User login
Christopher Palmer has been an associate editor at MDedge News since 2017. When he's not tidying grammar, he writes short pieces about breaking FDA announcements and approvals, as well as journal articles. He proudly holds a BA in English and philosophy. Follow him on Twitter @cmacmpalm.
Lung ultrasound works well in children with COVID-19
researchers wrote in Pediatrics.
They also noted the benefits that modality provides over other imaging techniques.
Marco Denina, MD, and colleagues from the pediatric infectious diseases unit at Regina Margherita Children’s Hospital in Turin, Italy, performed an observational study of eight children aged 0-17 years who were admitted to the hospital for COVID-19 between March 8 and 26, 2020. In seven of eight patients, the findings were concordant between imaging modalities; in the remaining patient, lung ultrasound (LUS) found an interstitial B-lines pattern that was not seen on radiography. In seven patients with pathologic ultrasound findings at baseline, the improvement or resolution of the subpleural consolidations or interstitial patterns was consistent with concomitant radiologic findings.
The authors cited the benefits of using point-of-care ultrasound instead of other modalities, such as CT. “First, it may reduce the number of radiologic examinations, lowering the radiation exposure of the patients,” they wrote. “Secondly, when performed at the bedside, LUS allows for the reduction of the patient’s movement within the hospital; thus, it lowers the number of health care workers and medical devices exposed to [SARS-CoV-2].”
One limitation of the study is the small sample size; however, the researchers felt the high concordance still suggests LUS is a reasonable method for COVID-19 patients.
There was no external funding for this study and the investigators had no relevant financial disclosures.
SOURCE: Denina M et al. Pediatrics. 2020 Jun. doi: 10.1542/peds.2020-1157.
researchers wrote in Pediatrics.
They also noted the benefits that modality provides over other imaging techniques.
Marco Denina, MD, and colleagues from the pediatric infectious diseases unit at Regina Margherita Children’s Hospital in Turin, Italy, performed an observational study of eight children aged 0-17 years who were admitted to the hospital for COVID-19 between March 8 and 26, 2020. In seven of eight patients, the findings were concordant between imaging modalities; in the remaining patient, lung ultrasound (LUS) found an interstitial B-lines pattern that was not seen on radiography. In seven patients with pathologic ultrasound findings at baseline, the improvement or resolution of the subpleural consolidations or interstitial patterns was consistent with concomitant radiologic findings.
The authors cited the benefits of using point-of-care ultrasound instead of other modalities, such as CT. “First, it may reduce the number of radiologic examinations, lowering the radiation exposure of the patients,” they wrote. “Secondly, when performed at the bedside, LUS allows for the reduction of the patient’s movement within the hospital; thus, it lowers the number of health care workers and medical devices exposed to [SARS-CoV-2].”
One limitation of the study is the small sample size; however, the researchers felt the high concordance still suggests LUS is a reasonable method for COVID-19 patients.
There was no external funding for this study and the investigators had no relevant financial disclosures.
SOURCE: Denina M et al. Pediatrics. 2020 Jun. doi: 10.1542/peds.2020-1157.
researchers wrote in Pediatrics.
They also noted the benefits that modality provides over other imaging techniques.
Marco Denina, MD, and colleagues from the pediatric infectious diseases unit at Regina Margherita Children’s Hospital in Turin, Italy, performed an observational study of eight children aged 0-17 years who were admitted to the hospital for COVID-19 between March 8 and 26, 2020. In seven of eight patients, the findings were concordant between imaging modalities; in the remaining patient, lung ultrasound (LUS) found an interstitial B-lines pattern that was not seen on radiography. In seven patients with pathologic ultrasound findings at baseline, the improvement or resolution of the subpleural consolidations or interstitial patterns was consistent with concomitant radiologic findings.
The authors cited the benefits of using point-of-care ultrasound instead of other modalities, such as CT. “First, it may reduce the number of radiologic examinations, lowering the radiation exposure of the patients,” they wrote. “Secondly, when performed at the bedside, LUS allows for the reduction of the patient’s movement within the hospital; thus, it lowers the number of health care workers and medical devices exposed to [SARS-CoV-2].”
One limitation of the study is the small sample size; however, the researchers felt the high concordance still suggests LUS is a reasonable method for COVID-19 patients.
There was no external funding for this study and the investigators had no relevant financial disclosures.
SOURCE: Denina M et al. Pediatrics. 2020 Jun. doi: 10.1542/peds.2020-1157.
FROM PEDIATRICS
FDA makes Ilaris the first approved treatment for adult-onset Still’s disease
The Food and Drug Administration has expanded the indications for canakinumab (Ilaris) to include all patients with active Still’s disease older than 2 years, adding adult-onset Still’s disease (AOSD) to a previous approval for juvenile-onset Still’s disease, also known as systemic juvenile idiopathic arthritis (sJIA), making it the first approved treatment for AOSD, according to an FDA announcement.
The approval comes under a Priority Review designation that used “comparable pharmacokinetic exposure and extrapolation of established efficacy of canakinumab in patients with sJIA, as well as the safety of canakinumab in patients with AOSD and other diseases,” the FDA said.
The results from a randomized, double-blind, placebo-controlled study of 36 patients with AOSD aged 22-70 years showed that the efficacy and safety data in AOSD were generally consistent with the results of a pooled analysis of sJIA patients, according to Novartis, which markets canakinumab.
AOSD and sJIA share certain similarities, such as fever, arthritis, rash, and elevated markers of inflammation, which has led to suspicion that they are part of a continuum rather than wholly distinct, according to the agency. In addition, the role of interleukin-1 is well established in both diseases and is blocked by canakinumab.
