User login
ILLUSTRATIVE CASE
A 32-year-old woman with a history of migraine with aura presents to your office for contraception management. She works full-time, has 2 children, and has transportation barriers. She previously used injectable DMPA (administered every 3 months at a health care facility) and would like to restart it. However, because she had to reschedule her last appointment due to a lack of transportation, she missed her injection window and subsequently became pregnant with her second child. She would still prefer injectable DMPA over the other contraceptive options offered—etonogestrel implant, oral contraceptive, or intrauterine device (IUD)—given her migraine history. However, she’s concerned she may have difficulty coming to the office every 3 months for her injection. What alternative injectable option can you offer?
When not pregnant or seeking to become pregnant, women may spend a significant amount of their lives trying to avoid pregnancy, and almost all women use contraception at some point.2 During the childbearing years of 15 to 49, 65% of women report using contraception.2 Although DMPA is a safe and effective option, only 2% of women report using it for contraception.2
For patients who have migraine with aura, there are fewer contraception options because their risk for ischemic stroke is increased 2- to 4-fold if they use combined hormonal contraceptives in pill, patch, or vaginal ring form.3 Safe options for these patients include the copper IUD, levonorgestrel-releasing intrauterine system, progestin implant, and DMPA injection.3
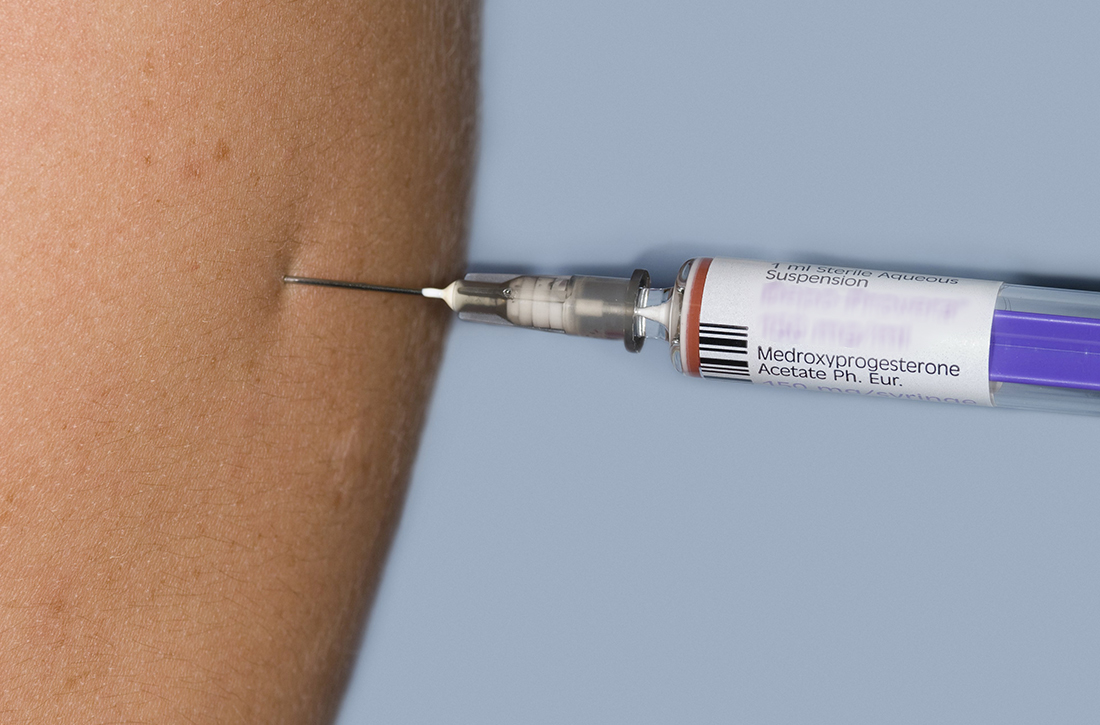
DMPA is a progestogen-only contraceptive approved by the US Food and Drug Administration to prevent pregnancy. It is available in an intramuscular formulation (DMPA-IM; 150 mg/mL every 13 weeks) and a subcutaneous formulation (DMPA-SC; 104 mg/0.65 mL every 12-14 weeks). DMPA-IM is administered by a health care provider and thus requires patients to present every 3 months for an injection. About 6% of DMPA-IM users have an unintended pregnancy in the first year due to inconsistent or incorrect use or late receipt of injection.4 DMPA-SC is produced as a prefilled needle that can be self-injected by patients.
