as a result of hepatic risks, other oral direct thrombin inhibitors are in phase 3 studies for use in orthopedic surgery and may be commercially available options for postoperative VTE prophylaxis before long.
GUIDELINES FOR VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
The ACCP guidelines referred to throughout this article are widely recognized as a practice standard for VTE prevention and treatment, and have been regularly updated throughout recent decades. The most recent version, issued in 2004, is formally known as the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.6 Key orthopedic surgery-related recommendations and notable changes from the previous version of the guidelines, issued in 2001, are outlined below, along with pertinent supportive or illustrative studies.
Hip replacement surgery
In a change from the previous guidelines, the Seventh ACCP Conference recommends extended prophylaxis, for up to 28 to 35 days after surgery, for patients undergoing hip replacement or hip fracture surgery. For hip replacement surgery, this is a Grade 1A recommendation for prophylaxis with either LMWH or warfarin and a Grade 1C+ recommendation (“no RCTs but strong RCT results can be unequivocally extrapolated, or overwhelming evidence from observational studies”) for prophylaxis with fondaparinux.6
Knee replacement surgery
The same three anticoagulant options that received Grade 1A recommendations for patients undergoing total hip replacement—LMWH, fondaparinux, and adjusted-dose warfarin—are also given Grade 1A recommendations as routine thromboprophylaxis in patients undergoing elective knee replacement (see Table 1 for dosing). In addition, optimal use of intermittent pneumatic compression devices is recommended as an alternative option to anticoagulant prophylaxis in these patients (Grade 1B, indicating a “strong recommendation” based on RCTs with important limitations). Use of UFH as the sole agent for prophylaxis is recommended against.6
For both hip and knee replacement surgery, the Seventh ACCP Conference does not endorse superiority of any one of its three recommended prophylaxis options—LMWH, fondaparinux, and adjusted-dose warfarin—over the other two. However, at least four large randomized trials have directly compared LMWH and adjusted-dose warfarin in the setting of arthroplasty—two in total hip replacement surgery19,20 and two in total knee replacement surgery.21,22 Each of these four studies found LMWH to be significantly more effective than warfarin in preventing VTE. In three of the four trials, there was no significant difference between the therapies in rates of major bleeding.19,21,22 In the remaining trial, which was conducted in hip replacement surgery patients and compared postoperative warfarin with dalteparin initiated either immediately before or early after surgery, patients who received preoperative dalteparin initiation (but not those who received postoperative dalteparin initiation) had an increased rate of major bleeding compared with warfarin recipients (P = .01).20
Hip fracture surgery
The supportive evidence for anticoagulant prophylaxis in hip fracture surgery is less robust than that in hip and knee replacement surgery. As a result, only fondaparinux has a Grade 1A recommendation as routine prophylaxis in patients undergoing hip fracture surgery. Options with less definitive recommendations are LMWH (Grade 1C+), low-dose UFH (Grade 1B), and adjusted-dose warfarin (Grade 2B, indicating a “weak recommendation” based on RCTs with important limitations) (see Table 1 for dosing of all agents).6
These differing recommendations are supported by the double-blind Pentasaccharide in Hip Fracture Surgery Study (PENTHIFRA) of 1,711 consecutive patients undergoing surgery for hip fracture repair.23 Patients were randomized to at least 5 days of fondaparinux 2.5 mg once daily, initiated postoperatively, or enoxaparin 40 mg once daily, initiated preoperatively. The incidence of DVT or PE by postoperative day 11 was 8.3% in the fondaparinux arm versus 19.1% in the enoxaparin arm, a statistically significant difference (P < .001) in favor of fondaparinux. There were no differences between the groups in rates of death or clinically relevant bleeding.
As noted above, the newly added recommendation in the Seventh ACCP Conference for extended prophylaxis, for up to 28 to 35 days after surgery, applies to patients undergoing hip fracture surgery as well as those undergoing hip replacement surgery. In the setting of hip fracture repair, extended prophylaxis is a Grade 1A recommendation with the use of fondaparinux and a Grade 1C+ recommendation with the use of either LMWH or adjusted-dose warfarin.6
Lower extremity fractures and trauma
Although lower extremity fractures are very common, the risk of DVT has been poorly studied in this setting. For patients with isolated lower extremity fractures, the Seventh ACCP Conference recommends that clinicians not use thromboprophylaxis routinely (Grade 2A, indicating an “intermediate-strength recommendation” based on RCTs without important limitations).6
Trauma patients, in contrast, are well recognized as being at very high risk for DVT and PE. The Seventh ACCP Conference gives a Grade 1A recommendation to thromboprophylaxis for all trauma patients who have at least one risk factor for VTE. LMWH is recommended (Grade 1A) as the agent of choice for this purpose, provided there are no contraindications to its use, and should be administered as soon as safely possible. Mechanical modalities are reserved for trauma patients with active bleeding or high risk for hemorrhage (Grade 1B). The guidelines recommend against use of inferior vena cava (IVC) filters as primary thromboprophylaxis in trauma patients (Grade 1C, indicating an “intermediate-strength recommendation” based on observational studies).6
Use of ultrasonography
Duplex ultrasonographic screening is recommended in orthopedic trauma patients who are at high risk for VTE and have received suboptimal or no prophylaxis (Grade 1C). In contrast, the Seventh ACCP Conference recommends against routine use of duplex ultrasonography to screen for VTE at hospital discharge in asymptomatic patients following major orthopedic surgery (Grade 1A).6
Knee arthroscopy
Arthroscopic knee procedures are increasing in frequency and raise the specter of a potential role for thromboprophylaxis. However, the clinical diagnosis of DVT is unreliable, and even diagnosis by ultrasonography is unreliable following knee arthroscopy, as interpreting scans of veins below the knee is challenging in this setting.24
The Seventh ACCP Conference recommends that clinicians not use routine thromboprophylaxis, other than early mobilization, for patients who undergo knee arthroscopy (Grade 2B). However, for arthroscopy patients who have inherent risk factors for VTE or who undergo a prolonged or complicated arthroscopy procedure, thromboprophylaxis with LMWH is suggested (Grade 2B).6
RECOMMENDED APPROACH TO VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
Drawing on the ACCP guidelines and the evidence reviewed above, we have outlined our evidence-based recommendations for pharmacologic VTE prophylaxis in patients undergoing orthopedic surgery, as presented in Table 1. All patients undergoing major orthopedic surgical procedures (ie, procedures other than arthroscopy) should routinely receive anticoagulant prophylaxis unless they have contraindications to anticoagulation. Recommended agents and their duration of use vary according to the type of surgery, as detailed in Table 1.
Extended-duration prophylaxis is recommended for patients undergoing total hip replacement and hip fracture surgery. Aspirin is not recommended as the sole agent for prophylaxis in any orthopedic surgery setting.
Importance of a postoperative prophylaxis protocol
In addition to these broad pharmacologic recommendations, it is important that a postoperative VTE prophylaxis protocol be in place at all hospitals.
At the Ochsner Medical Center in New Orleans, where one of us (S.B.D.) practices, postoperative orders include antithrombotic therapy for surgical patients, starting with placement of thigh-high antiembolism stockings on both legs on the day of surgery for patients undergoing hip replacement and on postoperative day 1 in those undergoing knee replacement. Plantar pneumatic compression devices are applied to both legs in the recovery room and kept on except when the patient is walking. The hospitalist team dictates further anticoagulation orders. If extended prophylaxis is prescribed, the discharge planner sets up drug delivery and reimbursement, provides a LMWH discharge kit, and teaches the patient to self-inject. If there is concern about increasing swelling at the surgical site while anticoagulant therapy continues, the protocol calls for prompt notification of the responsible physician. To minimize the risk that spinal or epidural hematomas will develop, all agents that increase bleeding propensity should be recognized and ordered accordingly.


SUMMARY
VTE in patients undergoing major orthopedic surgery is a serious health problem that is highly preventable, yet VTE prophylaxis remains underused in this patient population. Despite the availability of practice guidelines for VTE prevention in the orthopedic surgery setting, recommendations are not widely implemented in clinical practice. Recommended prophylactic options differ somewhat among various orthopedic procedures, and the supportive evidence differs for various anticoagulant options.
DISCUSSION: ADDITIONAL PERSPECTIVES FROM THE AUTHORS
Dr. Jaffer: The ACCP recommends against the routine use of aspirin as primary prophylaxis against VTE in major orthopedic surgery, yet orthopedic surgeons across the country still continue to use aspirin in this setting. What are your thoughts on this, Dr. McKean?
Dr. McKean: We agree with the ACCP’s recommendation against aspirin as primary VTE prophylaxis in orthopedic patients. The percentage of US knee arthroplasty patients who develop VTE after receiving no prophylaxis at all is roughly 64%; this percentage declines only slightly (to 56%) for knee arthroplasty patients who receive prophylaxis with aspirin.25 Since we clearly want to reduce VTE risk as much as possible, I would not use aspirin alone. I would use it only if the patient were already on aspirin, but then I would add either LMWH or fondaparinux.
Dr. Jaffer: Warfarin is another agent that is widely used for prophylaxis in major orthopedic surgery. In fact, the large registries of VTE prevention in major orthopedic surgery suggest that the use of warfarin may be slightly higher than the use of LMWH. If clinicians choose to use warfarin in their practice, what are your recommendations, Dr. Deitelzweig?
Dr. Deitelzweig: As primary prophylaxis for orthopedic surgery patients, warfarin must be dosed to achieve an INR of 2.0 to 3.0; the need for a value in this range is unequivocal. This is a challenging target to attain in the hospital setting.
Dr. Brotman: A study I was involved with a few years ago suggested that warfarin may be inadequate for VTE prevention in the first few days after orthopedic surgery.26 Orthopedic surgeons at the Cleveland Clinic, where I was practicing at the time, routinely used systematic ultrasonography to assess for thrombosis on postoperative day 2 or 3 following hip or knee arthroplasty, so we conducted a secondary analysis of a case-control study in these ultrasonographically screened arthroplasty patients to assess rates of early VTE and look for any associations with the type of prophylaxis used. We found that there was about a tenfold increase in the risk of VTE, both distal and proximal, on postoperative day 2 or 3 among patients who received warfarin compared with those who received LMWH. We concluded that warfarin’s delayed antithrombotic effects may not provide sufficient VTE prophylaxis in the immediate postoperative setting.26
Dr. Deitelzweig: That’s a good point. Although it’s important to achieve a therapeutic level of warfarin, we now have evidence that it takes some time to achieve that level, and in the interim, bad things can happen to patients.
