User login
Obstetricians work diligently to anticipate, diagnose, and treat preeclampsia because the maternal and perinatal health burden of the disease is enormous. Many meta-analyses have reported that aspirin treatment of women at high risk for preeclampsia reduces the risk of developing the disease by about 10% to 23%.1–5 In addition, for women at high risk for preeclampsia, aspirin treatment reduces the risk of preterm birth and intrauterine growth restriction (IUGR). In your practice you should start offering aspirin to pregnant women at high risk for preeclampsia.
Aspirin reduces the risk of preeclampsia, preterm birth, and IUGRBased on the results of multiple meta-analyses of clinical trials involving more than 35,000 women, investigators consistently have concluded that aspirin treatment reduces the risk of preeclampsia in women at high risk for the disease.1–5 The magnitude of the effect is difficult to define with precision, but the risk reduction is likely in the range of 10% to 23%.1
In addition to reducing the risk of preeclampsia, aspirin also reduces the risk of 2 associated problems: preterm birth and IUGR. For preterm birth, the risk reduction is estimated to be in the range of 11% to 31%. For IUGR, the estimation for risk reduction is in the range of 7% to 24%.1 Although these benefits are modest, the burden of maternal and perinatal morbidity associated with preeclampsia is great, making even a modest benefit clinically significant.
Potential harms of aspirin treatmentIn the most recent meta-analysis from the US Preventive Services Task Force (USPSTF),1 low-dose aspirin treatment was associated with no significant perinatal or maternal harms, but rare harms could not be ruled out. A small increase in the risk of placental abruption was noted, but this increase did not reach significance (relative risk [RR], 1.17; 95% confidence interval [CI], 0.93–1.48).1 There was no increased risk of maternal postpartum hemorrhage or blood loss at delivery.1 In one meta-analysis, aspirin treatment did not increase the risk of newborn intracranial hemorrhage.1
Other potential adverse effects of aspirin treatment include maternal gastrointestinal bleeding and exacerbation of respiratory disorders such as asthma, but these effects have not been reported as significant associations in clinical trials of preeclampsia prevention.
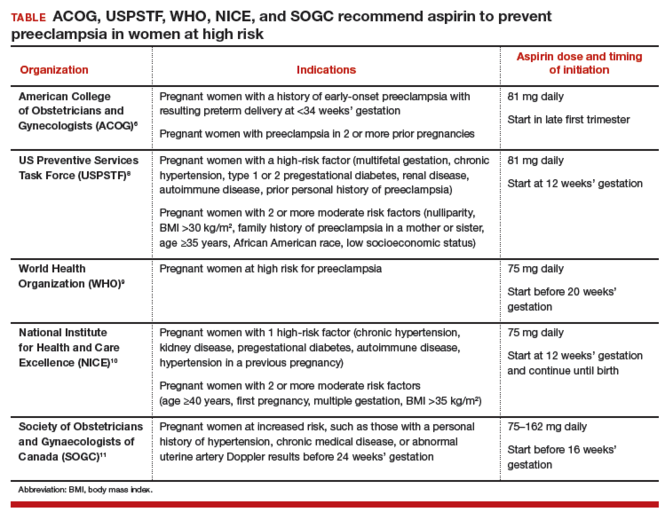
Dueling recommendations: Restrictive or liberal use of aspirin?The American College of Obstetricians and Gynecologists (ACOG) recommends use of aspirin to prevent preeclampsia in women who have a personal history of early-onset preeclampsia with delivery before 34 weeks of gestation and in women with preeclampsia in 2 or more prior pregnancies.6 The restrictive ACOG guideline recommends aspirin treatment for a very small group of women. In one analysis, using the ACOG guideline, only 0.35% of all pregnant women would be eligible for treatment with aspirin to prevent preeclampsia.7
The USPSTF recommends that all pregnant women with one major risk factor for preeclampsia—including multifetal gestation, chronic hypertension, type 1 or 2 pregestational diabetes, renal disease, autoimmune disease, or prior personal history of preeclampsia—receive treatment with aspirin to prevent preeclampsia.8 The Task Force also recommends that women with multiple moderate risk factors for preeclampsia, such as nulliparity, body mass index greater than 30 kg/m2, family history of preeclampsia in a mother or sister, age 35 years or older, and certain sociodemographic risk factors (African American race, low socioeconomic status) also be offered aspirin treatment.