Barriers to access are a growing concern. During the COVID-19 pandemic, one-third of women surveyed by the Guttmacher Institute (n = 2009) reported delaying or canceling a health care visit or having difficulty obtaining their contraception. Barriers to health care and contraception access were more common among Black and Hispanic women (vs White women), queer women (vs straight women), and low-income women (vs higher-income women).5
Following the overturning of Roe v Wade in June 2022, abortion access is now limited in parts of the United States. Given this significant policy change, physicians have an increasingly important role in providing contraception care and reducing barriers to contraception access. Since the SC forms of injectable contraception can be administered at home rather than in the health care setting, both the World Health Organization and the Centers for Disease Control and Prevention have recommended that self-administered injectable contraception be made widely available to expand access to contraception.6,7
STUDY SUMMARY
Higher contraceptive continuation rates with comparable safety and efficacy
This 2019 systematic review and meta-analysis evaluated the outcomes associated with use of self-administered DMPA-SC vs provider-administered DMPA in 5 countries.1 The authors searched several electronic databases for peer-reviewed studies of women who chose the option to self-administer DMPA-SC vs those who received DMPA injections from a health care provider.
Continue to: Outcomes included pregnancy
Outcomes included pregnancy; adverse effects or events (bleeding, injection site reactions, mental health concerns); initial use of injectable contraception (contraception uptake); and continuation rate of injectable contraception. Two reviewers extracted the data and assessed trials for bias. The authors used random-effects models to calculate pooled relative risk (RR) for studies with the same outcomes.
The analysis included a total of 6 trials (N = 3851): 3 RCTs (n = 1263) and 3 controlled cohort studies (n = 2588), conducted in the United States (2 trials), Malawi, Scotland, Uganda, and Senegal. All studies compared 12-month continuation rates of self-injected DMPA-SC vs provider-administered DMPA-SC or DMPA-IM every 3 months (12-13 weeks, with a window for early and late injections). Participants were at least 15 years of age (mean range, 26 to 29 years). In some studies, reminders (eg, texts, emails, calendar notifications) were provided to either the self-injection cohort only or to both cohorts of the trial. The RCTs were generally graded as having a low risk for bias, except for nonblinding of participants and personnel, given the nature of the interventions. The authors reported no evidence of significant heterogeneity in the studies.
The meta-analysis found higher continuation rates at 12 months with self-administrated DMPA compared with provider administration in the RCTs (RR = 1.27; 95% CI, 1.16-1.39) and in the observational cohort studies (RR = 1.18; 95% CI, 1.10-1.26). Pregnancy outcomes were reported in 4 studies, with the meta-analysis finding no significant difference in pregnancy rates in 2 RCTs (RR = 0.58; 95% CI, 0.15-2.22) or 2 observational cohort studies (RR = 1.1; 95% CI, 0.23-5.26).
Adverse effects or events were reported in 4 studies: 2 cohort studies reported increased injection site reactions with self-administration, and 1 RCT reported increased injection site pain or irritation with self-administration at 3 and 9 months. No other reported adverse effects occurred at higher rates with self-administration vs provider administration.
WHAT’S NEW
Demonstrated effectiveness of self-administered formulation
This systematic review and meta-analysis demonstrated that self-administration of DMPA-SC leads to higher contraception continuation rates at 12 months, without notable increased pregnancy rates or adverse effects, when compared with provider-administered DMPA.
