User login
Venous thromboembolism (VTE) is a common and dangerous disease, affecting 0.1%-0.2% of the population annually—a rate that might be underreported.1 VTE is a collective term for venous blood clots, including (1) deep vein thrombosis (DVT) of peripheral veins and (2) pulmonary embolism, which occurs after a clot travels through the heart and becomes lodged in the pulmonary vasculature. Two-thirds of VTE cases present clinically as DVT2; most mortality from VTE disease is caused by the 20% of cases of pulmonary embolism that present as sudden death.1
VTE is comparable to myocardial infarction (MI) in incidence and severity. In 2008, 208 of every 100,000 people had an MI, with a 30-day mortality of 16/100,0003; VTE disease has an annual incidence of 161 of every 100,000 people and a 28-day mortality of 18/100,000.4 Although the incidence and severity of MI are steadily decreasing, the rate of VTE appears constant.3,5 The high mortality of VTE suggests that primary prevention, which we discuss in this article, is valuable (see “Key points: Primary prevention of venous thromboembolism”).
SIDEBAR
Key points: Primary prevention of venous thromboembolism
- Primary prevention of venous thromboembolism (VTE), a disease with mortality similar to myocardial infarction, should be an important consideration in at-risk patients.
- Although statins reduce the risk of VTE, their use is justified only if they are also required for prevention of cardiovascular disease.
- The risk of travel-related VTE can be reduced by wearing compression stockings.
- The choice of particular methods of contraception and of hormone replacement therapy can reduce VTE risk.
- Because of the risk of bleeding, using anticoagulants for primary prevention of VTE is justified only in certain circumstances.
- Pregnancy is the only condition in which there is a guideline indication for thrombophilia testing, because test results in this setting can change recommendations for preventing VTE.
- Using a risk-stratification model is key to determining risk in both medically and surgically hospitalized patients. Trauma and major orthopedic surgery always place the patient at high risk of VTE.
Risk factors
Virchow’s triad of venous stasis, vascular injury, and hypercoagulability describes predisposing factors for VTE.6 Although venous valves promote blood flow, they produce isolated low-flow areas adjacent to valves that become concentrated and locally hypoxic, increasing the risk of clotting.7 The great majority of DVTs (≥ 96%) occur in the lower extremity,8 starting in the calf; there, 75% of cases resolve spontaneously before they extend into the deep veins of the proximal leg.7 One-half of DVTs that do move into the proximal leg eventually embolize.7
Major risk factors for VTE comprise inherited conditions, medical history, medical therapeutics, and behaviors (TABLE 1).9-11 Unlike the preventive management of coronary artery disease (CAD), there is no simple, generalized prevention algorithm to address VTE risk factors.
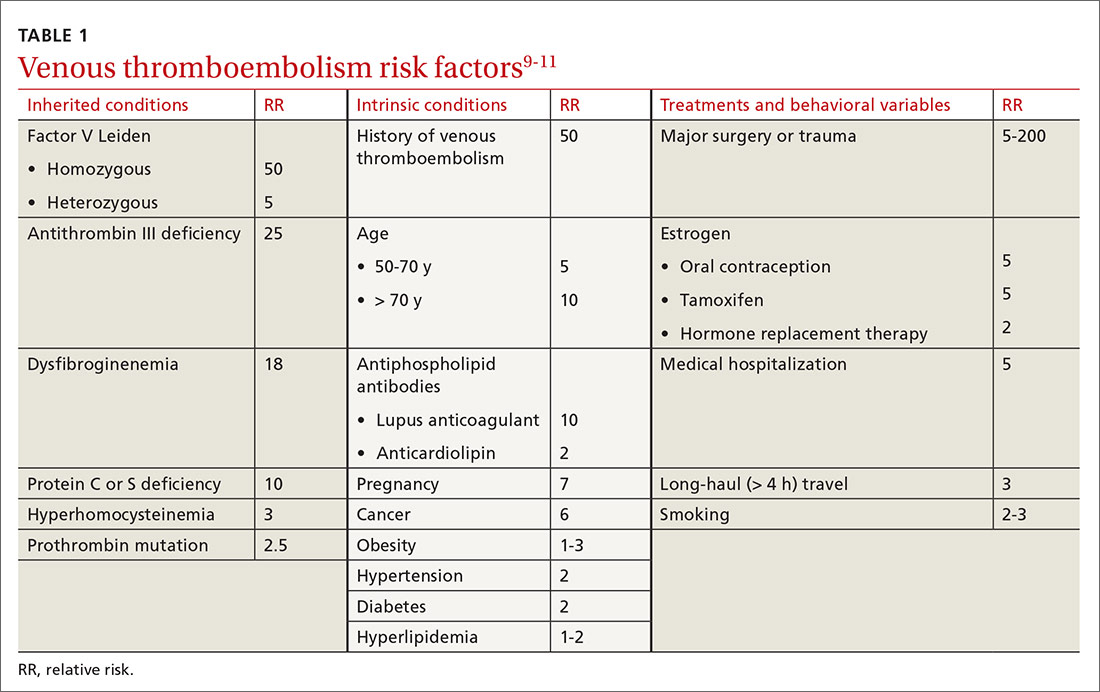
Risk factors for VTE and CAD overlap. Risk factors for atherosclerosis—obesity, diabetes, smoking, hypertension, hyperlipidemia—also increase the risk of VTE (TABLE 1).9-11 The association between risk factors for VTE and atherosclerosis is demonstrated by a doubling of the risk of MI and stroke in the year following VTE.11 Lifestyle changes are expected to reduce the risk of VTE, as they do for acute CAD, but studies are lacking to confirm this connection. There is no prospective evidence showing that weight loss or control of diabetes or hypertension reduces the risk of VTE.12 Smoking cessation does appear to reduce risk: Former smokers have the same VTE risk as never-smokers.13
Thrombophilia testing: Not generally useful
Inherited and acquired thrombophilic conditions define a group of disorders in which the risk of VTE is increased. Although thrombophilia testing was once considered for primary and secondary prevention of VTE, such testing is rarely used now because proof of benefit is lacking: A large case–control study showed that thrombophilia testing did not predict recurrence after a first VTE.14 Guidelines of the American College of Chest Physicians (ACCP) do not address thrombophilia, and the American Society of Hematology recommends against thrombophilia testing after a provoked VTE.15,16
Primary prophylaxis of patients with a family history of VTE and inherited thrombophilia is controversial. Patients with both a family history of VTE and demonstrated thrombophilia do have double the average incidence of VTE, but this increased risk does not offset the significant bleeding risk associated with anticoagulation.17 Recommendations for thrombophilia testing are limited to certain situations in pregnancy, discussed in a bit.16,18,19
Continue to: Primary prevention of VTE in the clinic
Primary prevention of VTE in the clinic
There is no single, overarching preventive strategy for VTE in an ambulatory patient (although statins, discussed in a moment, offer some benefit, broadly). There are, however, distinct behavioral characteristics and medical circumstances for which opportunities exist to reduce VTE risk—for example, when a person engages in long-distance travel, receives hormonal therapy, is pregnant, or has cancer. In each scenario, recognizing and mitigating risk are important.
Statins offer a (slight) benefit
There is evidence that statins reduce the risk of VTE—slightly20-23:
- A large randomized, controlled trial showed that rosuvastatin, 20 mg/d, reduced the rate of VTE, compared to placebo; however, the 2-year number needed to treat (NNT) was 349.20 The VTE benefit is minimal, however, compared to primary prevention of cardiovascular disease with statins (5-year NNT = 56).21 The sole significant adverse event associated with statins was new-onset type 2 diabetes (5-year number needed to harm = 235).21
- A subsequent meta-analysis confirmed a small reduction in VTE risk with statins.22 In its 2012 guidelines, ACCP declined to issue a recommendation on the use of statins for VTE prevention.23 When considering statins for primary cardiovascular disease prevention, take the additional VTE prevention into account.
Simple strategies can help prevent travel-related VTE
Travel is a common inciting factor for VTE. A systematic review showed that VTE risk triples after travel of ≥ 4 hours, increasing by 20% with each additional 2 hours.24 Most VTE occurs in travelers who have other VTE risk factors.25 Based on case–control studies,23 guidelines recommend these preventive measures:
- frequent calf exercises
- sitting in an aisle seat during air travel
- keeping hydrated.
