User login
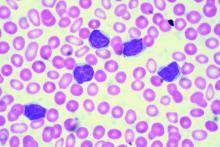
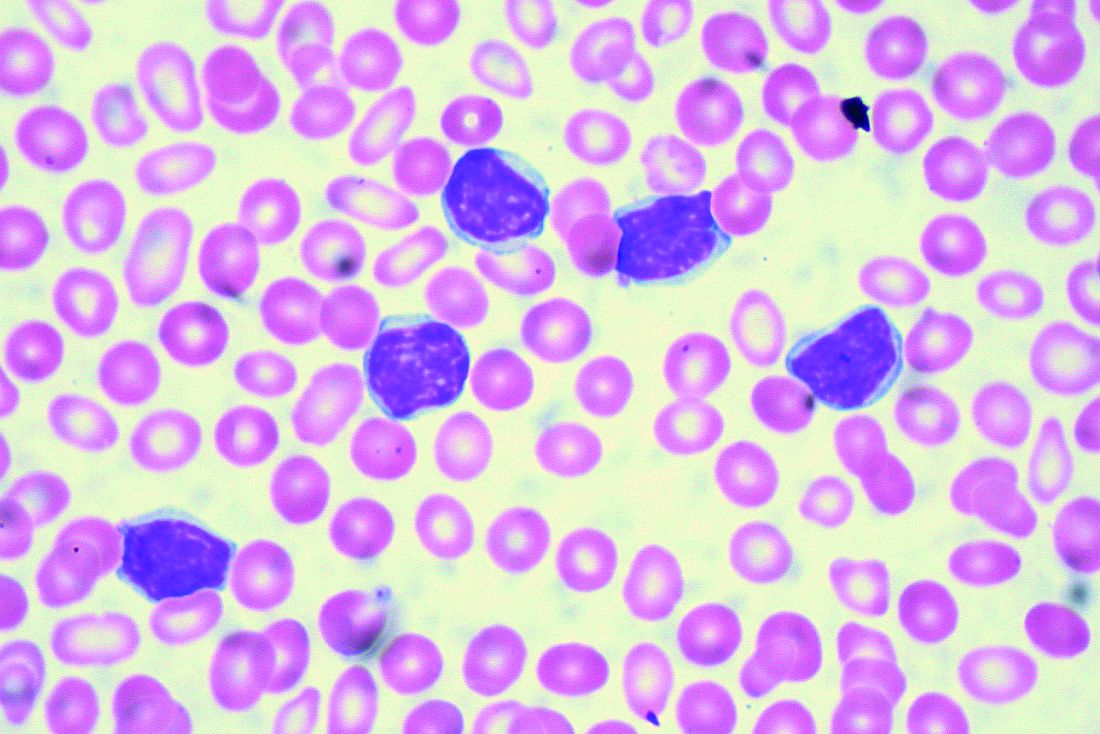
CHICAGO – A number of mutation tests – including immunoglobulin heavy chain gene (IgVH), fluorescence in situ hybridization (FISH), and TP53 – provide useful prognostic information at the time of chronic lymphocytic leukemia (CLL) diagnosis, according to Paul M. Barr, MD.
“It’s understood that IgVH mutation status is certainly prognostic,” Dr. Barr, associate professor of hematology/oncology at the University of Rochester (N.Y.), said during a presentation at the American Society of Hematology Meeting on Hematologic Malignancies.
The B-cell receptor of the CLL cells uses IgVH genes that may or may not have undergone somatic mutations, with unmutated being defined as 98% or more sequence homology to germline.
“This is indicative of stronger signaling through the B-cell receptor and, as we all know, predicts for an inferior prognosis,” he explained, citing a study that demonstrated superior survival rates with mutated IgVH genes (Blood. 1999;94[6]:1840-7).
“It’s also well understood and accepted that we should perform a FISH panel; we should look for interphase cytogenetics based on FISH in our patients,” Dr. Barr said. “Having said that, we, as medical oncologists, do not do a very good job of using this testing appropriately. Patterns of care studies have suggested that a significant number of patients don’t get FISH testing at diagnosis or before first-line therapy.”