The most common side effects (occurring in greater than 10% of patients) in sJIA studies included infections, abdominal pain, and injection-site reactions. Serious infections (e.g., pneumonia, varicella, gastroenteritis, measles, sepsis, otitis media, sinusitis, adenovirus, lymph node abscess, pharyngitis) were observed in approximately 4%-5%, according to the full prescribing information.
Canakinumab is also approved for the periodic fever syndromes of cryopyrin-associated periodic syndromes in adults and children aged 4 years and older (including familial cold auto-inflammatory syndrome and Muckle-Wells syndrome), tumor necrosis factor receptor associated periodic syndrome in adult and pediatric patients, hyperimmunoglobulin D syndrome/mevalonate kinase deficiency in adult and pediatric patients, and familial Mediterranean fever in adult and pediatric patients.
The Food and Drug Administration has expanded the indications for canakinumab (Ilaris) to include all patients with active Still’s disease older than 2 years, adding adult-onset Still’s disease (AOSD) to a previous approval for juvenile-onset Still’s disease, also known as systemic juvenile idiopathic arthritis (sJIA), making it the first approved treatment for AOSD, according to an FDA announcement.
The approval comes under a Priority Review designation that used “comparable pharmacokinetic exposure and extrapolation of established efficacy of canakinumab in patients with sJIA, as well as the safety of canakinumab in patients with AOSD and other diseases,” the FDA said.
The results from a randomized, double-blind, placebo-controlled study of 36 patients with AOSD aged 22-70 years showed that the efficacy and safety data in AOSD were generally consistent with the results of a pooled analysis of sJIA patients, according to Novartis, which markets canakinumab.
AOSD and sJIA share certain similarities, such as fever, arthritis, rash, and elevated markers of inflammation, which has led to suspicion that they are part of a continuum rather than wholly distinct, according to the agency. In addition, the role of interleukin-1 is well established in both diseases and is blocked by canakinumab.
The most common side effects (occurring in greater than 10% of patients) in sJIA studies included infections, abdominal pain, and injection-site reactions. Serious infections (e.g., pneumonia, varicella, gastroenteritis, measles, sepsis, otitis media, sinusitis, adenovirus, lymph node abscess, pharyngitis) were observed in approximately 4%-5%, according to the full prescribing information.
Canakinumab is also approved for the periodic fever syndromes of cryopyrin-associated periodic syndromes in adults and children aged 4 years and older (including familial cold auto-inflammatory syndrome and Muckle-Wells syndrome), tumor necrosis factor receptor associated periodic syndrome in adult and pediatric patients, hyperimmunoglobulin D syndrome/mevalonate kinase deficiency in adult and pediatric patients, and familial Mediterranean fever in adult and pediatric patients.
The Food and Drug Administration has expanded the indications for canakinumab (Ilaris) to include all patients with active Still’s disease older than 2 years, adding adult-onset Still’s disease (AOSD) to a previous approval for juvenile-onset Still’s disease, also known as systemic juvenile idiopathic arthritis (sJIA), making it the first approved treatment for AOSD, according to an FDA announcement.
The approval comes under a Priority Review designation that used “comparable pharmacokinetic exposure and extrapolation of established efficacy of canakinumab in patients with sJIA, as well as the safety of canakinumab in patients with AOSD and other diseases,” the FDA said.
The results from a randomized, double-blind, placebo-controlled study of 36 patients with AOSD aged 22-70 years showed that the efficacy and safety data in AOSD were generally consistent with the results of a pooled analysis of sJIA patients, according to Novartis, which markets canakinumab.
AOSD and sJIA share certain similarities, such as fever, arthritis, rash, and elevated markers of inflammation, which has led to suspicion that they are part of a continuum rather than wholly distinct, according to the agency. In addition, the role of interleukin-1 is well established in both diseases and is blocked by canakinumab.
The most common side effects (occurring in greater than 10% of patients) in sJIA studies included infections, abdominal pain, and injection-site reactions. Serious infections (e.g., pneumonia, varicella, gastroenteritis, measles, sepsis, otitis media, sinusitis, adenovirus, lymph node abscess, pharyngitis) were observed in approximately 4%-5%, according to the full prescribing information.
Canakinumab is also approved for the periodic fever syndromes of cryopyrin-associated periodic syndromes in adults and children aged 4 years and older (including familial cold auto-inflammatory syndrome and Muckle-Wells syndrome), tumor necrosis factor receptor associated periodic syndrome in adult and pediatric patients, hyperimmunoglobulin D syndrome/mevalonate kinase deficiency in adult and pediatric patients, and familial Mediterranean fever in adult and pediatric patients.
FDA approves ixekizumab for nonradiographic axSpA
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
Infectious disease specialists among lowest in pay
Between Oct. 4, 2019, and Feb. 10, 2020, infectious disease specialists reported making $246,000, which puts them at the fifth lowest paid specialty included in Medscape’s Physician Compensation Report 2020. Men earned $265,000 to women’s $211,000 and made up 64% of respondents.