The USPSTF guideline advises aspirin treatment for many women. According to one analysis, the USPSTFguideline would result in approximately 24% of all pregnant women being offered aspirin treatment.7
The USPSTF guideline would result in 67 times more pregnant women being treated with aspirin than the ACOG guideline. The narrowly focused ACOG recommendation is problematic because it recommends against aspirin treatment in women who are at very high risk for developing preeclampsia, for example, a 41-year-old woman in her first pregnancy with twins and pregestational diabetes. In addition, the ACOG recommendation is not consistent with the recommendations of most other major health organizations.
The World Health Organization,9 the United Kingdom’s National Institute for Health and Care Excellence (NICE),10 and the Society of Obstetricians and Gynaecologists of Canada11 all recommend aspirin treatment to prevent preeclampsia in pregnant women at high risk for the disease and utilize an expanded definition of “high risk” (TABLE). Some experts have observed that, in actual clinical practice, it is often difficult to consistently implement a prevention plan based on a complex assessment of clinical risk factors.7
An alternative to guidelines that use clinical risk factors to identify women at high risk is universal treatment. With universal treatment all pregnant women are prescribed aspirin, thereby maximizing the clinical benefit but unnecessarily treating many women with aspirin.7 Universal treatment of pregnant women with aspirin appears to be cost-effective and would be associated with annual health care savings of $365 million.7
Timing of aspirin initiationIn one meta-analysis, initiating aspirin before 16 weeks’ gestation resulted in a greater reduction in preeclampsia than starting aspirin after 16 weeks.12 The USPSTF cautions that meta-analysis of the available data is not well suited for identifying the optimal time to initiate aspirin therapy.13 ACOG, USPSTF, and NICE recommend initiating aspirin therapy at approximately 12 weeks’ gestation—the end of the first trimester.
Ideal aspirin doseThe optimal dose of aspirin to prevent preeclampsia is not precisely defined. Aspirin doses ranging from 50 mg to 162 mg have been proposed for the prevention of preeclampsia. Most authorities recommend a daily dose between 80 mg and less than 300 mg to prevent preeclampsia.14 ACOG and USPSTF recommend aspirin at a dose of 81 mg daily,6,8 because this dose is widely available in the United States.
Let’s close the gap between current and optimal practiceAccording to the USPSTF guidelines, approximately 24% of the pregnant women in our practices have risk factors that would justify the initiation of aspirin treatment for the prevention of preeclampsia.8 This approach would modestly reduce the rate of preeclampsia and the associated problems of preterm birth and IUGR with little cost and few adverse effects. Yet relatively few pregnant women in the United States are currently receiving aspirin therapy. We could close this clinical gap between current and optimal practice by reflecting on the USPSTF recommendations and implementing them in our practices, as appropriate.
Tell us…What are your thoughts about the use of aspirin in pregnant women who are at high risk for preeclampsia?
Send your letter to the editor to [email protected]. Please include the city and state in which you practice.
- Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(10):695-703.
- Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol. 2013;41(5):491-499.
- Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 pt 1):402-414.
- Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Askie LM, Duley L, Henderson-Smart DJ, Stewart LA; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791-1798.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131.
- Werner EF, Hauspurg AK, Rouse DJ. A cost-benefit analysis of low-dose aspirin prophylaxis for the prevention of preeclampsia in the United States. Obstet Gynecol. 2015;126(6):1242-1250.