Continue to: CAVEATS
CAVEATS
Outcome data limited to 12 months
Although self-administered DMPA-SC has the theoretical risk for user error and incorrect administration, this study did not find increased rates of pregnancy despite administration outside a health care center. However, the total number of pregnancies in each of the 4 studies measuring this outcome was low (< 5), and thus the authors noted that the effect size estimates may not be accurate.
Currently, there are no data on long-term outcomes beyond 12 months. Additionally, the health care visits for provider-administered DMPA every 3 months may afford other benefits, such as regular discussion of reproductive health concerns or testing for sexually transmitted infections, which must be weighed against the benefit of increased contraception access with self-administration. However, using the DMPA-SC self-administered formulation at home would not inhibit women from making separate health care visits as needed.
CHALLENGES TO IMPLEMENTATION
Limited resources to teach patients how to self-inject
Barriers to implementation include limited experience with prescribing DMPA-SC and changing practice culture to offer it to patients. Additionally, successful implementation of self-administered DMPA-SC is reliant on providing patients with appropriate information and training on self-injection, which requires knowledge, time, and other resources that may be limited in practices. Another potential barrier is product access, as not all insurers cover DMPA-SC and some pharmacies do not carry it.
1. Kennedy CE, Yeh PT, Gaffield ML, et al. Self-administration of injectable contraception: a systematic review and meta-analysis. BMJ Glob Health. 2019;4:e001350. doi: 10.1136/bmjgh-2018-001350
2. Daniels K, Abma J. Current contraceptive status among women aged 15-49: United States, 2017-2019. NCHS Data Brief. 2020;(388):1-8.
3. Paradise SL, Landis CA, Klein DA. Evidence-based contraception: common questions and answers. Am Fam Physician. 2022;106:251-259.
4. Marx M. Evidence‐based guidance for self‐administration of injectable contraception. J Midwifery Womens Health. 2021;66:108-112. doi: 10.1111/jmwh.13190
5. Lindberg LD, VandeVusse A, Mueller J, et al. Early Impacts of the COVID-19 Pandemic: Findings from the 2020 Guttmacher Survey of Reproductive Health Experiences. Guttmacher Institute; 2020. Accessed October 25, 2022. www.guttmacher.org/report/early-impacts-covid-19-pandemic-findings-2020-guttmacher-survey-reproductive-health
6. World Health Organization. WHO consolidated guidance on self-care interventions for health: sexual and reproductive health and rights. Published 2019. Accessed February 14, 2023. https://apps.who.int/iris/bitstream/handle/10665/325480/9789241550550-eng.pdf
7. Curtis KM, Nguyen A, Reeves JA, et al. Update to US selected practice recommendations for contraceptive use: self-administration of subcutaneous depot medroxyprogesterone acetate. MMWR Morb Mortal Wkly Rep. 2021;70:739-743. doi: 10.15585/mmwr.mm7020a2
ILLUSTRATIVE CASE
A 32-year-old woman with a history of migraine with aura presents to your office for contraception management. She works full-time, has 2 children, and has transportation barriers. She previously used injectable DMPA (administered every 3 months at a health care facility) and would like to restart it. However, because she had to reschedule her last appointment due to a lack of transportation, she missed her injection window and subsequently became pregnant with her second child. She would still prefer injectable DMPA over the other contraceptive options offered—etonogestrel implant, oral contraceptive, or intrauterine device (IUD)—given her migraine history. However, she’s concerned she may have difficulty coming to the office every 3 months for her injection. What alternative injectable option can you offer?