In fact, a typical interphase FISH panel identifies cytogenetic lesions, including del(17p), del(11q), del(13q), and trisomy 12 in more than 80% of CLL cases, with del(13q) being the most common.
Another marker that can be assessed in CLL patients and has maintained prognostic value across multiple analyses is serum beta-2 microglobulin, Dr. Barr noted.
TP53 sequencing is valuable as well and has been associated with outcomes similar to those seen in patients with del(17p), he said, citing data from a study that found similarly poor outcomes with TP53 mutations or deletions and del(17p), even when minor subclones are identified using next-generation sequencing (Blood. 2014;123:2139-47).
“One of the primary reasons for this is that the two aberrations go together. Most often, if you have del(17p) you’re also going to find a TP53 mutation, but in about 30% of patients or so, only one allele is affected, so this is why it’s still important to test for TP53 mutations when you’re looking for a 17p deletion,” he said.
Numerous other recurrent mutations in CLL have been associated with poor overall survival and/or progression-free survival, including SF3B1, ATM, NOTCH1, POT1, BIRC3, and NFKBIE.
“The gut instinct is that maybe we should start testing for all of these mutations now, but I would caution everybody that we still need further validation before we can apply these to the majority of patients,” Dr. Barr said. “We still don’t know exactly what to do with all of these mutations – when and how often we should test for them, if the novel agents are truly better – so while, again, they can predict for inferior outcomes, I would say these are not yet standard of care to be tested in all patients.”
It is likely, though, that new prognostic systems will evolve as more is learned about how to use these molecular aberrations. Attempts are already being made to incorporate novel mutations into a prognostic system. Dr. Barr pointed to a report that looked at the integration of mutations and cytogenetic lesions to improve the accuracy of survival prediction in CLL (Blood. 2013;121:1403-12).
“But this still requires prospective testing, especially in patients getting the novel agents,” he said.
Conventional karyotyping also has potential, though a limited role in this setting, he said, noting that it can be reliably performed with stimulation of CLL cells.
“We also know additional aberrations are prognostic and that a complex karyotype predicts for a very poor outcome,” he said. The International Workshop on CLL (iwCLL) guidelines, which were recently updated for the first time in a decade, state that further validation is needed.
“I think it’s potentially useful in a very young patient you are considering taking to transplant, but again, I agree with the stance that this is not something that should be performed in every patient across the board,” he said.
The tests currently recommended by iwCLL before CLL treatment include IgVH mutation status; FISH for del(13q), del(11q), del(17p), and trisomy 12 in peripheral blood lymphocytes; and TP53.
“Some folks... don’t check a lot of these markers at diagnosis, but wait for patients to require therapy, and that’s a reasonable way to practice,” Dr. Barr said, noting, however, that he prefers knowing patients’ risk up front – especially for those patients he will see just once before they are “managed closer to home for the majority of their course.
“But if you [wait], then knowing what to repeat later is important,” he added. Namely, the FISH and TP53 tests are worth repeating as patients can acquire additional molecular aberrations over time.
Dr. Barr reported serving as a consultant for Pharmacyclics, AbbVie, Celgene, Gilead Sciences, Infinity Pharmaceuticals, Novartis, and Seattle Genetics. He also reported receiving research funding from Pharmacyclics and AbbVie.
CHICAGO – A number of mutation tests – including immunoglobulin heavy chain gene (IgVH), fluorescence in situ hybridization (FISH), and TP53 – provide useful prognostic information at the time of chronic lymphocytic leukemia (CLL) diagnosis, according to Paul M. Barr, MD.
“It’s understood that IgVH mutation status is certainly prognostic,” Dr. Barr, associate professor of hematology/oncology at the University of Rochester (N.Y.), said during a presentation at the American Society of Hematology Meeting on Hematologic Malignancies.
The B-cell receptor of the CLL cells uses IgVH genes that may or may not have undergone somatic mutations, with unmutated being defined as 98% or more sequence homology to germline.
“This is indicative of stronger signaling through the B-cell receptor and, as we all know, predicts for an inferior prognosis,” he explained, citing a study that demonstrated superior survival rates with mutated IgVH genes (Blood. 1999;94[6]:1840-7).