Dr. Jaffer: Orthopedic surgery encompasses several types of procedures. Dr. Amin, which specific orthopedic surgery patients stand to benefit from extended prophylaxis, and how long should extended prophylaxis last?
Dr. Amin: Major orthopedic surgery comprises hip fracture repair, total hip replacement, and total knee replacement. For hip fracture, there are strong data to support the use of extended prophylaxis with fondaparinux 2.5 mg/day, which showed about an 88% relative reduction in the risk of symptomatic VTE compared with standard-duration fondaparinux (6 to 8 days) followed by matching placebo for the extended phase.27 The total duration of fondaparinux therapy in the extended-duration arm was 4 to 5 weeks.
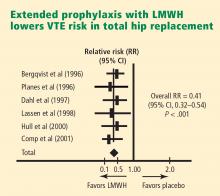
Likewise, data support extended prophylaxis in hip arthroplasty patients, for whom the recommended duration is also 4 to 5 weeks. The systematic review by Hull et al17 demonstrated a 0.41 relative risk of DVT with extended-duration LMWH prophylaxis versus placebo in hip replacement patients (Figure 1), which was a highly statistically significant result.
In contrast, we do not yet have good data to support extended prophylaxis for patients undergoing total knee replacement, which is a bit surprising. In this setting, prophylaxis is recommended for 7 to 14 days but not beyond that.
Dr. Jaffer: Arthroscopy is probably the most common orthopedic procedure performed in the United States today. Dr. Brotman, what is the role of prophylaxis in patients undergoing arthroscopy?
Dr. Brotman: Minor surgery such as arthroscopy can typically be performed safely without routine prophylaxis, other than having the patient ambulate as soon as possible after the procedure. There may be exceptions to this rule, however. I believe that there is potentially a role for pharmacologic prophylaxis in arthroscopy patients who have major risk factors for VTE, such as a personal history of VTE, or who are not expected to become mobile again in a normal rapid fashion after the operation, but prophylaxis has not been studied systematically in such patients.
Dr. Jaffer: Dr. Spyropoulos, there are several new anticoagulants in the pipeline, specifically agents such as the oral direct factor Xa inhibitors and the direct thrombin inhibitors. What do recent clinical trials suggest with regard to the efficacy of these two drug classes for thromboprophylaxis in major orthopedic surgery?
Dr. Spyropoulos: The agents with the most available data are the oral direct factor Xa inhibitors apixaban and rivaroxaban and the oral direct thrombin inhibitor dabigatran. For prophylaxis in orthopedic surgery populations, phase 2 studies have been completed for apixaban and phase 3 trials have been completed for rivaroxaban and dabigatran.
It appears that the factor Xa inhibitors, apixaban and rivaroxaban, are efficacious in comparison with both adjusted-dose warfarin and LMWH, which is the gold standard for this group of patients.28,29 So these indeed appear to be promising agents. Rivaroxaban has been submitted to European regulatory agencies for approval for the prevention of VTE in patients undergoing major orthopedic surgery, and its developer plans to submit it to the FDA in 2008 for a similar indication in the United States.
The data are more equivocal with dabigatran. There have been several positive phase 3 studies in orthopedic surgery comparing two dabigatran dosing schemes, 150 and 220 mg once daily, with the European regimen of enoxaparin (40 mg once daily),30 but a recent study that compared these doses with the North American enoxaparin regimen (30 mg twice daily) failed to meet the criteria for noninferiority.31 Further clinical trial development is necessary for dabigatran, although in January 2008 the European Medicines Agency recommended its marketing approval for thromboprophylaxis in patients undergoing orthopedic procedures.32
I believe that in the next 3 to 5 years our armamentarium will see the addition of at least one, if not more, of these new agents that offer the promise of oral anticoagulation with highly predictable pharmacokinetics and pharmacodynamics and no need for monitoring.
- Yu HT, Dylan ML, Lin J, Dubois RW. Hospitals’ compliance with prophylaxis guidelines for venous thromboembolism. Am J Health Syst Pharm 2007; 64:69–76.
- Stratton MA, Anderson FA, Bussey HI, et al. Prevention of venous thromboembolism: adherence to the 1995 ACCP consensus guidelines for surgical patients. Arch Intern Med 2000; 160:334–340.
- National Consensus Standards for Prevention and Care of Venous Thromboembolism (VTE). The Joint Commission Web site. http://www.jointcommission.org/PerformanceMeasurement/Perform anceMeasurement/VTE.htm. Accessed January 8, 2008.
- Surgical Care Improvement Project. MedQIC Web site. http://www.medqic.org/scip. Accessed January 8, 2008.
- SCIP process and outcome measures, October 2005. MedQIC Web site. http://www.medqic.org. Accessed January 1, 2007.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- Zimlich RH, Fulbright BM, Friedman RJ. Current status of anticoagulation therapy after total hip and total knee arthroplasty.J Am Acad Orthop Surg 1996; 4:54–62.
- Planes A, Vochelle N, Mazas F, et al. Prevention of postoperative venous thrombosis: a randomized trial comparing unfractionated heparin with low molecular weight heparin in patients undergoing total hip replacement. Thromb Haemost 1988; 60:407–410.
- Colwell CW Jr, Spiro TE, Trowbridge AA, et al. Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep venous thrombosis after elective knee arthroplasty. Clin Orthop Relat Res 1995; 321:19–27.
- Bauer KA, Eriksson BI, Lassen MR, Turpie AG. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med 2001; 345:1305–1310.
- Turpie AGG. Pentasaccharide Org31540/SR90107A clinical trials update: lessons for practice. Am Heart J 2001; 142(Suppl):S9–S15.
- Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med 1994; 120:897–902.
- Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med 1996; 335:540–546.
- Samsa GP, Matchar DB, Goldstein LB, et al. Quality of anticoagulation management among patients with atrial fibrillation: review of medical records from 2 communities. Arch Intern Med 2000; 160:967–973.
- PEP Trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000; 355:1295–1302.
- Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haemotol 2003; 121:535–555.
- Hull RD, Pineo GF, Stein PD, et al. Extended out-of-hospital low-molecular-weight heparin prophylaxis against deep venous thrombosis in patients after elective hip arthroplasty: a systematic review. Ann Intern Med 2001; 135:858–869.
- Haentjens P, De Groote K, Annemans L. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement: a cost-utility analysis. Arch Orthop Trauma Surg 2004; 124:507–517.
- Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty: evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am 1999; 81:932–940.
- Hull RD, Pineo GF, Francis C, et al. Low-molecular-weight heparin prophylaxis using dalteparin in close proximity to surgery vs warfarin in hip arthroplasty patients: a double-blind, randomized comparison. Arch Intern Med 2000; 160:2199–2207.
- Leclerc JR, Geerts WH, Desjardins L, et al. Prevention of venous thromboembolism after knee arthroplasty: a randomized, double-blind trial comparing enoxaparin with warfarin. Ann Intern Med 1996; 124:619–626.
- Fitzgerald RH Jr, Spiro TE, Trowbridge AA, et al. Prevention of venous thromboembolic disease following primary total knee arthroplasty: a randomized, multicenter, open-label, parallel-group comparison of enoxaparin and warfarin. J Bone Joint Surg Am 2001; 83-A:900–906.
- Eriksson BI, Bauer KA, Lassen MR, Turpie AG, Steering Committee of the Pentasaccharide in Hip-Fracture Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med 2001; 345:1298–1304.
- Demers C, Marcoux S, Ginsberg JS, Laroche F, Cloutier R, Poulin J. Incidence of venographically proved deep vein thrombosis after knee arthroscopy. Arch Intern Med 1998; 158:47–50.
- Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest 2001; 119(1 Suppl):132S–175S.
- Brotman DJ, Jaffer AK, Hurbanek JG, Morra N. Warfarin prophylaxis and venous thromboembolism in the first 5 days following hip and knee arthroplasty. Thromb Haemost 2004; 92:1012–1017.
- Eriksson BI, Lassen MR; Pentasaccharide in Hip-Fracture Surgery Plus Investigators. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med 2003; 163:1337–1342.
- The Botticelli Investigators. Late-breaking clinical trial: a dose-finding study of the oral direct factor Xa inhibitor apixaban in the treatment of patients with acute symptomatic deep vein thrombosis [abstract]. Presented at the 21st Congress of the International Society on Thrombosis and Haemostasis; July 2007; Geneva, Switzerland.
- Fisher WD, Eriksson BI, Bauer KA, et al. Rivaroxaban for thromboprophylaxis after orthopaedic surgery: pooled analysis of two studies. Thromb Haemost 2007; 97:931–937.
- Haas S. New oral Xa and IIa inhibitors: updates on clinical trial results. J Thromb Thrombolysis 2008; 25:52–60.
- Friedman RJ, Caprini JA, Comp PC, et al. Dabigatran etexilate vs enoxaparin in preventing venous thromboembolism following total knee arthroplasty. Presented at: 2007 Congress of the International Society on Thrombosis and Haemostasis; July 7–13, 2007; Geneva, Switzerland.
- Committee for Medicinal Products for Human Use summary of positive opinion for Pradaxa [news release]. London, UK: European Medicines Agency. January 24, 2008. http://www.emea.europa.eu/pdfs/human/ opinion/Pradaxa_3503008en.pdf. Accessed February 21, 2008.
- Goldhaber SZ, Grodstein F, Stampfer MJ. A prospective study of risk factors for pulmonary embolism in women. JAMA 1997; 277:642–645.
- Turpie AGG, Bauer KA, Eriksson BI, Lassen MR, for the Steering Committees of the Pentasaccharide Orthopedic Prophylaxis Studies. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery. Arch Intern Med 2002; 162:1833–1840.
- Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 2007; 120:871–879.
- Goldhaber SZ. Diagnosis of acute pulmonary embolism: always be vigilant. Am J Med 2007; 120:827–828.