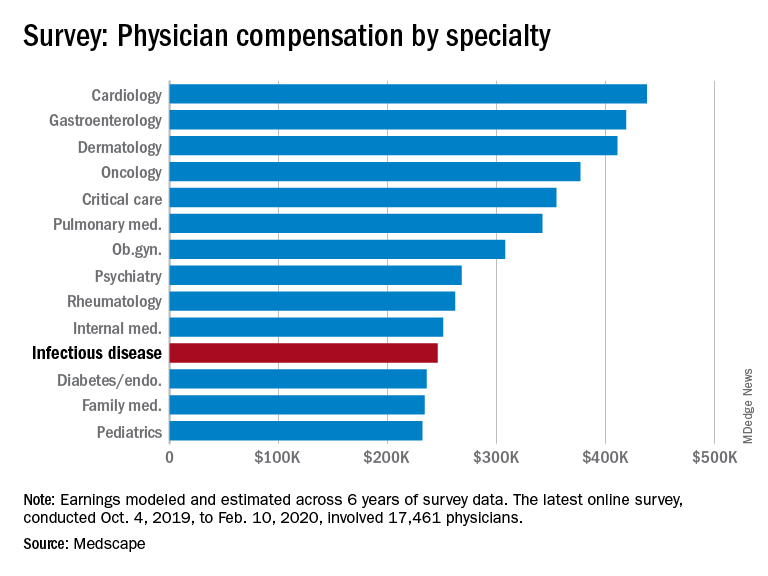
Infectious disease specialists are tied with internal medicine for time spent on paperwork at 18.5 hours/week, with only critical care beating them at 19.1 hours per week. Among infectious disease specialists, 41% reported that being very good at what they do/finding answers and diagnoses as the most rewarding part of their jobs, whereas rules and regulations, long hours, and difficulties with reimbursement were cited as the most challenging aspects (at 21%, 17%, and 15%, respectively).
About 51% report feeling they’re fairly compensated, with puts them in a tie with ob.gyns. for the fifth and sixth lowest positions in this regard.
The data in this report were gathered before COVID-19 had really taken hold in the United States – before states began issuing stay-at-home orders and before practices began implementing their own precautions. Although in the best interest of both patients and providers, switching to telemedicine, eliminating most elective procedures, and making other changes to improve safety will have significant financial consequences. It is unclear at this time how the ongoing pandemic will affect things like physician compensation and income.
The survey respondents were Medscape members who had been invited to participate. The sample size was 17,461 physicians, and compensation was modeled and estimated based on a range of variables across 6 years of survey data.
Between Oct. 4, 2019, and Feb. 10, 2020, infectious disease specialists reported making $246,000, which puts them at the fifth lowest paid specialty included in Medscape’s Physician Compensation Report 2020. Men earned $265,000 to women’s $211,000 and made up 64% of respondents.

Infectious disease specialists are tied with internal medicine for time spent on paperwork at 18.5 hours/week, with only critical care beating them at 19.1 hours per week. Among infectious disease specialists, 41% reported that being very good at what they do/finding answers and diagnoses as the most rewarding part of their jobs, whereas rules and regulations, long hours, and difficulties with reimbursement were cited as the most challenging aspects (at 21%, 17%, and 15%, respectively).
About 51% report feeling they’re fairly compensated, with puts them in a tie with ob.gyns. for the fifth and sixth lowest positions in this regard.
The data in this report were gathered before COVID-19 had really taken hold in the United States – before states began issuing stay-at-home orders and before practices began implementing their own precautions. Although in the best interest of both patients and providers, switching to telemedicine, eliminating most elective procedures, and making other changes to improve safety will have significant financial consequences. It is unclear at this time how the ongoing pandemic will affect things like physician compensation and income.
The survey respondents were Medscape members who had been invited to participate. The sample size was 17,461 physicians, and compensation was modeled and estimated based on a range of variables across 6 years of survey data.
Between Oct. 4, 2019, and Feb. 10, 2020, infectious disease specialists reported making $246,000, which puts them at the fifth lowest paid specialty included in Medscape’s Physician Compensation Report 2020. Men earned $265,000 to women’s $211,000 and made up 64% of respondents.

Infectious disease specialists are tied with internal medicine for time spent on paperwork at 18.5 hours/week, with only critical care beating them at 19.1 hours per week. Among infectious disease specialists, 41% reported that being very good at what they do/finding answers and diagnoses as the most rewarding part of their jobs, whereas rules and regulations, long hours, and difficulties with reimbursement were cited as the most challenging aspects (at 21%, 17%, and 15%, respectively).
About 51% report feeling they’re fairly compensated, with puts them in a tie with ob.gyns. for the fifth and sixth lowest positions in this regard.
The data in this report were gathered before COVID-19 had really taken hold in the United States – before states began issuing stay-at-home orders and before practices began implementing their own precautions. Although in the best interest of both patients and providers, switching to telemedicine, eliminating most elective procedures, and making other changes to improve safety will have significant financial consequences. It is unclear at this time how the ongoing pandemic will affect things like physician compensation and income.
The survey respondents were Medscape members who had been invited to participate. The sample size was 17,461 physicians, and compensation was modeled and estimated based on a range of variables across 6 years of survey data.
COVID-19 may cause subacute thyroiditis
Coronavirus disease of 2019 (COVID-19) may lead to subacute thyroiditis in some patients, which is suspected to have viral or postviral origin, especially with upper respiratory tract infections, according to a case study in the Journal of Clinical Endocrinology & Metabolism.
Alessandro Brancatella, a PhD student at the University Hospital Pisa (Italy), and colleagues described the case of an 18-year-old woman who was tested Feb. 21 for SARS-CoV-2 infection after her father was hospitalized because of COVID-19. Her results were positive for the virus, and not long after, she developed mild symptoms. By March 13 and again on March 14, test swabs for SARS-CoV-2 were both negative.
On March 17, she presented with fever, fatigue, palpitations, and neck pain that radiated to her jaw. Testing and physical examination pointed to subacute thyroiditis, and she was soon diagnosed and treated with prednisone. Her neck pain and fever disappeared within 2 days, and the remaining symptoms went away within a week.