“It’s also well understood and accepted that we should perform a FISH panel; we should look for interphase cytogenetics based on FISH in our patients,” Dr. Barr said. “Having said that, we, as medical oncologists, do not do a very good job of using this testing appropriately. Patterns of care studies have suggested that a significant number of patients don’t get FISH testing at diagnosis or before first-line therapy.”
In fact, a typical interphase FISH panel identifies cytogenetic lesions, including del(17p), del(11q), del(13q), and trisomy 12 in more than 80% of CLL cases, with del(13q) being the most common.
Another marker that can be assessed in CLL patients and has maintained prognostic value across multiple analyses is serum beta-2 microglobulin, Dr. Barr noted.
TP53 sequencing is valuable as well and has been associated with outcomes similar to those seen in patients with del(17p), he said, citing data from a study that found similarly poor outcomes with TP53 mutations or deletions and del(17p), even when minor subclones are identified using next-generation sequencing (Blood. 2014;123:2139-47).
“One of the primary reasons for this is that the two aberrations go together. Most often, if you have del(17p) you’re also going to find a TP53 mutation, but in about 30% of patients or so, only one allele is affected, so this is why it’s still important to test for TP53 mutations when you’re looking for a 17p deletion,” he said.
Numerous other recurrent mutations in CLL have been associated with poor overall survival and/or progression-free survival, including SF3B1, ATM, NOTCH1, POT1, BIRC3, and NFKBIE.
“The gut instinct is that maybe we should start testing for all of these mutations now, but I would caution everybody that we still need further validation before we can apply these to the majority of patients,” Dr. Barr said. “We still don’t know exactly what to do with all of these mutations – when and how often we should test for them, if the novel agents are truly better – so while, again, they can predict for inferior outcomes, I would say these are not yet standard of care to be tested in all patients.”
It is likely, though, that new prognostic systems will evolve as more is learned about how to use these molecular aberrations. Attempts are already being made to incorporate novel mutations into a prognostic system. Dr. Barr pointed to a report that looked at the integration of mutations and cytogenetic lesions to improve the accuracy of survival prediction in CLL (Blood. 2013;121:1403-12).
“But this still requires prospective testing, especially in patients getting the novel agents,” he said.
Conventional karyotyping also has potential, though a limited role in this setting, he said, noting that it can be reliably performed with stimulation of CLL cells.
“We also know additional aberrations are prognostic and that a complex karyotype predicts for a very poor outcome,” he said. The International Workshop on CLL (iwCLL) guidelines, which were recently updated for the first time in a decade, state that further validation is needed.
“I think it’s potentially useful in a very young patient you are considering taking to transplant, but again, I agree with the stance that this is not something that should be performed in every patient across the board,” he said.
The tests currently recommended by iwCLL before CLL treatment include IgVH mutation status; FISH for del(13q), del(11q), del(17p), and trisomy 12 in peripheral blood lymphocytes; and TP53.
“Some folks... don’t check a lot of these markers at diagnosis, but wait for patients to require therapy, and that’s a reasonable way to practice,” Dr. Barr said, noting, however, that he prefers knowing patients’ risk up front – especially for those patients he will see just once before they are “managed closer to home for the majority of their course.
“But if you [wait], then knowing what to repeat later is important,” he added. Namely, the FISH and TP53 tests are worth repeating as patients can acquire additional molecular aberrations over time.
Dr. Barr reported serving as a consultant for Pharmacyclics, AbbVie, Celgene, Gilead Sciences, Infinity Pharmaceuticals, Novartis, and Seattle Genetics. He also reported receiving research funding from Pharmacyclics and AbbVie.
CHICAGO – A number of mutation tests – including immunoglobulin heavy chain gene (IgVH), fluorescence in situ hybridization (FISH), and TP53 – provide useful prognostic information at the time of chronic lymphocytic leukemia (CLL) diagnosis, according to Paul M. Barr, MD.
“It’s understood that IgVH mutation status is certainly prognostic,” Dr. Barr, associate professor of hematology/oncology at the University of Rochester (N.Y.), said during a presentation at the American Society of Hematology Meeting on Hematologic Malignancies.