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819-826.
- World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva, Switzerland: WHO; 2011:13-15. https://www.preeclampsia.org/images/pdf/2011c-who_pe_final.pdf. Accessed January 4, 2016.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. Clinical guideline 107. Manchester, United Kingdom: NICE; 2010:7. https://www.nice.org.uk/guidance/cg107/resources/hypertension-in-pregnancy-diagnosis-and-management-35109334009285. Accessed April 4, 2016.
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416-441.
- Roberge S, Demers S, Bujold E. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613.
- Henderson JT, O'Connor E, Whitlock EP. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613-614.
- Bujold E, Roberge S, Nicolaides KH. Low-dose aspirin for prevention of adverse outcomes related to abnormal placentation. Prenat Diagn. 2014;34(7):642-648.
Obstetricians work diligently to anticipate, diagnose, and treat preeclampsia because the maternal and perinatal health burden of the disease is enormous. Many meta-analyses have reported that aspirin treatment of women at high risk for preeclampsia reduces the risk of developing the disease by about 10% to 23%.1–5 In addition, for women at high risk for preeclampsia, aspirin treatment reduces the risk of preterm birth and intrauterine growth restriction (IUGR). In your practice you should start offering aspirin to pregnant women at high risk for preeclampsia.
Aspirin reduces the risk of preeclampsia, preterm birth, and IUGRBased on the results of multiple meta-analyses of clinical trials involving more than 35,000 women, investigators consistently have concluded that aspirin treatment reduces the risk of preeclampsia in women at high risk for the disease.1–5 The magnitude of the effect is difficult to define with precision, but the risk reduction is likely in the range of 10% to 23%.1
In addition to reducing the risk of preeclampsia, aspirin also reduces the risk of 2 associated problems: preterm birth and IUGR. For preterm birth, the risk reduction is estimated to be in the range of 11% to 31%. For IUGR, the estimation for risk reduction is in the range of 7% to 24%.1 Although these benefits are modest, the burden of maternal and perinatal morbidity associated with preeclampsia is great, making even a modest benefit clinically significant.
Potential harms of aspirin treatmentIn the most recent meta-analysis from the US Preventive Services Task Force (USPSTF),1 low-dose aspirin treatment was associated with no significant perinatal or maternal harms, but rare harms could not be ruled out. A small increase in the risk of placental abruption was noted, but this increase did not reach significance (relative risk [RR], 1.17; 95% confidence interval [CI], 0.93–1.48).1 There was no increased risk of maternal postpartum hemorrhage or blood loss at delivery.1 In one meta-analysis, aspirin treatment did not increase the risk of newborn intracranial hemorrhage.1
Other potential adverse effects of aspirin treatment include maternal gastrointestinal bleeding and exacerbation of respiratory disorders such as asthma, but these effects have not been reported as significant associations in clinical trials of preeclampsia prevention.
Dueling recommendations: Restrictive or liberal use of aspirin?The American College of Obstetricians and Gynecologists (ACOG) recommends use of aspirin to prevent preeclampsia in women who have a personal history of early-onset preeclampsia with delivery before 34 weeks of gestation and in women with preeclampsia in 2 or more prior pregnancies.6 The restrictive ACOG guideline recommends aspirin treatment for a very small group of women. In one analysis, using the ACOG guideline, only 0.35% of all pregnant women would be eligible for treatment with aspirin to prevent preeclampsia.7
The USPSTF recommends that all pregnant women with one major risk factor for preeclampsia—including multifetal gestation, chronic hypertension, type 1 or 2 pregestational diabetes, renal disease, autoimmune disease, or prior personal history of preeclampsia—receive treatment with aspirin to prevent preeclampsia.8 The Task Force also recommends that women with multiple moderate risk factors for preeclampsia, such as nulliparity, body mass index greater than 30 kg/m2, family history of preeclampsia in a mother or sister, age 35 years or older, and certain sociodemographic risk factors (African American race, low socioeconomic status) also be offered aspirin treatment.
