User login
Editor's note: This paper was posted online prior to publication in print. To expedite publication, the paper was peer-reviewed by a CCJM physician editor.
The unexpected and well-publicized appearance of swine-origin influenza A (H1N1) virus (S-OIV, informally known as swine flu) has both physicians and the general public on edge. The health care system is mobilizing while the world watches to see if S-OIV will become a pandemic or will just fade away, like the swine flu outbreak of 1976.
In this update, written in mid-May 2009, I try to provide an overview of our current understanding of S-OIV, its diagnosis, treatment, and prevention, knowing that the information about the outbreak is being updated almost daily. To stay abreast of the latest developments, physicians should also consult Web sites of the US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO).
IS IT REALLY ‘SWINE’?
An unexpected surge in influenza A cases toward the end of the 2008–2009 influenza season occurring in and around Mexico City alerted health authorities to a type of influenza virus infection that does not commonly affect humans.
In most years, the annual influenza epidemics in the Northern Hemisphere wane by the end of April. S-OIV infection first appeared in Mexico in April 2009 and shortly after in California and Texas.
In the first few days, the specific viral genetic origin of the epidemic was unclear. But genetic analysis of the virus isolated from a patient in California found that this virus was a recent reassortant of previous triple-reassortants of viruses from pigs, humans, and birds, called triple-reassortant swine influenza A (H1) viruses, which have been circulating in pigs for about a decade, and a Eurasian swine influenza virus.1
Through the years, only a few influenza viruses have been successfully transmitted from birds to humans and then to swine.2 It is interesting that exposure to pigs is not a risk factor for infection with the current S-OIV, unlike in prior cases of swine influenza reported in the literature.3,4 Total reported cases of swine influenza in humans numbered only 50 from 1958 to 2005 and 11 from December 2005 through February 2009, but more cases must have occurred that were not readily identified.
The Veterinary Services of Canada announced on May 2, 2009, that a pig farm in Alberta had been infected with the current type of S-OIV. The infection was introduced to the farm by a carpenter who developed symptoms of influenza after a short stay in Mexico. It is reassuring to learn that, so far, the S-OIV causing illness in these pigs has not been transmitted to people living on that farm. The failure of the S-OIV to transmit back to people suggests that it did not come into the human population directly from swine.
AN EPIDEMIC IN MOTION
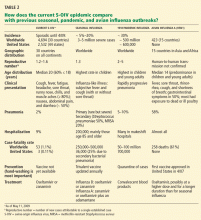
As of this writing, 2,532 cases of S-OIV have been confirmed in the United States by the CDC in 44 states, and 3 people have died, for a case-fatality rate of 0.11%. Simultaneously, the WHO reported 4,694 confirmed cases in 30 countries, with 53 deaths (a case-fatality rate of 1.1%), and with 48 of the deaths outside the United States occurring in Mexico.
It is unclear which direction this epidemic will take over the next several months. What happens in the annual influenza season in the Southern Hemisphere, which is just starting, and the early features of influenza activity in the Northern Hemisphere starting in September 2009 will indicate how this epidemic will materialize and the prospects of it’s progressing to an influenza pandemic.
While most adults today have some immunity against previously circulating H1 variants, it is not known if cross-reacting antibodies would provide any protection against the current S-OIV. An animal model showed that mice immunized against the neuraminidase of a human influenza A (H1N1) virus were partially protected from lethal challenge with H5N1 virus.5 In that same study, some humans also had serum antibodies that can inhibit sialidase activity of avian H5N1 viruses.
A remnant of the 1918 pandemic?
The two mechanisms by which pandemic influenza occurred in the 20th century were direct transmission of a novel virus and reassortment of avian and human viruses. In the 1918 pandemic, an influenza A (H1N1) virus closely related to avian viruses adapted to replicate efficiently in humans. Reassortment of an avian influenza A (H2N2) virus and a human influenza A (H1N1) virus resulted in the 1957 pandemic, and reassortment of an avian influenza A H3 virus and a human influenza A (H2N2) virus resulted in the 1968 pandemic.6 One could thus consider the current S-OIV epidemic as genetically a remnant or continuation of the 1918 pandemic, but so far it is less deadly.1
What should we be looking for?
Several characteristic features were seen in prior pandemics that we should be looking for in the next few months to better understand the pandemic potential of the current S-OIV epidemic.7
While the severity of prior pandemics varied significantly, they were all heralded by an antigenic shift in viral subtype. Young adults and previously healthy people were disproportionately affected and had a higher-than-expected death rate. This may be explained by partial protection in older people due to antigen recycling. Secondary bacterial pneumonia is believed to have been a significant cause of death in the 1918 pandemic,8 and bacterial pharyngeal carriage rates are higher in younger people.
Pandemic waves smoldered, lasting 2 to 5 years, but the pattern of deaths varied significantly in different parts of the world. For example, in 1968, most deaths in North America occurred during the first pandemic season, whereas most deaths in Europe and Asia occurred during the second pandemic season.9 This may be explained by geographic variation in preexisting immunity, intrapandemic antigenic drift, viral adaptation, demographic differences, or seasonality.
Of importance, influenza viruses that caused prior pandemics were highly transmissible between humans.
CLINICAL FEATURES OF THE CURRENT OUTBREAK
The current S-OIV epidemic in the United States is affecting mainly younger people: 60% of people affected have been 18 years of age or younger.10,11 It is unclear if this is due to transmission patterns or to possible immunity in older patients. Efficient human-to-human transmission within the United States is occurring, since only 18% of patients had recently traveled to Mexico. School outbreaks accounted for 16% of cases so far.
Patients have symptoms similar to those of seasonal influenza, with few exceptions. The most frequently reported symptoms are cough, fever, fatigue, headache, sore throat, runny nose, chills, and muscle aches, all occurring in 80% or more of patients. Almost all patients fit the CDC definition for influenza-like illness, consisting of subjective fever plus cough or sore throat.
Nausea, abdominal pain, and diarrhea, which are not common symptoms of seasonal influenza, have been reported in approximately 50% of patients with S-OIV. The spectrum of illness ranges from self-limited to severe, with 2% of patients developing pneumonia and 9% requiring hospitalization.
Continued analysis of the case-fatality rate highlights that people ages 20 to 29 are disproportionately represented among the fatalities.
A PCR test has been developed
Since clinical findings identify patients with influenza-like illness but cannot confirm or exclude the diagnosis of influenza,12 a specific diagnostic real-time reverse-transcriptase polymerase chain reaction (RT-PCR) test has been developed, and the CDC is currently distributing it to state health departments.
An interim case definition
An interim case definition for the purpose of epidemiologic investigation of cases of S-OIV infection includes acute fever (temperature ≥ 100°F, 37.8°C) and acute respiratory illness (rhinorrhea, sore throat, or cough), plus:
- For a confirmed case, S-OIV infection confirmed by RT-PCR or viral culture
- For a probable case, laboratory-confirmed influenza A, but negative for H1 and H3 by RT-PCR
- For a suspected case, onset of above illness within 7 days of close contact with a confirmed case of S-OIV infection; or travel within 7 days to a community within the United States or internationally where there are one or more confirmed cases of S-OIV infection; or residing in such a community.
