User login
, according to results of a pair of head-to-head trials published in the Lancet.
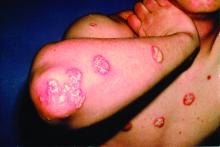
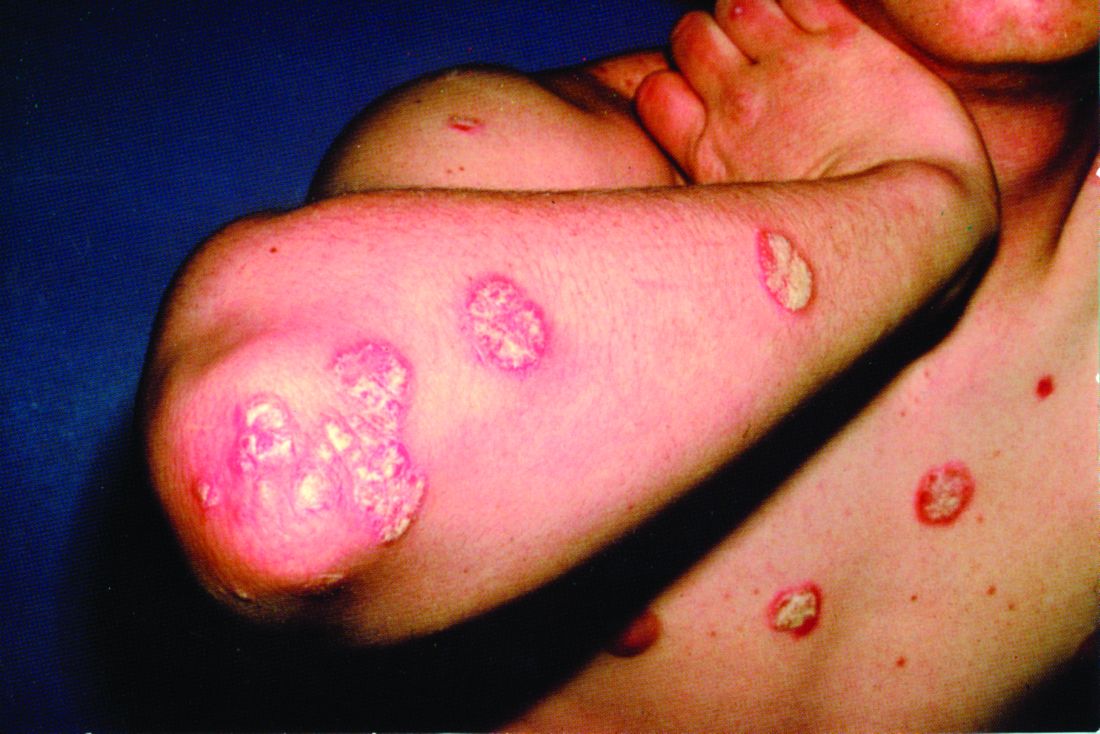
The replicate phase 3, randomized, double-blind, placebo- and active comparator–controlled trials, UltIMMa-1 (NCT02684370) and UltIMMa-2 (NCT02684375) altogether randomized 997 patients to risankizumab, ustekinumab, or placebo. The coprimary endpoints were the proportions of patients achieving 90% reduction in Psoriasis Area and Severity Index (PASI 90) at 16 weeks and a static Physician Global Assessment (sPGA) score of 0 or 1, and the 15 ranked secondary endpoints included proportions of those achieving PASI 100 or sPGA 0, both of which demonstrate total clearance of psoriasis, as well as measures of quality of life improvement.
Compared with those receiving either ustekinumab or placebo, a significantly higher proportion of patients receiving risankizumab achieved the coprimary endpoints, and all secondary endpoints were met. In UltIMMA-1, 75.3% of risankizumab patients achieved PASI 90, compared with 4.9% of placebo patients and 42% of ustekinumab patients (P less than .0001 when comparing it with both placebo and ustekinumab); sPGA of 0 or 1 was achieved by 87.8% of risankizumab patients and only 7.8% of placebo patients and 63% of ustekinumab patients (P less than .0001 when comparing it with both placebo and ustekinumab). Results were similar in UltIMMA-2: 74.8% of risankizumab patients achieved PASI 90, and 83.7% of them achieved sPGA 0 or 1 (P less than .0001 when comparing them with placebo and ustekinumab). According to results of the secondary endpoints, both studies also showed greater rates of clearance and improvements in quality of life among patients receiving risankizumab than among those receiving either placebo or ustekinumab.
The safety profiles across treatment groups were similar in both studies, with the most common adverse events including upper respiratory tract infection, headache, and diarrhea.
Risankizumab is a humanized IgG1 monoclonal antibody that targets the p19 subunit of only interleukin-23, unlike the studies’ active comparator, ustekinumab, which targets both interleukin-23 and interleukin-12. “Selectively blocking interleukin 23 with a p19 inhibitor appears to be one of the best ways to treat psoriasis,” commented Abigail Cline, MD, and Steven R. Feldman, MD, PhD, both of Wake Forest University, Winston-Salem, N.C., in an accompanying editorial (Lancet. 2018 Aug 7;392:616-71.).
The authors of the study reported relationships with various industry entities, including AbbVie, which sponsored the studies and developed risankizumab, and Boehringer Ingelheim, which collaborated in the studies. The authors of the editorial also disclosed relationships with entities, including AbbVie.