When not pregnant or seeking to become pregnant, women may spend a significant amount of their lives trying to avoid pregnancy, and almost all women use contraception at some point.2 During the childbearing years of 15 to 49, 65% of women report using contraception.2 Although DMPA is a safe and effective option, only 2% of women report using it for contraception.2
For patients who have migraine with aura, there are fewer contraception options because their risk for ischemic stroke is increased 2- to 4-fold if they use combined hormonal contraceptives in pill, patch, or vaginal ring form.3 Safe options for these patients include the copper IUD, levonorgestrel-releasing intrauterine system, progestin implant, and DMPA injection.3
DMPA is a progestogen-only contraceptive approved by the US Food and Drug Administration to prevent pregnancy. It is available in an intramuscular formulation (DMPA-IM; 150 mg/mL every 13 weeks) and a subcutaneous formulation (DMPA-SC; 104 mg/0.65 mL every 12-14 weeks). DMPA-IM is administered by a health care provider and thus requires patients to present every 3 months for an injection. About 6% of DMPA-IM users have an unintended pregnancy in the first year due to inconsistent or incorrect use or late receipt of injection.4 DMPA-SC is produced as a prefilled needle that can be self-injected by patients.
Barriers to access are a growing concern. During the COVID-19 pandemic, one-third of women surveyed by the Guttmacher Institute (n = 2009) reported delaying or canceling a health care visit or having difficulty obtaining their contraception. Barriers to health care and contraception access were more common among Black and Hispanic women (vs White women), queer women (vs straight women), and low-income women (vs higher-income women).5
Following the overturning of Roe v Wade in June 2022, abortion access is now limited in parts of the United States. Given this significant policy change, physicians have an increasingly important role in providing contraception care and reducing barriers to contraception access. Since the SC forms of injectable contraception can be administered at home rather than in the health care setting, both the World Health Organization and the Centers for Disease Control and Prevention have recommended that self-administered injectable contraception be made widely available to expand access to contraception.6,7
STUDY SUMMARY
Higher contraceptive continuation rates with comparable safety and efficacy
This 2019 systematic review and meta-analysis evaluated the outcomes associated with use of self-administered DMPA-SC vs provider-administered DMPA in 5 countries.1 The authors searched several electronic databases for peer-reviewed studies of women who chose the option to self-administer DMPA-SC vs those who received DMPA injections from a health care provider.
Continue to: Outcomes included pregnancy
Outcomes included pregnancy; adverse effects or events (bleeding, injection site reactions, mental health concerns); initial use of injectable contraception (contraception uptake); and continuation rate of injectable contraception. Two reviewers extracted the data and assessed trials for bias. The authors used random-effects models to calculate pooled relative risk (RR) for studies with the same outcomes.
The analysis included a total of 6 trials (N = 3851): 3 RCTs (n = 1263) and 3 controlled cohort studies (n = 2588), conducted in the United States (2 trials), Malawi, Scotland, Uganda, and Senegal. All studies compared 12-month continuation rates of self-injected DMPA-SC vs provider-administered DMPA-SC or DMPA-IM every 3 months (12-13 weeks, with a window for early and late injections). Participants were at least 15 years of age (mean range, 26 to 29 years). In some studies, reminders (eg, texts, emails, calendar notifications) were provided to either the self-injection cohort only or to both cohorts of the trial. The RCTs were generally graded as having a low risk for bias, except for nonblinding of participants and personnel, given the nature of the interventions. The authors reported no evidence of significant heterogeneity in the studies.
The meta-analysis found higher continuation rates at 12 months with self-administrated DMPA compared with provider administration in the RCTs (RR = 1.27; 95% CI, 1.16-1.39) and in the observational cohort studies (RR = 1.18; 95% CI, 1.10-1.26). Pregnancy outcomes were reported in 4 studies, with the meta-analysis finding no significant difference in pregnancy rates in 2 RCTs (RR = 0.58; 95% CI, 0.15-2.22) or 2 observational cohort studies (RR = 1.1; 95% CI, 0.23-5.26).
Adverse effects or events were reported in 4 studies: 2 cohort studies reported increased injection site reactions with self-administration, and 1 RCT reported increased injection site pain or irritation with self-administration at 3 and 9 months. No other reported adverse effects occurred at higher rates with self-administration vs provider administration.