- American Academy of Orthopaedic Surgeons Clinical Guideline on Prevention of Symptomatic Pulmonary Embolism in Patients Undergoing Total Hip or Knee Arthroplasty: Summary of Recommendations. http://www.aaos.org/Research/guidelines/PE_ summary.pdf. Accessed December 10, 2007.
Nearly half of orthopedic surgery patients do not receive appropriate prophylaxis for venous thromboembolism (VTE), as defined by American College of Chest Physicians (ACCP) consensus guidelines, according to a recent analysis of a nationwide database of hospital admissions.1 Even in teaching hospitals, compliance with consensus guidelines for thromboprophylaxis is suboptimal. In a study of adherence to the ACCP guidelines for VTE prevention among 1,907 surgical patients at 10 teaching hospitals, only 45.2% of hip fracture patients received optimal VTE prophylaxis.2 Rates of optimal prophylaxis were higher among patients undergoing hip arthroplasty and knee arthroplasty—84.3% and 75.9%, respectively—but were still in need of improvement.2
GROWING INTEREST IN POSTOPERATIVE VTE PROPHYLAXIS AS A QUALITY INDICATOR
As noted in the introductory article in this supplement, the Joint Commission on Accreditation of Healthcare Organizations has taken notice of these shortcomings and has proposed national consensus standards for VTE prevention and treatment.3 Among its proposed standards are two related to risk assessment and prophylaxis: whether risk assessment/prophylaxis is ordered within 24 hours of hospital admission and within 24 hours of transfer to the intensive care unit.
Other quality-monitoring initiatives are focused specifically on VTE in the surgical population. The Surgical Care Improvement Project (SCIP) has approved two quality measures with respect to VTE prevention: (1) the proportion of surgical patients for whom recommended VTE prophylaxis is ordered, and (2) the proportion of patients who receive appropriate VTE prophylaxis (based on ACCP guideline recommendations) within 24 hours before or after surgery.4
In the future, two other VTE-related quality measures from SCIP may be implemented by the Centers for Medicare and Medicaid Services: (1) how often intra- or postoperative pulmonary embolism (PE) is diagnosed during the index hospitalization and within 30 days of surgery, and (2) how often intra- or postoperative deep vein thrombosis (DVT) is diagnosed during the index hospitalization and within 30 days of surgery.5
VTE RISK IN ORTHOPEDIC SURGERY
Surgical patients can be stratified into four VTE risk levels—low, moderate, high, and highest—based on age, surgery type, surgery duration, duration of immobilization, and other risk factors.6 For patients undergoing orthopedic surgery, these levels may be defined according to the following patient and surgical characteristics:
- Low risk—surgery duration of less than 30 minutes, age less than 40 years, repair of small fractures
- Moderate risk—age of 40 to 60 years, arthroscopy or repair of lower leg fractures, postoperative plaster cast
- High risk—age greater than 60 years, or age 40 to 60 years with additional VTE risk factors, or immobilization for greater than 4 days
- Highest risk—hip or knee arthroplasty, hip fracture repair, repair of open lower leg fractures, major trauma or spinal cord injury, or multiple risk factors for VTE (age > 40 years, prior VTE, cancer, or hypercoagulable state).
For patients in the low-risk category, no specific prophylaxis is indicated beyond early and aggressive ambulation.6 For those in all other risk categories, prophylaxis with pharmacologic anticoagulant agents and/or mechanical devices is indicated, as reviewed below.
All major orthopedic procedures confer highest risk level
Notably, the “highest risk” category includes any patient undergoing hip or knee arthroplasty or hip fracture repair. Among orthopedic surgery patients in this highest-risk category, rates of VTE events in the absence of prophylaxis are as follows:6
- Calf DVT, 40% to 80%
- Proximal DVT, 10% to 20%
- Clinical PE, 4% to 10%
- Fatal PE, 0.2% to 5%.
Hip replacement poses greater risk than knee replacement
Within this overall highest-risk category, thromboembolic risk in the absence of prophylaxis differs among procedures. Although patients undergoing hip replacement and those undergoing knee replacement have similar rates of DVT of any type,6,7 hip replacement is associated with higher rates of the more clinically important events, specifically proximal DVT and PE. In the absence of prophylaxis, proximal DVT occurs in 23% to 36% of hip replacement patients as opposed to 9% to 20% of knee replacement patients; similarly, PE occurs in 0.7% to 30% of hip replacement patients as compared with 1.8% to 7.0% of knee replacement patients.6,7
What about bleeding risk?
For many orthopedic surgeons, the risk of bleeding as a result of anticoagulant prophylaxis of VTE looms larger than the risk of VTE itself. This is likely because bleeding, when it does occur, is likely to occur more acutely than VTE does and may directly compromise the result of the operation. For this reason, orthopedic surgeons may be more likely to actually witness bleeding events than VTE events (especially fatal PEs) while their patients are still under their care, leading to a misperception of the relative risks of anticoagulation-related bleeding and thromboembolism.
In reality, rates of major bleeding with pharmacologic prophylaxis of VTE are a tiny fraction of the above-listed rates of VTE events in the absence of prophylaxis in patients undergoing major orthopedic surgery. Reported 30-day rates of major bleeding in patients receiving VTE prophylaxis with heparins range from 0.2% to 1.7%; these rates barely differ from the rates among placebo recipients in the same VTE prophylaxis trials, which range from 0.2% to 1.5%.8,9 Additionally, within the continuum of risk of major bleeding from various medical interventions, VTE prophylaxis with heparins is one of the lowest-risk interventions, posing far less risk than, for example, the use of warfarin in ischemic stroke patients or in patients older than 75 years.
PHARMACOLOGIC OPTIONS FOR VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
As reviewed in the introductory article of this supplement, the arsenal of anticoagulants for use in VTE prophylaxis includes low-dose unfractionated heparin (UFH), low-molecular-weight heparin (LMWH) agents such as dalteparin and enoxaparin, and the factor Xa inhibitor fondaparinux. A few additional comments about these and other anticoagulant options is warranted in the specific context of orthopedic surgery.
Fondaparinux. Because most of its formal US indications are for use as VTE prophylaxis in major orthopedic surgery—including hip replacement, knee replacement, and hip fracture repair—fondaparinux has been studied more widely in orthopedic surgery patients than in the other populations reviewed earlier in this supplement. Nevertheless, its use even in these settings has remained somewhat limited. This may be because of concerns over possible increased bleeding risk relative to some other anticoagulants. Because of bleeding risk, fondaparinux is contraindicated in patients who weigh less than 50 kg, and its package insert recommends caution when it is used in the elderly due to an increased risk of bleeding in patients aged 65 or older. Additionally, the Pentasaccharide in Major Knee Surgery (PENTAMAKS) study found fondaparinux to be associated with a significantly higher incidence of major bleeding compared with enoxaparin (2.1% vs 0.2%; P = .006) in major knee surgery, although it was superior to enoxaparin in preventing VTE.10 Other possible reasons for slow adoption of fondaparinux include its long half-life, which results in a sustained antithrombotic effect, its lack of easy reversibility, and a contraindication in patients with renal insufficiency.11
Limited role for UFH. Low-dose UFH has a more limited role in orthopedic surgery than in other settings requiring VTE prophylaxis, as current ACCP guidelines for VTE prevention recognize it only as a possible option in hip fracture surgery and state that it is not to be considered as sole prophylaxis in patients undergoing hip or knee replacement.6
Warfarin. Although not indicated for use in other VTE prophylaxis settings, the vitamin K antagonist warfarin is recommended as an option for all three major orthopedic surgery indications—knee replacement, hip replacement, and hip fracture repair.6
The key to effective prophylaxis with warfarin is achieving the appropriate intensity of anticoagulation. In two separate analyses, Hylek et al demonstrated a balance between safety and efficacy with warfarin therapy targeted to an international normalized ratio (INR) of 2.0 to 3.0.12,13 An INR greater than 4.0 greatly increased the risk of intracranial hemorrhage, whereas thrombosis was not effectively prevented with an INR less than 2.0.12,13 This latter point should be stressed to orthopedic surgeons, who sometimes aim for INR values below 2.0.
Although anticoagulation clinics are superior to usual care at maintaining the INR within the window of 2.0 to 3.0, only about one-third of patients nationally who take warfarin receive care in such clinics.14 Even with optimal care in anticoagulation clinics, some patients will still receive subtherapeutic or supertherapeutic levels of warfarin, which is one of this agent’s limitations.
Aspirin not recommended as sole agent. Although aspirin is still used as thromboprophylaxis in orthopedic surgery patients, current ACCP guidelines recommend against its use as the sole means of VTE prophylaxis in any patient group.6 The limitations of the evidence for aspirin in this setting are illustrated by the Pulmonary Embolism Prevention study, a multicenter randomized trial in patients undergoing hip fracture (n = 13,356) or hip/knee replacement (n = 4,088).15 Patients received aspirin 160 mg/day or placebo for 5 weeks, starting preoperatively, and were evaluated for outcomes at day 35. Among the hip fracture patients, the rate of symptomatic DVT was lower in the aspirin group than in the placebo group (1.0% vs 1.5%; P = .03), as was the rate of PE (0.7% vs 1.2%, respectively; P = .002), but there was no significant difference in outcomes between the groups among the patients undergoing hip or knee replacement. Notably, 40% of patients in the study also received UFH or LMWH. Further confounding the results, some patients received nonpharmacologic VTE prophylaxis modalities, and others received nonsteroidal anti-inflammatory drugs other than aspirin.
Heparin-induced thrombocytopenia. As noted earlier in this supplement, the incidence of heparin-induced thrombocytopenia (HIT) is markedly higher in patients who receive UFH than in those who receive LMWH. This difference in frequency, which constitutes about a sixfold to eightfold differential, is due to the relationship between standard heparin and platelet factor IV, which can induce formation of IgG antibodies.16 A 50% or greater reduction in platelet count in heparin recipients should prompt consideration of HIT.
Oral direct thrombin inhibitors. Although the oral direct thrombin inhibitor ximelagatran was rejected for approval by the US Food and Drug Administration (FDA) and recently withdrawn from the market worldwide as a result of hepatic risks, other oral direct thrombin inhibitors are in phase 3 studies for use in orthopedic surgery and may be commercially available options for postoperative VTE prophylaxis before long.