The authors noted that the woman’s thyroid had been evaluated before she tested positive for SARS-CoV-2, and at that time, thyroid disease was ruled out. They also pointed out that, although the exact etiology for subacute thyroiditis is unknown, “it is common opinion that the disease is due to a viral infection or to a post-viral inflammatory reaction in genetically predisposed subjects.” They cited examples of viruses with suspected causal associations, including mumps, Epstein-Barr virus, and HIV, and they suggested that, based on the timing of the woman’s subacute thyroiditis and the normal results of her thyroid evaluation before developing COVID-19, SARS-CoV-2 be added to that list.
“To our knowledge, this is the first case of [subacute thyroiditis] related to SARS-CoV-2,” they concluded. “We therefore believe that physicians should be alerted about the possibility of this additional clinical manifestation related to SARS-CoV-2 infection.”
One author reported funding from the University of Pisa.
SOURCE: Brancatella A et al. J Clin Endocrinol Metab. 2020 May 21. doi: 10.1210/clinem/dgaa276.
Coronavirus disease of 2019 (COVID-19) may lead to subacute thyroiditis in some patients, which is suspected to have viral or postviral origin, especially with upper respiratory tract infections, according to a case study in the Journal of Clinical Endocrinology & Metabolism.
Alessandro Brancatella, a PhD student at the University Hospital Pisa (Italy), and colleagues described the case of an 18-year-old woman who was tested Feb. 21 for SARS-CoV-2 infection after her father was hospitalized because of COVID-19. Her results were positive for the virus, and not long after, she developed mild symptoms. By March 13 and again on March 14, test swabs for SARS-CoV-2 were both negative.
On March 17, she presented with fever, fatigue, palpitations, and neck pain that radiated to her jaw. Testing and physical examination pointed to subacute thyroiditis, and she was soon diagnosed and treated with prednisone. Her neck pain and fever disappeared within 2 days, and the remaining symptoms went away within a week.
The authors noted that the woman’s thyroid had been evaluated before she tested positive for SARS-CoV-2, and at that time, thyroid disease was ruled out. They also pointed out that, although the exact etiology for subacute thyroiditis is unknown, “it is common opinion that the disease is due to a viral infection or to a post-viral inflammatory reaction in genetically predisposed subjects.” They cited examples of viruses with suspected causal associations, including mumps, Epstein-Barr virus, and HIV, and they suggested that, based on the timing of the woman’s subacute thyroiditis and the normal results of her thyroid evaluation before developing COVID-19, SARS-CoV-2 be added to that list.
“To our knowledge, this is the first case of [subacute thyroiditis] related to SARS-CoV-2,” they concluded. “We therefore believe that physicians should be alerted about the possibility of this additional clinical manifestation related to SARS-CoV-2 infection.”
One author reported funding from the University of Pisa.
SOURCE: Brancatella A et al. J Clin Endocrinol Metab. 2020 May 21. doi: 10.1210/clinem/dgaa276.
Coronavirus disease of 2019 (COVID-19) may lead to subacute thyroiditis in some patients, which is suspected to have viral or postviral origin, especially with upper respiratory tract infections, according to a case study in the Journal of Clinical Endocrinology & Metabolism.
Alessandro Brancatella, a PhD student at the University Hospital Pisa (Italy), and colleagues described the case of an 18-year-old woman who was tested Feb. 21 for SARS-CoV-2 infection after her father was hospitalized because of COVID-19. Her results were positive for the virus, and not long after, she developed mild symptoms. By March 13 and again on March 14, test swabs for SARS-CoV-2 were both negative.
On March 17, she presented with fever, fatigue, palpitations, and neck pain that radiated to her jaw. Testing and physical examination pointed to subacute thyroiditis, and she was soon diagnosed and treated with prednisone. Her neck pain and fever disappeared within 2 days, and the remaining symptoms went away within a week.
The authors noted that the woman’s thyroid had been evaluated before she tested positive for SARS-CoV-2, and at that time, thyroid disease was ruled out. They also pointed out that, although the exact etiology for subacute thyroiditis is unknown, “it is common opinion that the disease is due to a viral infection or to a post-viral inflammatory reaction in genetically predisposed subjects.” They cited examples of viruses with suspected causal associations, including mumps, Epstein-Barr virus, and HIV, and they suggested that, based on the timing of the woman’s subacute thyroiditis and the normal results of her thyroid evaluation before developing COVID-19, SARS-CoV-2 be added to that list.
“To our knowledge, this is the first case of [subacute thyroiditis] related to SARS-CoV-2,” they concluded. “We therefore believe that physicians should be alerted about the possibility of this additional clinical manifestation related to SARS-CoV-2 infection.”
One author reported funding from the University of Pisa.
SOURCE: Brancatella A et al. J Clin Endocrinol Metab. 2020 May 21. doi: 10.1210/clinem/dgaa276.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Ob.gyns. income is in the middle of the pack of specialties
Obstetrician/gynecologists reported making $308,000 between Oct. 4, 2019, and Feb. 10, 2020, which is slightly below middle among the specialties included in Medscape’s Physician Compensation Report 2020.
