User login
CASE Withdrawn and confused
Mr. J, age 54, is brought to the emergency department from a correctional treatment facility where he is reported to be displaying new, unusual behavior. He has a history of schizoaffective disorder, which has been stable with haloperidol, 10 mg/d, for more than a year.
Although previously Mr. J openly discussed his long-standing delusions about the FBI coming to release him from prison, he no longer mentions this or any other delusional beliefs, and has become less communicative with staff and peers. Mr. J no longer accompanies the other patients to the cafeteria for meals and eats in his room alone and appears to be losing weight. He says, “I do not feel good,” but otherwise does not communicate spontaneously. Intermittently, he is irritable, without known triggers. The staff notices that Mr. J often lays on his bed, sometimes in a fetal position. Over time, he becomes confused and is seen attempting to open his room door with a toothbrush. His personal hygiene is poor, and he often urinates through his clothes, on the floor, and in his bed. Recently, Mr. J’s eczema has worsened. His gait has become unsteady, and he has orthostasis.
What could be causing these new symptoms?
a) worsening schizoaffective disorder
b) illicit drug use in the prison
c) atypical dementia
d) cardiac etiology
The author’s observations
The differential diagnosis for Mr. J appeared to be wide and without specific etiology. Because of the complex types of symptoms that Mr. J was experiencing, the emergency department managed his care and specialty clinic referrals were ordered.
It was reported that Mr. J started complaining of lightheadedness a few months ago, which worsened (unsteady gait, near falls). In the context of Mr. J’s history of lightheadedness and orthostasis, the cardiology clinic ordered a tilt table test, which was within normal limits:
- 70º head-up tilt: blood pressure, 91/67 to 102/62 mm Hg, and pulse, 70 to 79 beats per minute (bpm)
- with isoproterenol, 1 μg/minute: blood pressure, 90/66 to 110/70 mm Hg, and pulse, 77 to 124 bpm
- with isoproterenol, 2 μg/minute: blood pressure, 98/58 to 111/66 mm Hg, and pulse, 121 to 134 bpm.
The neurologist’s diagnostic impression was atypical dementia; however, Mr. J showed no memory deficits. Parkinsonism also was considered, but Mr. J had no unilateral tremor, masked facies, or micrographia. Mr. J showed some restriction in his movement, but he was not bradykinetic. The team suspected haloperidol could be causing his stiff movement.
Although it was possible that Mr. J’s schizoaffective disorder was worsening and led to the new symptoms, Mr. J appeared to be less delusional because he was no longer talking to the staff about his delusions. There seemed to be no outward evidence of progression of psychotic symptoms.
Mr. J had a history of substance abuse, including alcohol, cocaine, and Cannabis. Although prison inmates have been known to manufacture and drink “hooch,” the new symptoms Mr. J was experiencing were severe enough that his social interactions with other inmates diminished substantially. Because Mr. J had not been communicating with the other inmates and had no recent visitors, the team felt that it was unlikely that drugs were causing these symptoms. Also, a urine drug screen for cocaine, amphetamines, benzodiazepines, Cannabis, and opioids was negative.
HISTORY Substance use, violence
Mr. J was diagnosed with bipolar disorder at age 18. After later hospitalizations, his diagnosis was changed to schizoaffective disorder as a matter of diagnostic clarification. He has a long history of non-compliance with treatment, homelessness, and drug abuse.
Mr. J is serving a 20-year sentence for first-degree reckless homicide. A year after he was incarcerated, Mr. J was sent to a specialized mental health facility for inmates whose illness cannot be managed in a typical correctional setting. While at the treatment facility, Mr. J was non-compliant with medications and because of concerns about dangerousness and psychosis, the court found probable cause for involuntary commitment.
His medication regimen is trihexyphenidyl, 2 mg/d, for extrapyramidal symptoms; haloperidol, 10 mg/d, for psychosis; trazodone, 150 mg/d, for insomnia; vitamin D3, 2,000 IU/d; vitamin E, 400 IU/d, for symptoms of tardive dyskinesia; IM ziprasidone, 20 mg, because he refused oral haliperidol; and hydrocortisone cream 1% for eczema.
EVALUATION Additional tests
Mr. J’s blood pressure is 124/72 mm Hg, and pulse, 104 bpm, laying down; blood pressure, 110/84 mm Hg, and pulse, 112 bpm, sitting; and blood pressure, 108/82 mm Hg, and pulse, 129 bpm, standing. With repeated readings: blood pressure, 128/84 mm Hg, and pulse, 98 bpm, laying down; blood pressure, 125/86 mm Hg, and pulse, 113 bpm, sitting; and blood pressure, 105/76 mm Hg, and pulse, 130 bpm, standing.