In practice, is it seasonal flu or swine flu?
In clinical practice in United States, in the springtime, a person with influenza-like illness and microbiologically confirmed seasonal influenza B obviously would not raise any concern about the ongoing S-OIV epidemic. Sporadic cases of seasonal influenza A are still occurring, but these are the ones that create a diagnostic dilemma, since very few laboratories currently have the ability to differentiate between influenza A H1 and H3. Since S-OIV has been reported in almost all states in the United States, one can argue that most cases of influenza A currently being identified should be considered suspected S-OIV.
PREVENTIVE MEASURES
In response to this ongoing outbreak, the WHO raised its epidemic alert level from 4 to 5, one level shy of declaring a pandemic. Several measures have been implemented in an attempt to halt this outbreak, the most important of which is the rapid dissemination of information to health professionals,13 with the Internet playing a central role.14
The world is better prepared for a pandemic now than at any time in history. Seed virus for vaccine development has been provided to various governments and pharmaceutical manufacturers. Stockpiles of antiviral agents are being mobilized and distributed to various locations, and dispensing plans are being reviewed for potential execution. The US Food and Drug Administration (FDA) issued emergency-use authorizations for mass deployment of the strategic stockpile of oseltamivir (Tamiflu), including for children younger than 1 year, and of zanamivir (Relenza) for the treatment and prophylaxis of S-OIV infection. It also authorized the use of disposable N95 respiratory masks by the general public, as well as the RT-PCR diagnostic test.
General advice for healthy people in the community
- Maintain a distance of at least 1 meter from a person with influenza-like illness.
- Wear a mask while providing care for a person with influenza-like illness.
- Avoid touching your eyes, nose, or mouth, since these are potential portals of entry for the virus. This may be a difficult recommendation to follow, since it requires constant vigilance of a common human behavior.
- Wash your hands often with either soap and water or an alcohol-based hand rub for 20 to 30 seconds, particularly after touching your eyes, nose, or mouth or after contact with respiratory secretions from a person, including your child, with influenza-like illness.
- If possible, reduce the time spent in close contact with people with influenza-like illness and in crowded settings.
- If possible, open windows in your living space to improve airflow.
While the CDC has recommended avoiding nonessential travel to Mexico at the current time, the WHO is not recommending any travel restrictions, since the outbreak has already spread to many parts of the world and all continents.
There is no limitation on handling or consuming pork meat or other well-processed swine products.
Recommendations for school dismissal and social-distancing interventions are evolving. During the 1918 pandemic, nonpharmaceutical interventions were associated with a significant reduction in deaths,15 but it is unclear how much additional benefit these measures would add to effective immunization, antiviral treatment for patients, and chemoprophylaxis for their contacts.
General advice for people with influenza-like illness
- Stay home for 7 days after the onset of symptoms or 48 hours after symptoms resolve, whichever is longer.
- Maintain a distance of at least 1 meter from all people.
- Cover your mouth and nose with tissues when coughing or sneezing, and dispose of the tissues immediately after use.
- Avoid touching your eyes, nose, and mouth.
- Wash your hands often with either soap and water or an alcohol-based hand rub for 20 to 30 seconds, particularly after touching your eyes, nose, or mouth or after contact with your respiratory secretions during coughing or sneezing. Adding virucidal agents or antiseptics to hand-washing is not likely to have an incremental effect.16
- If possible, open windows in your living space to improve airflow.
- If possible, when you are in close contact with other people, wear a mask to help contain your respiratory secretions.
Masks
The designs and standards of masks vary from country to country. Masks have been shown to reduce the transmission of influenza in health care settings,16 but the benefit in the community has not been established. Advice on proper use of a mask:
- Cover your mouth and nose with the mask and tie it securely to minimize gaps.
- Avoid touching the mask while it is on your face.
- Wash your hands with soap and water or an alcohol-based hand rub for 20 to 30 seconds after removing the mask.
- If the mask becomes damp, replace it with a new one.
- Avoid reusing single-use masks, and dispose of them immediately after removing.
VACCINE DEVELOPMENT
The most difficult question about vaccine development for S-OIV at this time is whether to prepare it as a separate product or try to incorporate it in the seasonal influenza vaccine.
The problem is that the seasonal influenza vaccine for the Southern Hemisphere has already been made and distributed, and vaccination programs are already well under way. Although flu season in the Northern Hemisphere is not expected before September or October 2009, vaccine production and distribution take several months, leaving little time to observe which direction the S-OIV epidemic will take before making this decision.
Vaccine distribution also raises difficult questions, since a limited amount will be available initially and rationing to the most vulnerable people will be necessary. While health care workers are more likely to be exposed to people infected with S-OIV compared with the general population, mandating their immunization may pose other moral dilemmas.17
The current global capacity for production of seasonal influenza vaccine is approximately 400 million doses.18 Since the process of vaccine production takes at least 4 to 6 months, measures have been proposed to speed up the production of pandemic vaccine or immunogenicity; these include recombinant technology, reverse genetics, and the use of adjuvants. In April 2007, the FDA approved the first H5 subviron vaccine for people ages 19 to 64.
This topic brings back memories of the 1976 swine influenza immunization program, in which the rate of Guillain-Barré syndrome was 5 to 10 times the background rate, resulting in a halt in vaccine production.
Why this syndrome occurred is not known, but it is suspected to be due to cross-reacting antibodies against peripheral-nerve antigen that developed after the vaccine was given. Data since then have shown no association between vaccination and Guillain-Barré syndrome. 19 On the other hand, influenza viruses were found to trigger Guillain-Barré syndrome only infrequently, except during major outbreaks, in which they may play a significant role.20
TREATMENT
Antiviral drugs
Tests of current S-OIV isolates showed them to be susceptible to the neuraminidase inhibitors, ie, oseltamivir and zanamivir, but resistant to the adamantanes, ie, amantadine (Symmetrel) and rimantadine (Flumadine).21 All isolates contained the S31N mutation in the M2 protein, which confers resistance against the adamantanes and which has been detected in most influenza A (H3N2) isolates in the United States since 2006. Fortunately, the H274Y mutation in N1—which confers resistance to oseltamivir but not to zanamivir and which has been detected in almost all seasonal influenza A (H1N1) isolates since the early weeks of the current influenza season— has not been detected in any of the current S-OIV isolates.
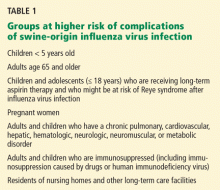
Patients who are otherwise healthy who present with an uncomplicated febrile illness due to S-OIV do not require antiviral treatment. Either oseltamivir or zanamivir is recommended for treatment of patients hospitalized for management of confirmed, probable, or suspected infection with S-OIV, or for those at high risk of influenza-related complications, defined similarly to seasonal influenza (Table 1).
