User login
The US Food and Drug Administration (FDA) has granted fast track designation to caplacizumab, an anti-von Willebrand factor (vWF) nanobody being developed for the treatment of acquired thrombotic thrombocytopenic purpura (aTTP).
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
About caplacizumab
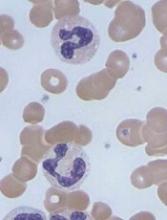
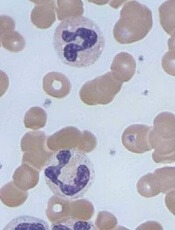
Caplacizumab is a bivalent anti-vWF nanobody being developed by Ablynx. Caplacizumab works by blocking the interaction of ultra-large vWF multimers with platelets, having an immediate effect on platelet aggregation and the ensuing formation and accumulation of the micro-clots that cause the severe thrombocytopenia, tissue ischemia, and organ dysfunction that occurs in patients with aTTP.
Researchers evaluated the efficacy and safety of caplacizumab, given with standard care for aTTP, in the phase 2 TITAN trial.
The trial enrolled 75 patients with aTTP. They all received the standard of care—daily plasma exchange and immunosuppressive therapy. Thirty-six patients were randomized to receive caplacizumab as well, and 39 were randomized to placebo.
The study’s primary endpoint was time to response (platelet count normalization). Patients in the caplacizumab arm had a 39% reduction in the median time to response compared to patients in the placebo arm (P=0.005).
The rate of confirmed response was 86.1% (n=31) in the caplacizumab arm and 71.8% (n=28) in the placebo arm.
There were more relapses in the caplacizumab arm than the placebo arm—8 (22.2%) and 0, respectively. Relapse was defined as a TTP event occurring more than 30 days after the end of daily plasma exchange.
There were fewer exacerbations in the caplacizumab arm than the placebo arm—3 (8.3%) and 11 (28.2%), respectively. Exacerbation was defined as recurrent thrombocytopenia within 30 days of the end of daily plasma exchange that required re-initiation of daily exchange.
The rate of adverse events thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of events that were possibly related was 54% and 8%, respectively.
A lower proportion of subjects in the caplacizumab arm experienced one or more major thromboembolic events or died, compared to the placebo arm—11.4% and 43.2%, respectively.
In addition, fewer caplacizumab-treated patients were refractory to treatment—5.7% vs 21.6%.
There were 2 deaths in the placebo arm, and both of those patients were refractory to treatment. There were no deaths reported in the caplacizumab arm.
Now, researchers are evaluating caplacizumab in the phase 3 HERCULES trial (NCT02553317). Results from this study are anticipated in the second half of 2017 are expected to support a planned biologics license application filing in the US in 2018. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to caplacizumab, an anti-von Willebrand factor (vWF) nanobody being developed for the treatment of acquired thrombotic thrombocytopenic purpura (aTTP).
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
About caplacizumab
Caplacizumab is a bivalent anti-vWF nanobody being developed by Ablynx. Caplacizumab works by blocking the interaction of ultra-large vWF multimers with platelets, having an immediate effect on platelet aggregation and the ensuing formation and accumulation of the micro-clots that cause the severe thrombocytopenia, tissue ischemia, and organ dysfunction that occurs in patients with aTTP.
Researchers evaluated the efficacy and safety of caplacizumab, given with standard care for aTTP, in the phase 2 TITAN trial.
The trial enrolled 75 patients with aTTP. They all received the standard of care—daily plasma exchange and immunosuppressive therapy. Thirty-six patients were randomized to receive caplacizumab as well, and 39 were randomized to placebo.
The study’s primary endpoint was time to response (platelet count normalization). Patients in the caplacizumab arm had a 39% reduction in the median time to response compared to patients in the placebo arm (P=0.005).
The rate of confirmed response was 86.1% (n=31) in the caplacizumab arm and 71.8% (n=28) in the placebo arm.
There were more relapses in the caplacizumab arm than the placebo arm—8 (22.2%) and 0, respectively. Relapse was defined as a TTP event occurring more than 30 days after the end of daily plasma exchange.
There were fewer exacerbations in the caplacizumab arm than the placebo arm—3 (8.3%) and 11 (28.2%), respectively. Exacerbation was defined as recurrent thrombocytopenia within 30 days of the end of daily plasma exchange that required re-initiation of daily exchange.
The rate of adverse events thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of events that were possibly related was 54% and 8%, respectively.
A lower proportion of subjects in the caplacizumab arm experienced one or more major thromboembolic events or died, compared to the placebo arm—11.4% and 43.2%, respectively.
In addition, fewer caplacizumab-treated patients were refractory to treatment—5.7% vs 21.6%.
There were 2 deaths in the placebo arm, and both of those patients were refractory to treatment. There were no deaths reported in the caplacizumab arm.
Now, researchers are evaluating caplacizumab in the phase 3 HERCULES trial (NCT02553317). Results from this study are anticipated in the second half of 2017 are expected to support a planned biologics license application filing in the US in 2018. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to caplacizumab, an anti-von Willebrand factor (vWF) nanobody being developed for the treatment of acquired thrombotic thrombocytopenic purpura (aTTP).
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
About caplacizumab
Caplacizumab is a bivalent anti-vWF nanobody being developed by Ablynx. Caplacizumab works by blocking the interaction of ultra-large vWF multimers with platelets, having an immediate effect on platelet aggregation and the ensuing formation and accumulation of the micro-clots that cause the severe thrombocytopenia, tissue ischemia, and organ dysfunction that occurs in patients with aTTP.
Researchers evaluated the efficacy and safety of caplacizumab, given with standard care for aTTP, in the phase 2 TITAN trial.
The trial enrolled 75 patients with aTTP. They all received the standard of care—daily plasma exchange and immunosuppressive therapy. Thirty-six patients were randomized to receive caplacizumab as well, and 39 were randomized to placebo.
The study’s primary endpoint was time to response (platelet count normalization). Patients in the caplacizumab arm had a 39% reduction in the median time to response compared to patients in the placebo arm (P=0.005).
The rate of confirmed response was 86.1% (n=31) in the caplacizumab arm and 71.8% (n=28) in the placebo arm.
There were more relapses in the caplacizumab arm than the placebo arm—8 (22.2%) and 0, respectively. Relapse was defined as a TTP event occurring more than 30 days after the end of daily plasma exchange.
There were fewer exacerbations in the caplacizumab arm than the placebo arm—3 (8.3%) and 11 (28.2%), respectively. Exacerbation was defined as recurrent thrombocytopenia within 30 days of the end of daily plasma exchange that required re-initiation of daily exchange.
The rate of adverse events thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of events that were possibly related was 54% and 8%, respectively.
A lower proportion of subjects in the caplacizumab arm experienced one or more major thromboembolic events or died, compared to the placebo arm—11.4% and 43.2%, respectively.
In addition, fewer caplacizumab-treated patients were refractory to treatment—5.7% vs 21.6%.
There were 2 deaths in the placebo arm, and both of those patients were refractory to treatment. There were no deaths reported in the caplacizumab arm.
Now, researchers are evaluating caplacizumab in the phase 3 HERCULES trial (NCT02553317). Results from this study are anticipated in the second half of 2017 are expected to support a planned biologics license application filing in the US in 2018. ![]()