Laboratory tests, including complete blood count, chemistry panel, thyroid-stimulating hormone, are within normal limits. The team feels that the investigation for an etiology for Mr. J’s symptoms needs to be more exhaustive and additional tests are ordered, including vitamin levels (C, B1, B12, B6), rapid plasma reagin for syphilis, and arbovirus testing (eastern equine encephalitis virus, western equine encephalitis, West Nile virus, La Crosse encephalitis, St. Louis encephalitis), which are negative.
What’s the next best step in managing Mr. J’s care?
a) adjust his medication
b) eliminate a mediation
c) order further testing
The author’s observations
To determine if Mr. J’s new-onset symptoms might be related to the progression of his psychiatric illness, the haloperidol dosage was increased to 20 mg/d; however, we saw no positive response to this change. His tardive dyskinesia symptoms (bruxism and other oral buccal movements) worsened. Haloperidol was reduced to 10 mg/d.
Trihexyphenidyl then was suspected to contribute to Mr. J’s confusion. Unfortunately, lowering the dosage of trihexyphenidyl to 1 mg/d, did not affect Mr. J’s current symptoms and exacerbated extrapyramidal symptoms.
The treatment team then questioned if porphyria—known as the “little imitator”—might be considered because of the variety of symptoms without an etiology, despite extensive testing. A 24-hour urine collection was ordered.
What is the correct method of collecting a urine sample for porphyrins?
a) collect a small sample and expose it to light before testing
b) collect a 24-hour sample with the sample kept in ambient temperature and light
c) collect a 24-hour sample with the sample kept on ice in a light-blocking container and frozen when sent to the laboratory
EVALUATION Diagnosis revealed
The 24-hour urine collection is obtained. However, it needed to be collected twice, because the first sample was not a full sample. Interestingly, the first sample, which is exposed to light and not kept on ice, turned dark in color. The second sample is obtained properly and sent to the laboratory. When the laboratory results are returned (Figure 1), Mr. J is diagnosed with hereditary coproporphyria (HCP).

The author’s observations
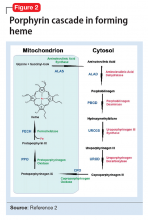
There are several types of porphyria, each associated with a different step in the chain of enzymes associated with synthesis of a heme molecule in the mitochondria. A defect in any single enzyme step will create a build up of porphyrins—a precursor to heme molecules—in erythrocytes or hepatic cells.
It is important to differentiate hepatic from erythropoietic porphyrias. The acute porphyrias (acute intermittent porphyria [AIP], HCP, and variegate porphyria generally are hepatic in origin with neuropsychiatric and neurovisceral symptoms. Cutaneous porphyrias originate in bone marrow and therefore are erythropoietic. However, there are exceptions such as porphyria cutanea tarda (PCT), which is hepatic in origin but the manifestations mainly are cutaneous1 (Figure 2).2
Although acute porphyria originates in the liver, it is a neuropsychiatric illness. In these cases, excess porphyrins cannot cross the blood–brain barrier and are neurotoxic. Clinicians can look for abnormalities in the liver via liver function tests, but liver parenchyma is not damaged by these enzyme precursors. During an acute porphyic attack, patients could experience symptoms such as:
- muscle spasms (commonly abdominal, but can be any muscle group)
- confusion
- disorientation
- autonomic instability
- lightheadness
- disorientation
- diarrhea
- light sensitivity
- dermatologic conditions
- weakness (particularly peripheral weakness)
- hypesthesia
- allodynia
- severe nausea and vomiting
- emotional lability
- psychosis as well as general malaise.
The attack could result in death.
Mr. J had many differing symptoms and was evaluated by several specialty providers. He had a chronic dermatologic condition; he was confused, disoriented, and complained of nausea, weakness, orthostasis, and loose stools. With the variety of possible symptoms that patients such as Mr. J could experience, one can see why it would lead to many different providers being involved in the diagnosis. It is not uncommon for psychiatrists to be the last providers to care for such patients who could have been evaluated by hematology, cardiology, gastroenterology, dermatology, and/or neurology.
