User login
“Moon face” is a term that’s become popular on social media, used to describe people with unusually round faces who are purported to have high levels of cortisol. But the term “moon face” isn’t new. It was actually coined in the 1930s by neurosurgeon Harvey Cushing, MD, who identified patients with a constellation of clinical characteristics — a condition that came to bear his name — which included rapidly developing facial adiposity. And indeed, elevated cortisol is a hallmark feature of Cushing syndrome (CS), but there are other reasons for elevated cortisol and other manifestations of CS.
Today, the term “moon face” has been replaced with “round face,” which is considered more encompassing and culturally sensitive, said Maria Fleseriu, MD, professor of medicine and neurological surgery and director of the Pituitary Center at Oregon Health and Science University in Portland, Oregon.
Facial roundness can lead clinicians to be suspicious that their patient is experiencing CS. But because a round face is associated with several other conditions, it’s important to be familiar with its particular presentation in CS, as well as how to diagnose and treat CS.
Pathophysiology of CS
Dr. Fleseriu defined CS as “prolonged nonphysiologic increase in cortisol, due either to exogenous use of steroids (oral, topical, or inhaled) or to excess endogenous cortisol production.” She added that it’s important “to always exclude exogenous causes before conducting a further workup to determine the type and cause of cortisol excess.”
Dr. Fleseriu said. Other causes of CS are ectopic (caused by neuroendocrine tumors) or adrenal. CS affects primarily females and typically has an onset between ages 20 and 50 years, depending on the CS type.
Diagnosis of CS is “substantially delayed for most patients, due to metabolic syndrome phenotypic overlap and lack of a single pathognomonic symptom,” according to Dr. Fleseriu.
An accurate diagnosis should be on the basis of signs and symptoms, biochemical screening, other laboratory testing, and diagnostic imaging.
Look for Clinical Signs and Symptoms of CS
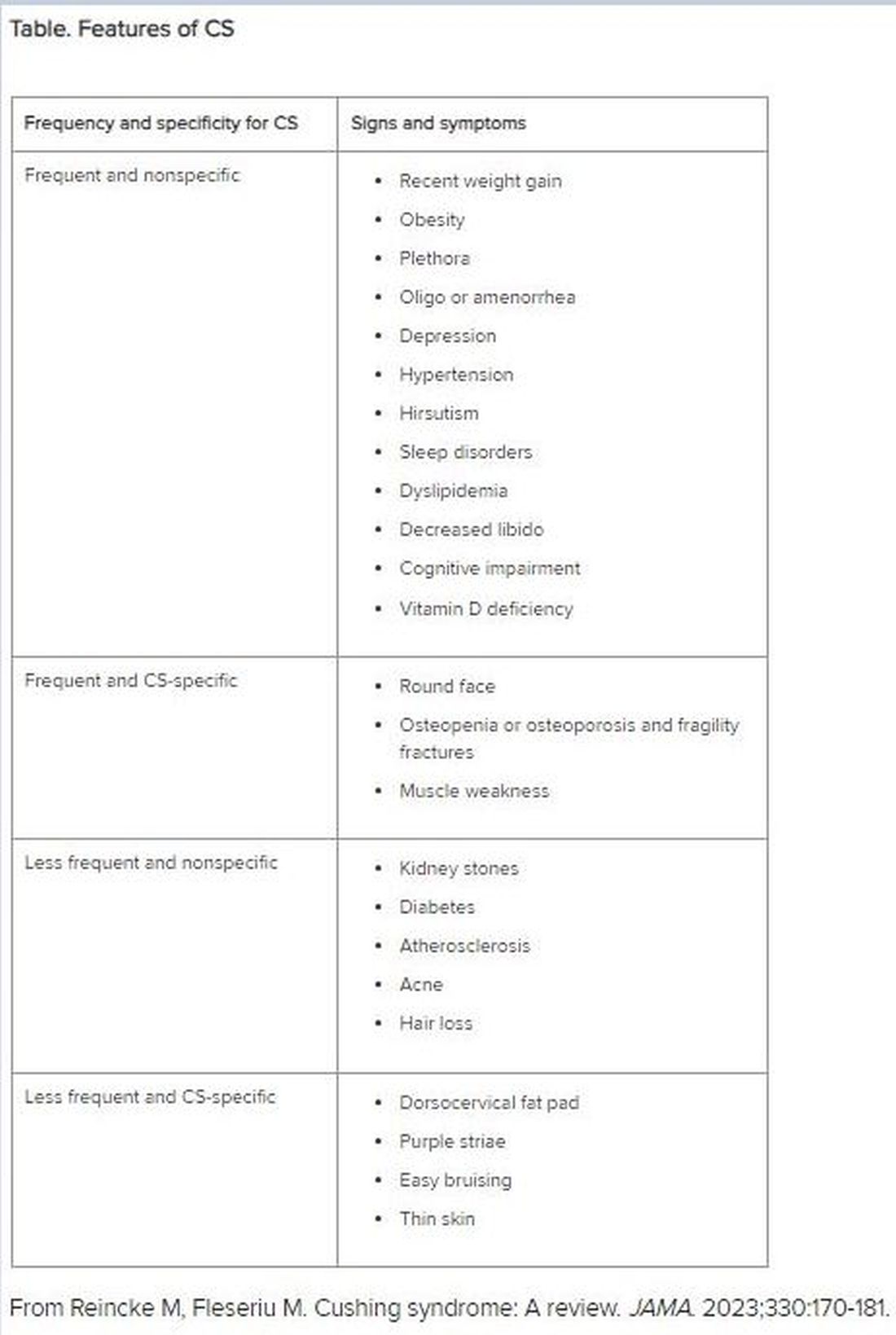
“CS mostly presents as a combination of two or more features,” Dr. Fleseriu stated. These include increased fat pads (in the face, neck, and trunk), skin changes, signs of protein catabolism, growth retardation and body weight increase in children, and metabolic dysregulations (Table).
“Biochemical screening should be performed in patients with a combination of symptoms, and therefore an increased pretest probability for CS,” Dr. Fleseriu advised.
A CS diagnosis requires not only biochemical confirmation of hypercortisolemia but also determination of the underlying cause of the excess endogenous cortisol production. This is a key step, as the management of CS is specific to its etiology.