GUIDELINES FOR VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
The ACCP guidelines referred to throughout this article are widely recognized as a practice standard for VTE prevention and treatment, and have been regularly updated throughout recent decades. The most recent version, issued in 2004, is formally known as the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.6 Key orthopedic surgery-related recommendations and notable changes from the previous version of the guidelines, issued in 2001, are outlined below, along with pertinent supportive or illustrative studies.
Hip replacement surgery
In a change from the previous guidelines, the Seventh ACCP Conference recommends extended prophylaxis, for up to 28 to 35 days after surgery, for patients undergoing hip replacement or hip fracture surgery. For hip replacement surgery, this is a Grade 1A recommendation for prophylaxis with either LMWH or warfarin and a Grade 1C+ recommendation (“no RCTs but strong RCT results can be unequivocally extrapolated, or overwhelming evidence from observational studies”) for prophylaxis with fondaparinux.6
Knee replacement surgery
The same three anticoagulant options that received Grade 1A recommendations for patients undergoing total hip replacement—LMWH, fondaparinux, and adjusted-dose warfarin—are also given Grade 1A recommendations as routine thromboprophylaxis in patients undergoing elective knee replacement (see Table 1 for dosing). In addition, optimal use of intermittent pneumatic compression devices is recommended as an alternative option to anticoagulant prophylaxis in these patients (Grade 1B, indicating a “strong recommendation” based on RCTs with important limitations). Use of UFH as the sole agent for prophylaxis is recommended against.6
For both hip and knee replacement surgery, the Seventh ACCP Conference does not endorse superiority of any one of its three recommended prophylaxis options—LMWH, fondaparinux, and adjusted-dose warfarin—over the other two. However, at least four large randomized trials have directly compared LMWH and adjusted-dose warfarin in the setting of arthroplasty—two in total hip replacement surgery19,20 and two in total knee replacement surgery.21,22 Each of these four studies found LMWH to be significantly more effective than warfarin in preventing VTE. In three of the four trials, there was no significant difference between the therapies in rates of major bleeding.19,21,22 In the remaining trial, which was conducted in hip replacement surgery patients and compared postoperative warfarin with dalteparin initiated either immediately before or early after surgery, patients who received preoperative dalteparin initiation (but not those who received postoperative dalteparin initiation) had an increased rate of major bleeding compared with warfarin recipients (P = .01).20
Hip fracture surgery
The supportive evidence for anticoagulant prophylaxis in hip fracture surgery is less robust than that in hip and knee replacement surgery. As a result, only fondaparinux has a Grade 1A recommendation as routine prophylaxis in patients undergoing hip fracture surgery. Options with less definitive recommendations are LMWH (Grade 1C+), low-dose UFH (Grade 1B), and adjusted-dose warfarin (Grade 2B, indicating a “weak recommendation” based on RCTs with important limitations) (see Table 1 for dosing of all agents).6
These differing recommendations are supported by the double-blind Pentasaccharide in Hip Fracture Surgery Study (PENTHIFRA) of 1,711 consecutive patients undergoing surgery for hip fracture repair.23 Patients were randomized to at least 5 days of fondaparinux 2.5 mg once daily, initiated postoperatively, or enoxaparin 40 mg once daily, initiated preoperatively. The incidence of DVT or PE by postoperative day 11 was 8.3% in the fondaparinux arm versus 19.1% in the enoxaparin arm, a statistically significant difference (P < .001) in favor of fondaparinux. There were no differences between the groups in rates of death or clinically relevant bleeding.
As noted above, the newly added recommendation in the Seventh ACCP Conference for extended prophylaxis, for up to 28 to 35 days after surgery, applies to patients undergoing hip fracture surgery as well as those undergoing hip replacement surgery. In the setting of hip fracture repair, extended prophylaxis is a Grade 1A recommendation with the use of fondaparinux and a Grade 1C+ recommendation with the use of either LMWH or adjusted-dose warfarin.6
Lower extremity fractures and trauma
Although lower extremity fractures are very common, the risk of DVT has been poorly studied in this setting. For patients with isolated lower extremity fractures, the Seventh ACCP Conference recommends that clinicians not use thromboprophylaxis routinely (Grade 2A, indicating an “intermediate-strength recommendation” based on RCTs without important limitations).6
Trauma patients, in contrast, are well recognized as being at very high risk for DVT and PE. The Seventh ACCP Conference gives a Grade 1A recommendation to thromboprophylaxis for all trauma patients who have at least one risk factor for VTE. LMWH is recommended (Grade 1A) as the agent of choice for this purpose, provided there are no contraindications to its use, and should be administered as soon as safely possible. Mechanical modalities are reserved for trauma patients with active bleeding or high risk for hemorrhage (Grade 1B). The guidelines recommend against use of inferior vena cava (IVC) filters as primary thromboprophylaxis in trauma patients (Grade 1C, indicating an “intermediate-strength recommendation” based on observational studies).6
Use of ultrasonography
Duplex ultrasonographic screening is recommended in orthopedic trauma patients who are at high risk for VTE and have received suboptimal or no prophylaxis (Grade 1C). In contrast, the Seventh ACCP Conference recommends against routine use of duplex ultrasonography to screen for VTE at hospital discharge in asymptomatic patients following major orthopedic surgery (Grade 1A).6
Knee arthroscopy
Arthroscopic knee procedures are increasing in frequency and raise the specter of a potential role for thromboprophylaxis. However, the clinical diagnosis of DVT is unreliable, and even diagnosis by ultrasonography is unreliable following knee arthroscopy, as interpreting scans of veins below the knee is challenging in this setting.24
The Seventh ACCP Conference recommends that clinicians not use routine thromboprophylaxis, other than early mobilization, for patients who undergo knee arthroscopy (Grade 2B). However, for arthroscopy patients who have inherent risk factors for VTE or who undergo a prolonged or complicated arthroscopy procedure, thromboprophylaxis with LMWH is suggested (Grade 2B).6
RECOMMENDED APPROACH TO VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
Drawing on the ACCP guidelines and the evidence reviewed above, we have outlined our evidence-based recommendations for pharmacologic VTE prophylaxis in patients undergoing orthopedic surgery, as presented in Table 1. All patients undergoing major orthopedic surgical procedures (ie, procedures other than arthroscopy) should routinely receive anticoagulant prophylaxis unless they have contraindications to anticoagulation. Recommended agents and their duration of use vary according to the type of surgery, as detailed in Table 1.
Extended-duration prophylaxis is recommended for patients undergoing total hip replacement and hip fracture surgery. Aspirin is not recommended as the sole agent for prophylaxis in any orthopedic surgery setting.
Importance of a postoperative prophylaxis protocol
In addition to these broad pharmacologic recommendations, it is important that a postoperative VTE prophylaxis protocol be in place at all hospitals.
At the Ochsner Medical Center in New Orleans, where one of us (S.B.D.) practices, postoperative orders include antithrombotic therapy for surgical patients, starting with placement of thigh-high antiembolism stockings on both legs on the day of surgery for patients undergoing hip replacement and on postoperative day 1 in those undergoing knee replacement. Plantar pneumatic compression devices are applied to both legs in the recovery room and kept on except when the patient is walking. The hospitalist team dictates further anticoagulation orders. If extended prophylaxis is prescribed, the discharge planner sets up drug delivery and reimbursement, provides a LMWH discharge kit, and teaches the patient to self-inject. If there is concern about increasing swelling at the surgical site while anticoagulant therapy continues, the protocol calls for prompt notification of the responsible physician. To minimize the risk that spinal or epidural hematomas will develop, all agents that increase bleeding propensity should be recognized and ordered accordingly.



SUMMARY
VTE in patients undergoing major orthopedic surgery is a serious health problem that is highly preventable, yet VTE prophylaxis remains underused in this patient population. Despite the availability of practice guidelines for VTE prevention in the orthopedic surgery setting, recommendations are not widely implemented in clinical practice. Recommended prophylactic options differ somewhat among various orthopedic procedures, and the supportive evidence differs for various anticoagulant options.
DISCUSSION: ADDITIONAL PERSPECTIVES FROM THE AUTHORS
Dr. Jaffer: The ACCP recommends against the routine use of aspirin as primary prophylaxis against VTE in major orthopedic surgery, yet orthopedic surgeons across the country still continue to use aspirin in this setting. What are your thoughts on this, Dr. McKean?
Dr. McKean: We agree with the ACCP’s recommendation against aspirin as primary VTE prophylaxis in orthopedic patients. The percentage of US knee arthroplasty patients who develop VTE after receiving no prophylaxis at all is roughly 64%; this percentage declines only slightly (to 56%) for knee arthroplasty patients who receive prophylaxis with aspirin.25 Since we clearly want to reduce VTE risk as much as possible, I would not use aspirin alone. I would use it only if the patient were already on aspirin, but then I would add either LMWH or fondaparinux.
Dr. Jaffer: Warfarin is another agent that is widely used for prophylaxis in major orthopedic surgery. In fact, the large registries of VTE prevention in major orthopedic surgery suggest that the use of warfarin may be slightly higher than the use of LMWH. If clinicians choose to use warfarin in their practice, what are your recommendations, Dr. Deitelzweig?
Dr. Deitelzweig: As primary prophylaxis for orthopedic surgery patients, warfarin must be dosed to achieve an INR of 2.0 to 3.0; the need for a value in this range is unequivocal. This is a challenging target to attain in the hospital setting.
Dr. Brotman: A study I was involved with a few years ago suggested that warfarin may be inadequate for VTE prevention in the first few days after orthopedic surgery.26 Orthopedic surgeons at the Cleveland Clinic, where I was practicing at the time, routinely used systematic ultrasonography to assess for thrombosis on postoperative day 2 or 3 following hip or knee arthroplasty, so we conducted a secondary analysis of a case-control study in these ultrasonographically screened arthroplasty patients to assess rates of early VTE and look for any associations with the type of prophylaxis used. We found that there was about a tenfold increase in the risk of VTE, both distal and proximal, on postoperative day 2 or 3 among patients who received warfarin compared with those who received LMWH. We concluded that warfarin’s delayed antithrombotic effects may not provide sufficient VTE prophylaxis in the immediate postoperative setting.26
Dr. Deitelzweig: That’s a good point. Although it’s important to achieve a therapeutic level of warfarin, we now have evidence that it takes some time to achieve that level, and in the interim, bad things can happen to patients.