A Cochrane review showed that graded compression stockings reduce asymptomatic DVT in travelers by a factor of 10, in high- and low-risk patients.26
VTE risk varies with type of hormonal contraception
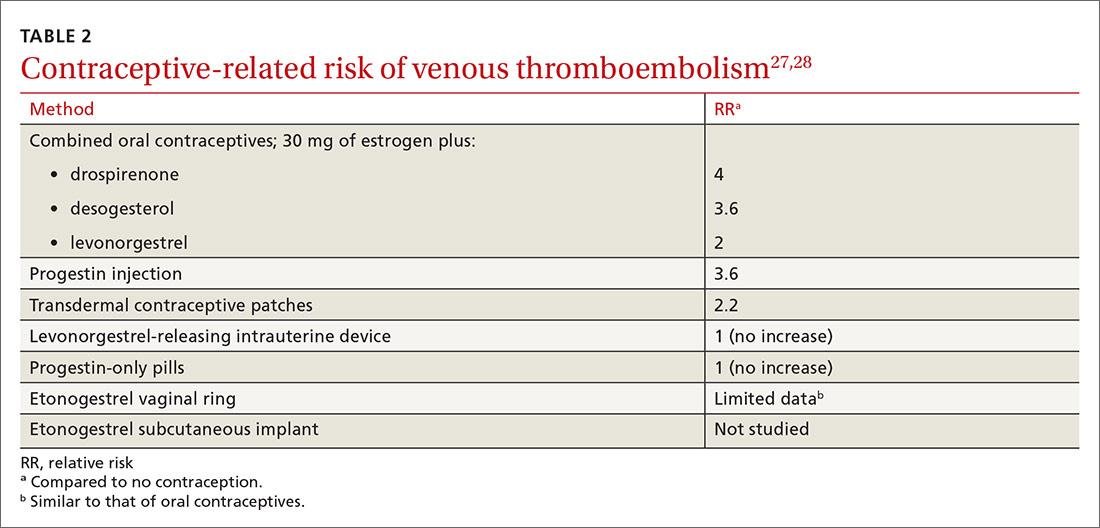
Most contraceptives increase VTE risk (TABLE 227,28). Risk with combined oral contraceptives varies with the amount of estrogen and progesterone. To reduce VTE risk with oral contraceptives, patients can use an agent that contains a lower dose of estrogen or one in which levonorgestrel replaces other progesterones.27
Continue to: Studies suggest that the levonorgestrel-releasing...
Studies suggest that the levonorgestrel-releasing intrauterine device and progestin-only pills are not associated with an increase in VTE risk.27 Although the quality of evidence varies, most nonoral hormonal contraceptives have been determined to carry a risk of VTE that is similar to that of combined oral contraceptives.28
In hormone replacement, avoid pills to lower risk
Hormone replacement therapy (HRT) for postmenopausal women increases VTE risk when administered in oral form, with combined estrogen and progestin HRT doubling the risk and estrogen-only formulations having a lower risk.29 VTE risk is highest in the first 6 months of HRT, declining to that of a non-HRT user within 5 years.29 Neither transdermal HRT nor estrogen creams increase the risk of VTE, according to a systematic review.30 The estradiol-containing vaginal ring also does not confer increased risk.29
Pregnancy, thrombophilia, and VTE prevention
VTE affects as many as 0.2% of pregnancies but causes 9% of pregnancy-related deaths.18 The severity of VTE in pregnancy led the American College of Obstetricians and Gynecologists (ACOG) to recommend primary VTE prophylaxis in patients with certain thrombophilias.18 Thrombophilia testing is recommended in patients with proven high-risk thrombophilia in a first-degree relative.18 ACOG recognizes 5 thrombophilias considered to carry a high risk of VTE in pregnancy18:
- homozygous Factor V Leiden
- homozygous prothrombin G20210A mutation
- antithrombin deficiency
- heterozygous Factor V Leiden and prothrombin G20210A mutation
- antiphospholipid antibody syndrome.
ACOG recommends limiting thrombophilia testing to (1) any specific thrombophilia carried by a relative and (2) possibly, the antiphospholipid antibodies anticardiolipin and lupus anticoagulant.18,19 Antiphospholipid testing is recommended when there is a history of stillbirth, 3 early pregnancy losses, or delivery earlier than 34 weeks secondary to preeclampsia.19
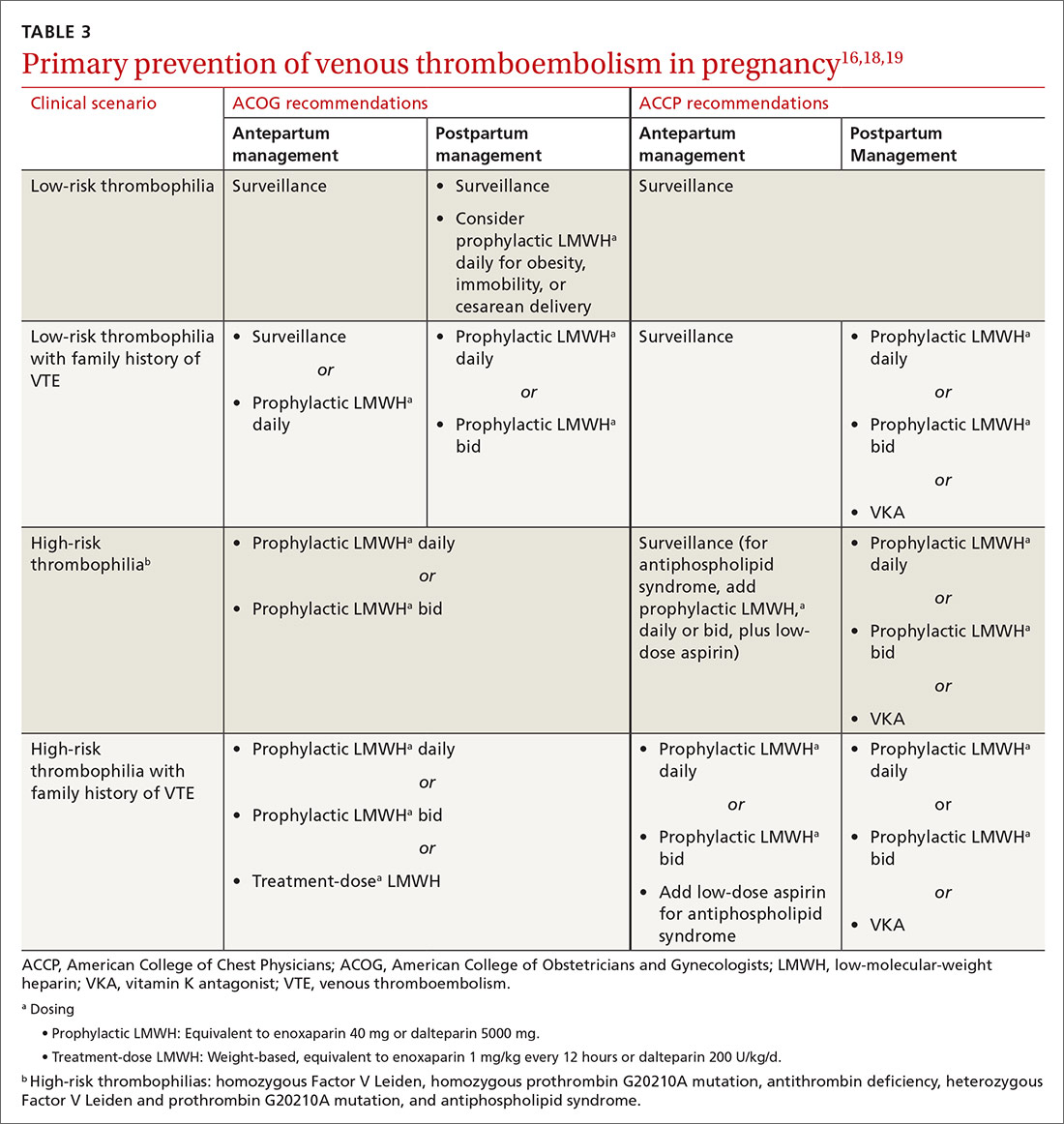
Primary VTE prophylaxis is recommended for pregnant patients with a high-risk thrombophilia; low-molecular-weight heparin (LMWH) is safe and its effects are predictable.18 Because postpartum risk of VTE is higher than antepartum risk, postpartum prophylaxis is also recommended with lower-risk thrombophilias18; a vitamin K antagonist or LMWH can be used.18 ACCP and ACOG recommendations for VTE prophylaxis in pregnancy differ slightly (TABLE 316,18,19).
Continue to: Cancer increases risks of VTE and bleeding
Cancer increases risks of VTE and bleeding
Cancer increases VTE risk > 6-fold31; metastases, chemotherapy, and radiotherapy further increase risk. Cancer also greatly increases the risk of bleeding: Cancer patients with VTE have an annual major bleeding rate ≥ 20%.32 Guidelines do not recommend primary VTE prophylaxis for cancer, although American Society of Clinical Oncology guidelines discuss consideration of prophylaxis for select, high-risk patients,33,34 including those with multiple myeloma, metastatic gastrointestinal cancer, or metastatic brain cancer.31,34 Recent evidence (discussed in a moment) supports the use of apixaban for primary VTE prevention during chemotherapy for high-risk cancer.