The duration of shedding of S-OIV is unknown, but starting an antiviral agent early in the course of illness is expected to reduce contagiousness. Extrapolating from data in seasonal influenza, infected persons are assumed to be shedding virus from 1 day prior to illness onset until resolution of symptoms, usually 7 days, and up to 10 days in younger children.
Oseltamivir accounts for the lion’s share of the stockpile of antiviral drugs against pandemic influenza. However, with mass utilization, antiviral resistance to a single agent may develop. A mathematical model showed that adding a smaller stockpile of a second agent, such as zanamivir, to be used either in combination with or sequential to oseltamivir, can effectively prevent or at least delay the development of resistance.22
Other potential measures for management
Since secondary bacterial pneumonia is expected to play a significant role in influenza-related death during the next pandemic, stockpiling antibacterial agents may also be prudent.8 The death rate in methicillin-resistant Staphylococcus aureus pneumonia secondary to seasonal influenza is 50%, further complicating the choice of stockpiling for antibacterial agents.
A meta-analysis of 11 studies involving 1,703 patients during the 1918 pandemic showed that those who received influenza-convalescent human blood products were less likely to die than those who did not.23 Anti-influenza drugs and advanced techniques to care for critically ill patients were not available at that time, so extrapolating these data to the current era may not be appropriate.
The cost of vaccine and antiviral drugs is an expected limitation to mass implementation during a pandemic, particularly in developing countries. Certain inexpensive generic drugs that have been shown to have some activity against influenza, such statins, fibrates, and chloroquine, deserve further attention.24
PUTING THE CURRENT EPIDEMIC IN PERSPECTIVE
In summary, the world is now better prepared, vaccine is in development, and antiviral treatment is available. For more information, readers are directed to go to www.cdc.gov/h1n1flu/ or www.who.int/csr/don/2009_05_11/en/index.html.
- Belshe RB. Implications of the emergence of a novel H1 influenza virus. N Engl J Med 2009 May 7; doi:10.1056/NEJMe0903995.
- Ducatez MF, Webster RG, Webby RJ. Animal influenza epidemiology. Vaccine 2008; 26(suppl 4):D67–D69.
- Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis 2007; 44:1084–1088.
- Shinde V, Bridges CB, Uyeki TM, et al. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N Engl J Med 2009 May 7; doi:10.1056/NEJMoa0903812.
- Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med 2007; 4( 2):e59. doi:10.1371/journal. pmed.0040059.
- Belshe RB. The origins of pandemic influenza—lessons from the 1918 virus. N Engl J Med 2005; 353:2209–2211.
- Miller MA, Viboud C, Balinska M, Simonsen L. The signature features of influenza pandemics—implications for policy. N Engl J Med 2009 May 7; doi:10.1056/NEJMp0903906.
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–970.
- Viboud C, Grais RF, Lafont BAP, Miller MA, Simonsen L. Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis 2005; 192:233–248.
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009 May 7; doi:10.1056/NEJMoa0903810.
- US Centers for Disease Control and PreventionC. Swine-origin influenza A (H1N1) virus infections in a school—New York City, April 2009. MMWR 2009; 58(Dispatch):1–3.
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA 2005: 293:987–997.
- Baden LR, Drazen JM, Kritek PA, Curfman GD, Morrissey S, Campion EW. H1N1 influenza A disease—information for health professionals. N Engl J Med 2009 May 7; doi:10.1056/NEJMe0903992.
- Brownstein JS, Freifeld CC, Madoff LC. Influenza A (H1N1) virus, 2009—online monitoring. N Engl J Med 2009 May 7; doi:10.1056/NEJMp0904012.
- Markel H, Lipman HB, Navarro JA, et al. Nonpharmaceutical interventions implemented by US cities during the 1918–1919 influenza pandemic. JAMA 2007; 298:644–654.
- Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 336( 7635):77–80.
- Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med 2009; 360:1981–1988.
- Sahni R, Mossad SB. Controlling pandemic influenza through vaccination programs. Future Virol 2009; 4:271–276.
- Hughes RA, Charlton J, Latinovic R, Gulliford MC. No association between immunization and Guillain-Barré syndrome in the United Kingdom, 1992 to 2000. Arch Intern Med 2006; 166:1301–1304.
- Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Guillain-Barré syndrome and influenza virus infection. Clin Infect Dis 2009; 48:48–56.
- US Centers for Disease Control and Prevention. Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR May 1, 2009; 58( 16):433–435.
- Wu JT, Leung GM, Lipsitch M, Cooper BS, Riley S. Hedging against antiviral resistance during the next influenza pandemic using small stockpiles of an alternative chemotherapy. PLoS Med 2009;e1000085. doi:10.1371/journal.pmed.1000085.
- Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med 2006; 145:599–609.
- Fedson DS. Confronting an influenza pandemic with inexpensive generic agents: can it be done? Lancet Infect Dis 2008; 8:571–576.
Editor's note: This paper was posted online prior to publication in print. To expedite publication, the paper was peer-reviewed by a CCJM physician editor.
The unexpected and well-publicized appearance of swine-origin influenza A (H1N1) virus (S-OIV, informally known as swine flu) has both physicians and the general public on edge. The health care system is mobilizing while the world watches to see if S-OIV will become a pandemic or will just fade away, like the swine flu outbreak of 1976.
In this update, written in mid-May 2009, I try to provide an overview of our current understanding of S-OIV, its diagnosis, treatment, and prevention, knowing that the information about the outbreak is being updated almost daily. To stay abreast of the latest developments, physicians should also consult Web sites of the US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO).
IS IT REALLY ‘SWINE’?
An unexpected surge in influenza A cases toward the end of the 2008–2009 influenza season occurring in and around Mexico City alerted health authorities to a type of influenza virus infection that does not commonly affect humans.
In most years, the annual influenza epidemics in the Northern Hemisphere wane by the end of April. S-OIV infection first appeared in Mexico in April 2009 and shortly after in California and Texas.
In the first few days, the specific viral genetic origin of the epidemic was unclear. But genetic analysis of the virus isolated from a patient in California found that this virus was a recent reassortant of previous triple-reassortants of viruses from pigs, humans, and birds, called triple-reassortant swine influenza A (H1) viruses, which have been circulating in pigs for about a decade, and a Eurasian swine influenza virus.1
Through the years, only a few influenza viruses have been successfully transmitted from birds to humans and then to swine.2 It is interesting that exposure to pigs is not a risk factor for infection with the current S-OIV, unlike in prior cases of swine influenza reported in the literature.3,4 Total reported cases of swine influenza in humans numbered only 50 from 1958 to 2005 and 11 from December 2005 through February 2009, but more cases must have occurred that were not readily identified.
The Veterinary Services of Canada announced on May 2, 2009, that a pig farm in Alberta had been infected with the current type of S-OIV. The infection was introduced to the farm by a carpenter who developed symptoms of influenza after a short stay in Mexico. It is reassuring to learn that, so far, the S-OIV causing illness in these pigs has not been transmitted to people living on that farm. The failure of the S-OIV to transmit back to people suggests that it did not come into the human population directly from swine.