SOURCE: Gordon KB et al. Lancet. 2018 Aug 7;392:650-61.
, according to results of a pair of head-to-head trials published in the Lancet.
The replicate phase 3, randomized, double-blind, placebo- and active comparator–controlled trials, UltIMMa-1 (NCT02684370) and UltIMMa-2 (NCT02684375) altogether randomized 997 patients to risankizumab, ustekinumab, or placebo. The coprimary endpoints were the proportions of patients achieving 90% reduction in Psoriasis Area and Severity Index (PASI 90) at 16 weeks and a static Physician Global Assessment (sPGA) score of 0 or 1, and the 15 ranked secondary endpoints included proportions of those achieving PASI 100 or sPGA 0, both of which demonstrate total clearance of psoriasis, as well as measures of quality of life improvement.
Compared with those receiving either ustekinumab or placebo, a significantly higher proportion of patients receiving risankizumab achieved the coprimary endpoints, and all secondary endpoints were met. In UltIMMA-1, 75.3% of risankizumab patients achieved PASI 90, compared with 4.9% of placebo patients and 42% of ustekinumab patients (P less than .0001 when comparing it with both placebo and ustekinumab); sPGA of 0 or 1 was achieved by 87.8% of risankizumab patients and only 7.8% of placebo patients and 63% of ustekinumab patients (P less than .0001 when comparing it with both placebo and ustekinumab). Results were similar in UltIMMA-2: 74.8% of risankizumab patients achieved PASI 90, and 83.7% of them achieved sPGA 0 or 1 (P less than .0001 when comparing them with placebo and ustekinumab). According to results of the secondary endpoints, both studies also showed greater rates of clearance and improvements in quality of life among patients receiving risankizumab than among those receiving either placebo or ustekinumab.
The safety profiles across treatment groups were similar in both studies, with the most common adverse events including upper respiratory tract infection, headache, and diarrhea.
Risankizumab is a humanized IgG1 monoclonal antibody that targets the p19 subunit of only interleukin-23, unlike the studies’ active comparator, ustekinumab, which targets both interleukin-23 and interleukin-12. “Selectively blocking interleukin 23 with a p19 inhibitor appears to be one of the best ways to treat psoriasis,” commented Abigail Cline, MD, and Steven R. Feldman, MD, PhD, both of Wake Forest University, Winston-Salem, N.C., in an accompanying editorial (Lancet. 2018 Aug 7;392:616-71.).
The authors of the study reported relationships with various industry entities, including AbbVie, which sponsored the studies and developed risankizumab, and Boehringer Ingelheim, which collaborated in the studies. The authors of the editorial also disclosed relationships with entities, including AbbVie.
SOURCE: Gordon KB et al. Lancet. 2018 Aug 7;392:650-61.
, according to results of a pair of head-to-head trials published in the Lancet.
The replicate phase 3, randomized, double-blind, placebo- and active comparator–controlled trials, UltIMMa-1 (NCT02684370) and UltIMMa-2 (NCT02684375) altogether randomized 997 patients to risankizumab, ustekinumab, or placebo. The coprimary endpoints were the proportions of patients achieving 90% reduction in Psoriasis Area and Severity Index (PASI 90) at 16 weeks and a static Physician Global Assessment (sPGA) score of 0 or 1, and the 15 ranked secondary endpoints included proportions of those achieving PASI 100 or sPGA 0, both of which demonstrate total clearance of psoriasis, as well as measures of quality of life improvement.
Compared with those receiving either ustekinumab or placebo, a significantly higher proportion of patients receiving risankizumab achieved the coprimary endpoints, and all secondary endpoints were met. In UltIMMA-1, 75.3% of risankizumab patients achieved PASI 90, compared with 4.9% of placebo patients and 42% of ustekinumab patients (P less than .0001 when comparing it with both placebo and ustekinumab); sPGA of 0 or 1 was achieved by 87.8% of risankizumab patients and only 7.8% of placebo patients and 63% of ustekinumab patients (P less than .0001 when comparing it with both placebo and ustekinumab). Results were similar in UltIMMA-2: 74.8% of risankizumab patients achieved PASI 90, and 83.7% of them achieved sPGA 0 or 1 (P less than .0001 when comparing them with placebo and ustekinumab). According to results of the secondary endpoints, both studies also showed greater rates of clearance and improvements in quality of life among patients receiving risankizumab than among those receiving either placebo or ustekinumab.
The safety profiles across treatment groups were similar in both studies, with the most common adverse events including upper respiratory tract infection, headache, and diarrhea.
Risankizumab is a humanized IgG1 monoclonal antibody that targets the p19 subunit of only interleukin-23, unlike the studies’ active comparator, ustekinumab, which targets both interleukin-23 and interleukin-12. “Selectively blocking interleukin 23 with a p19 inhibitor appears to be one of the best ways to treat psoriasis,” commented Abigail Cline, MD, and Steven R. Feldman, MD, PhD, both of Wake Forest University, Winston-Salem, N.C., in an accompanying editorial (Lancet. 2018 Aug 7;392:616-71.).
The authors of the study reported relationships with various industry entities, including AbbVie, which sponsored the studies and developed risankizumab, and Boehringer Ingelheim, which collaborated in the studies. The authors of the editorial also disclosed relationships with entities, including AbbVie.
SOURCE: Gordon KB et al. Lancet. 2018 Aug 7;392:650-61.
FROM THE LANCET