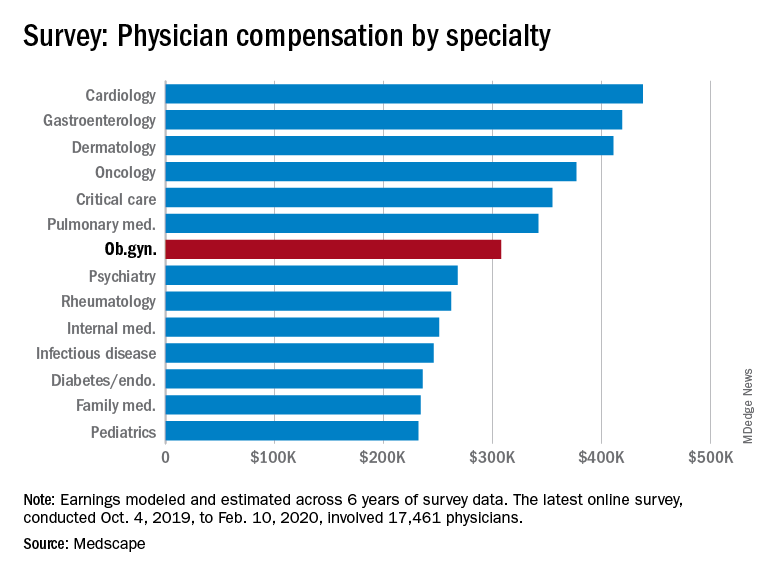
This occurs although male and female ob.gyns. reported working about the same hours per week (40.2 vs. 39).
The average incentive bonus for ob.gyns. was about $44,000, which is on the low side among specialties included in the report. Although 42% of ob.gyns. achieve 100% of this bonus and 17% achieve 76%-99% of their bonus, slightly less than a quarter (22%) achieve only 25% or less.
About 51% of ob.gyns. reported feeling fairly compensated, which put them in the bottom fifth of the 29 specialties asked that question.
Among ob.gyns., 38% reported that gratitude and relationships with patients is the most rewarding part of their job, while 20% said that helping others or being good at what they do is the most rewarding aspect of their job. About even proportions of ob.gyns. complained that the most challenging part of their job is dealing with EHRs (18%), working long hours (17%), or navigating rules and regulations (16%).
The data in the Medscape report were gathered before COVID-19 had really taken hold in the United States – before states began issuing stay-at-home orders and before practices began implementing their own precautions. Although in the best interest of patients and providers, switching to telemedicine, eliminating most elective procedures, and making other changes to improve safety will have significant financial consequences. It is unclear at this time how this ongoing pandemic will affect physician compensation and income.
The survey respondents were Medscape members who had been invited to participate. The sample size was 17,461 physicians, and compensation was modeled and estimated based on a range of variables across 6 years of survey data. The sampling error was ±0.74%.
Obstetrician/gynecologists reported making $308,000 between Oct. 4, 2019, and Feb. 10, 2020, which is slightly below middle among the specialties included in Medscape’s Physician Compensation Report 2020.

This occurs although male and female ob.gyns. reported working about the same hours per week (40.2 vs. 39).
The average incentive bonus for ob.gyns. was about $44,000, which is on the low side among specialties included in the report. Although 42% of ob.gyns. achieve 100% of this bonus and 17% achieve 76%-99% of their bonus, slightly less than a quarter (22%) achieve only 25% or less.
About 51% of ob.gyns. reported feeling fairly compensated, which put them in the bottom fifth of the 29 specialties asked that question.
Among ob.gyns., 38% reported that gratitude and relationships with patients is the most rewarding part of their job, while 20% said that helping others or being good at what they do is the most rewarding aspect of their job. About even proportions of ob.gyns. complained that the most challenging part of their job is dealing with EHRs (18%), working long hours (17%), or navigating rules and regulations (16%).
The data in the Medscape report were gathered before COVID-19 had really taken hold in the United States – before states began issuing stay-at-home orders and before practices began implementing their own precautions. Although in the best interest of patients and providers, switching to telemedicine, eliminating most elective procedures, and making other changes to improve safety will have significant financial consequences. It is unclear at this time how this ongoing pandemic will affect physician compensation and income.
The survey respondents were Medscape members who had been invited to participate. The sample size was 17,461 physicians, and compensation was modeled and estimated based on a range of variables across 6 years of survey data. The sampling error was ±0.74%.
Obstetrician/gynecologists reported making $308,000 between Oct. 4, 2019, and Feb. 10, 2020, which is slightly below middle among the specialties included in Medscape’s Physician Compensation Report 2020.

This occurs although male and female ob.gyns. reported working about the same hours per week (40.2 vs. 39).
The average incentive bonus for ob.gyns. was about $44,000, which is on the low side among specialties included in the report. Although 42% of ob.gyns. achieve 100% of this bonus and 17% achieve 76%-99% of their bonus, slightly less than a quarter (22%) achieve only 25% or less.
About 51% of ob.gyns. reported feeling fairly compensated, which put them in the bottom fifth of the 29 specialties asked that question.
Among ob.gyns., 38% reported that gratitude and relationships with patients is the most rewarding part of their job, while 20% said that helping others or being good at what they do is the most rewarding aspect of their job. About even proportions of ob.gyns. complained that the most challenging part of their job is dealing with EHRs (18%), working long hours (17%), or navigating rules and regulations (16%).
The data in the Medscape report were gathered before COVID-19 had really taken hold in the United States – before states began issuing stay-at-home orders and before practices began implementing their own precautions. Although in the best interest of patients and providers, switching to telemedicine, eliminating most elective procedures, and making other changes to improve safety will have significant financial consequences. It is unclear at this time how this ongoing pandemic will affect physician compensation and income.
The survey respondents were Medscape members who had been invited to participate. The sample size was 17,461 physicians, and compensation was modeled and estimated based on a range of variables across 6 years of survey data. The sampling error was ±0.74%.
Sensitizer prevalent in many hypoallergenic products for children
(AD) and allergic contact dermatitis, according to a research letter in the Journal of the American Academy of Dermatology.