Elevated plasma cortisol alone is insufficient for diagnosing CS, as several conditions can be associated with physiologic, nonneoplastic endogenous hypercortisolemia, according to the 2021 updated CS guidelines for which Dr. Fleseriu served as a coauthor. These include depression, alcohol dependence, glucocorticoid resistance, obesity, diabetes, pregnancy, prolonged physical exertion, malnutrition, and cortisol-binding globulin excess.
The diagnosis begins with the following screening tests:
- Late-night salivary cortisol (LNSC) to assess an abnormal circadian rhythm
According to the 2021 guideline, this is “based on the assumption that patients with CS lose the normal circadian nadir of cortisol secretion.”
- Overnight 1-mg dexamethasone suppression test (DST) to assess impaired glucocorticoid feedback
The authors noted that in healthy individuals, a supraphysiologic dexamethasone dose inhibits vasopressin and adrenocorticotropic hormone (ACTH) secretion, leading to decreased cortisol concentration. Cortisol concentrations of < 1-8 μg/dL in the morning (after administration of the dexamethasone between 11 p.m. and midnight) are considered “normal,” and a negative result “strongly predicts” the absence of CS. But false-positive and false-negative results can occur. Thus, “it is imperative that first-line testing is elected on the basis of physiologic conditions and drug intake — for example, use of CYP2A4/5 inhibitors or stimulators and oral estrogen — as well as laboratory quality control measure, and special attention to night shift workers,” Dr. Fleseriu emphasized.
- A 24-hour urinary free cortisol (UFC) test to assess increased bioavailable cortisol
The guideline encourages conducting several 24-hour urine collections to account for intra-patient variability.
Dr. Fleseriu recommended utilizing at least two of the three screening tests, all of which have reasonable sensitivity and specificity.
“Two normal test results usually exclude the presence of CS, except in rare cyclic CS,” she added.
Conduct Additional Laboratory Testing
Additional laboratory abnormalities suggestive of CS include:
- Increased leukocytes with decreased lymphocytes, eosinophils, monocytes, and basophils
- Elevated glucose and insulin levels
- Hypokalemia
- Increased triglycerides and total cholesterol levels
- Elevated liver enzymes
- Changes in activated thromboplastin time and plasma concentrations of pro- and anticoagulant factors
- Hypercalciuria, hypocalcemia (rare), hypophosphatemia, decreased phosphate maximum resorption, and increased alkaline phosphatase activity
Dr. Fleseriu noted that, in most cases, a final CS diagnosis can be reached after confirmation of biochemical hypercortisolism, which is done after an initial positive screening test.