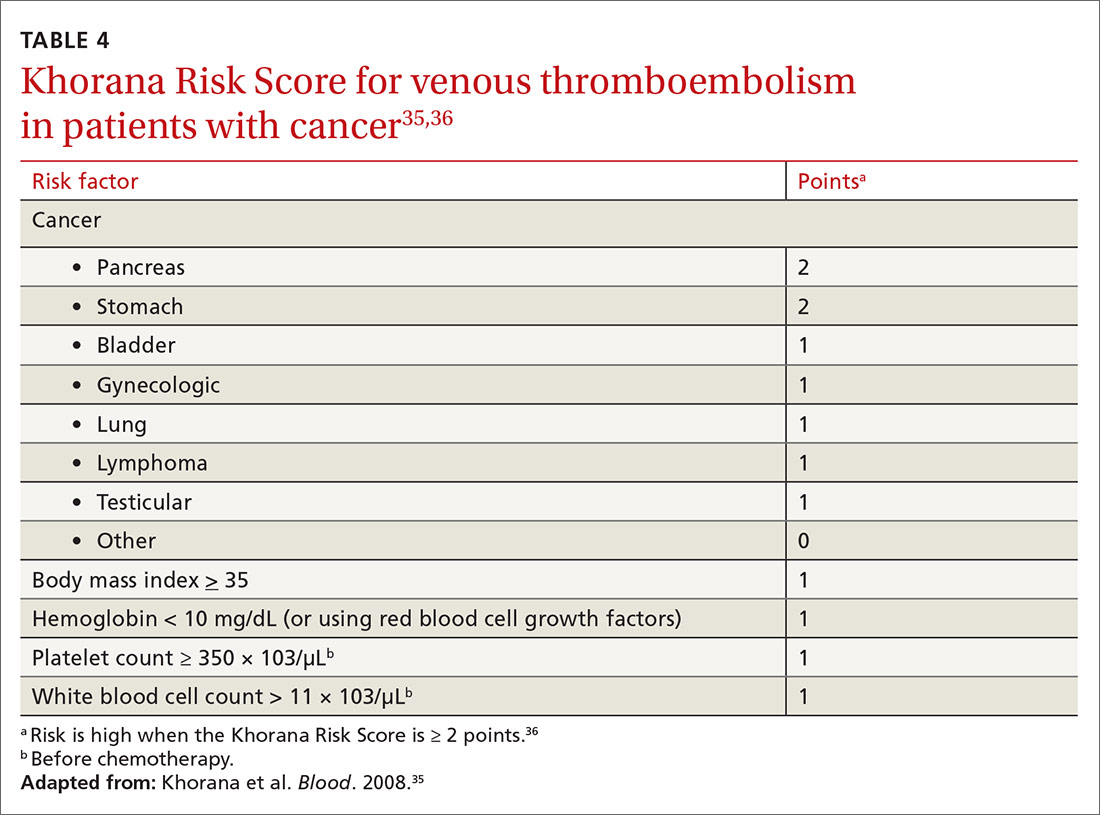
The Khorana Risk Score (TABLE 435,36) for VTE was developed and validated for use in patients with solid cancer35: A score of 2 conveys nearly a 10% risk of VTE over 6 months.36 A recent study of 550 cancer patients with a Khorana score of ≥ 2—the first evidence of risk-guided primary VTE prevention in cancer—showed that primary prophylaxis with 2.5 mg of apixaban, bid, reduced the risk of VTE (NNT = 17); however, the number needed to harm (for major bleeding) was 59.37 Mortality was not changed with apixaban treatment
Primary VTE prevention in med-surg hospitalizations
The risk of VTE increases significantly during hospitalization, although not enough to justify universal prophylaxis. Recommended prevention strategies for different classes of hospitalized patients are summarized below.
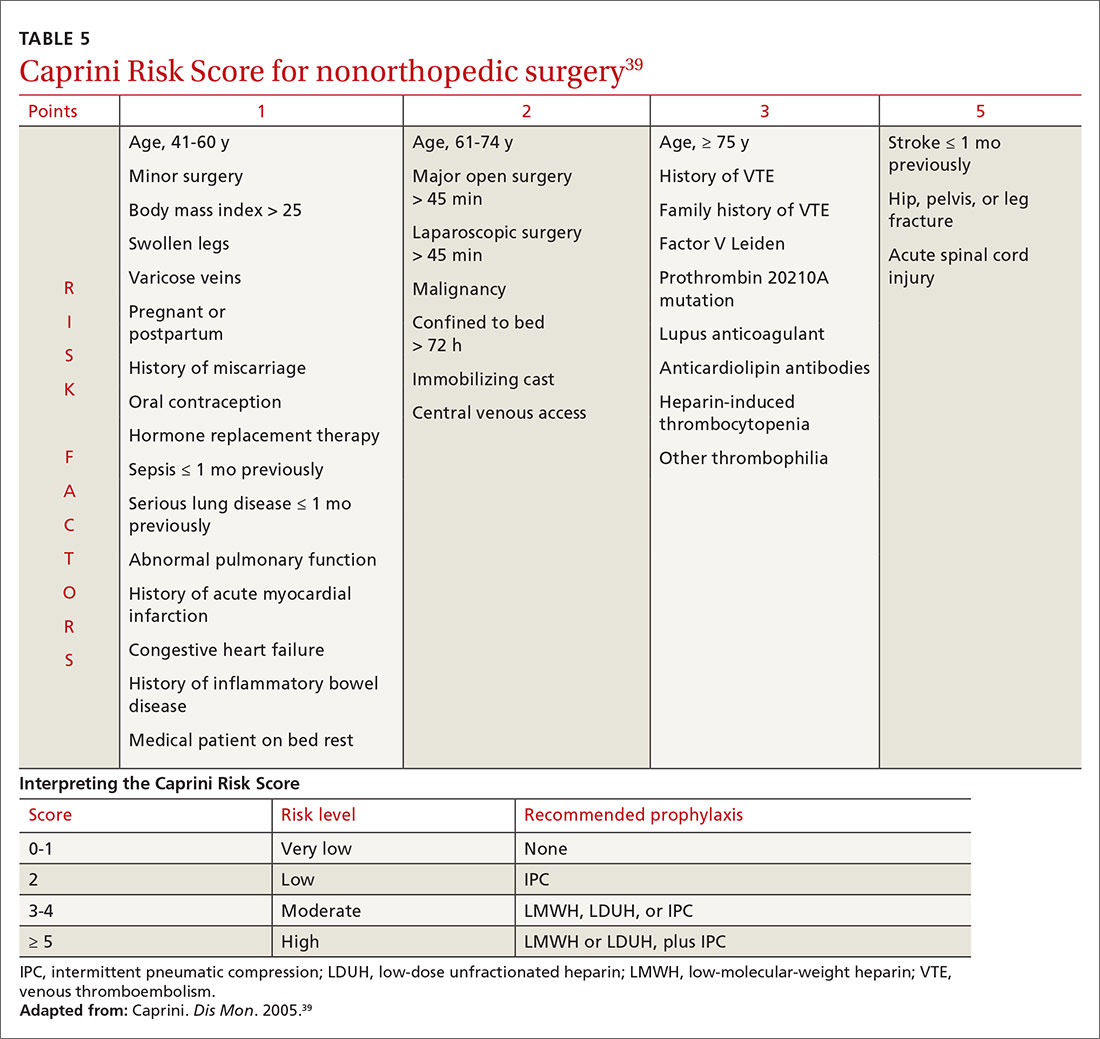
In medically hospitalized patients, risk is stratified with a risk-assessment model. Medically hospitalized patients have, on average, a VTE risk of 1.2%23; 12 risk-assessment models designed to stratify risk were recently compared.38 Two models, the Caprini Score (TABLE 5)39 and the IMPROVE VTE Risk Calculator,40 were best able to identify low-risk patients (negative predictive value, > 99%).38 American Society of Hematology guidelines recommend IMPROVE VTE or the Padua Prediction Score for risk stratification.41 While the Caprini score only designates 11% of eventual VTE cases as low risk, both the IMPROVE VTE and Padua scores miss more than 35% of eventual VTE.38
Because LMWH prophylaxis has been shown to reduce VTE by 40% without increasing the risk of major bleeding, using Caprini should prevent 2 VTEs for every 1000 patients, without an increase in major bleeding and with 13 additional minor bleeding events.42
Continue to: Critically ill patients
Critically ill patients are assumed to be at high risk of VTE and do not require stratification.23 For high-risk patients, prophylaxis with LMWH, low-dose unfractionated heparin (LDUH), or fondaparinux is recommended for the duration of admission.23 For patients at high risk of both VTE and bleeding, mechanical prophylaxis with intermittent pneumatic compression (IPC) is recommended instead of LMWH, LDUH, or fondaparinux.23
Surgery, like trauma (see next page), increases the risk of VTE and has been well studied. Prophylaxis after orthopedic surgery differs from that of other types of surgery.