In the letter, the authors, Reid W. Collis, of Washington University in St. Louis, and David M. Sheinbein, MD, of the division of dermatology at the university, referred to a previous study showing an association between contact sensitivity with CAPB and people with a history of AD. This was supported by the results of their own recent study in pediatric patients, they wrote, which found that reactions to CAPB were “exclusively” in patients with AD.
In the survey, they looked at children’s shampoo and soap products available on online databases of six of the biggest retailers, and analyzed the top 20 best-selling products for each retailer in 2018. Of the unique products, CAPB was found to be an ingredient in 52% (39 of 75) of the shampoos and 44% (29 of 66) of the soap products. But each of these products “contained the term ‘hypoallergenic; on the product itself or in the product’s description,” they noted.
“CAPB is a prevalent sensitizer in pediatric patients and should be avoided in patients with AD,” the investigators wrote. That said, it’s not included among the 35 prevalent allergens in the T.R.U.E. test, and they recommended that pediatricians and dermatologists “be aware of common products containing CAPB when counseling patients about their product choices,” considering that CAPB sensitivity is more likely in patients with AD.
The study had no funding source, and the authors had no disclosures.
[email protected]
SOURCE: Cho SI et al. J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2019.12.036.
(AD) and allergic contact dermatitis, according to a research letter in the Journal of the American Academy of Dermatology.
In the letter, the authors, Reid W. Collis, of Washington University in St. Louis, and David M. Sheinbein, MD, of the division of dermatology at the university, referred to a previous study showing an association between contact sensitivity with CAPB and people with a history of AD. This was supported by the results of their own recent study in pediatric patients, they wrote, which found that reactions to CAPB were “exclusively” in patients with AD.
In the survey, they looked at children’s shampoo and soap products available on online databases of six of the biggest retailers, and analyzed the top 20 best-selling products for each retailer in 2018. Of the unique products, CAPB was found to be an ingredient in 52% (39 of 75) of the shampoos and 44% (29 of 66) of the soap products. But each of these products “contained the term ‘hypoallergenic; on the product itself or in the product’s description,” they noted.
“CAPB is a prevalent sensitizer in pediatric patients and should be avoided in patients with AD,” the investigators wrote. That said, it’s not included among the 35 prevalent allergens in the T.R.U.E. test, and they recommended that pediatricians and dermatologists “be aware of common products containing CAPB when counseling patients about their product choices,” considering that CAPB sensitivity is more likely in patients with AD.
The study had no funding source, and the authors had no disclosures.
[email protected]
SOURCE: Cho SI et al. J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2019.12.036.
(AD) and allergic contact dermatitis, according to a research letter in the Journal of the American Academy of Dermatology.
In the letter, the authors, Reid W. Collis, of Washington University in St. Louis, and David M. Sheinbein, MD, of the division of dermatology at the university, referred to a previous study showing an association between contact sensitivity with CAPB and people with a history of AD. This was supported by the results of their own recent study in pediatric patients, they wrote, which found that reactions to CAPB were “exclusively” in patients with AD.
In the survey, they looked at children’s shampoo and soap products available on online databases of six of the biggest retailers, and analyzed the top 20 best-selling products for each retailer in 2018. Of the unique products, CAPB was found to be an ingredient in 52% (39 of 75) of the shampoos and 44% (29 of 66) of the soap products. But each of these products “contained the term ‘hypoallergenic; on the product itself or in the product’s description,” they noted.
“CAPB is a prevalent sensitizer in pediatric patients and should be avoided in patients with AD,” the investigators wrote. That said, it’s not included among the 35 prevalent allergens in the T.R.U.E. test, and they recommended that pediatricians and dermatologists “be aware of common products containing CAPB when counseling patients about their product choices,” considering that CAPB sensitivity is more likely in patients with AD.
The study had no funding source, and the authors had no disclosures.
[email protected]
SOURCE: Cho SI et al. J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2019.12.036.
Suicide increased 35% during 1999-2018 in the U.S.
Age-adjusted suicide rate rose from 10.5 per 100,000 to 14.2 from 1999 to 2018, according to trends reported by the Centers for Disease Control and Prevention in a data brief.
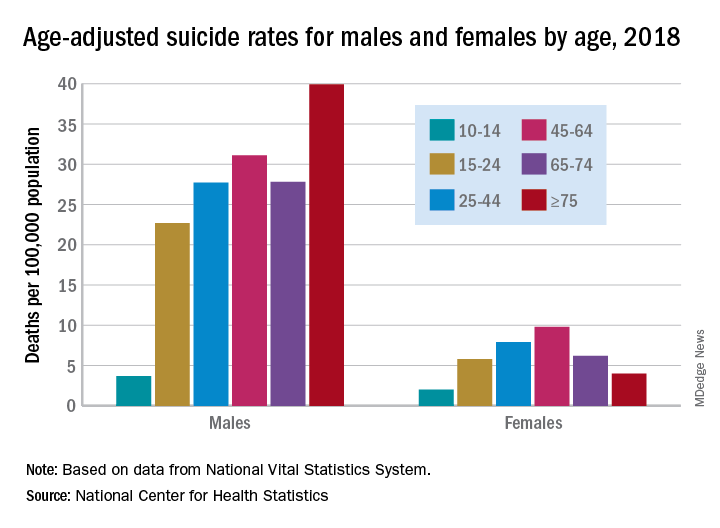
Holly Hedegaard, MD, and colleagues from the National Center for Health Statistics within the CDC analyzed final mortality data from the National Vital Statistics System. As the second most common cause of death among Americans aged 10-34 years and the fourth most common among those aged 35-54 years, suicide is a major contributer to premature mortality.
at 22.8 and 6.2 per 100,000, respectively. Young people aged 10-14 years among both genders had the lowest rates of completing suicide, but it was men aged 75 years and older and women aged 45-64 years who had the highest rates. All of these trends were consistent throughout the study period.