She added that plasma ACTH levels are “instrumental” in distinguishing ACTH-depending forms of CS — such as Cushing disease and ectopic CS — from adrenal cases. Bilateral inferior petrosal sinus sampling is necessary in ACTH-dependent CS.
Utilize Diagnostic Imaging
There are several diagnostic imaging techniques that localize the origin of the hypercortisolism, thus informing the course of treatment.
- Pituitary MRI to detect corticotropin-secreting corticotroph adenomas, which are typically small lesions (< 6 mm in diameter)
- CT evaluation of the neck, thoracic cavity, and abdomen to diagnose ectopic CS, including lung neuroendocrine tumors and bronchial neuroendocrine tumors
- Cervical and thyroid ultrasonography to identify primary or metastatic medullary thyroid carcinoma, and PET scans, which have greater sensitivity in detecting tumors, compared with CT scans
- Contrast-enhanced CT scans to detect adrenal adenomas and adrenocortical carcinomas
Management of CS
“The primary aim of treatment is eucortisolemia, and in those with endogenous CS, complete surgical resection of the underlying tumor is the primary method,” Dr. Fleseriu said.
It’s critical to monitor for biochemical remission following surgery, utilizing 24-hour UFC, LNSC, and DST “because clinical manifestations may lag behind biochemical evidence.”
In Cushing disease, almost half of patients will have either persistent or recurrent hypercortisolemia after surgery. In those cases, individualized adjuvant treatments are recommended. These include repeat surgery, bilateral adrenalectomy, radiation, or medical treatments, including pituitary-directed drugs, adrenal steroidogenesis inhibitors, or glucocorticoid receptor-blocking agents. The last two groups are used for other types of CS.
Dr. Fleseriu pointed out that CS is “associated with increased metabolic, cardiovascular, psychiatric, infectious, and musculoskeletal morbidity, which are only partially reversible with successful [CS] treatment.” These comorbidities need to be addressed via individualized therapies. Moreover, long-term mortality is increased in all forms of CS. Thus, patients require lifelong follow-up to detect recurrence at an early stage and to treat comorbidities.
“It is likely that delayed diagnosis might explain the long-term consequences of CS, including increased morbidity and mortality despite remission,” she said.
Familiarity with the presenting signs and symptoms of CS and ordering recommended screening and confirmatory tests will enable appropriate management of the condition, leading to better outcomes.
Dr. Fleseriu reported receiving research grants from Sparrow Pharmaceuticals to Oregon Health and Science University as principal investigator and receiving occasional fees for scientific consulting/advisory boards from Sparrow Pharmaceuticals, Recordati Rare Diseases Inc., and Xeris Biopharma Holdings Inc.
A version of this article first appeared on Medscape.com.
“Moon face” is a term that’s become popular on social media, used to describe people with unusually round faces who are purported to have high levels of cortisol. But the term “moon face” isn’t new. It was actually coined in the 1930s by neurosurgeon Harvey Cushing, MD, who identified patients with a constellation of clinical characteristics — a condition that came to bear his name — which included rapidly developing facial adiposity. And indeed, elevated cortisol is a hallmark feature of Cushing syndrome (CS), but there are other reasons for elevated cortisol and other manifestations of CS.
Today, the term “moon face” has been replaced with “round face,” which is considered more encompassing and culturally sensitive, said Maria Fleseriu, MD, professor of medicine and neurological surgery and director of the Pituitary Center at Oregon Health and Science University in Portland, Oregon.
Facial roundness can lead clinicians to be suspicious that their patient is experiencing CS. But because a round face is associated with several other conditions, it’s important to be familiar with its particular presentation in CS, as well as how to diagnose and treat CS.
Pathophysiology of CS
Dr. Fleseriu defined CS as “prolonged nonphysiologic increase in cortisol, due either to exogenous use of steroids (oral, topical, or inhaled) or to excess endogenous cortisol production.” She added that it’s important “to always exclude exogenous causes before conducting a further workup to determine the type and cause of cortisol excess.”