Dr. Jaffer: Orthopedic surgery encompasses several types of procedures. Dr. Amin, which specific orthopedic surgery patients stand to benefit from extended prophylaxis, and how long should extended prophylaxis last?
Dr. Amin: Major orthopedic surgery comprises hip fracture repair, total hip replacement, and total knee replacement. For hip fracture, there are strong data to support the use of extended prophylaxis with fondaparinux 2.5 mg/day, which showed about an 88% relative reduction in the risk of symptomatic VTE compared with standard-duration fondaparinux (6 to 8 days) followed by matching placebo for the extended phase.27 The total duration of fondaparinux therapy in the extended-duration arm was 4 to 5 weeks.
Likewise, data support extended prophylaxis in hip arthroplasty patients, for whom the recommended duration is also 4 to 5 weeks. The systematic review by Hull et al17 demonstrated a 0.41 relative risk of DVT with extended-duration LMWH prophylaxis versus placebo in hip replacement patients (Figure 1), which was a highly statistically significant result.
In contrast, we do not yet have good data to support extended prophylaxis for patients undergoing total knee replacement, which is a bit surprising. In this setting, prophylaxis is recommended for 7 to 14 days but not beyond that.
Dr. Jaffer: Arthroscopy is probably the most common orthopedic procedure performed in the United States today. Dr. Brotman, what is the role of prophylaxis in patients undergoing arthroscopy?
Dr. Brotman: Minor surgery such as arthroscopy can typically be performed safely without routine prophylaxis, other than having the patient ambulate as soon as possible after the procedure. There may be exceptions to this rule, however. I believe that there is potentially a role for pharmacologic prophylaxis in arthroscopy patients who have major risk factors for VTE, such as a personal history of VTE, or who are not expected to become mobile again in a normal rapid fashion after the operation, but prophylaxis has not been studied systematically in such patients.
Dr. Jaffer: Dr. Spyropoulos, there are several new anticoagulants in the pipeline, specifically agents such as the oral direct factor Xa inhibitors and the direct thrombin inhibitors. What do recent clinical trials suggest with regard to the efficacy of these two drug classes for thromboprophylaxis in major orthopedic surgery?
Dr. Spyropoulos: The agents with the most available data are the oral direct factor Xa inhibitors apixaban and rivaroxaban and the oral direct thrombin inhibitor dabigatran. For prophylaxis in orthopedic surgery populations, phase 2 studies have been completed for apixaban and phase 3 trials have been completed for rivaroxaban and dabigatran.
It appears that the factor Xa inhibitors, apixaban and rivaroxaban, are efficacious in comparison with both adjusted-dose warfarin and LMWH, which is the gold standard for this group of patients.28,29 So these indeed appear to be promising agents. Rivaroxaban has been submitted to European regulatory agencies for approval for the prevention of VTE in patients undergoing major orthopedic surgery, and its developer plans to submit it to the FDA in 2008 for a similar indication in the United States.
The data are more equivocal with dabigatran. There have been several positive phase 3 studies in orthopedic surgery comparing two dabigatran dosing schemes, 150 and 220 mg once daily, with the European regimen of enoxaparin (40 mg once daily),30 but a recent study that compared these doses with the North American enoxaparin regimen (30 mg twice daily) failed to meet the criteria for noninferiority.31 Further clinical trial development is necessary for dabigatran, although in January 2008 the European Medicines Agency recommended its marketing approval for thromboprophylaxis in patients undergoing orthopedic procedures.32
I believe that in the next 3 to 5 years our armamentarium will see the addition of at least one, if not more, of these new agents that offer the promise of oral anticoagulation with highly predictable pharmacokinetics and pharmacodynamics and no need for monitoring.
Nearly half of orthopedic surgery patients do not receive appropriate prophylaxis for venous thromboembolism (VTE), as defined by American College of Chest Physicians (ACCP) consensus guidelines, according to a recent analysis of a nationwide database of hospital admissions.1 Even in teaching hospitals, compliance with consensus guidelines for thromboprophylaxis is suboptimal. In a study of adherence to the ACCP guidelines for VTE prevention among 1,907 surgical patients at 10 teaching hospitals, only 45.2% of hip fracture patients received optimal VTE prophylaxis.2 Rates of optimal prophylaxis were higher among patients undergoing hip arthroplasty and knee arthroplasty—84.3% and 75.9%, respectively—but were still in need of improvement.2
GROWING INTEREST IN POSTOPERATIVE VTE PROPHYLAXIS AS A QUALITY INDICATOR
As noted in the introductory article in this supplement, the Joint Commission on Accreditation of Healthcare Organizations has taken notice of these shortcomings and has proposed national consensus standards for VTE prevention and treatment.3 Among its proposed standards are two related to risk assessment and prophylaxis: whether risk assessment/prophylaxis is ordered within 24 hours of hospital admission and within 24 hours of transfer to the intensive care unit.
Other quality-monitoring initiatives are focused specifically on VTE in the surgical population. The Surgical Care Improvement Project (SCIP) has approved two quality measures with respect to VTE prevention: (1) the proportion of surgical patients for whom recommended VTE prophylaxis is ordered, and (2) the proportion of patients who receive appropriate VTE prophylaxis (based on ACCP guideline recommendations) within 24 hours before or after surgery.4
In the future, two other VTE-related quality measures from SCIP may be implemented by the Centers for Medicare and Medicaid Services: (1) how often intra- or postoperative pulmonary embolism (PE) is diagnosed during the index hospitalization and within 30 days of surgery, and (2) how often intra- or postoperative deep vein thrombosis (DVT) is diagnosed during the index hospitalization and within 30 days of surgery.5
VTE RISK IN ORTHOPEDIC SURGERY
Surgical patients can be stratified into four VTE risk levels—low, moderate, high, and highest—based on age, surgery type, surgery duration, duration of immobilization, and other risk factors.6 For patients undergoing orthopedic surgery, these levels may be defined according to the following patient and surgical characteristics:
- Low risk—surgery duration of less than 30 minutes, age less than 40 years, repair of small fractures
- Moderate risk—age of 40 to 60 years, arthroscopy or repair of lower leg fractures, postoperative plaster cast
- High risk—age greater than 60 years, or age 40 to 60 years with additional VTE risk factors, or immobilization for greater than 4 days
- Highest risk—hip or knee arthroplasty, hip fracture repair, repair of open lower leg fractures, major trauma or spinal cord injury, or multiple risk factors for VTE (age > 40 years, prior VTE, cancer, or hypercoagulable state).
For patients in the low-risk category, no specific prophylaxis is indicated beyond early and aggressive ambulation.6 For those in all other risk categories, prophylaxis with pharmacologic anticoagulant agents and/or mechanical devices is indicated, as reviewed below.
All major orthopedic procedures confer highest risk level
Notably, the “highest risk” category includes any patient undergoing hip or knee arthroplasty or hip fracture repair. Among orthopedic surgery patients in this highest-risk category, rates of VTE events in the absence of prophylaxis are as follows:6
- Calf DVT, 40% to 80%
- Proximal DVT, 10% to 20%
- Clinical PE, 4% to 10%
- Fatal PE, 0.2% to 5%.
Hip replacement poses greater risk than knee replacement
Within this overall highest-risk category, thromboembolic risk in the absence of prophylaxis differs among procedures. Although patients undergoing hip replacement and those undergoing knee replacement have similar rates of DVT of any type,6,7 hip replacement is associated with higher rates of the more clinically important events, specifically proximal DVT and PE. In the absence of prophylaxis, proximal DVT occurs in 23% to 36% of hip replacement patients as opposed to 9% to 20% of knee replacement patients; similarly, PE occurs in 0.7% to 30% of hip replacement patients as compared with 1.8% to 7.0% of knee replacement patients.6,7
What about bleeding risk?
For many orthopedic surgeons, the risk of bleeding as a result of anticoagulant prophylaxis of VTE looms larger than the risk of VTE itself. This is likely because bleeding, when it does occur, is likely to occur more acutely than VTE does and may directly compromise the result of the operation. For this reason, orthopedic surgeons may be more likely to actually witness bleeding events than VTE events (especially fatal PEs) while their patients are still under their care, leading to a misperception of the relative risks of anticoagulation-related bleeding and thromboembolism.
In reality, rates of major bleeding with pharmacologic prophylaxis of VTE are a tiny fraction of the above-listed rates of VTE events in the absence of prophylaxis in patients undergoing major orthopedic surgery. Reported 30-day rates of major bleeding in patients receiving VTE prophylaxis with heparins range from 0.2% to 1.7%; these rates barely differ from the rates among placebo recipients in the same VTE prophylaxis trials, which range from 0.2% to 1.5%.8,9 Additionally, within the continuum of risk of major bleeding from various medical interventions, VTE prophylaxis with heparins is one of the lowest-risk interventions, posing far less risk than, for example, the use of warfarin in ischemic stroke patients or in patients older than 75 years.
PHARMACOLOGIC OPTIONS FOR VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
As reviewed in the introductory article of this supplement, the arsenal of anticoagulants for use in VTE prophylaxis includes low-dose unfractionated heparin (UFH), low-molecular-weight heparin (LMWH) agents such as dalteparin and enoxaparin, and the factor Xa inhibitor fondaparinux. A few additional comments about these and other anticoagulant options is warranted in the specific context of orthopedic surgery.