Hereditary coproporphyria
The team considered hepatic porphyias because of new-onset symptoms of mood lability, confusion, orthostasis, unsteady gait, weakness, dermatologic conditions on hands not responsive to treatment, and general malaise. Mr. J was diagnosed with HCP, a type of porphyria caused by a defect in coproporphyrinogen oxidase that leads to an accumulation of coproporphyrinogen III. This precursor, as are many porphyrin precursors, is neurotoxic, leading to neurovisceral or neuropsychiatric effects. Although in Mr. J’s case the coproporphyrinogen III value from the 24-hour drug screen was only modestly elevated, it has been noted that levels of excreted prophyrins do not necessarily correlate with symptom severity.3
In the past, porphyria testing was performed using the Watson-Schwartz test, which used Ehrlich’s reagent to precipitate porphyrins in a urine sample,4 and was used as a “bedside” test. Interestingly, porphyrins—not the iron found in the heme molecule—are precipitated in this test and cause the reddish-purple coloration of the urine sample. When quantitative testing was developed, a 24-hour sample of urine—kept on ice and away from ambient light, later to be frozen when sent to the laboratory—became the standard tool for testing for porphyrins. Now DNA testing can be used to diagnose HCP.
OUTCOME Symptoms resolve
Mr. J is started on loxapine, 20 mg at bedtime, and his symptoms resolve within 2 weeks. He maintains some baseline delusional ideation consistent with his history of schizoaffective disorder, but he is more social, his personal hygiene improves, he attends groups, eats in the cafeteria with his peers, and is no longer confused.
The author’s observations
In the 1950s, chlorpromazine was used to treat AIP.5 Mr. J received loxapine, a mid-potency first-generation antipsychotic, although it has been this author’s observation that high-potency first-generation antipsychotics are not effective for treating porphyria.
1. NIH: National Human Genome Research Institute. Learning about porphyria. https://www.genome.gov/19016728/learning-about-porphyria/learning-about-porphyria. Accessed February 23, 2017.
2. Ajioka RS, Phillips JD, Kushner JP. Biosynthesis of heme in mammals. Biochim Biophys Acta. 2006;1763(7):723-736.
3. Peters HA, Gocmen A, Cripps DJ, et al. Epidemiology of hexachlorobenzene-induced porphyria in Turkey: clinical and laboratory follow-up after 25 years. Arch Neurol. 1982;39(12):744-749.
4. The Watsonschwartz test. JAMA. 1966;195(6):481.
5. Brunton L, Chabner BA, Knollman B. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw-Hill Professional; 2010.
6. Broomfield B. Acute Intermittent porphyria treated with chlorpromazine. Proc R Soc Med. 1962;55(9):799-800.
7. Hunter JA, Khan SA, Hope E, et al. Hereditary coproporphyria. Photosensitivity, jaundice and neuropsychiatric manifestations associated with pregnancy. Br J Dermatol. 1971;84(4):301-310.
8. Bonkovsky HL, Maddukuri V. Merck Manual. http://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/porphyrias/overview-of-porphyrias. Accessed February 2, 2017.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24 (suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4): 247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1): 64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.
CASE Withdrawn and confused
Mr. J, age 54, is brought to the emergency department from a correctional treatment facility where he is reported to be displaying new, unusual behavior. He has a history of schizoaffective disorder, which has been stable with haloperidol, 10 mg/d, for more than a year.
Although previously Mr. J openly discussed his long-standing delusions about the FBI coming to release him from prison, he no longer mentions this or any other delusional beliefs, and has become less communicative with staff and peers. Mr. J no longer accompanies the other patients to the cafeteria for meals and eats in his room alone and appears to be losing weight. He says, “I do not feel good,” but otherwise does not communicate spontaneously. Intermittently, he is irritable, without known triggers. The staff notices that Mr. J often lays on his bed, sometimes in a fetal position. Over time, he becomes confused and is seen attempting to open his room door with a toothbrush. His personal hygiene is poor, and he often urinates through his clothes, on the floor, and in his bed. Recently, Mr. J’s eczema has worsened. His gait has become unsteady, and he has orthostasis.
What could be causing these new symptoms?
a) worsening schizoaffective disorder
b) illicit drug use in the prison
c) atypical dementia
d) cardiac etiology
The author’s observations
The differential diagnosis for Mr. J appeared to be wide and without specific etiology. Because of the complex types of symptoms that Mr. J was experiencing, the emergency department managed his care and specialty clinic referrals were ordered.