WHAT’S NEW
Demonstrated effectiveness of self-administered formulation
This systematic review and meta-analysis demonstrated that self-administration of DMPA-SC leads to higher contraception continuation rates at 12 months, without notable increased pregnancy rates or adverse effects, when compared with provider-administered DMPA.
Continue to: CAVEATS
CAVEATS
Outcome data limited to 12 months
Although self-administered DMPA-SC has the theoretical risk for user error and incorrect administration, this study did not find increased rates of pregnancy despite administration outside a health care center. However, the total number of pregnancies in each of the 4 studies measuring this outcome was low (< 5), and thus the authors noted that the effect size estimates may not be accurate.
Currently, there are no data on long-term outcomes beyond 12 months. Additionally, the health care visits for provider-administered DMPA every 3 months may afford other benefits, such as regular discussion of reproductive health concerns or testing for sexually transmitted infections, which must be weighed against the benefit of increased contraception access with self-administration. However, using the DMPA-SC self-administered formulation at home would not inhibit women from making separate health care visits as needed.
CHALLENGES TO IMPLEMENTATION
Limited resources to teach patients how to self-inject
Barriers to implementation include limited experience with prescribing DMPA-SC and changing practice culture to offer it to patients. Additionally, successful implementation of self-administered DMPA-SC is reliant on providing patients with appropriate information and training on self-injection, which requires knowledge, time, and other resources that may be limited in practices. Another potential barrier is product access, as not all insurers cover DMPA-SC and some pharmacies do not carry it.
ILLUSTRATIVE CASE
A 32-year-old woman with a history of migraine with aura presents to your office for contraception management. She works full-time, has 2 children, and has transportation barriers. She previously used injectable DMPA (administered every 3 months at a health care facility) and would like to restart it. However, because she had to reschedule her last appointment due to a lack of transportation, she missed her injection window and subsequently became pregnant with her second child. She would still prefer injectable DMPA over the other contraceptive options offered—etonogestrel implant, oral contraceptive, or intrauterine device (IUD)—given her migraine history. However, she’s concerned she may have difficulty coming to the office every 3 months for her injection. What alternative injectable option can you offer?
When not pregnant or seeking to become pregnant, women may spend a significant amount of their lives trying to avoid pregnancy, and almost all women use contraception at some point.2 During the childbearing years of 15 to 49, 65% of women report using contraception.2 Although DMPA is a safe and effective option, only 2% of women report using it for contraception.2
For patients who have migraine with aura, there are fewer contraception options because their risk for ischemic stroke is increased 2- to 4-fold if they use combined hormonal contraceptives in pill, patch, or vaginal ring form.3 Safe options for these patients include the copper IUD, levonorgestrel-releasing intrauterine system, progestin implant, and DMPA injection.3
DMPA is a progestogen-only contraceptive approved by the US Food and Drug Administration to prevent pregnancy. It is available in an intramuscular formulation (DMPA-IM; 150 mg/mL every 13 weeks) and a subcutaneous formulation (DMPA-SC; 104 mg/0.65 mL every 12-14 weeks). DMPA-IM is administered by a health care provider and thus requires patients to present every 3 months for an injection. About 6% of DMPA-IM users have an unintended pregnancy in the first year due to inconsistent or incorrect use or late receipt of injection.4 DMPA-SC is produced as a prefilled needle that can be self-injected by patients.
Barriers to access are a growing concern. During the COVID-19 pandemic, one-third of women surveyed by the Guttmacher Institute (n = 2009) reported delaying or canceling a health care visit or having difficulty obtaining their contraception. Barriers to health care and contraception access were more common among Black and Hispanic women (vs White women), queer women (vs straight women), and low-income women (vs higher-income women).5
Following the overturning of Roe v Wade in June 2022, abortion access is now limited in parts of the United States. Given this significant policy change, physicians have an increasingly important role in providing contraception care and reducing barriers to contraception access. Since the SC forms of injectable contraception can be administered at home rather than in the health care setting, both the World Health Organization and the Centers for Disease Control and Prevention have recommended that self-administered injectable contraception be made widely available to expand access to contraception.6,7
STUDY SUMMARY
Higher contraceptive continuation rates with comparable safety and efficacy
This 2019 systematic review and meta-analysis evaluated the outcomes associated with use of self-administered DMPA-SC vs provider-administered DMPA in 5 countries.1 The authors searched several electronic databases for peer-reviewed studies of women who chose the option to self-administer DMPA-SC vs those who received DMPA injections from a health care provider.