In orthopedic surgery, risk depends on the procedure. For major orthopedic surgery, including total hip or knee arthroplasty and hip fracture surgery, VTE prophylaxis is recommended for 35 days postsurgically.43 LMWH is the preferred agent, although many other means have been shown to be beneficial.44 A recent systematic review demonstrated that aspirin is not inferior to other medications after hip or knee arthroplasty.45 No mechanical or pharmacotherapeutic prophylaxis is generally recommended after nonmajor orthopedic surgery.43
Nonorthopedic surgery is stratified by risk factors, using Caprini44 (TABLE 539). For medium-risk patients (Caprini score, 3-4) LDUH, LMWH, or IPC is recommended; for high-risk patients (Caprini score, ≥ 5) preventive treatment should combine pharmacotherapeutic and mechanical prophylaxis.46 A recent meta-analysis, comprising 14,776 patients, showed that surgical patients with a Caprini score ≥ 7 had a reduced incidence of VTE when given chemoprophylaxis, whereas patients whose score is < 7 do not benefit from chemoprophylaxis.43 When bleeding risk is high, IPC is recommended as sole therapy.43 Prophylaxis is not recommended when risk (determined by the Caprini score) is low.46
Post-hospitalization. Risk of VTE can persist for as long as 90 days after hospitalization; this finding has led to evaluation of the benefit of prolonged chemoprophylaxis.23 Extended-duration LMWH prophylaxis decreases the incidence of VTE, but at the cost of increased risk of major bleeding.47 Based on this evidence, guidelines recommend against prolonged-duration anticoagulation.23 A 2016 trial showed that 35 days of the direct-acting anticoagulant betrixaban reduced the risk of symptomatic VTE events, compared to 10 days of LMWH (NNT = 167), without increased risk of bleeding.48 This is a limited benefit, however, that is unlikely to change guideline recommendations.
Continue to: Trauma
Trauma: VTE risk increases with severity
Trauma increases the risk of VTE considerably. A national study showed that 1.5% of admitted trauma patients experienced VTE during hospitalization and that 1.2% were readmitted for VTE within 1 year.49 As many as 32% of trauma patients admitted to the intensive care unit experience VTE despite appropriate prophylaxis.50 A Cochrane Review51 found that:
- prophylaxis significantly reduces DVT risk
- pharmacotherapeutic prophylaxis is more effective than mechanical prophylaxis
- LMWH is more effective than LDUH.
Guidelines recommend that major trauma patients receive prophylaxis with LMWH, LDUH, or IPC.46
CORRESPONDENCE
Michael J. Arnold, MD, CDR, MC, USN; Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Jacksonville, FL 32214; [email protected].
1. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010. 38(4 suppl):S495-S501.
2. Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126:832.e13-e21.
3. Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010. 362:2155-2165.
4. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
5. Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25:235-242.
6. Stone J, Hangge P, Albadawi H, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S276-S284.
7. Olaf M, Cooney R. Deep venous thrombosis. Emerg Med Clin North Am. 2017;35:743-770.
8. Sajid MS, Ahmed N, Desai M, et al. Upper limb deep vein thrombosis: a literature review to streamline the protocol for management. Acta Haematol. 2007;118:10-18.
9. Bates SM, Ginsberg JS. Clinical practice. Treatment of deep-vein thrombosis. N Engl J Med. 2004;351:268-277.
10. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
11. Goldhaber SZ. Risk factors for venous thromboembolism. J Am Col Cardiol. 2010;56:1-7.
12. Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2:499-509.
13. Severinsen MT, Kristensen SR, Johnsen SP, et al. Smoking and venous thromboembolism: a Danish follow-up study. J Thromb Haemost. 2009;7:1297-1303.
14. Coppens M, Reijnders JH, Middeldorp S, et al. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6:1474-1477.
15. Choosing Wisely. American Society of Hematology. Ten things physicians and patients should question. www.choosingwisely.org/societies/american-society-of-hematology/. Accessed September 28, 2020.
16. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S-e736S.
17. Vossen CY, Conard J, Fontcuberta J, et al. Risk of a first venous thrombotic event in carriers of a familial thrombophilic defect. The European Prospective Cohort on Thrombophilia (EPCOT). J Thromb Haemost. 2005;3:459-464.
18. Practice Bulletin No. 197: Inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34.
19. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice Bulletin No. 132: Antiphospholipid syndrome. Obstet Gynecol. 2012;120:1514-1521.
20. Glynn RJ, Danielson E, Fonseca FAH, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851-1861.
21. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013(1):CD004816.
22. Squizzato A, Galli M, Romualdi E, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31:1248-1256.
23. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S.
24. Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ. 2003;327:1072.
25. Johnston RV, Hudson MF; . Travelers’ thrombosis. Aviat Space Environ Med. 2014;85:191-194.
26. Clarke MJ, Broderick C, Hopewell S, et al. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database Syst Rev. 2016;9:CD004002.
27. van Hylckama Vlieg A, Middledorp S. Hormone therapies and venous thromboembolism: where are we now? J Thromb Haemost. 2011;9:257-266.
28. Tepper NK, Dragoman MV, Gaffield ME, et al. Nonoral combined hormonal contraceptives and thromboembolism: a systematic review. Contraception. 2017;95:130-139.
29. Lekovic D, Miljic P, Dmitrovic A, et al. How do you decide on hormone replacement therapy in women with risk of venous thromboembolism? Blood Rev. 2017;31:151-157.
30. Rovinski D, Ramos RB, Fighera TM, et al. Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: a systematic review and meta-analysis. Thromb Res. 2018;168:83-95.
31. Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001275.
32. Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res. 2014;133(suppl 2):S49-S55.
33. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352.
34. Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2013;31:2189-2204.
35. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907.
36. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377-5382.
37. Carrier M, Abou-Nassar K, Mallick R, et al; AVERT Investigators. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711-719.
38. Cobben MRR, Nemeth B, Lijfering WM, et al. Validation of risk assessment models for venous thrombosis in hospitalized medical patients. Res Pract Thromb Haemost. 2019;3:217-225.
39. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70-78.
40. Spyropoulos AC, Anderson FA Jr, FitzGerald G, et al; IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706-714.
41. Kanaan AO, Silva MA, Donovan JL, et al. Meta-analysis of venous thromboembolism prophylaxis in medically Ill patients. Clin Ther. 2007;29:2395-2405.
42. HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198-3225.
43. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
44. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini Score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1103.
45. Matharu GS, Kunutsor SK, Judge A, et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2020;180:376-384.
46. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S-e277S.
47. Hull RD, Schellong SM, Tapson VF, et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recent reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8-18.
48. Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534-544.
49. Rattan R, Parreco J, Eidelson SA, et al. Hidden burden of venous thromboembolism after trauma: a national analysis. J Trauma Acute Care Surg. 2018;85:899-906.
50. Yumoto T, Naito H, Yamakawa Y, et al. Venous thromboembolism in major trauma patients: a single-center retrospective cohort study of the epidemiology and utility of D-dimer for screening. Acute Med Surg. 2017;4:394-400.
51. Barrera LM, Perel P, Ker K, et al. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013(3):CD008303.
Venous thromboembolism (VTE) is a common and dangerous disease, affecting 0.1%-0.2% of the population annually—a rate that might be underreported.1 VTE is a collective term for venous blood clots, including (1) deep vein thrombosis (DVT) of peripheral veins and (2) pulmonary embolism, which occurs after a clot travels through the heart and becomes lodged in the pulmonary vasculature. Two-thirds of VTE cases present clinically as DVT2; most mortality from VTE disease is caused by the 20% of cases of pulmonary embolism that present as sudden death.1
VTE is comparable to myocardial infarction (MI) in incidence and severity. In 2008, 208 of every 100,000 people had an MI, with a 30-day mortality of 16/100,0003; VTE disease has an annual incidence of 161 of every 100,000 people and a 28-day mortality of 18/100,000.4 Although the incidence and severity of MI are steadily decreasing, the rate of VTE appears constant.3,5 The high mortality of VTE suggests that primary prevention, which we discuss in this article, is valuable (see “Key points: Primary prevention of venous thromboembolism”).
SIDEBAR
Key points: Primary prevention of venous thromboembolism
- Primary prevention of venous thromboembolism (VTE), a disease with mortality similar to myocardial infarction, should be an important consideration in at-risk patients.
- Although statins reduce the risk of VTE, their use is justified only if they are also required for prevention of cardiovascular disease.
- The risk of travel-related VTE can be reduced by wearing compression stockings.
- The choice of particular methods of contraception and of hormone replacement therapy can reduce VTE risk.
- Because of the risk of bleeding, using anticoagulants for primary prevention of VTE is justified only in certain circumstances.
- Pregnancy is the only condition in which there is a guideline indication for thrombophilia testing, because test results in this setting can change recommendations for preventing VTE.
- Using a risk-stratification model is key to determining risk in both medically and surgically hospitalized patients. Trauma and major orthopedic surgery always place the patient at high risk of VTE.