The B-cell receptor of the CLL cells uses IgVH genes that may or may not have undergone somatic mutations, with unmutated being defined as 98% or more sequence homology to germline.
“This is indicative of stronger signaling through the B-cell receptor and, as we all know, predicts for an inferior prognosis,” he explained, citing a study that demonstrated superior survival rates with mutated IgVH genes (Blood. 1999;94[6]:1840-7).
“It’s also well understood and accepted that we should perform a FISH panel; we should look for interphase cytogenetics based on FISH in our patients,” Dr. Barr said. “Having said that, we, as medical oncologists, do not do a very good job of using this testing appropriately. Patterns of care studies have suggested that a significant number of patients don’t get FISH testing at diagnosis or before first-line therapy.”
In fact, a typical interphase FISH panel identifies cytogenetic lesions, including del(17p), del(11q), del(13q), and trisomy 12 in more than 80% of CLL cases, with del(13q) being the most common.
Another marker that can be assessed in CLL patients and has maintained prognostic value across multiple analyses is serum beta-2 microglobulin, Dr. Barr noted.
TP53 sequencing is valuable as well and has been associated with outcomes similar to those seen in patients with del(17p), he said, citing data from a study that found similarly poor outcomes with TP53 mutations or deletions and del(17p), even when minor subclones are identified using next-generation sequencing (Blood. 2014;123:2139-47).
“One of the primary reasons for this is that the two aberrations go together. Most often, if you have del(17p) you’re also going to find a TP53 mutation, but in about 30% of patients or so, only one allele is affected, so this is why it’s still important to test for TP53 mutations when you’re looking for a 17p deletion,” he said.
Numerous other recurrent mutations in CLL have been associated with poor overall survival and/or progression-free survival, including SF3B1, ATM, NOTCH1, POT1, BIRC3, and NFKBIE.
“The gut instinct is that maybe we should start testing for all of these mutations now, but I would caution everybody that we still need further validation before we can apply these to the majority of patients,” Dr. Barr said. “We still don’t know exactly what to do with all of these mutations – when and how often we should test for them, if the novel agents are truly better – so while, again, they can predict for inferior outcomes, I would say these are not yet standard of care to be tested in all patients.”
It is likely, though, that new prognostic systems will evolve as more is learned about how to use these molecular aberrations. Attempts are already being made to incorporate novel mutations into a prognostic system. Dr. Barr pointed to a report that looked at the integration of mutations and cytogenetic lesions to improve the accuracy of survival prediction in CLL (Blood. 2013;121:1403-12).
“But this still requires prospective testing, especially in patients getting the novel agents,” he said.
Conventional karyotyping also has potential, though a limited role in this setting, he said, noting that it can be reliably performed with stimulation of CLL cells.
“We also know additional aberrations are prognostic and that a complex karyotype predicts for a very poor outcome,” he said. The International Workshop on CLL (iwCLL) guidelines, which were recently updated for the first time in a decade, state that further validation is needed.
“I think it’s potentially useful in a very young patient you are considering taking to transplant, but again, I agree with the stance that this is not something that should be performed in every patient across the board,” he said.
The tests currently recommended by iwCLL before CLL treatment include IgVH mutation status; FISH for del(13q), del(11q), del(17p), and trisomy 12 in peripheral blood lymphocytes; and TP53.
“Some folks... don’t check a lot of these markers at diagnosis, but wait for patients to require therapy, and that’s a reasonable way to practice,” Dr. Barr said, noting, however, that he prefers knowing patients’ risk up front – especially for those patients he will see just once before they are “managed closer to home for the majority of their course.
“But if you [wait], then knowing what to repeat later is important,” he added. Namely, the FISH and TP53 tests are worth repeating as patients can acquire additional molecular aberrations over time.
Dr. Barr reported serving as a consultant for Pharmacyclics, AbbVie, Celgene, Gilead Sciences, Infinity Pharmaceuticals, Novartis, and Seattle Genetics. He also reported receiving research funding from Pharmacyclics and AbbVie.
EXPERT ANALYSIS FROM MHM 2018