Fondaparinux. Because most of its formal US indications are for use as VTE prophylaxis in major orthopedic surgery—including hip replacement, knee replacement, and hip fracture repair—fondaparinux has been studied more widely in orthopedic surgery patients than in the other populations reviewed earlier in this supplement. Nevertheless, its use even in these settings has remained somewhat limited. This may be because of concerns over possible increased bleeding risk relative to some other anticoagulants. Because of bleeding risk, fondaparinux is contraindicated in patients who weigh less than 50 kg, and its package insert recommends caution when it is used in the elderly due to an increased risk of bleeding in patients aged 65 or older. Additionally, the Pentasaccharide in Major Knee Surgery (PENTAMAKS) study found fondaparinux to be associated with a significantly higher incidence of major bleeding compared with enoxaparin (2.1% vs 0.2%; P = .006) in major knee surgery, although it was superior to enoxaparin in preventing VTE.10 Other possible reasons for slow adoption of fondaparinux include its long half-life, which results in a sustained antithrombotic effect, its lack of easy reversibility, and a contraindication in patients with renal insufficiency.11
Limited role for UFH. Low-dose UFH has a more limited role in orthopedic surgery than in other settings requiring VTE prophylaxis, as current ACCP guidelines for VTE prevention recognize it only as a possible option in hip fracture surgery and state that it is not to be considered as sole prophylaxis in patients undergoing hip or knee replacement.6
Warfarin. Although not indicated for use in other VTE prophylaxis settings, the vitamin K antagonist warfarin is recommended as an option for all three major orthopedic surgery indications—knee replacement, hip replacement, and hip fracture repair.6
The key to effective prophylaxis with warfarin is achieving the appropriate intensity of anticoagulation. In two separate analyses, Hylek et al demonstrated a balance between safety and efficacy with warfarin therapy targeted to an international normalized ratio (INR) of 2.0 to 3.0.12,13 An INR greater than 4.0 greatly increased the risk of intracranial hemorrhage, whereas thrombosis was not effectively prevented with an INR less than 2.0.12,13 This latter point should be stressed to orthopedic surgeons, who sometimes aim for INR values below 2.0.
Although anticoagulation clinics are superior to usual care at maintaining the INR within the window of 2.0 to 3.0, only about one-third of patients nationally who take warfarin receive care in such clinics.14 Even with optimal care in anticoagulation clinics, some patients will still receive subtherapeutic or supertherapeutic levels of warfarin, which is one of this agent’s limitations.
Aspirin not recommended as sole agent. Although aspirin is still used as thromboprophylaxis in orthopedic surgery patients, current ACCP guidelines recommend against its use as the sole means of VTE prophylaxis in any patient group.6 The limitations of the evidence for aspirin in this setting are illustrated by the Pulmonary Embolism Prevention study, a multicenter randomized trial in patients undergoing hip fracture (n = 13,356) or hip/knee replacement (n = 4,088).15 Patients received aspirin 160 mg/day or placebo for 5 weeks, starting preoperatively, and were evaluated for outcomes at day 35. Among the hip fracture patients, the rate of symptomatic DVT was lower in the aspirin group than in the placebo group (1.0% vs 1.5%; P = .03), as was the rate of PE (0.7% vs 1.2%, respectively; P = .002), but there was no significant difference in outcomes between the groups among the patients undergoing hip or knee replacement. Notably, 40% of patients in the study also received UFH or LMWH. Further confounding the results, some patients received nonpharmacologic VTE prophylaxis modalities, and others received nonsteroidal anti-inflammatory drugs other than aspirin.
Heparin-induced thrombocytopenia. As noted earlier in this supplement, the incidence of heparin-induced thrombocytopenia (HIT) is markedly higher in patients who receive UFH than in those who receive LMWH. This difference in frequency, which constitutes about a sixfold to eightfold differential, is due to the relationship between standard heparin and platelet factor IV, which can induce formation of IgG antibodies.16 A 50% or greater reduction in platelet count in heparin recipients should prompt consideration of HIT.
Oral direct thrombin inhibitors. Although the oral direct thrombin inhibitor ximelagatran was rejected for approval by the US Food and Drug Administration (FDA) and recently withdrawn from the market worldwide as a result of hepatic risks, other oral direct thrombin inhibitors are in phase 3 studies for use in orthopedic surgery and may be commercially available options for postoperative VTE prophylaxis before long.
GUIDELINES FOR VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
The ACCP guidelines referred to throughout this article are widely recognized as a practice standard for VTE prevention and treatment, and have been regularly updated throughout recent decades. The most recent version, issued in 2004, is formally known as the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.6 Key orthopedic surgery-related recommendations and notable changes from the previous version of the guidelines, issued in 2001, are outlined below, along with pertinent supportive or illustrative studies.
Hip replacement surgery
In a change from the previous guidelines, the Seventh ACCP Conference recommends extended prophylaxis, for up to 28 to 35 days after surgery, for patients undergoing hip replacement or hip fracture surgery. For hip replacement surgery, this is a Grade 1A recommendation for prophylaxis with either LMWH or warfarin and a Grade 1C+ recommendation (“no RCTs but strong RCT results can be unequivocally extrapolated, or overwhelming evidence from observational studies”) for prophylaxis with fondaparinux.6
Knee replacement surgery
The same three anticoagulant options that received Grade 1A recommendations for patients undergoing total hip replacement—LMWH, fondaparinux, and adjusted-dose warfarin—are also given Grade 1A recommendations as routine thromboprophylaxis in patients undergoing elective knee replacement (see Table 1 for dosing). In addition, optimal use of intermittent pneumatic compression devices is recommended as an alternative option to anticoagulant prophylaxis in these patients (Grade 1B, indicating a “strong recommendation” based on RCTs with important limitations). Use of UFH as the sole agent for prophylaxis is recommended against.6
For both hip and knee replacement surgery, the Seventh ACCP Conference does not endorse superiority of any one of its three recommended prophylaxis options—LMWH, fondaparinux, and adjusted-dose warfarin—over the other two. However, at least four large randomized trials have directly compared LMWH and adjusted-dose warfarin in the setting of arthroplasty—two in total hip replacement surgery19,20 and two in total knee replacement surgery.21,22 Each of these four studies found LMWH to be significantly more effective than warfarin in preventing VTE. In three of the four trials, there was no significant difference between the therapies in rates of major bleeding.19,21,22 In the remaining trial, which was conducted in hip replacement surgery patients and compared postoperative warfarin with dalteparin initiated either immediately before or early after surgery, patients who received preoperative dalteparin initiation (but not those who received postoperative dalteparin initiation) had an increased rate of major bleeding compared with warfarin recipients (P = .01).20
Hip fracture surgery
The supportive evidence for anticoagulant prophylaxis in hip fracture surgery is less robust than that in hip and knee replacement surgery. As a result, only fondaparinux has a Grade 1A recommendation as routine prophylaxis in patients undergoing hip fracture surgery. Options with less definitive recommendations are LMWH (Grade 1C+), low-dose UFH (Grade 1B), and adjusted-dose warfarin (Grade 2B, indicating a “weak recommendation” based on RCTs with important limitations) (see Table 1 for dosing of all agents).6
These differing recommendations are supported by the double-blind Pentasaccharide in Hip Fracture Surgery Study (PENTHIFRA) of 1,711 consecutive patients undergoing surgery for hip fracture repair.23 Patients were randomized to at least 5 days of fondaparinux 2.5 mg once daily, initiated postoperatively, or enoxaparin 40 mg once daily, initiated preoperatively. The incidence of DVT or PE by postoperative day 11 was 8.3% in the fondaparinux arm versus 19.1% in the enoxaparin arm, a statistically significant difference (P < .001) in favor of fondaparinux. There were no differences between the groups in rates of death or clinically relevant bleeding.
As noted above, the newly added recommendation in the Seventh ACCP Conference for extended prophylaxis, for up to 28 to 35 days after surgery, applies to patients undergoing hip fracture surgery as well as those undergoing hip replacement surgery. In the setting of hip fracture repair, extended prophylaxis is a Grade 1A recommendation with the use of fondaparinux and a Grade 1C+ recommendation with the use of either LMWH or adjusted-dose warfarin.6
Lower extremity fractures and trauma
Although lower extremity fractures are very common, the risk of DVT has been poorly studied in this setting. For patients with isolated lower extremity fractures, the Seventh ACCP Conference recommends that clinicians not use thromboprophylaxis routinely (Grade 2A, indicating an “intermediate-strength recommendation” based on RCTs without important limitations).6
Trauma patients, in contrast, are well recognized as being at very high risk for DVT and PE. The Seventh ACCP Conference gives a Grade 1A recommendation to thromboprophylaxis for all trauma patients who have at least one risk factor for VTE. LMWH is recommended (Grade 1A) as the agent of choice for this purpose, provided there are no contraindications to its use, and should be administered as soon as safely possible. Mechanical modalities are reserved for trauma patients with active bleeding or high risk for hemorrhage (Grade 1B). The guidelines recommend against use of inferior vena cava (IVC) filters as primary thromboprophylaxis in trauma patients (Grade 1C, indicating an “intermediate-strength recommendation” based on observational studies).6
Use of ultrasonography
Duplex ultrasonographic screening is recommended in orthopedic trauma patients who are at high risk for VTE and have received suboptimal or no prophylaxis (Grade 1C). In contrast, the Seventh ACCP Conference recommends against routine use of duplex ultrasonography to screen for VTE at hospital discharge in asymptomatic patients following major orthopedic surgery (Grade 1A).6
Knee arthroscopy
Arthroscopic knee procedures are increasing in frequency and raise the specter of a potential role for thromboprophylaxis. However, the clinical diagnosis of DVT is unreliable, and even diagnosis by ultrasonography is unreliable following knee arthroscopy, as interpreting scans of veins below the knee is challenging in this setting.24
The Seventh ACCP Conference recommends that clinicians not use routine thromboprophylaxis, other than early mobilization, for patients who undergo knee arthroscopy (Grade 2B). However, for arthroscopy patients who have inherent risk factors for VTE or who undergo a prolonged or complicated arthroscopy procedure, thromboprophylaxis with LMWH is suggested (Grade 2B).6
RECOMMENDED APPROACH TO VTE PROPHYLAXIS IN ORTHOPEDIC SURGERY
Drawing on the ACCP guidelines and the evidence reviewed above, we have outlined our evidence-based recommendations for pharmacologic VTE prophylaxis in patients undergoing orthopedic surgery, as presented in Table 1. All patients undergoing major orthopedic surgical procedures (ie, procedures other than arthroscopy) should routinely receive anticoagulant prophylaxis unless they have contraindications to anticoagulation. Recommended agents and their duration of use vary according to the type of surgery, as detailed in Table 1.