The USPSTF guideline advises aspirin treatment for many women. According to one analysis, the USPSTFguideline would result in approximately 24% of all pregnant women being offered aspirin treatment.7
The USPSTF guideline would result in 67 times more pregnant women being treated with aspirin than the ACOG guideline. The narrowly focused ACOG recommendation is problematic because it recommends against aspirin treatment in women who are at very high risk for developing preeclampsia, for example, a 41-year-old woman in her first pregnancy with twins and pregestational diabetes. In addition, the ACOG recommendation is not consistent with the recommendations of most other major health organizations.
The World Health Organization,9 the United Kingdom’s National Institute for Health and Care Excellence (NICE),10 and the Society of Obstetricians and Gynaecologists of Canada11 all recommend aspirin treatment to prevent preeclampsia in pregnant women at high risk for the disease and utilize an expanded definition of “high risk” (TABLE). Some experts have observed that, in actual clinical practice, it is often difficult to consistently implement a prevention plan based on a complex assessment of clinical risk factors.7

An alternative to guidelines that use clinical risk factors to identify women at high risk is universal treatment. With universal treatment all pregnant women are prescribed aspirin, thereby maximizing the clinical benefit but unnecessarily treating many women with aspirin.7 Universal treatment of pregnant women with aspirin appears to be cost-effective and would be associated with annual health care savings of $365 million.7
Timing of aspirin initiationIn one meta-analysis, initiating aspirin before 16 weeks’ gestation resulted in a greater reduction in preeclampsia than starting aspirin after 16 weeks.12 The USPSTF cautions that meta-analysis of the available data is not well suited for identifying the optimal time to initiate aspirin therapy.13 ACOG, USPSTF, and NICE recommend initiating aspirin therapy at approximately 12 weeks’ gestation—the end of the first trimester.
Ideal aspirin doseThe optimal dose of aspirin to prevent preeclampsia is not precisely defined. Aspirin doses ranging from 50 mg to 162 mg have been proposed for the prevention of preeclampsia. Most authorities recommend a daily dose between 80 mg and less than 300 mg to prevent preeclampsia.14 ACOG and USPSTF recommend aspirin at a dose of 81 mg daily,6,8 because this dose is widely available in the United States.
Let’s close the gap between current and optimal practiceAccording to the USPSTF guidelines, approximately 24% of the pregnant women in our practices have risk factors that would justify the initiation of aspirin treatment for the prevention of preeclampsia.8 This approach would modestly reduce the rate of preeclampsia and the associated problems of preterm birth and IUGR with little cost and few adverse effects. Yet relatively few pregnant women in the United States are currently receiving aspirin therapy. We could close this clinical gap between current and optimal practice by reflecting on the USPSTF recommendations and implementing them in our practices, as appropriate.
Tell us…What are your thoughts about the use of aspirin in pregnant women who are at high risk for preeclampsia?
Send your letter to the editor to [email protected]. Please include the city and state in which you practice.
Obstetricians work diligently to anticipate, diagnose, and treat preeclampsia because the maternal and perinatal health burden of the disease is enormous. Many meta-analyses have reported that aspirin treatment of women at high risk for preeclampsia reduces the risk of developing the disease by about 10% to 23%.1–5 In addition, for women at high risk for preeclampsia, aspirin treatment reduces the risk of preterm birth and intrauterine growth restriction (IUGR). In your practice you should start offering aspirin to pregnant women at high risk for preeclampsia.