Risk factors
Virchow’s triad of venous stasis, vascular injury, and hypercoagulability describes predisposing factors for VTE.6 Although venous valves promote blood flow, they produce isolated low-flow areas adjacent to valves that become concentrated and locally hypoxic, increasing the risk of clotting.7 The great majority of DVTs (≥ 96%) occur in the lower extremity,8 starting in the calf; there, 75% of cases resolve spontaneously before they extend into the deep veins of the proximal leg.7 One-half of DVTs that do move into the proximal leg eventually embolize.7
Major risk factors for VTE comprise inherited conditions, medical history, medical therapeutics, and behaviors (TABLE 1).9-11 Unlike the preventive management of coronary artery disease (CAD), there is no simple, generalized prevention algorithm to address VTE risk factors.

Risk factors for VTE and CAD overlap. Risk factors for atherosclerosis—obesity, diabetes, smoking, hypertension, hyperlipidemia—also increase the risk of VTE (TABLE 1).9-11 The association between risk factors for VTE and atherosclerosis is demonstrated by a doubling of the risk of MI and stroke in the year following VTE.11 Lifestyle changes are expected to reduce the risk of VTE, as they do for acute CAD, but studies are lacking to confirm this connection. There is no prospective evidence showing that weight loss or control of diabetes or hypertension reduces the risk of VTE.12 Smoking cessation does appear to reduce risk: Former smokers have the same VTE risk as never-smokers.13
Thrombophilia testing: Not generally useful
Inherited and acquired thrombophilic conditions define a group of disorders in which the risk of VTE is increased. Although thrombophilia testing was once considered for primary and secondary prevention of VTE, such testing is rarely used now because proof of benefit is lacking: A large case–control study showed that thrombophilia testing did not predict recurrence after a first VTE.14 Guidelines of the American College of Chest Physicians (ACCP) do not address thrombophilia, and the American Society of Hematology recommends against thrombophilia testing after a provoked VTE.15,16
Primary prophylaxis of patients with a family history of VTE and inherited thrombophilia is controversial. Patients with both a family history of VTE and demonstrated thrombophilia do have double the average incidence of VTE, but this increased risk does not offset the significant bleeding risk associated with anticoagulation.17 Recommendations for thrombophilia testing are limited to certain situations in pregnancy, discussed in a bit.16,18,19
Continue to: Primary prevention of VTE in the clinic
Primary prevention of VTE in the clinic
There is no single, overarching preventive strategy for VTE in an ambulatory patient (although statins, discussed in a moment, offer some benefit, broadly). There are, however, distinct behavioral characteristics and medical circumstances for which opportunities exist to reduce VTE risk—for example, when a person engages in long-distance travel, receives hormonal therapy, is pregnant, or has cancer. In each scenario, recognizing and mitigating risk are important.
Statins offer a (slight) benefit
There is evidence that statins reduce the risk of VTE—slightly20-23:
- A large randomized, controlled trial showed that rosuvastatin, 20 mg/d, reduced the rate of VTE, compared to placebo; however, the 2-year number needed to treat (NNT) was 349.20 The VTE benefit is minimal, however, compared to primary prevention of cardiovascular disease with statins (5-year NNT = 56).21 The sole significant adverse event associated with statins was new-onset type 2 diabetes (5-year number needed to harm = 235).21
- A subsequent meta-analysis confirmed a small reduction in VTE risk with statins.22 In its 2012 guidelines, ACCP declined to issue a recommendation on the use of statins for VTE prevention.23 When considering statins for primary cardiovascular disease prevention, take the additional VTE prevention into account.
Simple strategies can help prevent travel-related VTE
Travel is a common inciting factor for VTE. A systematic review showed that VTE risk triples after travel of ≥ 4 hours, increasing by 20% with each additional 2 hours.24 Most VTE occurs in travelers who have other VTE risk factors.25 Based on case–control studies,23 guidelines recommend these preventive measures:
- frequent calf exercises
- sitting in an aisle seat during air travel
- keeping hydrated.
A Cochrane review showed that graded compression stockings reduce asymptomatic DVT in travelers by a factor of 10, in high- and low-risk patients.26
VTE risk varies with type of hormonal contraception
Most contraceptives increase VTE risk (TABLE 227,28). Risk with combined oral contraceptives varies with the amount of estrogen and progesterone. To reduce VTE risk with oral contraceptives, patients can use an agent that contains a lower dose of estrogen or one in which levonorgestrel replaces other progesterones.27

Continue to: Studies suggest that the levonorgestrel-releasing...
Studies suggest that the levonorgestrel-releasing intrauterine device and progestin-only pills are not associated with an increase in VTE risk.27 Although the quality of evidence varies, most nonoral hormonal contraceptives have been determined to carry a risk of VTE that is similar to that of combined oral contraceptives.28
In hormone replacement, avoid pills to lower risk
Hormone replacement therapy (HRT) for postmenopausal women increases VTE risk when administered in oral form, with combined estrogen and progestin HRT doubling the risk and estrogen-only formulations having a lower risk.29 VTE risk is highest in the first 6 months of HRT, declining to that of a non-HRT user within 5 years.29 Neither transdermal HRT nor estrogen creams increase the risk of VTE, according to a systematic review.30 The estradiol-containing vaginal ring also does not confer increased risk.29
Pregnancy, thrombophilia, and VTE prevention
VTE affects as many as 0.2% of pregnancies but causes 9% of pregnancy-related deaths.18 The severity of VTE in pregnancy led the American College of Obstetricians and Gynecologists (ACOG) to recommend primary VTE prophylaxis in patients with certain thrombophilias.18 Thrombophilia testing is recommended in patients with proven high-risk thrombophilia in a first-degree relative.18 ACOG recognizes 5 thrombophilias considered to carry a high risk of VTE in pregnancy18:
- homozygous Factor V Leiden
- homozygous prothrombin G20210A mutation
- antithrombin deficiency
- heterozygous Factor V Leiden and prothrombin G20210A mutation
- antiphospholipid antibody syndrome.
ACOG recommends limiting thrombophilia testing to (1) any specific thrombophilia carried by a relative and (2) possibly, the antiphospholipid antibodies anticardiolipin and lupus anticoagulant.18,19 Antiphospholipid testing is recommended when there is a history of stillbirth, 3 early pregnancy losses, or delivery earlier than 34 weeks secondary to preeclampsia.19
Primary VTE prophylaxis is recommended for pregnant patients with a high-risk thrombophilia; low-molecular-weight heparin (LMWH) is safe and its effects are predictable.18 Because postpartum risk of VTE is higher than antepartum risk, postpartum prophylaxis is also recommended with lower-risk thrombophilias18; a vitamin K antagonist or LMWH can be used.18 ACCP and ACOG recommendations for VTE prophylaxis in pregnancy differ slightly (TABLE 316,18,19).

Continue to: Cancer increases risks of VTE and bleeding
Cancer increases risks of VTE and bleeding
Cancer increases VTE risk > 6-fold31; metastases, chemotherapy, and radiotherapy further increase risk. Cancer also greatly increases the risk of bleeding: Cancer patients with VTE have an annual major bleeding rate ≥ 20%.32 Guidelines do not recommend primary VTE prophylaxis for cancer, although American Society of Clinical Oncology guidelines discuss consideration of prophylaxis for select, high-risk patients,33,34 including those with multiple myeloma, metastatic gastrointestinal cancer, or metastatic brain cancer.31,34 Recent evidence (discussed in a moment) supports the use of apixaban for primary VTE prevention during chemotherapy for high-risk cancer.
The Khorana Risk Score (TABLE 435,36) for VTE was developed and validated for use in patients with solid cancer35: A score of 2 conveys nearly a 10% risk of VTE over 6 months.36 A recent study of 550 cancer patients with a Khorana score of ≥ 2—the first evidence of risk-guided primary VTE prevention in cancer—showed that primary prophylaxis with 2.5 mg of apixaban, bid, reduced the risk of VTE (NNT = 17); however, the number needed to harm (for major bleeding) was 59.37 Mortality was not changed with apixaban treatment

Primary VTE prevention in med-surg hospitalizations
The risk of VTE increases significantly during hospitalization, although not enough to justify universal prophylaxis. Recommended prevention strategies for different classes of hospitalized patients are summarized below.
In medically hospitalized patients, risk is stratified with a risk-assessment model. Medically hospitalized patients have, on average, a VTE risk of 1.2%23; 12 risk-assessment models designed to stratify risk were recently compared.38 Two models, the Caprini Score (TABLE 5)39 and the IMPROVE VTE Risk Calculator,40 were best able to identify low-risk patients (negative predictive value, > 99%).38 American Society of Hematology guidelines recommend IMPROVE VTE or the Padua Prediction Score for risk stratification.41 While the Caprini score only designates 11% of eventual VTE cases as low risk, both the IMPROVE VTE and Padua scores miss more than 35% of eventual VTE.38

Because LMWH prophylaxis has been shown to reduce VTE by 40% without increasing the risk of major bleeding, using Caprini should prevent 2 VTEs for every 1000 patients, without an increase in major bleeding and with 13 additional minor bleeding events.42
Continue to: Critically ill patients
Critically ill patients are assumed to be at high risk of VTE and do not require stratification.23 For high-risk patients, prophylaxis with LMWH, low-dose unfractionated heparin (LDUH), or fondaparinux is recommended for the duration of admission.23 For patients at high risk of both VTE and bleeding, mechanical prophylaxis with intermittent pneumatic compression (IPC) is recommended instead of LMWH, LDUH, or fondaparinux.23
Surgery, like trauma (see next page), increases the risk of VTE and has been well studied. Prophylaxis after orthopedic surgery differs from that of other types of surgery.