It was reported that Mr. J started complaining of lightheadedness a few months ago, which worsened (unsteady gait, near falls). In the context of Mr. J’s history of lightheadedness and orthostasis, the cardiology clinic ordered a tilt table test, which was within normal limits:
- 70º head-up tilt: blood pressure, 91/67 to 102/62 mm Hg, and pulse, 70 to 79 beats per minute (bpm)
- with isoproterenol, 1 μg/minute: blood pressure, 90/66 to 110/70 mm Hg, and pulse, 77 to 124 bpm
- with isoproterenol, 2 μg/minute: blood pressure, 98/58 to 111/66 mm Hg, and pulse, 121 to 134 bpm.
The neurologist’s diagnostic impression was atypical dementia; however, Mr. J showed no memory deficits. Parkinsonism also was considered, but Mr. J had no unilateral tremor, masked facies, or micrographia. Mr. J showed some restriction in his movement, but he was not bradykinetic. The team suspected haloperidol could be causing his stiff movement.
Although it was possible that Mr. J’s schizoaffective disorder was worsening and led to the new symptoms, Mr. J appeared to be less delusional because he was no longer talking to the staff about his delusions. There seemed to be no outward evidence of progression of psychotic symptoms.
Mr. J had a history of substance abuse, including alcohol, cocaine, and Cannabis. Although prison inmates have been known to manufacture and drink “hooch,” the new symptoms Mr. J was experiencing were severe enough that his social interactions with other inmates diminished substantially. Because Mr. J had not been communicating with the other inmates and had no recent visitors, the team felt that it was unlikely that drugs were causing these symptoms. Also, a urine drug screen for cocaine, amphetamines, benzodiazepines, Cannabis, and opioids was negative.
HISTORY Substance use, violence
Mr. J was diagnosed with bipolar disorder at age 18. After later hospitalizations, his diagnosis was changed to schizoaffective disorder as a matter of diagnostic clarification. He has a long history of non-compliance with treatment, homelessness, and drug abuse.
Mr. J is serving a 20-year sentence for first-degree reckless homicide. A year after he was incarcerated, Mr. J was sent to a specialized mental health facility for inmates whose illness cannot be managed in a typical correctional setting. While at the treatment facility, Mr. J was non-compliant with medications and because of concerns about dangerousness and psychosis, the court found probable cause for involuntary commitment.
His medication regimen is trihexyphenidyl, 2 mg/d, for extrapyramidal symptoms; haloperidol, 10 mg/d, for psychosis; trazodone, 150 mg/d, for insomnia; vitamin D3, 2,000 IU/d; vitamin E, 400 IU/d, for symptoms of tardive dyskinesia; IM ziprasidone, 20 mg, because he refused oral haliperidol; and hydrocortisone cream 1% for eczema.
EVALUATION Additional tests
Mr. J’s blood pressure is 124/72 mm Hg, and pulse, 104 bpm, laying down; blood pressure, 110/84 mm Hg, and pulse, 112 bpm, sitting; and blood pressure, 108/82 mm Hg, and pulse, 129 bpm, standing. With repeated readings: blood pressure, 128/84 mm Hg, and pulse, 98 bpm, laying down; blood pressure, 125/86 mm Hg, and pulse, 113 bpm, sitting; and blood pressure, 105/76 mm Hg, and pulse, 130 bpm, standing.
Laboratory tests, including complete blood count, chemistry panel, thyroid-stimulating hormone, are within normal limits. The team feels that the investigation for an etiology for Mr. J’s symptoms needs to be more exhaustive and additional tests are ordered, including vitamin levels (C, B1, B12, B6), rapid plasma reagin for syphilis, and arbovirus testing (eastern equine encephalitis virus, western equine encephalitis, West Nile virus, La Crosse encephalitis, St. Louis encephalitis), which are negative.
What’s the next best step in managing Mr. J’s care?
a) adjust his medication
b) eliminate a mediation
c) order further testing
The author’s observations
To determine if Mr. J’s new-onset symptoms might be related to the progression of his psychiatric illness, the haloperidol dosage was increased to 20 mg/d; however, we saw no positive response to this change. His tardive dyskinesia symptoms (bruxism and other oral buccal movements) worsened. Haloperidol was reduced to 10 mg/d.
Trihexyphenidyl then was suspected to contribute to Mr. J’s confusion. Unfortunately, lowering the dosage of trihexyphenidyl to 1 mg/d, did not affect Mr. J’s current symptoms and exacerbated extrapyramidal symptoms.