Dr. Fleseriu said. Other causes of CS are ectopic (caused by neuroendocrine tumors) or adrenal. CS affects primarily females and typically has an onset between ages 20 and 50 years, depending on the CS type.
Diagnosis of CS is “substantially delayed for most patients, due to metabolic syndrome phenotypic overlap and lack of a single pathognomonic symptom,” according to Dr. Fleseriu.
An accurate diagnosis should be on the basis of signs and symptoms, biochemical screening, other laboratory testing, and diagnostic imaging.
Look for Clinical Signs and Symptoms of CS
“CS mostly presents as a combination of two or more features,” Dr. Fleseriu stated. These include increased fat pads (in the face, neck, and trunk), skin changes, signs of protein catabolism, growth retardation and body weight increase in children, and metabolic dysregulations (Table).
“Biochemical screening should be performed in patients with a combination of symptoms, and therefore an increased pretest probability for CS,” Dr. Fleseriu advised.
A CS diagnosis requires not only biochemical confirmation of hypercortisolemia but also determination of the underlying cause of the excess endogenous cortisol production. This is a key step, as the management of CS is specific to its etiology.
Elevated plasma cortisol alone is insufficient for diagnosing CS, as several conditions can be associated with physiologic, nonneoplastic endogenous hypercortisolemia, according to the 2021 updated CS guidelines for which Dr. Fleseriu served as a coauthor. These include depression, alcohol dependence, glucocorticoid resistance, obesity, diabetes, pregnancy, prolonged physical exertion, malnutrition, and cortisol-binding globulin excess.
The diagnosis begins with the following screening tests:
- Late-night salivary cortisol (LNSC) to assess an abnormal circadian rhythm
According to the 2021 guideline, this is “based on the assumption that patients with CS lose the normal circadian nadir of cortisol secretion.”
- Overnight 1-mg dexamethasone suppression test (DST) to assess impaired glucocorticoid feedback
The authors noted that in healthy individuals, a supraphysiologic dexamethasone dose inhibits vasopressin and adrenocorticotropic hormone (ACTH) secretion, leading to decreased cortisol concentration. Cortisol concentrations of < 1-8 μg/dL in the morning (after administration of the dexamethasone between 11 p.m. and midnight) are considered “normal,” and a negative result “strongly predicts” the absence of CS. But false-positive and false-negative results can occur. Thus, “it is imperative that first-line testing is elected on the basis of physiologic conditions and drug intake — for example, use of CYP2A4/5 inhibitors or stimulators and oral estrogen — as well as laboratory quality control measure, and special attention to night shift workers,” Dr. Fleseriu emphasized.
- A 24-hour urinary free cortisol (UFC) test to assess increased bioavailable cortisol
The guideline encourages conducting several 24-hour urine collections to account for intra-patient variability.
Dr. Fleseriu recommended utilizing at least two of the three screening tests, all of which have reasonable sensitivity and specificity.
“Two normal test results usually exclude the presence of CS, except in rare cyclic CS,” she added.
Conduct Additional Laboratory Testing
Additional laboratory abnormalities suggestive of CS include:
- Increased leukocytes with decreased lymphocytes, eosinophils, monocytes, and basophils
- Elevated glucose and insulin levels
- Hypokalemia
- Increased triglycerides and total cholesterol levels
- Elevated liver enzymes
- Changes in activated thromboplastin time and plasma concentrations of pro- and anticoagulant factors
- Hypercalciuria, hypocalcemia (rare), hypophosphatemia, decreased phosphate maximum resorption, and increased alkaline phosphatase activity
Dr. Fleseriu noted that, in most cases, a final CS diagnosis can be reached after confirmation of biochemical hypercortisolism, which is done after an initial positive screening test.
She added that plasma ACTH levels are “instrumental” in distinguishing ACTH-depending forms of CS — such as Cushing disease and ectopic CS — from adrenal cases. Bilateral inferior petrosal sinus sampling is necessary in ACTH-dependent CS.
Utilize Diagnostic Imaging
There are several diagnostic imaging techniques that localize the origin of the hypercortisolism, thus informing the course of treatment.