Aspirin reduces the risk of preeclampsia, preterm birth, and IUGRBased on the results of multiple meta-analyses of clinical trials involving more than 35,000 women, investigators consistently have concluded that aspirin treatment reduces the risk of preeclampsia in women at high risk for the disease.1–5 The magnitude of the effect is difficult to define with precision, but the risk reduction is likely in the range of 10% to 23%.1
In addition to reducing the risk of preeclampsia, aspirin also reduces the risk of 2 associated problems: preterm birth and IUGR. For preterm birth, the risk reduction is estimated to be in the range of 11% to 31%. For IUGR, the estimation for risk reduction is in the range of 7% to 24%.1 Although these benefits are modest, the burden of maternal and perinatal morbidity associated with preeclampsia is great, making even a modest benefit clinically significant.
Potential harms of aspirin treatmentIn the most recent meta-analysis from the US Preventive Services Task Force (USPSTF),1 low-dose aspirin treatment was associated with no significant perinatal or maternal harms, but rare harms could not be ruled out. A small increase in the risk of placental abruption was noted, but this increase did not reach significance (relative risk [RR], 1.17; 95% confidence interval [CI], 0.93–1.48).1 There was no increased risk of maternal postpartum hemorrhage or blood loss at delivery.1 In one meta-analysis, aspirin treatment did not increase the risk of newborn intracranial hemorrhage.1
Other potential adverse effects of aspirin treatment include maternal gastrointestinal bleeding and exacerbation of respiratory disorders such as asthma, but these effects have not been reported as significant associations in clinical trials of preeclampsia prevention.
Dueling recommendations: Restrictive or liberal use of aspirin?The American College of Obstetricians and Gynecologists (ACOG) recommends use of aspirin to prevent preeclampsia in women who have a personal history of early-onset preeclampsia with delivery before 34 weeks of gestation and in women with preeclampsia in 2 or more prior pregnancies.6 The restrictive ACOG guideline recommends aspirin treatment for a very small group of women. In one analysis, using the ACOG guideline, only 0.35% of all pregnant women would be eligible for treatment with aspirin to prevent preeclampsia.7
The USPSTF recommends that all pregnant women with one major risk factor for preeclampsia—including multifetal gestation, chronic hypertension, type 1 or 2 pregestational diabetes, renal disease, autoimmune disease, or prior personal history of preeclampsia—receive treatment with aspirin to prevent preeclampsia.8 The Task Force also recommends that women with multiple moderate risk factors for preeclampsia, such as nulliparity, body mass index greater than 30 kg/m2, family history of preeclampsia in a mother or sister, age 35 years or older, and certain sociodemographic risk factors (African American race, low socioeconomic status) also be offered aspirin treatment.
The USPSTF guideline advises aspirin treatment for many women. According to one analysis, the USPSTFguideline would result in approximately 24% of all pregnant women being offered aspirin treatment.7
The USPSTF guideline would result in 67 times more pregnant women being treated with aspirin than the ACOG guideline. The narrowly focused ACOG recommendation is problematic because it recommends against aspirin treatment in women who are at very high risk for developing preeclampsia, for example, a 41-year-old woman in her first pregnancy with twins and pregestational diabetes. In addition, the ACOG recommendation is not consistent with the recommendations of most other major health organizations.
The World Health Organization,9 the United Kingdom’s National Institute for Health and Care Excellence (NICE),10 and the Society of Obstetricians and Gynaecologists of Canada11 all recommend aspirin treatment to prevent preeclampsia in pregnant women at high risk for the disease and utilize an expanded definition of “high risk” (TABLE). Some experts have observed that, in actual clinical practice, it is often difficult to consistently implement a prevention plan based on a complex assessment of clinical risk factors.7

An alternative to guidelines that use clinical risk factors to identify women at high risk is universal treatment. With universal treatment all pregnant women are prescribed aspirin, thereby maximizing the clinical benefit but unnecessarily treating many women with aspirin.7 Universal treatment of pregnant women with aspirin appears to be cost-effective and would be associated with annual health care savings of $365 million.7
Timing of aspirin initiationIn one meta-analysis, initiating aspirin before 16 weeks’ gestation resulted in a greater reduction in preeclampsia than starting aspirin after 16 weeks.12 The USPSTF cautions that meta-analysis of the available data is not well suited for identifying the optimal time to initiate aspirin therapy.13 ACOG, USPSTF, and NICE recommend initiating aspirin therapy at approximately 12 weeks’ gestation—the end of the first trimester.