The treatment team then questioned if porphyria—known as the “little imitator”—might be considered because of the variety of symptoms without an etiology, despite extensive testing. A 24-hour urine collection was ordered.
What is the correct method of collecting a urine sample for porphyrins?
a) collect a small sample and expose it to light before testing
b) collect a 24-hour sample with the sample kept in ambient temperature and light
c) collect a 24-hour sample with the sample kept on ice in a light-blocking container and frozen when sent to the laboratory
EVALUATION Diagnosis revealed
The 24-hour urine collection is obtained. However, it needed to be collected twice, because the first sample was not a full sample. Interestingly, the first sample, which is exposed to light and not kept on ice, turned dark in color. The second sample is obtained properly and sent to the laboratory. When the laboratory results are returned (Figure 1), Mr. J is diagnosed with hereditary coproporphyria (HCP).

The author’s observations
There are several types of porphyria, each associated with a different step in the chain of enzymes associated with synthesis of a heme molecule in the mitochondria. A defect in any single enzyme step will create a build up of porphyrins—a precursor to heme molecules—in erythrocytes or hepatic cells.
It is important to differentiate hepatic from erythropoietic porphyrias. The acute porphyrias (acute intermittent porphyria [AIP], HCP, and variegate porphyria generally are hepatic in origin with neuropsychiatric and neurovisceral symptoms. Cutaneous porphyrias originate in bone marrow and therefore are erythropoietic. However, there are exceptions such as porphyria cutanea tarda (PCT), which is hepatic in origin but the manifestations mainly are cutaneous1 (Figure 2).2
Although acute porphyria originates in the liver, it is a neuropsychiatric illness. In these cases, excess porphyrins cannot cross the blood–brain barrier and are neurotoxic. Clinicians can look for abnormalities in the liver via liver function tests, but liver parenchyma is not damaged by these enzyme precursors. During an acute porphyic attack, patients could experience symptoms such as:
- muscle spasms (commonly abdominal, but can be any muscle group)
- confusion
- disorientation
- autonomic instability
- lightheadness
- disorientation
- diarrhea
- light sensitivity
- dermatologic conditions
- weakness (particularly peripheral weakness)
- hypesthesia
- allodynia
- severe nausea and vomiting
- emotional lability
- psychosis as well as general malaise.
The attack could result in death.
Mr. J had many differing symptoms and was evaluated by several specialty providers. He had a chronic dermatologic condition; he was confused, disoriented, and complained of nausea, weakness, orthostasis, and loose stools. With the variety of possible symptoms that patients such as Mr. J could experience, one can see why it would lead to many different providers being involved in the diagnosis. It is not uncommon for psychiatrists to be the last providers to care for such patients who could have been evaluated by hematology, cardiology, gastroenterology, dermatology, and/or neurology.
Hereditary coproporphyria
The team considered hepatic porphyias because of new-onset symptoms of mood lability, confusion, orthostasis, unsteady gait, weakness, dermatologic conditions on hands not responsive to treatment, and general malaise. Mr. J was diagnosed with HCP, a type of porphyria caused by a defect in coproporphyrinogen oxidase that leads to an accumulation of coproporphyrinogen III. This precursor, as are many porphyrin precursors, is neurotoxic, leading to neurovisceral or neuropsychiatric effects. Although in Mr. J’s case the coproporphyrinogen III value from the 24-hour drug screen was only modestly elevated, it has been noted that levels of excreted prophyrins do not necessarily correlate with symptom severity.3
In the past, porphyria testing was performed using the Watson-Schwartz test, which used Ehrlich’s reagent to precipitate porphyrins in a urine sample,4 and was used as a “bedside” test. Interestingly, porphyrins—not the iron found in the heme molecule—are precipitated in this test and cause the reddish-purple coloration of the urine sample. When quantitative testing was developed, a 24-hour sample of urine—kept on ice and away from ambient light, later to be frozen when sent to the laboratory—became the standard tool for testing for porphyrins. Now DNA testing can be used to diagnose HCP.
OUTCOME Symptoms resolve
Mr. J is started on loxapine, 20 mg at bedtime, and his symptoms resolve within 2 weeks. He maintains some baseline delusional ideation consistent with his history of schizoaffective disorder, but he is more social, his personal hygiene improves, he attends groups, eats in the cafeteria with his peers, and is no longer confused.