- Pituitary MRI to detect corticotropin-secreting corticotroph adenomas, which are typically small lesions (< 6 mm in diameter)
- CT evaluation of the neck, thoracic cavity, and abdomen to diagnose ectopic CS, including lung neuroendocrine tumors and bronchial neuroendocrine tumors
- Cervical and thyroid ultrasonography to identify primary or metastatic medullary thyroid carcinoma, and PET scans, which have greater sensitivity in detecting tumors, compared with CT scans
- Contrast-enhanced CT scans to detect adrenal adenomas and adrenocortical carcinomas
Management of CS
“The primary aim of treatment is eucortisolemia, and in those with endogenous CS, complete surgical resection of the underlying tumor is the primary method,” Dr. Fleseriu said.
It’s critical to monitor for biochemical remission following surgery, utilizing 24-hour UFC, LNSC, and DST “because clinical manifestations may lag behind biochemical evidence.”
In Cushing disease, almost half of patients will have either persistent or recurrent hypercortisolemia after surgery. In those cases, individualized adjuvant treatments are recommended. These include repeat surgery, bilateral adrenalectomy, radiation, or medical treatments, including pituitary-directed drugs, adrenal steroidogenesis inhibitors, or glucocorticoid receptor-blocking agents. The last two groups are used for other types of CS.
Dr. Fleseriu pointed out that CS is “associated with increased metabolic, cardiovascular, psychiatric, infectious, and musculoskeletal morbidity, which are only partially reversible with successful [CS] treatment.” These comorbidities need to be addressed via individualized therapies. Moreover, long-term mortality is increased in all forms of CS. Thus, patients require lifelong follow-up to detect recurrence at an early stage and to treat comorbidities.
“It is likely that delayed diagnosis might explain the long-term consequences of CS, including increased morbidity and mortality despite remission,” she said.
Familiarity with the presenting signs and symptoms of CS and ordering recommended screening and confirmatory tests will enable appropriate management of the condition, leading to better outcomes.
Dr. Fleseriu reported receiving research grants from Sparrow Pharmaceuticals to Oregon Health and Science University as principal investigator and receiving occasional fees for scientific consulting/advisory boards from Sparrow Pharmaceuticals, Recordati Rare Diseases Inc., and Xeris Biopharma Holdings Inc.
A version of this article first appeared on Medscape.com.
“Moon face” is a term that’s become popular on social media, used to describe people with unusually round faces who are purported to have high levels of cortisol. But the term “moon face” isn’t new. It was actually coined in the 1930s by neurosurgeon Harvey Cushing, MD, who identified patients with a constellation of clinical characteristics — a condition that came to bear his name — which included rapidly developing facial adiposity. And indeed, elevated cortisol is a hallmark feature of Cushing syndrome (CS), but there are other reasons for elevated cortisol and other manifestations of CS.
Today, the term “moon face” has been replaced with “round face,” which is considered more encompassing and culturally sensitive, said Maria Fleseriu, MD, professor of medicine and neurological surgery and director of the Pituitary Center at Oregon Health and Science University in Portland, Oregon.
Facial roundness can lead clinicians to be suspicious that their patient is experiencing CS. But because a round face is associated with several other conditions, it’s important to be familiar with its particular presentation in CS, as well as how to diagnose and treat CS.
Pathophysiology of CS
Dr. Fleseriu defined CS as “prolonged nonphysiologic increase in cortisol, due either to exogenous use of steroids (oral, topical, or inhaled) or to excess endogenous cortisol production.” She added that it’s important “to always exclude exogenous causes before conducting a further workup to determine the type and cause of cortisol excess.”
Dr. Fleseriu said. Other causes of CS are ectopic (caused by neuroendocrine tumors) or adrenal. CS affects primarily females and typically has an onset between ages 20 and 50 years, depending on the CS type.
Diagnosis of CS is “substantially delayed for most patients, due to metabolic syndrome phenotypic overlap and lack of a single pathognomonic symptom,” according to Dr. Fleseriu.
An accurate diagnosis should be on the basis of signs and symptoms, biochemical screening, other laboratory testing, and diagnostic imaging.
Look for Clinical Signs and Symptoms of CS
“CS mostly presents as a combination of two or more features,” Dr. Fleseriu stated. These include increased fat pads (in the face, neck, and trunk), skin changes, signs of protein catabolism, growth retardation and body weight increase in children, and metabolic dysregulations (Table).