AN EPIDEMIC IN MOTION
As of this writing, 2,532 cases of S-OIV have been confirmed in the United States by the CDC in 44 states, and 3 people have died, for a case-fatality rate of 0.11%. Simultaneously, the WHO reported 4,694 confirmed cases in 30 countries, with 53 deaths (a case-fatality rate of 1.1%), and with 48 of the deaths outside the United States occurring in Mexico.
It is unclear which direction this epidemic will take over the next several months. What happens in the annual influenza season in the Southern Hemisphere, which is just starting, and the early features of influenza activity in the Northern Hemisphere starting in September 2009 will indicate how this epidemic will materialize and the prospects of it’s progressing to an influenza pandemic.
While most adults today have some immunity against previously circulating H1 variants, it is not known if cross-reacting antibodies would provide any protection against the current S-OIV. An animal model showed that mice immunized against the neuraminidase of a human influenza A (H1N1) virus were partially protected from lethal challenge with H5N1 virus.5 In that same study, some humans also had serum antibodies that can inhibit sialidase activity of avian H5N1 viruses.
A remnant of the 1918 pandemic?
The two mechanisms by which pandemic influenza occurred in the 20th century were direct transmission of a novel virus and reassortment of avian and human viruses. In the 1918 pandemic, an influenza A (H1N1) virus closely related to avian viruses adapted to replicate efficiently in humans. Reassortment of an avian influenza A (H2N2) virus and a human influenza A (H1N1) virus resulted in the 1957 pandemic, and reassortment of an avian influenza A H3 virus and a human influenza A (H2N2) virus resulted in the 1968 pandemic.6 One could thus consider the current S-OIV epidemic as genetically a remnant or continuation of the 1918 pandemic, but so far it is less deadly.1
What should we be looking for?
Several characteristic features were seen in prior pandemics that we should be looking for in the next few months to better understand the pandemic potential of the current S-OIV epidemic.7
While the severity of prior pandemics varied significantly, they were all heralded by an antigenic shift in viral subtype. Young adults and previously healthy people were disproportionately affected and had a higher-than-expected death rate. This may be explained by partial protection in older people due to antigen recycling. Secondary bacterial pneumonia is believed to have been a significant cause of death in the 1918 pandemic,8 and bacterial pharyngeal carriage rates are higher in younger people.
Pandemic waves smoldered, lasting 2 to 5 years, but the pattern of deaths varied significantly in different parts of the world. For example, in 1968, most deaths in North America occurred during the first pandemic season, whereas most deaths in Europe and Asia occurred during the second pandemic season.9 This may be explained by geographic variation in preexisting immunity, intrapandemic antigenic drift, viral adaptation, demographic differences, or seasonality.
Of importance, influenza viruses that caused prior pandemics were highly transmissible between humans.
CLINICAL FEATURES OF THE CURRENT OUTBREAK
The current S-OIV epidemic in the United States is affecting mainly younger people: 60% of people affected have been 18 years of age or younger.10,11 It is unclear if this is due to transmission patterns or to possible immunity in older patients. Efficient human-to-human transmission within the United States is occurring, since only 18% of patients had recently traveled to Mexico. School outbreaks accounted for 16% of cases so far.
Patients have symptoms similar to those of seasonal influenza, with few exceptions. The most frequently reported symptoms are cough, fever, fatigue, headache, sore throat, runny nose, chills, and muscle aches, all occurring in 80% or more of patients. Almost all patients fit the CDC definition for influenza-like illness, consisting of subjective fever plus cough or sore throat.
Nausea, abdominal pain, and diarrhea, which are not common symptoms of seasonal influenza, have been reported in approximately 50% of patients with S-OIV. The spectrum of illness ranges from self-limited to severe, with 2% of patients developing pneumonia and 9% requiring hospitalization.
Continued analysis of the case-fatality rate highlights that people ages 20 to 29 are disproportionately represented among the fatalities.
A PCR test has been developed
Since clinical findings identify patients with influenza-like illness but cannot confirm or exclude the diagnosis of influenza,12 a specific diagnostic real-time reverse-transcriptase polymerase chain reaction (RT-PCR) test has been developed, and the CDC is currently distributing it to state health departments.
An interim case definition
An interim case definition for the purpose of epidemiologic investigation of cases of S-OIV infection includes acute fever (temperature ≥ 100°F, 37.8°C) and acute respiratory illness (rhinorrhea, sore throat, or cough), plus:
- For a confirmed case, S-OIV infection confirmed by RT-PCR or viral culture
- For a probable case, laboratory-confirmed influenza A, but negative for H1 and H3 by RT-PCR
- For a suspected case, onset of above illness within 7 days of close contact with a confirmed case of S-OIV infection; or travel within 7 days to a community within the United States or internationally where there are one or more confirmed cases of S-OIV infection; or residing in such a community.
In practice, is it seasonal flu or swine flu?
In clinical practice in United States, in the springtime, a person with influenza-like illness and microbiologically confirmed seasonal influenza B obviously would not raise any concern about the ongoing S-OIV epidemic. Sporadic cases of seasonal influenza A are still occurring, but these are the ones that create a diagnostic dilemma, since very few laboratories currently have the ability to differentiate between influenza A H1 and H3. Since S-OIV has been reported in almost all states in the United States, one can argue that most cases of influenza A currently being identified should be considered suspected S-OIV.
PREVENTIVE MEASURES
In response to this ongoing outbreak, the WHO raised its epidemic alert level from 4 to 5, one level shy of declaring a pandemic. Several measures have been implemented in an attempt to halt this outbreak, the most important of which is the rapid dissemination of information to health professionals,13 with the Internet playing a central role.14
The world is better prepared for a pandemic now than at any time in history. Seed virus for vaccine development has been provided to various governments and pharmaceutical manufacturers. Stockpiles of antiviral agents are being mobilized and distributed to various locations, and dispensing plans are being reviewed for potential execution. The US Food and Drug Administration (FDA) issued emergency-use authorizations for mass deployment of the strategic stockpile of oseltamivir (Tamiflu), including for children younger than 1 year, and of zanamivir (Relenza) for the treatment and prophylaxis of S-OIV infection. It also authorized the use of disposable N95 respiratory masks by the general public, as well as the RT-PCR diagnostic test.
General advice for healthy people in the community
- Maintain a distance of at least 1 meter from a person with influenza-like illness.
- Wear a mask while providing care for a person with influenza-like illness.
- Avoid touching your eyes, nose, or mouth, since these are potential portals of entry for the virus. This may be a difficult recommendation to follow, since it requires constant vigilance of a common human behavior.
- Wash your hands often with either soap and water or an alcohol-based hand rub for 20 to 30 seconds, particularly after touching your eyes, nose, or mouth or after contact with respiratory secretions from a person, including your child, with influenza-like illness.
- If possible, reduce the time spent in close contact with people with influenza-like illness and in crowded settings.
- If possible, open windows in your living space to improve airflow.
While the CDC has recommended avoiding nonessential travel to Mexico at the current time, the WHO is not recommending any travel restrictions, since the outbreak has already spread to many parts of the world and all continents.
There is no limitation on handling or consuming pork meat or other well-processed swine products.