Drawing from the 2013 National Center for Health Statistics Urban-Rural Classification Scheme for Counties, the researchers found that rural counties had significantly higher rates of suicide than did urban counties in 2018, and this was true for men and women. That said, suicide rates were still 3.5-3.9 times higher among men than among women regardless of urbanicity or rurality that year.
The full data brief can be found on the CDC website.
Age-adjusted suicide rate rose from 10.5 per 100,000 to 14.2 from 1999 to 2018, according to trends reported by the Centers for Disease Control and Prevention in a data brief.

Holly Hedegaard, MD, and colleagues from the National Center for Health Statistics within the CDC analyzed final mortality data from the National Vital Statistics System. As the second most common cause of death among Americans aged 10-34 years and the fourth most common among those aged 35-54 years, suicide is a major contributer to premature mortality.
at 22.8 and 6.2 per 100,000, respectively. Young people aged 10-14 years among both genders had the lowest rates of completing suicide, but it was men aged 75 years and older and women aged 45-64 years who had the highest rates. All of these trends were consistent throughout the study period.
Drawing from the 2013 National Center for Health Statistics Urban-Rural Classification Scheme for Counties, the researchers found that rural counties had significantly higher rates of suicide than did urban counties in 2018, and this was true for men and women. That said, suicide rates were still 3.5-3.9 times higher among men than among women regardless of urbanicity or rurality that year.
The full data brief can be found on the CDC website.
Age-adjusted suicide rate rose from 10.5 per 100,000 to 14.2 from 1999 to 2018, according to trends reported by the Centers for Disease Control and Prevention in a data brief.

Holly Hedegaard, MD, and colleagues from the National Center for Health Statistics within the CDC analyzed final mortality data from the National Vital Statistics System. As the second most common cause of death among Americans aged 10-34 years and the fourth most common among those aged 35-54 years, suicide is a major contributer to premature mortality.
at 22.8 and 6.2 per 100,000, respectively. Young people aged 10-14 years among both genders had the lowest rates of completing suicide, but it was men aged 75 years and older and women aged 45-64 years who had the highest rates. All of these trends were consistent throughout the study period.
Drawing from the 2013 National Center for Health Statistics Urban-Rural Classification Scheme for Counties, the researchers found that rural counties had significantly higher rates of suicide than did urban counties in 2018, and this was true for men and women. That said, suicide rates were still 3.5-3.9 times higher among men than among women regardless of urbanicity or rurality that year.
The full data brief can be found on the CDC website.
FDA approves first generic albuterol inhaler
The Food and Drug Administration has approved the first generic of Proventil HFA (albuterol sulfate) metered-dose inhaler, 90 mcg/inhalation, according to a release from the agency. This inhaler is indicated for prevention of bronchospasm in patients aged 4 years and older. Specifically, these are patients with reversible obstructive airway disease or exercise-induced bronchospasm.
“The FDA recognizes the increased demand for albuterol products during the novel coronavirus pandemic,” said FDA Commissioner Stephen M. Hahn, MD.
The most common side effects include upper respiratory tract infection, rhinitis, nausea, vomiting, rapid heart rate, tremor, and nervousness.
This approval comes as part of FDA’s efforts to guide industry through the development process of generic products, according to the release. Complex combination products – such as this inhaler, which comprises both medication and a delivery system – can be more challenging to develop than solid oral dosage forms, such as tablets.
The FDA released a draft guidance in March 2020 specific to proposed generic albuterol sulfate metered-dose inhalers, including drug products referencing Proventil HFA. As with other similar guidances, it details the steps companies need to take in developing generics in order to submit complete applications for those products. The full news release regarding this approval is available on the FDA website.
This article was updated 4/8/20.
The Food and Drug Administration has approved the first generic of Proventil HFA (albuterol sulfate) metered-dose inhaler, 90 mcg/inhalation, according to a release from the agency. This inhaler is indicated for prevention of bronchospasm in patients aged 4 years and older. Specifically, these are patients with reversible obstructive airway disease or exercise-induced bronchospasm.
“The FDA recognizes the increased demand for albuterol products during the novel coronavirus pandemic,” said FDA Commissioner Stephen M. Hahn, MD.
The most common side effects include upper respiratory tract infection, rhinitis, nausea, vomiting, rapid heart rate, tremor, and nervousness.
This approval comes as part of FDA’s efforts to guide industry through the development process of generic products, according to the release. Complex combination products – such as this inhaler, which comprises both medication and a delivery system – can be more challenging to develop than solid oral dosage forms, such as tablets.
The FDA released a draft guidance in March 2020 specific to proposed generic albuterol sulfate metered-dose inhalers, including drug products referencing Proventil HFA. As with other similar guidances, it details the steps companies need to take in developing generics in order to submit complete applications for those products. The full news release regarding this approval is available on the FDA website.
This article was updated 4/8/20.
The Food and Drug Administration has approved the first generic of Proventil HFA (albuterol sulfate) metered-dose inhaler, 90 mcg/inhalation, according to a release from the agency. This inhaler is indicated for prevention of bronchospasm in patients aged 4 years and older. Specifically, these are patients with reversible obstructive airway disease or exercise-induced bronchospasm.
“The FDA recognizes the increased demand for albuterol products during the novel coronavirus pandemic,” said FDA Commissioner Stephen M. Hahn, MD.