The author’s observations
In the 1950s, chlorpromazine was used to treat AIP.5 Mr. J received loxapine, a mid-potency first-generation antipsychotic, although it has been this author’s observation that high-potency first-generation antipsychotics are not effective for treating porphyria.

CASE Withdrawn and confused
Mr. J, age 54, is brought to the emergency department from a correctional treatment facility where he is reported to be displaying new, unusual behavior. He has a history of schizoaffective disorder, which has been stable with haloperidol, 10 mg/d, for more than a year.
Although previously Mr. J openly discussed his long-standing delusions about the FBI coming to release him from prison, he no longer mentions this or any other delusional beliefs, and has become less communicative with staff and peers. Mr. J no longer accompanies the other patients to the cafeteria for meals and eats in his room alone and appears to be losing weight. He says, “I do not feel good,” but otherwise does not communicate spontaneously. Intermittently, he is irritable, without known triggers. The staff notices that Mr. J often lays on his bed, sometimes in a fetal position. Over time, he becomes confused and is seen attempting to open his room door with a toothbrush. His personal hygiene is poor, and he often urinates through his clothes, on the floor, and in his bed. Recently, Mr. J’s eczema has worsened. His gait has become unsteady, and he has orthostasis.
What could be causing these new symptoms?
a) worsening schizoaffective disorder
b) illicit drug use in the prison
c) atypical dementia
d) cardiac etiology
The author’s observations
The differential diagnosis for Mr. J appeared to be wide and without specific etiology. Because of the complex types of symptoms that Mr. J was experiencing, the emergency department managed his care and specialty clinic referrals were ordered.
It was reported that Mr. J started complaining of lightheadedness a few months ago, which worsened (unsteady gait, near falls). In the context of Mr. J’s history of lightheadedness and orthostasis, the cardiology clinic ordered a tilt table test, which was within normal limits:
- 70º head-up tilt: blood pressure, 91/67 to 102/62 mm Hg, and pulse, 70 to 79 beats per minute (bpm)
- with isoproterenol, 1 μg/minute: blood pressure, 90/66 to 110/70 mm Hg, and pulse, 77 to 124 bpm
- with isoproterenol, 2 μg/minute: blood pressure, 98/58 to 111/66 mm Hg, and pulse, 121 to 134 bpm.
The neurologist’s diagnostic impression was atypical dementia; however, Mr. J showed no memory deficits. Parkinsonism also was considered, but Mr. J had no unilateral tremor, masked facies, or micrographia. Mr. J showed some restriction in his movement, but he was not bradykinetic. The team suspected haloperidol could be causing his stiff movement.
Although it was possible that Mr. J’s schizoaffective disorder was worsening and led to the new symptoms, Mr. J appeared to be less delusional because he was no longer talking to the staff about his delusions. There seemed to be no outward evidence of progression of psychotic symptoms.
Mr. J had a history of substance abuse, including alcohol, cocaine, and Cannabis. Although prison inmates have been known to manufacture and drink “hooch,” the new symptoms Mr. J was experiencing were severe enough that his social interactions with other inmates diminished substantially. Because Mr. J had not been communicating with the other inmates and had no recent visitors, the team felt that it was unlikely that drugs were causing these symptoms. Also, a urine drug screen for cocaine, amphetamines, benzodiazepines, Cannabis, and opioids was negative.
HISTORY Substance use, violence
Mr. J was diagnosed with bipolar disorder at age 18. After later hospitalizations, his diagnosis was changed to schizoaffective disorder as a matter of diagnostic clarification. He has a long history of non-compliance with treatment, homelessness, and drug abuse.
Mr. J is serving a 20-year sentence for first-degree reckless homicide. A year after he was incarcerated, Mr. J was sent to a specialized mental health facility for inmates whose illness cannot be managed in a typical correctional setting. While at the treatment facility, Mr. J was non-compliant with medications and because of concerns about dangerousness and psychosis, the court found probable cause for involuntary commitment.
His medication regimen is trihexyphenidyl, 2 mg/d, for extrapyramidal symptoms; haloperidol, 10 mg/d, for psychosis; trazodone, 150 mg/d, for insomnia; vitamin D3, 2,000 IU/d; vitamin E, 400 IU/d, for symptoms of tardive dyskinesia; IM ziprasidone, 20 mg, because he refused oral haliperidol; and hydrocortisone cream 1% for eczema.