Recommendations for school dismissal and social-distancing interventions are evolving. During the 1918 pandemic, nonpharmaceutical interventions were associated with a significant reduction in deaths,15 but it is unclear how much additional benefit these measures would add to effective immunization, antiviral treatment for patients, and chemoprophylaxis for their contacts.
General advice for people with influenza-like illness
- Stay home for 7 days after the onset of symptoms or 48 hours after symptoms resolve, whichever is longer.
- Maintain a distance of at least 1 meter from all people.
- Cover your mouth and nose with tissues when coughing or sneezing, and dispose of the tissues immediately after use.
- Avoid touching your eyes, nose, and mouth.
- Wash your hands often with either soap and water or an alcohol-based hand rub for 20 to 30 seconds, particularly after touching your eyes, nose, or mouth or after contact with your respiratory secretions during coughing or sneezing. Adding virucidal agents or antiseptics to hand-washing is not likely to have an incremental effect.16
- If possible, open windows in your living space to improve airflow.
- If possible, when you are in close contact with other people, wear a mask to help contain your respiratory secretions.
Masks
The designs and standards of masks vary from country to country. Masks have been shown to reduce the transmission of influenza in health care settings,16 but the benefit in the community has not been established. Advice on proper use of a mask:
- Cover your mouth and nose with the mask and tie it securely to minimize gaps.
- Avoid touching the mask while it is on your face.
- Wash your hands with soap and water or an alcohol-based hand rub for 20 to 30 seconds after removing the mask.
- If the mask becomes damp, replace it with a new one.
- Avoid reusing single-use masks, and dispose of them immediately after removing.
VACCINE DEVELOPMENT
The most difficult question about vaccine development for S-OIV at this time is whether to prepare it as a separate product or try to incorporate it in the seasonal influenza vaccine.
The problem is that the seasonal influenza vaccine for the Southern Hemisphere has already been made and distributed, and vaccination programs are already well under way. Although flu season in the Northern Hemisphere is not expected before September or October 2009, vaccine production and distribution take several months, leaving little time to observe which direction the S-OIV epidemic will take before making this decision.
Vaccine distribution also raises difficult questions, since a limited amount will be available initially and rationing to the most vulnerable people will be necessary. While health care workers are more likely to be exposed to people infected with S-OIV compared with the general population, mandating their immunization may pose other moral dilemmas.17
The current global capacity for production of seasonal influenza vaccine is approximately 400 million doses.18 Since the process of vaccine production takes at least 4 to 6 months, measures have been proposed to speed up the production of pandemic vaccine or immunogenicity; these include recombinant technology, reverse genetics, and the use of adjuvants. In April 2007, the FDA approved the first H5 subviron vaccine for people ages 19 to 64.
This topic brings back memories of the 1976 swine influenza immunization program, in which the rate of Guillain-Barré syndrome was 5 to 10 times the background rate, resulting in a halt in vaccine production.
Why this syndrome occurred is not known, but it is suspected to be due to cross-reacting antibodies against peripheral-nerve antigen that developed after the vaccine was given. Data since then have shown no association between vaccination and Guillain-Barré syndrome. 19 On the other hand, influenza viruses were found to trigger Guillain-Barré syndrome only infrequently, except during major outbreaks, in which they may play a significant role.20
TREATMENT
Antiviral drugs
Tests of current S-OIV isolates showed them to be susceptible to the neuraminidase inhibitors, ie, oseltamivir and zanamivir, but resistant to the adamantanes, ie, amantadine (Symmetrel) and rimantadine (Flumadine).21 All isolates contained the S31N mutation in the M2 protein, which confers resistance against the adamantanes and which has been detected in most influenza A (H3N2) isolates in the United States since 2006. Fortunately, the H274Y mutation in N1—which confers resistance to oseltamivir but not to zanamivir and which has been detected in almost all seasonal influenza A (H1N1) isolates since the early weeks of the current influenza season— has not been detected in any of the current S-OIV isolates.
Patients who are otherwise healthy who present with an uncomplicated febrile illness due to S-OIV do not require antiviral treatment. Either oseltamivir or zanamivir is recommended for treatment of patients hospitalized for management of confirmed, probable, or suspected infection with S-OIV, or for those at high risk of influenza-related complications, defined similarly to seasonal influenza (Table 1).
The duration of shedding of S-OIV is unknown, but starting an antiviral agent early in the course of illness is expected to reduce contagiousness. Extrapolating from data in seasonal influenza, infected persons are assumed to be shedding virus from 1 day prior to illness onset until resolution of symptoms, usually 7 days, and up to 10 days in younger children.
Oseltamivir accounts for the lion’s share of the stockpile of antiviral drugs against pandemic influenza. However, with mass utilization, antiviral resistance to a single agent may develop. A mathematical model showed that adding a smaller stockpile of a second agent, such as zanamivir, to be used either in combination with or sequential to oseltamivir, can effectively prevent or at least delay the development of resistance.22
Other potential measures for management
Since secondary bacterial pneumonia is expected to play a significant role in influenza-related death during the next pandemic, stockpiling antibacterial agents may also be prudent.8 The death rate in methicillin-resistant Staphylococcus aureus pneumonia secondary to seasonal influenza is 50%, further complicating the choice of stockpiling for antibacterial agents.
A meta-analysis of 11 studies involving 1,703 patients during the 1918 pandemic showed that those who received influenza-convalescent human blood products were less likely to die than those who did not.23 Anti-influenza drugs and advanced techniques to care for critically ill patients were not available at that time, so extrapolating these data to the current era may not be appropriate.
The cost of vaccine and antiviral drugs is an expected limitation to mass implementation during a pandemic, particularly in developing countries. Certain inexpensive generic drugs that have been shown to have some activity against influenza, such statins, fibrates, and chloroquine, deserve further attention.24
PUTING THE CURRENT EPIDEMIC IN PERSPECTIVE
In summary, the world is now better prepared, vaccine is in development, and antiviral treatment is available. For more information, readers are directed to go to www.cdc.gov/h1n1flu/ or www.who.int/csr/don/2009_05_11/en/index.html.
Editor's note: This paper was posted online prior to publication in print. To expedite publication, the paper was peer-reviewed by a CCJM physician editor.
The unexpected and well-publicized appearance of swine-origin influenza A (H1N1) virus (S-OIV, informally known as swine flu) has both physicians and the general public on edge. The health care system is mobilizing while the world watches to see if S-OIV will become a pandemic or will just fade away, like the swine flu outbreak of 1976.
In this update, written in mid-May 2009, I try to provide an overview of our current understanding of S-OIV, its diagnosis, treatment, and prevention, knowing that the information about the outbreak is being updated almost daily. To stay abreast of the latest developments, physicians should also consult Web sites of the US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO).
IS IT REALLY ‘SWINE’?
An unexpected surge in influenza A cases toward the end of the 2008–2009 influenza season occurring in and around Mexico City alerted health authorities to a type of influenza virus infection that does not commonly affect humans.
In most years, the annual influenza epidemics in the Northern Hemisphere wane by the end of April. S-OIV infection first appeared in Mexico in April 2009 and shortly after in California and Texas.