The most common side effects include upper respiratory tract infection, rhinitis, nausea, vomiting, rapid heart rate, tremor, and nervousness.
This approval comes as part of FDA’s efforts to guide industry through the development process of generic products, according to the release. Complex combination products – such as this inhaler, which comprises both medication and a delivery system – can be more challenging to develop than solid oral dosage forms, such as tablets.
The FDA released a draft guidance in March 2020 specific to proposed generic albuterol sulfate metered-dose inhalers, including drug products referencing Proventil HFA. As with other similar guidances, it details the steps companies need to take in developing generics in order to submit complete applications for those products. The full news release regarding this approval is available on the FDA website.
This article was updated 4/8/20.
FDA approves recombinant treatment for hemophilia A or B with inhibitors
The Food and Drug Administration has approved a new rabbit-derived recombinant analog of human coagulation factor VII (Sevenfact) for the treatment of hemophilia A or B, according to a release from the agency.
The treatment’s approval is for use in patients aged 12 years and older who’ve developed inhibitors (neutralizing antibodies). This factor VII (FVII) analog bypasses the FVIII and FIX reactions, which are no longer effective pathways for treatment in these patients because of the inhibitors they’ve developed.
According to the release, “the active ingredient of Sevenfact is a recombinant analog of human FVII, which is expressed in the mammary gland of genetically engineered rabbits and secreted into the rabbits’ milk. During purification and processing of the milk, FVII is converted into activated FVII.” The FDA’s Center for Veterinary Medicine has, after “comprehensive analysis of the scientific evidence,” determined that the process is safe for both the rabbits and handlers and that it is an effective means of producing the coagulation factor.
The approval is based on a clinical study that evaluated 27 patients with hemophilia A or B and included treatment for 465 mild to moderate events and 3 severe ones. The low (75 mcg/kg) or high (225 mcg/kg) dose was able to successfully treat 86% of the mild to moderate episodes, and all three of the severe ones were successfully treated with the high dose.
The most common side effects were headache, dizziness, infusion-site discomfort, infusion-related reaction, infusion-site hematoma, and fever. This product is contraindicated in patients with known allergies or hypersensitivities to rabbits or rabbit proteins. There is potential for increased risk of serious arterial or venous thrombotic events among patients with other risk factors for blood clots. More safety information can be found in the prescribing information, and more information about the approval can be found in the full release.
The Food and Drug Administration has approved a new rabbit-derived recombinant analog of human coagulation factor VII (Sevenfact) for the treatment of hemophilia A or B, according to a release from the agency.
The treatment’s approval is for use in patients aged 12 years and older who’ve developed inhibitors (neutralizing antibodies). This factor VII (FVII) analog bypasses the FVIII and FIX reactions, which are no longer effective pathways for treatment in these patients because of the inhibitors they’ve developed.
According to the release, “the active ingredient of Sevenfact is a recombinant analog of human FVII, which is expressed in the mammary gland of genetically engineered rabbits and secreted into the rabbits’ milk. During purification and processing of the milk, FVII is converted into activated FVII.” The FDA’s Center for Veterinary Medicine has, after “comprehensive analysis of the scientific evidence,” determined that the process is safe for both the rabbits and handlers and that it is an effective means of producing the coagulation factor.
The approval is based on a clinical study that evaluated 27 patients with hemophilia A or B and included treatment for 465 mild to moderate events and 3 severe ones. The low (75 mcg/kg) or high (225 mcg/kg) dose was able to successfully treat 86% of the mild to moderate episodes, and all three of the severe ones were successfully treated with the high dose.
The most common side effects were headache, dizziness, infusion-site discomfort, infusion-related reaction, infusion-site hematoma, and fever. This product is contraindicated in patients with known allergies or hypersensitivities to rabbits or rabbit proteins. There is potential for increased risk of serious arterial or venous thrombotic events among patients with other risk factors for blood clots. More safety information can be found in the prescribing information, and more information about the approval can be found in the full release.
The Food and Drug Administration has approved a new rabbit-derived recombinant analog of human coagulation factor VII (Sevenfact) for the treatment of hemophilia A or B, according to a release from the agency.
The treatment’s approval is for use in patients aged 12 years and older who’ve developed inhibitors (neutralizing antibodies). This factor VII (FVII) analog bypasses the FVIII and FIX reactions, which are no longer effective pathways for treatment in these patients because of the inhibitors they’ve developed.
According to the release, “the active ingredient of Sevenfact is a recombinant analog of human FVII, which is expressed in the mammary gland of genetically engineered rabbits and secreted into the rabbits’ milk. During purification and processing of the milk, FVII is converted into activated FVII.” The FDA’s Center for Veterinary Medicine has, after “comprehensive analysis of the scientific evidence,” determined that the process is safe for both the rabbits and handlers and that it is an effective means of producing the coagulation factor.
The approval is based on a clinical study that evaluated 27 patients with hemophilia A or B and included treatment for 465 mild to moderate events and 3 severe ones. The low (75 mcg/kg) or high (225 mcg/kg) dose was able to successfully treat 86% of the mild to moderate episodes, and all three of the severe ones were successfully treated with the high dose.
The most common side effects were headache, dizziness, infusion-site discomfort, infusion-related reaction, infusion-site hematoma, and fever. This product is contraindicated in patients with known allergies or hypersensitivities to rabbits or rabbit proteins. There is potential for increased risk of serious arterial or venous thrombotic events among patients with other risk factors for blood clots. More safety information can be found in the prescribing information, and more information about the approval can be found in the full release.