EVALUATION Additional tests
Mr. J’s blood pressure is 124/72 mm Hg, and pulse, 104 bpm, laying down; blood pressure, 110/84 mm Hg, and pulse, 112 bpm, sitting; and blood pressure, 108/82 mm Hg, and pulse, 129 bpm, standing. With repeated readings: blood pressure, 128/84 mm Hg, and pulse, 98 bpm, laying down; blood pressure, 125/86 mm Hg, and pulse, 113 bpm, sitting; and blood pressure, 105/76 mm Hg, and pulse, 130 bpm, standing.
Laboratory tests, including complete blood count, chemistry panel, thyroid-stimulating hormone, are within normal limits. The team feels that the investigation for an etiology for Mr. J’s symptoms needs to be more exhaustive and additional tests are ordered, including vitamin levels (C, B1, B12, B6), rapid plasma reagin for syphilis, and arbovirus testing (eastern equine encephalitis virus, western equine encephalitis, West Nile virus, La Crosse encephalitis, St. Louis encephalitis), which are negative.
What’s the next best step in managing Mr. J’s care?
a) adjust his medication
b) eliminate a mediation
c) order further testing
The author’s observations
To determine if Mr. J’s new-onset symptoms might be related to the progression of his psychiatric illness, the haloperidol dosage was increased to 20 mg/d; however, we saw no positive response to this change. His tardive dyskinesia symptoms (bruxism and other oral buccal movements) worsened. Haloperidol was reduced to 10 mg/d.
Trihexyphenidyl then was suspected to contribute to Mr. J’s confusion. Unfortunately, lowering the dosage of trihexyphenidyl to 1 mg/d, did not affect Mr. J’s current symptoms and exacerbated extrapyramidal symptoms.
The treatment team then questioned if porphyria—known as the “little imitator”—might be considered because of the variety of symptoms without an etiology, despite extensive testing. A 24-hour urine collection was ordered.
What is the correct method of collecting a urine sample for porphyrins?
a) collect a small sample and expose it to light before testing
b) collect a 24-hour sample with the sample kept in ambient temperature and light
c) collect a 24-hour sample with the sample kept on ice in a light-blocking container and frozen when sent to the laboratory
EVALUATION Diagnosis revealed
The 24-hour urine collection is obtained. However, it needed to be collected twice, because the first sample was not a full sample. Interestingly, the first sample, which is exposed to light and not kept on ice, turned dark in color. The second sample is obtained properly and sent to the laboratory. When the laboratory results are returned (Figure 1), Mr. J is diagnosed with hereditary coproporphyria (HCP).

The author’s observations
There are several types of porphyria, each associated with a different step in the chain of enzymes associated with synthesis of a heme molecule in the mitochondria. A defect in any single enzyme step will create a build up of porphyrins—a precursor to heme molecules—in erythrocytes or hepatic cells.
It is important to differentiate hepatic from erythropoietic porphyrias. The acute porphyrias (acute intermittent porphyria [AIP], HCP, and variegate porphyria generally are hepatic in origin with neuropsychiatric and neurovisceral symptoms. Cutaneous porphyrias originate in bone marrow and therefore are erythropoietic. However, there are exceptions such as porphyria cutanea tarda (PCT), which is hepatic in origin but the manifestations mainly are cutaneous1 (Figure 2).2
Although acute porphyria originates in the liver, it is a neuropsychiatric illness. In these cases, excess porphyrins cannot cross the blood–brain barrier and are neurotoxic. Clinicians can look for abnormalities in the liver via liver function tests, but liver parenchyma is not damaged by these enzyme precursors. During an acute porphyic attack, patients could experience symptoms such as:
- muscle spasms (commonly abdominal, but can be any muscle group)
- confusion
- disorientation
- autonomic instability
- lightheadness
- disorientation
- diarrhea
- light sensitivity
- dermatologic conditions
- weakness (particularly peripheral weakness)
- hypesthesia
- allodynia
- severe nausea and vomiting
- emotional lability
- psychosis as well as general malaise.
The attack could result in death.
Mr. J had many differing symptoms and was evaluated by several specialty providers. He had a chronic dermatologic condition; he was confused, disoriented, and complained of nausea, weakness, orthostasis, and loose stools. With the variety of possible symptoms that patients such as Mr. J could experience, one can see why it would lead to many different providers being involved in the diagnosis. It is not uncommon for psychiatrists to be the last providers to care for such patients who could have been evaluated by hematology, cardiology, gastroenterology, dermatology, and/or neurology.