Continue to: Outcomes included pregnancy
Outcomes included pregnancy; adverse effects or events (bleeding, injection site reactions, mental health concerns); initial use of injectable contraception (contraception uptake); and continuation rate of injectable contraception. Two reviewers extracted the data and assessed trials for bias. The authors used random-effects models to calculate pooled relative risk (RR) for studies with the same outcomes.
The analysis included a total of 6 trials (N = 3851): 3 RCTs (n = 1263) and 3 controlled cohort studies (n = 2588), conducted in the United States (2 trials), Malawi, Scotland, Uganda, and Senegal. All studies compared 12-month continuation rates of self-injected DMPA-SC vs provider-administered DMPA-SC or DMPA-IM every 3 months (12-13 weeks, with a window for early and late injections). Participants were at least 15 years of age (mean range, 26 to 29 years). In some studies, reminders (eg, texts, emails, calendar notifications) were provided to either the self-injection cohort only or to both cohorts of the trial. The RCTs were generally graded as having a low risk for bias, except for nonblinding of participants and personnel, given the nature of the interventions. The authors reported no evidence of significant heterogeneity in the studies.
The meta-analysis found higher continuation rates at 12 months with self-administrated DMPA compared with provider administration in the RCTs (RR = 1.27; 95% CI, 1.16-1.39) and in the observational cohort studies (RR = 1.18; 95% CI, 1.10-1.26). Pregnancy outcomes were reported in 4 studies, with the meta-analysis finding no significant difference in pregnancy rates in 2 RCTs (RR = 0.58; 95% CI, 0.15-2.22) or 2 observational cohort studies (RR = 1.1; 95% CI, 0.23-5.26).
Adverse effects or events were reported in 4 studies: 2 cohort studies reported increased injection site reactions with self-administration, and 1 RCT reported increased injection site pain or irritation with self-administration at 3 and 9 months. No other reported adverse effects occurred at higher rates with self-administration vs provider administration.
WHAT’S NEW
Demonstrated effectiveness of self-administered formulation
This systematic review and meta-analysis demonstrated that self-administration of DMPA-SC leads to higher contraception continuation rates at 12 months, without notable increased pregnancy rates or adverse effects, when compared with provider-administered DMPA.
Continue to: CAVEATS
CAVEATS
Outcome data limited to 12 months
Although self-administered DMPA-SC has the theoretical risk for user error and incorrect administration, this study did not find increased rates of pregnancy despite administration outside a health care center. However, the total number of pregnancies in each of the 4 studies measuring this outcome was low (< 5), and thus the authors noted that the effect size estimates may not be accurate.
Currently, there are no data on long-term outcomes beyond 12 months. Additionally, the health care visits for provider-administered DMPA every 3 months may afford other benefits, such as regular discussion of reproductive health concerns or testing for sexually transmitted infections, which must be weighed against the benefit of increased contraception access with self-administration. However, using the DMPA-SC self-administered formulation at home would not inhibit women from making separate health care visits as needed.
CHALLENGES TO IMPLEMENTATION
Limited resources to teach patients how to self-inject
Barriers to implementation include limited experience with prescribing DMPA-SC and changing practice culture to offer it to patients. Additionally, successful implementation of self-administered DMPA-SC is reliant on providing patients with appropriate information and training on self-injection, which requires knowledge, time, and other resources that may be limited in practices. Another potential barrier is product access, as not all insurers cover DMPA-SC and some pharmacies do not carry it.