In orthopedic surgery, risk depends on the procedure. For major orthopedic surgery, including total hip or knee arthroplasty and hip fracture surgery, VTE prophylaxis is recommended for 35 days postsurgically.43 LMWH is the preferred agent, although many other means have been shown to be beneficial.44 A recent systematic review demonstrated that aspirin is not inferior to other medications after hip or knee arthroplasty.45 No mechanical or pharmacotherapeutic prophylaxis is generally recommended after nonmajor orthopedic surgery.43
Nonorthopedic surgery is stratified by risk factors, using Caprini44 (TABLE 539). For medium-risk patients (Caprini score, 3-4) LDUH, LMWH, or IPC is recommended; for high-risk patients (Caprini score, ≥ 5) preventive treatment should combine pharmacotherapeutic and mechanical prophylaxis.46 A recent meta-analysis, comprising 14,776 patients, showed that surgical patients with a Caprini score ≥ 7 had a reduced incidence of VTE when given chemoprophylaxis, whereas patients whose score is < 7 do not benefit from chemoprophylaxis.43 When bleeding risk is high, IPC is recommended as sole therapy.43 Prophylaxis is not recommended when risk (determined by the Caprini score) is low.46
Post-hospitalization. Risk of VTE can persist for as long as 90 days after hospitalization; this finding has led to evaluation of the benefit of prolonged chemoprophylaxis.23 Extended-duration LMWH prophylaxis decreases the incidence of VTE, but at the cost of increased risk of major bleeding.47 Based on this evidence, guidelines recommend against prolonged-duration anticoagulation.23 A 2016 trial showed that 35 days of the direct-acting anticoagulant betrixaban reduced the risk of symptomatic VTE events, compared to 10 days of LMWH (NNT = 167), without increased risk of bleeding.48 This is a limited benefit, however, that is unlikely to change guideline recommendations.
Continue to: Trauma
Trauma: VTE risk increases with severity
Trauma increases the risk of VTE considerably. A national study showed that 1.5% of admitted trauma patients experienced VTE during hospitalization and that 1.2% were readmitted for VTE within 1 year.49 As many as 32% of trauma patients admitted to the intensive care unit experience VTE despite appropriate prophylaxis.50 A Cochrane Review51 found that:
- prophylaxis significantly reduces DVT risk
- pharmacotherapeutic prophylaxis is more effective than mechanical prophylaxis
- LMWH is more effective than LDUH.
Guidelines recommend that major trauma patients receive prophylaxis with LMWH, LDUH, or IPC.46
CORRESPONDENCE
Michael J. Arnold, MD, CDR, MC, USN; Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Jacksonville, FL 32214; [email protected].
Venous thromboembolism (VTE) is a common and dangerous disease, affecting 0.1%-0.2% of the population annually—a rate that might be underreported.1 VTE is a collective term for venous blood clots, including (1) deep vein thrombosis (DVT) of peripheral veins and (2) pulmonary embolism, which occurs after a clot travels through the heart and becomes lodged in the pulmonary vasculature. Two-thirds of VTE cases present clinically as DVT2; most mortality from VTE disease is caused by the 20% of cases of pulmonary embolism that present as sudden death.1
VTE is comparable to myocardial infarction (MI) in incidence and severity. In 2008, 208 of every 100,000 people had an MI, with a 30-day mortality of 16/100,0003; VTE disease has an annual incidence of 161 of every 100,000 people and a 28-day mortality of 18/100,000.4 Although the incidence and severity of MI are steadily decreasing, the rate of VTE appears constant.3,5 The high mortality of VTE suggests that primary prevention, which we discuss in this article, is valuable (see “Key points: Primary prevention of venous thromboembolism”).
SIDEBAR
Key points: Primary prevention of venous thromboembolism
- Primary prevention of venous thromboembolism (VTE), a disease with mortality similar to myocardial infarction, should be an important consideration in at-risk patients.
- Although statins reduce the risk of VTE, their use is justified only if they are also required for prevention of cardiovascular disease.
- The risk of travel-related VTE can be reduced by wearing compression stockings.
- The choice of particular methods of contraception and of hormone replacement therapy can reduce VTE risk.
- Because of the risk of bleeding, using anticoagulants for primary prevention of VTE is justified only in certain circumstances.
- Pregnancy is the only condition in which there is a guideline indication for thrombophilia testing, because test results in this setting can change recommendations for preventing VTE.
- Using a risk-stratification model is key to determining risk in both medically and surgically hospitalized patients. Trauma and major orthopedic surgery always place the patient at high risk of VTE.
Risk factors
Virchow’s triad of venous stasis, vascular injury, and hypercoagulability describes predisposing factors for VTE.6 Although venous valves promote blood flow, they produce isolated low-flow areas adjacent to valves that become concentrated and locally hypoxic, increasing the risk of clotting.7 The great majority of DVTs (≥ 96%) occur in the lower extremity,8 starting in the calf; there, 75% of cases resolve spontaneously before they extend into the deep veins of the proximal leg.7 One-half of DVTs that do move into the proximal leg eventually embolize.7
Major risk factors for VTE comprise inherited conditions, medical history, medical therapeutics, and behaviors (TABLE 1).9-11 Unlike the preventive management of coronary artery disease (CAD), there is no simple, generalized prevention algorithm to address VTE risk factors.

Risk factors for VTE and CAD overlap. Risk factors for atherosclerosis—obesity, diabetes, smoking, hypertension, hyperlipidemia—also increase the risk of VTE (TABLE 1).9-11 The association between risk factors for VTE and atherosclerosis is demonstrated by a doubling of the risk of MI and stroke in the year following VTE.11 Lifestyle changes are expected to reduce the risk of VTE, as they do for acute CAD, but studies are lacking to confirm this connection. There is no prospective evidence showing that weight loss or control of diabetes or hypertension reduces the risk of VTE.12 Smoking cessation does appear to reduce risk: Former smokers have the same VTE risk as never-smokers.13
Thrombophilia testing: Not generally useful
Inherited and acquired thrombophilic conditions define a group of disorders in which the risk of VTE is increased. Although thrombophilia testing was once considered for primary and secondary prevention of VTE, such testing is rarely used now because proof of benefit is lacking: A large case–control study showed that thrombophilia testing did not predict recurrence after a first VTE.14 Guidelines of the American College of Chest Physicians (ACCP) do not address thrombophilia, and the American Society of Hematology recommends against thrombophilia testing after a provoked VTE.15,16
Primary prophylaxis of patients with a family history of VTE and inherited thrombophilia is controversial. Patients with both a family history of VTE and demonstrated thrombophilia do have double the average incidence of VTE, but this increased risk does not offset the significant bleeding risk associated with anticoagulation.17 Recommendations for thrombophilia testing are limited to certain situations in pregnancy, discussed in a bit.16,18,19
Continue to: Primary prevention of VTE in the clinic
Primary prevention of VTE in the clinic
There is no single, overarching preventive strategy for VTE in an ambulatory patient (although statins, discussed in a moment, offer some benefit, broadly). There are, however, distinct behavioral characteristics and medical circumstances for which opportunities exist to reduce VTE risk—for example, when a person engages in long-distance travel, receives hormonal therapy, is pregnant, or has cancer. In each scenario, recognizing and mitigating risk are important.
Statins offer a (slight) benefit
There is evidence that statins reduce the risk of VTE—slightly20-23:
- A large randomized, controlled trial showed that rosuvastatin, 20 mg/d, reduced the rate of VTE, compared to placebo; however, the 2-year number needed to treat (NNT) was 349.20 The VTE benefit is minimal, however, compared to primary prevention of cardiovascular disease with statins (5-year NNT = 56).21 The sole significant adverse event associated with statins was new-onset type 2 diabetes (5-year number needed to harm = 235).21
- A subsequent meta-analysis confirmed a small reduction in VTE risk with statins.22 In its 2012 guidelines, ACCP declined to issue a recommendation on the use of statins for VTE prevention.23 When considering statins for primary cardiovascular disease prevention, take the additional VTE prevention into account.
Simple strategies can help prevent travel-related VTE
Travel is a common inciting factor for VTE. A systematic review showed that VTE risk triples after travel of ≥ 4 hours, increasing by 20% with each additional 2 hours.24 Most VTE occurs in travelers who have other VTE risk factors.25 Based on case–control studies,23 guidelines recommend these preventive measures:
- frequent calf exercises
- sitting in an aisle seat during air travel
- keeping hydrated.