Hereditary coproporphyria
The team considered hepatic porphyias because of new-onset symptoms of mood lability, confusion, orthostasis, unsteady gait, weakness, dermatologic conditions on hands not responsive to treatment, and general malaise. Mr. J was diagnosed with HCP, a type of porphyria caused by a defect in coproporphyrinogen oxidase that leads to an accumulation of coproporphyrinogen III. This precursor, as are many porphyrin precursors, is neurotoxic, leading to neurovisceral or neuropsychiatric effects. Although in Mr. J’s case the coproporphyrinogen III value from the 24-hour drug screen was only modestly elevated, it has been noted that levels of excreted prophyrins do not necessarily correlate with symptom severity.3
In the past, porphyria testing was performed using the Watson-Schwartz test, which used Ehrlich’s reagent to precipitate porphyrins in a urine sample,4 and was used as a “bedside” test. Interestingly, porphyrins—not the iron found in the heme molecule—are precipitated in this test and cause the reddish-purple coloration of the urine sample. When quantitative testing was developed, a 24-hour sample of urine—kept on ice and away from ambient light, later to be frozen when sent to the laboratory—became the standard tool for testing for porphyrins. Now DNA testing can be used to diagnose HCP.
OUTCOME Symptoms resolve
Mr. J is started on loxapine, 20 mg at bedtime, and his symptoms resolve within 2 weeks. He maintains some baseline delusional ideation consistent with his history of schizoaffective disorder, but he is more social, his personal hygiene improves, he attends groups, eats in the cafeteria with his peers, and is no longer confused.
The author’s observations
In the 1950s, chlorpromazine was used to treat AIP.5 Mr. J received loxapine, a mid-potency first-generation antipsychotic, although it has been this author’s observation that high-potency first-generation antipsychotics are not effective for treating porphyria.

1. NIH: National Human Genome Research Institute. Learning about porphyria. https://www.genome.gov/19016728/learning-about-porphyria/learning-about-porphyria. Accessed February 23, 2017.
2. Ajioka RS, Phillips JD, Kushner JP. Biosynthesis of heme in mammals. Biochim Biophys Acta. 2006;1763(7):723-736.
3. Peters HA, Gocmen A, Cripps DJ, et al. Epidemiology of hexachlorobenzene-induced porphyria in Turkey: clinical and laboratory follow-up after 25 years. Arch Neurol. 1982;39(12):744-749.
4. The Watsonschwartz test. JAMA. 1966;195(6):481.
5. Brunton L, Chabner BA, Knollman B. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw-Hill Professional; 2010.
6. Broomfield B. Acute Intermittent porphyria treated with chlorpromazine. Proc R Soc Med. 1962;55(9):799-800.
7. Hunter JA, Khan SA, Hope E, et al. Hereditary coproporphyria. Photosensitivity, jaundice and neuropsychiatric manifestations associated with pregnancy. Br J Dermatol. 1971;84(4):301-310.
8. Bonkovsky HL, Maddukuri V. Merck Manual. http://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/porphyrias/overview-of-porphyrias. Accessed February 2, 2017.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24 (suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4): 247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1): 64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.
1. NIH: National Human Genome Research Institute. Learning about porphyria. https://www.genome.gov/19016728/learning-about-porphyria/learning-about-porphyria. Accessed February 23, 2017.
2. Ajioka RS, Phillips JD, Kushner JP. Biosynthesis of heme in mammals. Biochim Biophys Acta. 2006;1763(7):723-736.
3. Peters HA, Gocmen A, Cripps DJ, et al. Epidemiology of hexachlorobenzene-induced porphyria in Turkey: clinical and laboratory follow-up after 25 years. Arch Neurol. 1982;39(12):744-749.
4. The Watsonschwartz test. JAMA. 1966;195(6):481.
5. Brunton L, Chabner BA, Knollman B. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York, NY: McGraw-Hill Professional; 2010.
6. Broomfield B. Acute Intermittent porphyria treated with chlorpromazine. Proc R Soc Med. 1962;55(9):799-800.
7. Hunter JA, Khan SA, Hope E, et al. Hereditary coproporphyria. Photosensitivity, jaundice and neuropsychiatric manifestations associated with pregnancy. Br J Dermatol. 1971;84(4):301-310.
8. Bonkovsky HL, Maddukuri V. Merck Manual. http://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/porphyrias/overview-of-porphyrias. Accessed February 2, 2017.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24 (suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4): 247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1): 64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.