1. Kennedy CE, Yeh PT, Gaffield ML, et al. Self-administration of injectable contraception: a systematic review and meta-analysis. BMJ Glob Health. 2019;4:e001350. doi: 10.1136/bmjgh-2018-001350
2. Daniels K, Abma J. Current contraceptive status among women aged 15-49: United States, 2017-2019. NCHS Data Brief. 2020;(388):1-8.
3. Paradise SL, Landis CA, Klein DA. Evidence-based contraception: common questions and answers. Am Fam Physician. 2022;106:251-259.
4. Marx M. Evidence‐based guidance for self‐administration of injectable contraception. J Midwifery Womens Health. 2021;66:108-112. doi: 10.1111/jmwh.13190
5. Lindberg LD, VandeVusse A, Mueller J, et al. Early Impacts of the COVID-19 Pandemic: Findings from the 2020 Guttmacher Survey of Reproductive Health Experiences. Guttmacher Institute; 2020. Accessed October 25, 2022. www.guttmacher.org/report/early-impacts-covid-19-pandemic-findings-2020-guttmacher-survey-reproductive-health
6. World Health Organization. WHO consolidated guidance on self-care interventions for health: sexual and reproductive health and rights. Published 2019. Accessed February 14, 2023. https://apps.who.int/iris/bitstream/handle/10665/325480/9789241550550-eng.pdf
7. Curtis KM, Nguyen A, Reeves JA, et al. Update to US selected practice recommendations for contraceptive use: self-administration of subcutaneous depot medroxyprogesterone acetate. MMWR Morb Mortal Wkly Rep. 2021;70:739-743. doi: 10.15585/mmwr.mm7020a2
1. Kennedy CE, Yeh PT, Gaffield ML, et al. Self-administration of injectable contraception: a systematic review and meta-analysis. BMJ Glob Health. 2019;4:e001350. doi: 10.1136/bmjgh-2018-001350
2. Daniels K, Abma J. Current contraceptive status among women aged 15-49: United States, 2017-2019. NCHS Data Brief. 2020;(388):1-8.
3. Paradise SL, Landis CA, Klein DA. Evidence-based contraception: common questions and answers. Am Fam Physician. 2022;106:251-259.
4. Marx M. Evidence‐based guidance for self‐administration of injectable contraception. J Midwifery Womens Health. 2021;66:108-112. doi: 10.1111/jmwh.13190
5. Lindberg LD, VandeVusse A, Mueller J, et al. Early Impacts of the COVID-19 Pandemic: Findings from the 2020 Guttmacher Survey of Reproductive Health Experiences. Guttmacher Institute; 2020. Accessed October 25, 2022. www.guttmacher.org/report/early-impacts-covid-19-pandemic-findings-2020-guttmacher-survey-reproductive-health
6. World Health Organization. WHO consolidated guidance on self-care interventions for health: sexual and reproductive health and rights. Published 2019. Accessed February 14, 2023. https://apps.who.int/iris/bitstream/handle/10665/325480/9789241550550-eng.pdf
7. Curtis KM, Nguyen A, Reeves JA, et al. Update to US selected practice recommendations for contraceptive use: self-administration of subcutaneous depot medroxyprogesterone acetate. MMWR Morb Mortal Wkly Rep. 2021;70:739-743. doi: 10.15585/mmwr.mm7020a2
PRACTICE CHANGER
Consider prescribing self-administered subcutaneous depot medroxyprogesterone acetate (DMPA) for contraception instead of provider-administered DMPA. Self-administration improves contraception continuation rates without notable increases in pregnancy or adverse effects.
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of randomized controlled trials (RCTs) and cohort studies.1
Kennedy CE, Yeh PT, Gaffield ML, et al. Self-administration of injectable contraception: a systematic review and meta-analysis. BMJ Glob Health. 2019;4:e001350. doi: 10.1136/bmjgh-2018-001350