In the first few days, the specific viral genetic origin of the epidemic was unclear. But genetic analysis of the virus isolated from a patient in California found that this virus was a recent reassortant of previous triple-reassortants of viruses from pigs, humans, and birds, called triple-reassortant swine influenza A (H1) viruses, which have been circulating in pigs for about a decade, and a Eurasian swine influenza virus.1
Through the years, only a few influenza viruses have been successfully transmitted from birds to humans and then to swine.2 It is interesting that exposure to pigs is not a risk factor for infection with the current S-OIV, unlike in prior cases of swine influenza reported in the literature.3,4 Total reported cases of swine influenza in humans numbered only 50 from 1958 to 2005 and 11 from December 2005 through February 2009, but more cases must have occurred that were not readily identified.
The Veterinary Services of Canada announced on May 2, 2009, that a pig farm in Alberta had been infected with the current type of S-OIV. The infection was introduced to the farm by a carpenter who developed symptoms of influenza after a short stay in Mexico. It is reassuring to learn that, so far, the S-OIV causing illness in these pigs has not been transmitted to people living on that farm. The failure of the S-OIV to transmit back to people suggests that it did not come into the human population directly from swine.
AN EPIDEMIC IN MOTION
As of this writing, 2,532 cases of S-OIV have been confirmed in the United States by the CDC in 44 states, and 3 people have died, for a case-fatality rate of 0.11%. Simultaneously, the WHO reported 4,694 confirmed cases in 30 countries, with 53 deaths (a case-fatality rate of 1.1%), and with 48 of the deaths outside the United States occurring in Mexico.
It is unclear which direction this epidemic will take over the next several months. What happens in the annual influenza season in the Southern Hemisphere, which is just starting, and the early features of influenza activity in the Northern Hemisphere starting in September 2009 will indicate how this epidemic will materialize and the prospects of it’s progressing to an influenza pandemic.
While most adults today have some immunity against previously circulating H1 variants, it is not known if cross-reacting antibodies would provide any protection against the current S-OIV. An animal model showed that mice immunized against the neuraminidase of a human influenza A (H1N1) virus were partially protected from lethal challenge with H5N1 virus.5 In that same study, some humans also had serum antibodies that can inhibit sialidase activity of avian H5N1 viruses.
A remnant of the 1918 pandemic?
The two mechanisms by which pandemic influenza occurred in the 20th century were direct transmission of a novel virus and reassortment of avian and human viruses. In the 1918 pandemic, an influenza A (H1N1) virus closely related to avian viruses adapted to replicate efficiently in humans. Reassortment of an avian influenza A (H2N2) virus and a human influenza A (H1N1) virus resulted in the 1957 pandemic, and reassortment of an avian influenza A H3 virus and a human influenza A (H2N2) virus resulted in the 1968 pandemic.6 One could thus consider the current S-OIV epidemic as genetically a remnant or continuation of the 1918 pandemic, but so far it is less deadly.1
What should we be looking for?
Several characteristic features were seen in prior pandemics that we should be looking for in the next few months to better understand the pandemic potential of the current S-OIV epidemic.7
While the severity of prior pandemics varied significantly, they were all heralded by an antigenic shift in viral subtype. Young adults and previously healthy people were disproportionately affected and had a higher-than-expected death rate. This may be explained by partial protection in older people due to antigen recycling. Secondary bacterial pneumonia is believed to have been a significant cause of death in the 1918 pandemic,8 and bacterial pharyngeal carriage rates are higher in younger people.
Pandemic waves smoldered, lasting 2 to 5 years, but the pattern of deaths varied significantly in different parts of the world. For example, in 1968, most deaths in North America occurred during the first pandemic season, whereas most deaths in Europe and Asia occurred during the second pandemic season.9 This may be explained by geographic variation in preexisting immunity, intrapandemic antigenic drift, viral adaptation, demographic differences, or seasonality.
Of importance, influenza viruses that caused prior pandemics were highly transmissible between humans.
CLINICAL FEATURES OF THE CURRENT OUTBREAK
The current S-OIV epidemic in the United States is affecting mainly younger people: 60% of people affected have been 18 years of age or younger.10,11 It is unclear if this is due to transmission patterns or to possible immunity in older patients. Efficient human-to-human transmission within the United States is occurring, since only 18% of patients had recently traveled to Mexico. School outbreaks accounted for 16% of cases so far.
Patients have symptoms similar to those of seasonal influenza, with few exceptions. The most frequently reported symptoms are cough, fever, fatigue, headache, sore throat, runny nose, chills, and muscle aches, all occurring in 80% or more of patients. Almost all patients fit the CDC definition for influenza-like illness, consisting of subjective fever plus cough or sore throat.
Nausea, abdominal pain, and diarrhea, which are not common symptoms of seasonal influenza, have been reported in approximately 50% of patients with S-OIV. The spectrum of illness ranges from self-limited to severe, with 2% of patients developing pneumonia and 9% requiring hospitalization.
Continued analysis of the case-fatality rate highlights that people ages 20 to 29 are disproportionately represented among the fatalities.
A PCR test has been developed
Since clinical findings identify patients with influenza-like illness but cannot confirm or exclude the diagnosis of influenza,12 a specific diagnostic real-time reverse-transcriptase polymerase chain reaction (RT-PCR) test has been developed, and the CDC is currently distributing it to state health departments.
An interim case definition
An interim case definition for the purpose of epidemiologic investigation of cases of S-OIV infection includes acute fever (temperature ≥ 100°F, 37.8°C) and acute respiratory illness (rhinorrhea, sore throat, or cough), plus:
- For a confirmed case, S-OIV infection confirmed by RT-PCR or viral culture
- For a probable case, laboratory-confirmed influenza A, but negative for H1 and H3 by RT-PCR
- For a suspected case, onset of above illness within 7 days of close contact with a confirmed case of S-OIV infection; or travel within 7 days to a community within the United States or internationally where there are one or more confirmed cases of S-OIV infection; or residing in such a community.
In practice, is it seasonal flu or swine flu?
In clinical practice in United States, in the springtime, a person with influenza-like illness and microbiologically confirmed seasonal influenza B obviously would not raise any concern about the ongoing S-OIV epidemic. Sporadic cases of seasonal influenza A are still occurring, but these are the ones that create a diagnostic dilemma, since very few laboratories currently have the ability to differentiate between influenza A H1 and H3. Since S-OIV has been reported in almost all states in the United States, one can argue that most cases of influenza A currently being identified should be considered suspected S-OIV.
PREVENTIVE MEASURES
In response to this ongoing outbreak, the WHO raised its epidemic alert level from 4 to 5, one level shy of declaring a pandemic. Several measures have been implemented in an attempt to halt this outbreak, the most important of which is the rapid dissemination of information to health professionals,13 with the Internet playing a central role.14
The world is better prepared for a pandemic now than at any time in history. Seed virus for vaccine development has been provided to various governments and pharmaceutical manufacturers. Stockpiles of antiviral agents are being mobilized and distributed to various locations, and dispensing plans are being reviewed for potential execution. The US Food and Drug Administration (FDA) issued emergency-use authorizations for mass deployment of the strategic stockpile of oseltamivir (Tamiflu), including for children younger than 1 year, and of zanamivir (Relenza) for the treatment and prophylaxis of S-OIV infection. It also authorized the use of disposable N95 respiratory masks by the general public, as well as the RT-PCR diagnostic test.