Extended-duration prophylaxis is recommended for patients undergoing total hip replacement and hip fracture surgery. Aspirin is not recommended as the sole agent for prophylaxis in any orthopedic surgery setting.
Importance of a postoperative prophylaxis protocol
In addition to these broad pharmacologic recommendations, it is important that a postoperative VTE prophylaxis protocol be in place at all hospitals.
At the Ochsner Medical Center in New Orleans, where one of us (S.B.D.) practices, postoperative orders include antithrombotic therapy for surgical patients, starting with placement of thigh-high antiembolism stockings on both legs on the day of surgery for patients undergoing hip replacement and on postoperative day 1 in those undergoing knee replacement. Plantar pneumatic compression devices are applied to both legs in the recovery room and kept on except when the patient is walking. The hospitalist team dictates further anticoagulation orders. If extended prophylaxis is prescribed, the discharge planner sets up drug delivery and reimbursement, provides a LMWH discharge kit, and teaches the patient to self-inject. If there is concern about increasing swelling at the surgical site while anticoagulant therapy continues, the protocol calls for prompt notification of the responsible physician. To minimize the risk that spinal or epidural hematomas will develop, all agents that increase bleeding propensity should be recognized and ordered accordingly.



SUMMARY
VTE in patients undergoing major orthopedic surgery is a serious health problem that is highly preventable, yet VTE prophylaxis remains underused in this patient population. Despite the availability of practice guidelines for VTE prevention in the orthopedic surgery setting, recommendations are not widely implemented in clinical practice. Recommended prophylactic options differ somewhat among various orthopedic procedures, and the supportive evidence differs for various anticoagulant options.
DISCUSSION: ADDITIONAL PERSPECTIVES FROM THE AUTHORS
Dr. Jaffer: The ACCP recommends against the routine use of aspirin as primary prophylaxis against VTE in major orthopedic surgery, yet orthopedic surgeons across the country still continue to use aspirin in this setting. What are your thoughts on this, Dr. McKean?
Dr. McKean: We agree with the ACCP’s recommendation against aspirin as primary VTE prophylaxis in orthopedic patients. The percentage of US knee arthroplasty patients who develop VTE after receiving no prophylaxis at all is roughly 64%; this percentage declines only slightly (to 56%) for knee arthroplasty patients who receive prophylaxis with aspirin.25 Since we clearly want to reduce VTE risk as much as possible, I would not use aspirin alone. I would use it only if the patient were already on aspirin, but then I would add either LMWH or fondaparinux.
Dr. Jaffer: Warfarin is another agent that is widely used for prophylaxis in major orthopedic surgery. In fact, the large registries of VTE prevention in major orthopedic surgery suggest that the use of warfarin may be slightly higher than the use of LMWH. If clinicians choose to use warfarin in their practice, what are your recommendations, Dr. Deitelzweig?
Dr. Deitelzweig: As primary prophylaxis for orthopedic surgery patients, warfarin must be dosed to achieve an INR of 2.0 to 3.0; the need for a value in this range is unequivocal. This is a challenging target to attain in the hospital setting.
Dr. Brotman: A study I was involved with a few years ago suggested that warfarin may be inadequate for VTE prevention in the first few days after orthopedic surgery.26 Orthopedic surgeons at the Cleveland Clinic, where I was practicing at the time, routinely used systematic ultrasonography to assess for thrombosis on postoperative day 2 or 3 following hip or knee arthroplasty, so we conducted a secondary analysis of a case-control study in these ultrasonographically screened arthroplasty patients to assess rates of early VTE and look for any associations with the type of prophylaxis used. We found that there was about a tenfold increase in the risk of VTE, both distal and proximal, on postoperative day 2 or 3 among patients who received warfarin compared with those who received LMWH. We concluded that warfarin’s delayed antithrombotic effects may not provide sufficient VTE prophylaxis in the immediate postoperative setting.26
Dr. Deitelzweig: That’s a good point. Although it’s important to achieve a therapeutic level of warfarin, we now have evidence that it takes some time to achieve that level, and in the interim, bad things can happen to patients.
Dr. Jaffer: Orthopedic surgery encompasses several types of procedures. Dr. Amin, which specific orthopedic surgery patients stand to benefit from extended prophylaxis, and how long should extended prophylaxis last?
Dr. Amin: Major orthopedic surgery comprises hip fracture repair, total hip replacement, and total knee replacement. For hip fracture, there are strong data to support the use of extended prophylaxis with fondaparinux 2.5 mg/day, which showed about an 88% relative reduction in the risk of symptomatic VTE compared with standard-duration fondaparinux (6 to 8 days) followed by matching placebo for the extended phase.27 The total duration of fondaparinux therapy in the extended-duration arm was 4 to 5 weeks.
Likewise, data support extended prophylaxis in hip arthroplasty patients, for whom the recommended duration is also 4 to 5 weeks. The systematic review by Hull et al17 demonstrated a 0.41 relative risk of DVT with extended-duration LMWH prophylaxis versus placebo in hip replacement patients (Figure 1), which was a highly statistically significant result.
In contrast, we do not yet have good data to support extended prophylaxis for patients undergoing total knee replacement, which is a bit surprising. In this setting, prophylaxis is recommended for 7 to 14 days but not beyond that.
Dr. Jaffer: Arthroscopy is probably the most common orthopedic procedure performed in the United States today. Dr. Brotman, what is the role of prophylaxis in patients undergoing arthroscopy?
Dr. Brotman: Minor surgery such as arthroscopy can typically be performed safely without routine prophylaxis, other than having the patient ambulate as soon as possible after the procedure. There may be exceptions to this rule, however. I believe that there is potentially a role for pharmacologic prophylaxis in arthroscopy patients who have major risk factors for VTE, such as a personal history of VTE, or who are not expected to become mobile again in a normal rapid fashion after the operation, but prophylaxis has not been studied systematically in such patients.
Dr. Jaffer: Dr. Spyropoulos, there are several new anticoagulants in the pipeline, specifically agents such as the oral direct factor Xa inhibitors and the direct thrombin inhibitors. What do recent clinical trials suggest with regard to the efficacy of these two drug classes for thromboprophylaxis in major orthopedic surgery?
Dr. Spyropoulos: The agents with the most available data are the oral direct factor Xa inhibitors apixaban and rivaroxaban and the oral direct thrombin inhibitor dabigatran. For prophylaxis in orthopedic surgery populations, phase 2 studies have been completed for apixaban and phase 3 trials have been completed for rivaroxaban and dabigatran.
It appears that the factor Xa inhibitors, apixaban and rivaroxaban, are efficacious in comparison with both adjusted-dose warfarin and LMWH, which is the gold standard for this group of patients.28,29 So these indeed appear to be promising agents. Rivaroxaban has been submitted to European regulatory agencies for approval for the prevention of VTE in patients undergoing major orthopedic surgery, and its developer plans to submit it to the FDA in 2008 for a similar indication in the United States.
The data are more equivocal with dabigatran. There have been several positive phase 3 studies in orthopedic surgery comparing two dabigatran dosing schemes, 150 and 220 mg once daily, with the European regimen of enoxaparin (40 mg once daily),30 but a recent study that compared these doses with the North American enoxaparin regimen (30 mg twice daily) failed to meet the criteria for noninferiority.31 Further clinical trial development is necessary for dabigatran, although in January 2008 the European Medicines Agency recommended its marketing approval for thromboprophylaxis in patients undergoing orthopedic procedures.32
I believe that in the next 3 to 5 years our armamentarium will see the addition of at least one, if not more, of these new agents that offer the promise of oral anticoagulation with highly predictable pharmacokinetics and pharmacodynamics and no need for monitoring.
- Yu HT, Dylan ML, Lin J, Dubois RW. Hospitals’ compliance with prophylaxis guidelines for venous thromboembolism. Am J Health Syst Pharm 2007; 64:69–76.
- Stratton MA, Anderson FA, Bussey HI, et al. Prevention of venous thromboembolism: adherence to the 1995 ACCP consensus guidelines for surgical patients. Arch Intern Med 2000; 160:334–340.
- National Consensus Standards for Prevention and Care of Venous Thromboembolism (VTE). The Joint Commission Web site. http://www.jointcommission.org/PerformanceMeasurement/Perform anceMeasurement/VTE.htm. Accessed January 8, 2008.
- Surgical Care Improvement Project. MedQIC Web site. http://www.medqic.org/scip. Accessed January 8, 2008.
- SCIP process and outcome measures, October 2005. MedQIC Web site. http://www.medqic.org. Accessed January 1, 2007.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- Zimlich RH, Fulbright BM, Friedman RJ. Current status of anticoagulation therapy after total hip and total knee arthroplasty.J Am Acad Orthop Surg 1996; 4:54–62.
- Planes A, Vochelle N, Mazas F, et al. Prevention of postoperative venous thrombosis: a randomized trial comparing unfractionated heparin with low molecular weight heparin in patients undergoing total hip replacement. Thromb Haemost 1988; 60:407–410.
- Colwell CW Jr, Spiro TE, Trowbridge AA, et al. Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep venous thrombosis after elective knee arthroplasty. Clin Orthop Relat Res 1995; 321:19–27.
- Bauer KA, Eriksson BI, Lassen MR, Turpie AG. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med 2001; 345:1305–1310.
- Turpie AGG. Pentasaccharide Org31540/SR90107A clinical trials update: lessons for practice. Am Heart J 2001; 142(Suppl):S9–S15.
- Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med 1994; 120:897–902.
- Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med 1996; 335:540–546.
- Samsa GP, Matchar DB, Goldstein LB, et al. Quality of anticoagulation management among patients with atrial fibrillation: review of medical records from 2 communities. Arch Intern Med 2000; 160:967–973.
- PEP Trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000; 355:1295–1302.
- Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haemotol 2003; 121:535–555.
- Hull RD, Pineo GF, Stein PD, et al. Extended out-of-hospital low-molecular-weight heparin prophylaxis against deep venous thrombosis in patients after elective hip arthroplasty: a systematic review. Ann Intern Med 2001; 135:858–869.
- Haentjens P, De Groote K, Annemans L. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement: a cost-utility analysis. Arch Orthop Trauma Surg 2004; 124:507–517.
- Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty: evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am 1999; 81:932–940.
- Hull RD, Pineo GF, Francis C, et al. Low-molecular-weight heparin prophylaxis using dalteparin in close proximity to surgery vs warfarin in hip arthroplasty patients: a double-blind, randomized comparison. Arch Intern Med 2000; 160:2199–2207.
- Leclerc JR, Geerts WH, Desjardins L, et al. Prevention of venous thromboembolism after knee arthroplasty: a randomized, double-blind trial comparing enoxaparin with warfarin. Ann Intern Med 1996; 124:619–626.
- Fitzgerald RH Jr, Spiro TE, Trowbridge AA, et al. Prevention of venous thromboembolic disease following primary total knee arthroplasty: a randomized, multicenter, open-label, parallel-group comparison of enoxaparin and warfarin. J Bone Joint Surg Am 2001; 83-A:900–906.
- Eriksson BI, Bauer KA, Lassen MR, Turpie AG, Steering Committee of the Pentasaccharide in Hip-Fracture Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med 2001; 345:1298–1304.
- Demers C, Marcoux S, Ginsberg JS, Laroche F, Cloutier R, Poulin J. Incidence of venographically proved deep vein thrombosis after knee arthroscopy. Arch Intern Med 1998; 158:47–50.
- Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest 2001; 119(1 Suppl):132S–175S.
- Brotman DJ, Jaffer AK, Hurbanek JG, Morra N. Warfarin prophylaxis and venous thromboembolism in the first 5 days following hip and knee arthroplasty. Thromb Haemost 2004; 92:1012–1017.
- Eriksson BI, Lassen MR; Pentasaccharide in Hip-Fracture Surgery Plus Investigators. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med 2003; 163:1337–1342.
- The Botticelli Investigators. Late-breaking clinical trial: a dose-finding study of the oral direct factor Xa inhibitor apixaban in the treatment of patients with acute symptomatic deep vein thrombosis [abstract]. Presented at the 21st Congress of the International Society on Thrombosis and Haemostasis; July 2007; Geneva, Switzerland.
- Fisher WD, Eriksson BI, Bauer KA, et al. Rivaroxaban for thromboprophylaxis after orthopaedic surgery: pooled analysis of two studies. Thromb Haemost 2007; 97:931–937.
- Haas S. New oral Xa and IIa inhibitors: updates on clinical trial results. J Thromb Thrombolysis 2008; 25:52–60.
- Friedman RJ, Caprini JA, Comp PC, et al. Dabigatran etexilate vs enoxaparin in preventing venous thromboembolism following total knee arthroplasty. Presented at: 2007 Congress of the International Society on Thrombosis and Haemostasis; July 7–13, 2007; Geneva, Switzerland.
- Committee for Medicinal Products for Human Use summary of positive opinion for Pradaxa [news release]. London, UK: European Medicines Agency. January 24, 2008. http://www.emea.europa.eu/pdfs/human/ opinion/Pradaxa_3503008en.pdf. Accessed February 21, 2008.
- Goldhaber SZ, Grodstein F, Stampfer MJ. A prospective study of risk factors for pulmonary embolism in women. JAMA 1997; 277:642–645.
- Turpie AGG, Bauer KA, Eriksson BI, Lassen MR, for the Steering Committees of the Pentasaccharide Orthopedic Prophylaxis Studies. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery. Arch Intern Med 2002; 162:1833–1840.
- Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 2007; 120:871–879.
- Goldhaber SZ. Diagnosis of acute pulmonary embolism: always be vigilant. Am J Med 2007; 120:827–828.
- American Academy of Orthopaedic Surgeons Clinical Guideline on Prevention of Symptomatic Pulmonary Embolism in Patients Undergoing Total Hip or Knee Arthroplasty: Summary of Recommendations. http://www.aaos.org/Research/guidelines/PE_ summary.pdf. Accessed December 10, 2007.
- Yu HT, Dylan ML, Lin J, Dubois RW. Hospitals’ compliance with prophylaxis guidelines for venous thromboembolism. Am J Health Syst Pharm 2007; 64:69–76.
- Stratton MA, Anderson FA, Bussey HI, et al. Prevention of venous thromboembolism: adherence to the 1995 ACCP consensus guidelines for surgical patients. Arch Intern Med 2000; 160:334–340.
- National Consensus Standards for Prevention and Care of Venous Thromboembolism (VTE). The Joint Commission Web site. http://www.jointcommission.org/PerformanceMeasurement/Perform anceMeasurement/VTE.htm. Accessed January 8, 2008.
- Surgical Care Improvement Project. MedQIC Web site. http://www.medqic.org/scip. Accessed January 8, 2008.
- SCIP process and outcome measures, October 2005. MedQIC Web site. http://www.medqic.org. Accessed January 1, 2007.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- Zimlich RH, Fulbright BM, Friedman RJ. Current status of anticoagulation therapy after total hip and total knee arthroplasty.J Am Acad Orthop Surg 1996; 4:54–62.
- Planes A, Vochelle N, Mazas F, et al. Prevention of postoperative venous thrombosis: a randomized trial comparing unfractionated heparin with low molecular weight heparin in patients undergoing total hip replacement. Thromb Haemost 1988; 60:407–410.
- Colwell CW Jr, Spiro TE, Trowbridge AA, et al. Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep venous thrombosis after elective knee arthroplasty. Clin Orthop Relat Res 1995; 321:19–27.
- Bauer KA, Eriksson BI, Lassen MR, Turpie AG. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med 2001; 345:1305–1310.
- Turpie AGG. Pentasaccharide Org31540/SR90107A clinical trials update: lessons for practice. Am Heart J 2001; 142(Suppl):S9–S15.
- Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med 1994; 120:897–902.
- Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med 1996; 335:540–546.
- Samsa GP, Matchar DB, Goldstein LB, et al. Quality of anticoagulation management among patients with atrial fibrillation: review of medical records from 2 communities. Arch Intern Med 2000; 160:967–973.
- PEP Trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000; 355:1295–1302.
- Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haemotol 2003; 121:535–555.
- Hull RD, Pineo GF, Stein PD, et al. Extended out-of-hospital low-molecular-weight heparin prophylaxis against deep venous thrombosis in patients after elective hip arthroplasty: a systematic review. Ann Intern Med 2001; 135:858–869.
- Haentjens P, De Groote K, Annemans L. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement: a cost-utility analysis. Arch Orthop Trauma Surg 2004; 124:507–517.
- Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty: evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am 1999; 81:932–940.
- Hull RD, Pineo GF, Francis C, et al. Low-molecular-weight heparin prophylaxis using dalteparin in close proximity to surgery vs warfarin in hip arthroplasty patients: a double-blind, randomized comparison. Arch Intern Med 2000; 160:2199–2207.
- Leclerc JR, Geerts WH, Desjardins L, et al. Prevention of venous thromboembolism after knee arthroplasty: a randomized, double-blind trial comparing enoxaparin with warfarin. Ann Intern Med 1996; 124:619–626.
- Fitzgerald RH Jr, Spiro TE, Trowbridge AA, et al. Prevention of venous thromboembolic disease following primary total knee arthroplasty: a randomized, multicenter, open-label, parallel-group comparison of enoxaparin and warfarin. J Bone Joint Surg Am 2001; 83-A:900–906.
- Eriksson BI, Bauer KA, Lassen MR, Turpie AG, Steering Committee of the Pentasaccharide in Hip-Fracture Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med 2001; 345:1298–1304.
- Demers C, Marcoux S, Ginsberg JS, Laroche F, Cloutier R, Poulin J. Incidence of venographically proved deep vein thrombosis after knee arthroscopy. Arch Intern Med 1998; 158:47–50.
- Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest 2001; 119(1 Suppl):132S–175S.
- Brotman DJ, Jaffer AK, Hurbanek JG, Morra N. Warfarin prophylaxis and venous thromboembolism in the first 5 days following hip and knee arthroplasty. Thromb Haemost 2004; 92:1012–1017.
- Eriksson BI, Lassen MR; Pentasaccharide in Hip-Fracture Surgery Plus Investigators. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med 2003; 163:1337–1342.
- The Botticelli Investigators. Late-breaking clinical trial: a dose-finding study of the oral direct factor Xa inhibitor apixaban in the treatment of patients with acute symptomatic deep vein thrombosis [abstract]. Presented at the 21st Congress of the International Society on Thrombosis and Haemostasis; July 2007; Geneva, Switzerland.
- Fisher WD, Eriksson BI, Bauer KA, et al. Rivaroxaban for thromboprophylaxis after orthopaedic surgery: pooled analysis of two studies. Thromb Haemost 2007; 97:931–937.
- Haas S. New oral Xa and IIa inhibitors: updates on clinical trial results. J Thromb Thrombolysis 2008; 25:52–60.
- Friedman RJ, Caprini JA, Comp PC, et al. Dabigatran etexilate vs enoxaparin in preventing venous thromboembolism following total knee arthroplasty. Presented at: 2007 Congress of the International Society on Thrombosis and Haemostasis; July 7–13, 2007; Geneva, Switzerland.
- Committee for Medicinal Products for Human Use summary of positive opinion for Pradaxa [news release]. London, UK: European Medicines Agency. January 24, 2008. http://www.emea.europa.eu/pdfs/human/ opinion/Pradaxa_3503008en.pdf. Accessed February 21, 2008.
- Goldhaber SZ, Grodstein F, Stampfer MJ. A prospective study of risk factors for pulmonary embolism in women. JAMA 1997; 277:642–645.
- Turpie AGG, Bauer KA, Eriksson BI, Lassen MR, for the Steering Committees of the Pentasaccharide Orthopedic Prophylaxis Studies. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery. Arch Intern Med 2002; 162:1833–1840.
- Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 2007; 120:871–879.
- Goldhaber SZ. Diagnosis of acute pulmonary embolism: always be vigilant. Am J Med 2007; 120:827–828.
- American Academy of Orthopaedic Surgeons Clinical Guideline on Prevention of Symptomatic Pulmonary Embolism in Patients Undergoing Total Hip or Knee Arthroplasty: Summary of Recommendations. http://www.aaos.org/Research/guidelines/PE_ summary.pdf. Accessed December 10, 2007.