Ideal aspirin doseThe optimal dose of aspirin to prevent preeclampsia is not precisely defined. Aspirin doses ranging from 50 mg to 162 mg have been proposed for the prevention of preeclampsia. Most authorities recommend a daily dose between 80 mg and less than 300 mg to prevent preeclampsia.14 ACOG and USPSTF recommend aspirin at a dose of 81 mg daily,6,8 because this dose is widely available in the United States.
Let’s close the gap between current and optimal practiceAccording to the USPSTF guidelines, approximately 24% of the pregnant women in our practices have risk factors that would justify the initiation of aspirin treatment for the prevention of preeclampsia.8 This approach would modestly reduce the rate of preeclampsia and the associated problems of preterm birth and IUGR with little cost and few adverse effects. Yet relatively few pregnant women in the United States are currently receiving aspirin therapy. We could close this clinical gap between current and optimal practice by reflecting on the USPSTF recommendations and implementing them in our practices, as appropriate.
Tell us…What are your thoughts about the use of aspirin in pregnant women who are at high risk for preeclampsia?
Send your letter to the editor to [email protected]. Please include the city and state in which you practice.
- Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(10):695-703.
- Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol. 2013;41(5):491-499.
- Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 pt 1):402-414.
- Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Askie LM, Duley L, Henderson-Smart DJ, Stewart LA; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791-1798.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131.
- Werner EF, Hauspurg AK, Rouse DJ. A cost-benefit analysis of low-dose aspirin prophylaxis for the prevention of preeclampsia in the United States. Obstet Gynecol. 2015;126(6):1242-1250.
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819-826.
- World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva, Switzerland: WHO; 2011:13-15. https://www.preeclampsia.org/images/pdf/2011c-who_pe_final.pdf. Accessed January 4, 2016.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. Clinical guideline 107. Manchester, United Kingdom: NICE; 2010:7. https://www.nice.org.uk/guidance/cg107/resources/hypertension-in-pregnancy-diagnosis-and-management-35109334009285. Accessed April 4, 2016.
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416-441.
- Roberge S, Demers S, Bujold E. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613.
- Henderson JT, O'Connor E, Whitlock EP. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613-614.
- Bujold E, Roberge S, Nicolaides KH. Low-dose aspirin for prevention of adverse outcomes related to abnormal placentation. Prenat Diagn. 2014;34(7):642-648.
- Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(10):695-703.
- Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol. 2013;41(5):491-499.
- Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 pt 1):402-414.
- Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Askie LM, Duley L, Henderson-Smart DJ, Stewart LA; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791-1798.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131.
- Werner EF, Hauspurg AK, Rouse DJ. A cost-benefit analysis of low-dose aspirin prophylaxis for the prevention of preeclampsia in the United States. Obstet Gynecol. 2015;126(6):1242-1250.
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819-826.
- World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva, Switzerland: WHO; 2011:13-15. https://www.preeclampsia.org/images/pdf/2011c-who_pe_final.pdf. Accessed January 4, 2016.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. Clinical guideline 107. Manchester, United Kingdom: NICE; 2010:7. https://www.nice.org.uk/guidance/cg107/resources/hypertension-in-pregnancy-diagnosis-and-management-35109334009285. Accessed April 4, 2016.
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416-441.
- Roberge S, Demers S, Bujold E. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613.
- Henderson JT, O'Connor E, Whitlock EP. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613-614.
- Bujold E, Roberge S, Nicolaides KH. Low-dose aspirin for prevention of adverse outcomes related to abnormal placentation. Prenat Diagn. 2014;34(7):642-648.