General advice for healthy people in the community
- Maintain a distance of at least 1 meter from a person with influenza-like illness.
- Wear a mask while providing care for a person with influenza-like illness.
- Avoid touching your eyes, nose, or mouth, since these are potential portals of entry for the virus. This may be a difficult recommendation to follow, since it requires constant vigilance of a common human behavior.
- Wash your hands often with either soap and water or an alcohol-based hand rub for 20 to 30 seconds, particularly after touching your eyes, nose, or mouth or after contact with respiratory secretions from a person, including your child, with influenza-like illness.
- If possible, reduce the time spent in close contact with people with influenza-like illness and in crowded settings.
- If possible, open windows in your living space to improve airflow.
While the CDC has recommended avoiding nonessential travel to Mexico at the current time, the WHO is not recommending any travel restrictions, since the outbreak has already spread to many parts of the world and all continents.
There is no limitation on handling or consuming pork meat or other well-processed swine products.
Recommendations for school dismissal and social-distancing interventions are evolving. During the 1918 pandemic, nonpharmaceutical interventions were associated with a significant reduction in deaths,15 but it is unclear how much additional benefit these measures would add to effective immunization, antiviral treatment for patients, and chemoprophylaxis for their contacts.
General advice for people with influenza-like illness
- Stay home for 7 days after the onset of symptoms or 48 hours after symptoms resolve, whichever is longer.
- Maintain a distance of at least 1 meter from all people.
- Cover your mouth and nose with tissues when coughing or sneezing, and dispose of the tissues immediately after use.
- Avoid touching your eyes, nose, and mouth.
- Wash your hands often with either soap and water or an alcohol-based hand rub for 20 to 30 seconds, particularly after touching your eyes, nose, or mouth or after contact with your respiratory secretions during coughing or sneezing. Adding virucidal agents or antiseptics to hand-washing is not likely to have an incremental effect.16
- If possible, open windows in your living space to improve airflow.
- If possible, when you are in close contact with other people, wear a mask to help contain your respiratory secretions.
Masks
The designs and standards of masks vary from country to country. Masks have been shown to reduce the transmission of influenza in health care settings,16 but the benefit in the community has not been established. Advice on proper use of a mask:
- Cover your mouth and nose with the mask and tie it securely to minimize gaps.
- Avoid touching the mask while it is on your face.
- Wash your hands with soap and water or an alcohol-based hand rub for 20 to 30 seconds after removing the mask.
- If the mask becomes damp, replace it with a new one.
- Avoid reusing single-use masks, and dispose of them immediately after removing.
VACCINE DEVELOPMENT
The most difficult question about vaccine development for S-OIV at this time is whether to prepare it as a separate product or try to incorporate it in the seasonal influenza vaccine.
The problem is that the seasonal influenza vaccine for the Southern Hemisphere has already been made and distributed, and vaccination programs are already well under way. Although flu season in the Northern Hemisphere is not expected before September or October 2009, vaccine production and distribution take several months, leaving little time to observe which direction the S-OIV epidemic will take before making this decision.
Vaccine distribution also raises difficult questions, since a limited amount will be available initially and rationing to the most vulnerable people will be necessary. While health care workers are more likely to be exposed to people infected with S-OIV compared with the general population, mandating their immunization may pose other moral dilemmas.17
The current global capacity for production of seasonal influenza vaccine is approximately 400 million doses.18 Since the process of vaccine production takes at least 4 to 6 months, measures have been proposed to speed up the production of pandemic vaccine or immunogenicity; these include recombinant technology, reverse genetics, and the use of adjuvants. In April 2007, the FDA approved the first H5 subviron vaccine for people ages 19 to 64.
This topic brings back memories of the 1976 swine influenza immunization program, in which the rate of Guillain-Barré syndrome was 5 to 10 times the background rate, resulting in a halt in vaccine production.
Why this syndrome occurred is not known, but it is suspected to be due to cross-reacting antibodies against peripheral-nerve antigen that developed after the vaccine was given. Data since then have shown no association between vaccination and Guillain-Barré syndrome. 19 On the other hand, influenza viruses were found to trigger Guillain-Barré syndrome only infrequently, except during major outbreaks, in which they may play a significant role.20
TREATMENT
Antiviral drugs
Tests of current S-OIV isolates showed them to be susceptible to the neuraminidase inhibitors, ie, oseltamivir and zanamivir, but resistant to the adamantanes, ie, amantadine (Symmetrel) and rimantadine (Flumadine).21 All isolates contained the S31N mutation in the M2 protein, which confers resistance against the adamantanes and which has been detected in most influenza A (H3N2) isolates in the United States since 2006. Fortunately, the H274Y mutation in N1—which confers resistance to oseltamivir but not to zanamivir and which has been detected in almost all seasonal influenza A (H1N1) isolates since the early weeks of the current influenza season— has not been detected in any of the current S-OIV isolates.
Patients who are otherwise healthy who present with an uncomplicated febrile illness due to S-OIV do not require antiviral treatment. Either oseltamivir or zanamivir is recommended for treatment of patients hospitalized for management of confirmed, probable, or suspected infection with S-OIV, or for those at high risk of influenza-related complications, defined similarly to seasonal influenza (Table 1).
The duration of shedding of S-OIV is unknown, but starting an antiviral agent early in the course of illness is expected to reduce contagiousness. Extrapolating from data in seasonal influenza, infected persons are assumed to be shedding virus from 1 day prior to illness onset until resolution of symptoms, usually 7 days, and up to 10 days in younger children.
Oseltamivir accounts for the lion’s share of the stockpile of antiviral drugs against pandemic influenza. However, with mass utilization, antiviral resistance to a single agent may develop. A mathematical model showed that adding a smaller stockpile of a second agent, such as zanamivir, to be used either in combination with or sequential to oseltamivir, can effectively prevent or at least delay the development of resistance.22
Other potential measures for management
Since secondary bacterial pneumonia is expected to play a significant role in influenza-related death during the next pandemic, stockpiling antibacterial agents may also be prudent.8 The death rate in methicillin-resistant Staphylococcus aureus pneumonia secondary to seasonal influenza is 50%, further complicating the choice of stockpiling for antibacterial agents.
A meta-analysis of 11 studies involving 1,703 patients during the 1918 pandemic showed that those who received influenza-convalescent human blood products were less likely to die than those who did not.23 Anti-influenza drugs and advanced techniques to care for critically ill patients were not available at that time, so extrapolating these data to the current era may not be appropriate.