A Cochrane review showed that graded compression stockings reduce asymptomatic DVT in travelers by a factor of 10, in high- and low-risk patients.26
VTE risk varies with type of hormonal contraception
Most contraceptives increase VTE risk (TABLE 227,28). Risk with combined oral contraceptives varies with the amount of estrogen and progesterone. To reduce VTE risk with oral contraceptives, patients can use an agent that contains a lower dose of estrogen or one in which levonorgestrel replaces other progesterones.27

Continue to: Studies suggest that the levonorgestrel-releasing...
Studies suggest that the levonorgestrel-releasing intrauterine device and progestin-only pills are not associated with an increase in VTE risk.27 Although the quality of evidence varies, most nonoral hormonal contraceptives have been determined to carry a risk of VTE that is similar to that of combined oral contraceptives.28
In hormone replacement, avoid pills to lower risk
Hormone replacement therapy (HRT) for postmenopausal women increases VTE risk when administered in oral form, with combined estrogen and progestin HRT doubling the risk and estrogen-only formulations having a lower risk.29 VTE risk is highest in the first 6 months of HRT, declining to that of a non-HRT user within 5 years.29 Neither transdermal HRT nor estrogen creams increase the risk of VTE, according to a systematic review.30 The estradiol-containing vaginal ring also does not confer increased risk.29
Pregnancy, thrombophilia, and VTE prevention
VTE affects as many as 0.2% of pregnancies but causes 9% of pregnancy-related deaths.18 The severity of VTE in pregnancy led the American College of Obstetricians and Gynecologists (ACOG) to recommend primary VTE prophylaxis in patients with certain thrombophilias.18 Thrombophilia testing is recommended in patients with proven high-risk thrombophilia in a first-degree relative.18 ACOG recognizes 5 thrombophilias considered to carry a high risk of VTE in pregnancy18:
- homozygous Factor V Leiden
- homozygous prothrombin G20210A mutation
- antithrombin deficiency
- heterozygous Factor V Leiden and prothrombin G20210A mutation
- antiphospholipid antibody syndrome.
ACOG recommends limiting thrombophilia testing to (1) any specific thrombophilia carried by a relative and (2) possibly, the antiphospholipid antibodies anticardiolipin and lupus anticoagulant.18,19 Antiphospholipid testing is recommended when there is a history of stillbirth, 3 early pregnancy losses, or delivery earlier than 34 weeks secondary to preeclampsia.19
Primary VTE prophylaxis is recommended for pregnant patients with a high-risk thrombophilia; low-molecular-weight heparin (LMWH) is safe and its effects are predictable.18 Because postpartum risk of VTE is higher than antepartum risk, postpartum prophylaxis is also recommended with lower-risk thrombophilias18; a vitamin K antagonist or LMWH can be used.18 ACCP and ACOG recommendations for VTE prophylaxis in pregnancy differ slightly (TABLE 316,18,19).

Continue to: Cancer increases risks of VTE and bleeding
Cancer increases risks of VTE and bleeding
Cancer increases VTE risk > 6-fold31; metastases, chemotherapy, and radiotherapy further increase risk. Cancer also greatly increases the risk of bleeding: Cancer patients with VTE have an annual major bleeding rate ≥ 20%.32 Guidelines do not recommend primary VTE prophylaxis for cancer, although American Society of Clinical Oncology guidelines discuss consideration of prophylaxis for select, high-risk patients,33,34 including those with multiple myeloma, metastatic gastrointestinal cancer, or metastatic brain cancer.31,34 Recent evidence (discussed in a moment) supports the use of apixaban for primary VTE prevention during chemotherapy for high-risk cancer.
The Khorana Risk Score (TABLE 435,36) for VTE was developed and validated for use in patients with solid cancer35: A score of 2 conveys nearly a 10% risk of VTE over 6 months.36 A recent study of 550 cancer patients with a Khorana score of ≥ 2—the first evidence of risk-guided primary VTE prevention in cancer—showed that primary prophylaxis with 2.5 mg of apixaban, bid, reduced the risk of VTE (NNT = 17); however, the number needed to harm (for major bleeding) was 59.37 Mortality was not changed with apixaban treatment

Primary VTE prevention in med-surg hospitalizations
The risk of VTE increases significantly during hospitalization, although not enough to justify universal prophylaxis. Recommended prevention strategies for different classes of hospitalized patients are summarized below.
In medically hospitalized patients, risk is stratified with a risk-assessment model. Medically hospitalized patients have, on average, a VTE risk of 1.2%23; 12 risk-assessment models designed to stratify risk were recently compared.38 Two models, the Caprini Score (TABLE 5)39 and the IMPROVE VTE Risk Calculator,40 were best able to identify low-risk patients (negative predictive value, > 99%).38 American Society of Hematology guidelines recommend IMPROVE VTE or the Padua Prediction Score for risk stratification.41 While the Caprini score only designates 11% of eventual VTE cases as low risk, both the IMPROVE VTE and Padua scores miss more than 35% of eventual VTE.38

Because LMWH prophylaxis has been shown to reduce VTE by 40% without increasing the risk of major bleeding, using Caprini should prevent 2 VTEs for every 1000 patients, without an increase in major bleeding and with 13 additional minor bleeding events.42
Continue to: Critically ill patients
Critically ill patients are assumed to be at high risk of VTE and do not require stratification.23 For high-risk patients, prophylaxis with LMWH, low-dose unfractionated heparin (LDUH), or fondaparinux is recommended for the duration of admission.23 For patients at high risk of both VTE and bleeding, mechanical prophylaxis with intermittent pneumatic compression (IPC) is recommended instead of LMWH, LDUH, or fondaparinux.23
Surgery, like trauma (see next page), increases the risk of VTE and has been well studied. Prophylaxis after orthopedic surgery differs from that of other types of surgery.
In orthopedic surgery, risk depends on the procedure. For major orthopedic surgery, including total hip or knee arthroplasty and hip fracture surgery, VTE prophylaxis is recommended for 35 days postsurgically.43 LMWH is the preferred agent, although many other means have been shown to be beneficial.44 A recent systematic review demonstrated that aspirin is not inferior to other medications after hip or knee arthroplasty.45 No mechanical or pharmacotherapeutic prophylaxis is generally recommended after nonmajor orthopedic surgery.43
Nonorthopedic surgery is stratified by risk factors, using Caprini44 (TABLE 539). For medium-risk patients (Caprini score, 3-4) LDUH, LMWH, or IPC is recommended; for high-risk patients (Caprini score, ≥ 5) preventive treatment should combine pharmacotherapeutic and mechanical prophylaxis.46 A recent meta-analysis, comprising 14,776 patients, showed that surgical patients with a Caprini score ≥ 7 had a reduced incidence of VTE when given chemoprophylaxis, whereas patients whose score is < 7 do not benefit from chemoprophylaxis.43 When bleeding risk is high, IPC is recommended as sole therapy.43 Prophylaxis is not recommended when risk (determined by the Caprini score) is low.46
Post-hospitalization. Risk of VTE can persist for as long as 90 days after hospitalization; this finding has led to evaluation of the benefit of prolonged chemoprophylaxis.23 Extended-duration LMWH prophylaxis decreases the incidence of VTE, but at the cost of increased risk of major bleeding.47 Based on this evidence, guidelines recommend against prolonged-duration anticoagulation.23 A 2016 trial showed that 35 days of the direct-acting anticoagulant betrixaban reduced the risk of symptomatic VTE events, compared to 10 days of LMWH (NNT = 167), without increased risk of bleeding.48 This is a limited benefit, however, that is unlikely to change guideline recommendations.
Continue to: Trauma
Trauma: VTE risk increases with severity
Trauma increases the risk of VTE considerably. A national study showed that 1.5% of admitted trauma patients experienced VTE during hospitalization and that 1.2% were readmitted for VTE within 1 year.49 As many as 32% of trauma patients admitted to the intensive care unit experience VTE despite appropriate prophylaxis.50 A Cochrane Review51 found that:
- prophylaxis significantly reduces DVT risk
- pharmacotherapeutic prophylaxis is more effective than mechanical prophylaxis
- LMWH is more effective than LDUH.
Guidelines recommend that major trauma patients receive prophylaxis with LMWH, LDUH, or IPC.46
CORRESPONDENCE
Michael J. Arnold, MD, CDR, MC, USN; Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Jacksonville, FL 32214; [email protected].
1. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010. 38(4 suppl):S495-S501.
2. Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126:832.e13-e21.
3. Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010. 362:2155-2165.
4. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
5. Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25:235-242.
6. Stone J, Hangge P, Albadawi H, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S276-S284.
7. Olaf M, Cooney R. Deep venous thrombosis. Emerg Med Clin North Am. 2017;35:743-770.
8. Sajid MS, Ahmed N, Desai M, et al. Upper limb deep vein thrombosis: a literature review to streamline the protocol for management. Acta Haematol. 2007;118:10-18.
9. Bates SM, Ginsberg JS. Clinical practice. Treatment of deep-vein thrombosis. N Engl J Med. 2004;351:268-277.
10. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
11. Goldhaber SZ. Risk factors for venous thromboembolism. J Am Col Cardiol. 2010;56:1-7.
12. Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2:499-509.
13. Severinsen MT, Kristensen SR, Johnsen SP, et al. Smoking and venous thromboembolism: a Danish follow-up study. J Thromb Haemost. 2009;7:1297-1303.
14. Coppens M, Reijnders JH, Middeldorp S, et al. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6:1474-1477.
15. Choosing Wisely. American Society of Hematology. Ten things physicians and patients should question. www.choosingwisely.org/societies/american-society-of-hematology/. Accessed September 28, 2020.
16. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S-e736S.
17. Vossen CY, Conard J, Fontcuberta J, et al. Risk of a first venous thrombotic event in carriers of a familial thrombophilic defect. The European Prospective Cohort on Thrombophilia (EPCOT). J Thromb Haemost. 2005;3:459-464.
18. Practice Bulletin No. 197: Inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34.
19. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice Bulletin No. 132: Antiphospholipid syndrome. Obstet Gynecol. 2012;120:1514-1521.
20. Glynn RJ, Danielson E, Fonseca FAH, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851-1861.
21. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013(1):CD004816.
22. Squizzato A, Galli M, Romualdi E, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31:1248-1256.
23. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S.
24. Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ. 2003;327:1072.
25. Johnston RV, Hudson MF; . Travelers’ thrombosis. Aviat Space Environ Med. 2014;85:191-194.
26. Clarke MJ, Broderick C, Hopewell S, et al. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database Syst Rev. 2016;9:CD004002.
27. van Hylckama Vlieg A, Middledorp S. Hormone therapies and venous thromboembolism: where are we now? J Thromb Haemost. 2011;9:257-266.
28. Tepper NK, Dragoman MV, Gaffield ME, et al. Nonoral combined hormonal contraceptives and thromboembolism: a systematic review. Contraception. 2017;95:130-139.
29. Lekovic D, Miljic P, Dmitrovic A, et al. How do you decide on hormone replacement therapy in women with risk of venous thromboembolism? Blood Rev. 2017;31:151-157.
30. Rovinski D, Ramos RB, Fighera TM, et al. Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: a systematic review and meta-analysis. Thromb Res. 2018;168:83-95.
31. Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001275.
32. Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res. 2014;133(suppl 2):S49-S55.
33. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352.
34. Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2013;31:2189-2204.
35. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907.
36. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377-5382.
37. Carrier M, Abou-Nassar K, Mallick R, et al; AVERT Investigators. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711-719.
38. Cobben MRR, Nemeth B, Lijfering WM, et al. Validation of risk assessment models for venous thrombosis in hospitalized medical patients. Res Pract Thromb Haemost. 2019;3:217-225.
39. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70-78.
40. Spyropoulos AC, Anderson FA Jr, FitzGerald G, et al; IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706-714.
41. Kanaan AO, Silva MA, Donovan JL, et al. Meta-analysis of venous thromboembolism prophylaxis in medically Ill patients. Clin Ther. 2007;29:2395-2405.
42. HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198-3225.
43. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
44. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini Score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1103.
45. Matharu GS, Kunutsor SK, Judge A, et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2020;180:376-384.
46. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S-e277S.
47. Hull RD, Schellong SM, Tapson VF, et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recent reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8-18.
48. Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534-544.
49. Rattan R, Parreco J, Eidelson SA, et al. Hidden burden of venous thromboembolism after trauma: a national analysis. J Trauma Acute Care Surg. 2018;85:899-906.
50. Yumoto T, Naito H, Yamakawa Y, et al. Venous thromboembolism in major trauma patients: a single-center retrospective cohort study of the epidemiology and utility of D-dimer for screening. Acute Med Surg. 2017;4:394-400.
51. Barrera LM, Perel P, Ker K, et al. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013(3):CD008303.
1. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010. 38(4 suppl):S495-S501.
2. Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126:832.e13-e21.
3. Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010. 362:2155-2165.
4. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
5. Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25:235-242.
6. Stone J, Hangge P, Albadawi H, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S276-S284.
7. Olaf M, Cooney R. Deep venous thrombosis. Emerg Med Clin North Am. 2017;35:743-770.
8. Sajid MS, Ahmed N, Desai M, et al. Upper limb deep vein thrombosis: a literature review to streamline the protocol for management. Acta Haematol. 2007;118:10-18.
9. Bates SM, Ginsberg JS. Clinical practice. Treatment of deep-vein thrombosis. N Engl J Med. 2004;351:268-277.
10. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
11. Goldhaber SZ. Risk factors for venous thromboembolism. J Am Col Cardiol. 2010;56:1-7.
12. Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2:499-509.
13. Severinsen MT, Kristensen SR, Johnsen SP, et al. Smoking and venous thromboembolism: a Danish follow-up study. J Thromb Haemost. 2009;7:1297-1303.
14. Coppens M, Reijnders JH, Middeldorp S, et al. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6:1474-1477.
15. Choosing Wisely. American Society of Hematology. Ten things physicians and patients should question. www.choosingwisely.org/societies/american-society-of-hematology/. Accessed September 28, 2020.
16. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S-e736S.
17. Vossen CY, Conard J, Fontcuberta J, et al. Risk of a first venous thrombotic event in carriers of a familial thrombophilic defect. The European Prospective Cohort on Thrombophilia (EPCOT). J Thromb Haemost. 2005;3:459-464.
18. Practice Bulletin No. 197: Inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34.
19. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice Bulletin No. 132: Antiphospholipid syndrome. Obstet Gynecol. 2012;120:1514-1521.
20. Glynn RJ, Danielson E, Fonseca FAH, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851-1861.
21. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013(1):CD004816.
22. Squizzato A, Galli M, Romualdi E, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31:1248-1256.
23. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S.
24. Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ. 2003;327:1072.
25. Johnston RV, Hudson MF; . Travelers’ thrombosis. Aviat Space Environ Med. 2014;85:191-194.
26. Clarke MJ, Broderick C, Hopewell S, et al. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database Syst Rev. 2016;9:CD004002.
27. van Hylckama Vlieg A, Middledorp S. Hormone therapies and venous thromboembolism: where are we now? J Thromb Haemost. 2011;9:257-266.
28. Tepper NK, Dragoman MV, Gaffield ME, et al. Nonoral combined hormonal contraceptives and thromboembolism: a systematic review. Contraception. 2017;95:130-139.
29. Lekovic D, Miljic P, Dmitrovic A, et al. How do you decide on hormone replacement therapy in women with risk of venous thromboembolism? Blood Rev. 2017;31:151-157.
30. Rovinski D, Ramos RB, Fighera TM, et al. Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: a systematic review and meta-analysis. Thromb Res. 2018;168:83-95.
31. Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001275.
32. Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res. 2014;133(suppl 2):S49-S55.
33. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352.
34. Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2013;31:2189-2204.
35. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907.
36. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377-5382.
37. Carrier M, Abou-Nassar K, Mallick R, et al; AVERT Investigators. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711-719.
38. Cobben MRR, Nemeth B, Lijfering WM, et al. Validation of risk assessment models for venous thrombosis in hospitalized medical patients. Res Pract Thromb Haemost. 2019;3:217-225.
39. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70-78.
40. Spyropoulos AC, Anderson FA Jr, FitzGerald G, et al; IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706-714.
41. Kanaan AO, Silva MA, Donovan JL, et al. Meta-analysis of venous thromboembolism prophylaxis in medically Ill patients. Clin Ther. 2007;29:2395-2405.
42. HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198-3225.
43. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
44. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini Score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1103.
45. Matharu GS, Kunutsor SK, Judge A, et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2020;180:376-384.
46. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S-e277S.
47. Hull RD, Schellong SM, Tapson VF, et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recent reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8-18.
48. Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534-544.
49. Rattan R, Parreco J, Eidelson SA, et al. Hidden burden of venous thromboembolism after trauma: a national analysis. J Trauma Acute Care Surg. 2018;85:899-906.
50. Yumoto T, Naito H, Yamakawa Y, et al. Venous thromboembolism in major trauma patients: a single-center retrospective cohort study of the epidemiology and utility of D-dimer for screening. Acute Med Surg. 2017;4:394-400.
51. Barrera LM, Perel P, Ker K, et al. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013(3):CD008303.
PRACTICE RECOMMENDATIONS
› Consider the mild reduction in the risk of venous thromboembolism (VTE) provided by statins when contemplating their use for cardiovascular disease prevention. B
› Avoid testing for thrombophilia to determine the risk of VTE, except in pregnant patients who meet criteria for antiphospholipid syndrome or have a family history of VTE. B
› Recommend an intrauterine device or progestin-only pill for contraception if the patient’s risk of VTE is high. B
› Stratify hospitalized medical and nonorthopedic surgical patients by risk score to determine the need for VTE prophylaxis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series