The cost of vaccine and antiviral drugs is an expected limitation to mass implementation during a pandemic, particularly in developing countries. Certain inexpensive generic drugs that have been shown to have some activity against influenza, such statins, fibrates, and chloroquine, deserve further attention.24
PUTING THE CURRENT EPIDEMIC IN PERSPECTIVE
In summary, the world is now better prepared, vaccine is in development, and antiviral treatment is available. For more information, readers are directed to go to www.cdc.gov/h1n1flu/ or www.who.int/csr/don/2009_05_11/en/index.html.
- Belshe RB. Implications of the emergence of a novel H1 influenza virus. N Engl J Med 2009 May 7; doi:10.1056/NEJMe0903995.
- Ducatez MF, Webster RG, Webby RJ. Animal influenza epidemiology. Vaccine 2008; 26(suppl 4):D67–D69.
- Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis 2007; 44:1084–1088.
- Shinde V, Bridges CB, Uyeki TM, et al. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N Engl J Med 2009 May 7; doi:10.1056/NEJMoa0903812.
- Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med 2007; 4( 2):e59. doi:10.1371/journal. pmed.0040059.
- Belshe RB. The origins of pandemic influenza—lessons from the 1918 virus. N Engl J Med 2005; 353:2209–2211.
- Miller MA, Viboud C, Balinska M, Simonsen L. The signature features of influenza pandemics—implications for policy. N Engl J Med 2009 May 7; doi:10.1056/NEJMp0903906.
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–970.
- Viboud C, Grais RF, Lafont BAP, Miller MA, Simonsen L. Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis 2005; 192:233–248.
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009 May 7; doi:10.1056/NEJMoa0903810.
- US Centers for Disease Control and PreventionC. Swine-origin influenza A (H1N1) virus infections in a school—New York City, April 2009. MMWR 2009; 58(Dispatch):1–3.
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA 2005: 293:987–997.
- Baden LR, Drazen JM, Kritek PA, Curfman GD, Morrissey S, Campion EW. H1N1 influenza A disease—information for health professionals. N Engl J Med 2009 May 7; doi:10.1056/NEJMe0903992.
- Brownstein JS, Freifeld CC, Madoff LC. Influenza A (H1N1) virus, 2009—online monitoring. N Engl J Med 2009 May 7; doi:10.1056/NEJMp0904012.
- Markel H, Lipman HB, Navarro JA, et al. Nonpharmaceutical interventions implemented by US cities during the 1918–1919 influenza pandemic. JAMA 2007; 298:644–654.
- Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 336( 7635):77–80.
- Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med 2009; 360:1981–1988.
- Sahni R, Mossad SB. Controlling pandemic influenza through vaccination programs. Future Virol 2009; 4:271–276.
- Hughes RA, Charlton J, Latinovic R, Gulliford MC. No association between immunization and Guillain-Barré syndrome in the United Kingdom, 1992 to 2000. Arch Intern Med 2006; 166:1301–1304.
- Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Guillain-Barré syndrome and influenza virus infection. Clin Infect Dis 2009; 48:48–56.
- US Centers for Disease Control and Prevention. Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR May 1, 2009; 58( 16):433–435.
- Wu JT, Leung GM, Lipsitch M, Cooper BS, Riley S. Hedging against antiviral resistance during the next influenza pandemic using small stockpiles of an alternative chemotherapy. PLoS Med 2009;e1000085. doi:10.1371/journal.pmed.1000085.
- Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med 2006; 145:599–609.
- Fedson DS. Confronting an influenza pandemic with inexpensive generic agents: can it be done? Lancet Infect Dis 2008; 8:571–576.
- Belshe RB. Implications of the emergence of a novel H1 influenza virus. N Engl J Med 2009 May 7; doi:10.1056/NEJMe0903995.
- Ducatez MF, Webster RG, Webby RJ. Animal influenza epidemiology. Vaccine 2008; 26(suppl 4):D67–D69.
- Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis 2007; 44:1084–1088.
- Shinde V, Bridges CB, Uyeki TM, et al. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N Engl J Med 2009 May 7; doi:10.1056/NEJMoa0903812.
- Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med 2007; 4( 2):e59. doi:10.1371/journal. pmed.0040059.
- Belshe RB. The origins of pandemic influenza—lessons from the 1918 virus. N Engl J Med 2005; 353:2209–2211.
- Miller MA, Viboud C, Balinska M, Simonsen L. The signature features of influenza pandemics—implications for policy. N Engl J Med 2009 May 7; doi:10.1056/NEJMp0903906.
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–970.
- Viboud C, Grais RF, Lafont BAP, Miller MA, Simonsen L. Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis 2005; 192:233–248.
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009 May 7; doi:10.1056/NEJMoa0903810.
- US Centers for Disease Control and PreventionC. Swine-origin influenza A (H1N1) virus infections in a school—New York City, April 2009. MMWR 2009; 58(Dispatch):1–3.
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA 2005: 293:987–997.
- Baden LR, Drazen JM, Kritek PA, Curfman GD, Morrissey S, Campion EW. H1N1 influenza A disease—information for health professionals. N Engl J Med 2009 May 7; doi:10.1056/NEJMe0903992.
- Brownstein JS, Freifeld CC, Madoff LC. Influenza A (H1N1) virus, 2009—online monitoring. N Engl J Med 2009 May 7; doi:10.1056/NEJMp0904012.
- Markel H, Lipman HB, Navarro JA, et al. Nonpharmaceutical interventions implemented by US cities during the 1918–1919 influenza pandemic. JAMA 2007; 298:644–654.
- Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 336( 7635):77–80.
- Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med 2009; 360:1981–1988.
- Sahni R, Mossad SB. Controlling pandemic influenza through vaccination programs. Future Virol 2009; 4:271–276.
- Hughes RA, Charlton J, Latinovic R, Gulliford MC. No association between immunization and Guillain-Barré syndrome in the United Kingdom, 1992 to 2000. Arch Intern Med 2006; 166:1301–1304.
- Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Guillain-Barré syndrome and influenza virus infection. Clin Infect Dis 2009; 48:48–56.
- US Centers for Disease Control and Prevention. Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR May 1, 2009; 58( 16):433–435.
- Wu JT, Leung GM, Lipsitch M, Cooper BS, Riley S. Hedging against antiviral resistance during the next influenza pandemic using small stockpiles of an alternative chemotherapy. PLoS Med 2009;e1000085. doi:10.1371/journal.pmed.1000085.
- Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med 2006; 145:599–609.
- Fedson DS. Confronting an influenza pandemic with inexpensive generic agents: can it be done? Lancet Infect Dis 2008; 8:571–576.
KEY POINTS
- What happens in the annual influenza season in the Southern Hemisphere will indicate the prospects of S-OIV progressing to a pandemic.
- Oseltamivir (Tamiflu) and zanamivir (Relenza) are active against S-OIV and are recommended for hospitalized patients or people at higher risk of influenza-related complications.
- Otherwise-healthy patients who present with an uncomplicated febrile illness due to S-OIV do not require antiviral treatment.
- Hand-washing is the most important preventive measure.
- Vaccine development may take 4 to 6 months. The most difficult question about vaccine development for S-OIV is whether to prepare it as a separate product or incorporate it in the seasonal influenza vaccine.