“Biochemical screening should be performed in patients with a combination of symptoms, and therefore an increased pretest probability for CS,” Dr. Fleseriu advised.
A CS diagnosis requires not only biochemical confirmation of hypercortisolemia but also determination of the underlying cause of the excess endogenous cortisol production. This is a key step, as the management of CS is specific to its etiology.
Elevated plasma cortisol alone is insufficient for diagnosing CS, as several conditions can be associated with physiologic, nonneoplastic endogenous hypercortisolemia, according to the 2021 updated CS guidelines for which Dr. Fleseriu served as a coauthor. These include depression, alcohol dependence, glucocorticoid resistance, obesity, diabetes, pregnancy, prolonged physical exertion, malnutrition, and cortisol-binding globulin excess.
The diagnosis begins with the following screening tests:
- Late-night salivary cortisol (LNSC) to assess an abnormal circadian rhythm
According to the 2021 guideline, this is “based on the assumption that patients with CS lose the normal circadian nadir of cortisol secretion.”
- Overnight 1-mg dexamethasone suppression test (DST) to assess impaired glucocorticoid feedback
The authors noted that in healthy individuals, a supraphysiologic dexamethasone dose inhibits vasopressin and adrenocorticotropic hormone (ACTH) secretion, leading to decreased cortisol concentration. Cortisol concentrations of < 1-8 μg/dL in the morning (after administration of the dexamethasone between 11 p.m. and midnight) are considered “normal,” and a negative result “strongly predicts” the absence of CS. But false-positive and false-negative results can occur. Thus, “it is imperative that first-line testing is elected on the basis of physiologic conditions and drug intake — for example, use of CYP2A4/5 inhibitors or stimulators and oral estrogen — as well as laboratory quality control measure, and special attention to night shift workers,” Dr. Fleseriu emphasized.
- A 24-hour urinary free cortisol (UFC) test to assess increased bioavailable cortisol
The guideline encourages conducting several 24-hour urine collections to account for intra-patient variability.
Dr. Fleseriu recommended utilizing at least two of the three screening tests, all of which have reasonable sensitivity and specificity.
“Two normal test results usually exclude the presence of CS, except in rare cyclic CS,” she added.
Conduct Additional Laboratory Testing
Additional laboratory abnormalities suggestive of CS include:
- Increased leukocytes with decreased lymphocytes, eosinophils, monocytes, and basophils
- Elevated glucose and insulin levels
- Hypokalemia
- Increased triglycerides and total cholesterol levels
- Elevated liver enzymes
- Changes in activated thromboplastin time and plasma concentrations of pro- and anticoagulant factors
- Hypercalciuria, hypocalcemia (rare), hypophosphatemia, decreased phosphate maximum resorption, and increased alkaline phosphatase activity
Dr. Fleseriu noted that, in most cases, a final CS diagnosis can be reached after confirmation of biochemical hypercortisolism, which is done after an initial positive screening test.
She added that plasma ACTH levels are “instrumental” in distinguishing ACTH-depending forms of CS — such as Cushing disease and ectopic CS — from adrenal cases. Bilateral inferior petrosal sinus sampling is necessary in ACTH-dependent CS.
Utilize Diagnostic Imaging
There are several diagnostic imaging techniques that localize the origin of the hypercortisolism, thus informing the course of treatment.
- Pituitary MRI to detect corticotropin-secreting corticotroph adenomas, which are typically small lesions (< 6 mm in diameter)
- CT evaluation of the neck, thoracic cavity, and abdomen to diagnose ectopic CS, including lung neuroendocrine tumors and bronchial neuroendocrine tumors
- Cervical and thyroid ultrasonography to identify primary or metastatic medullary thyroid carcinoma, and PET scans, which have greater sensitivity in detecting tumors, compared with CT scans
- Contrast-enhanced CT scans to detect adrenal adenomas and adrenocortical carcinomas
Management of CS
“The primary aim of treatment is eucortisolemia, and in those with endogenous CS, complete surgical resection of the underlying tumor is the primary method,” Dr. Fleseriu said.
It’s critical to monitor for biochemical remission following surgery, utilizing 24-hour UFC, LNSC, and DST “because clinical manifestations may lag behind biochemical evidence.”
In Cushing disease, almost half of patients will have either persistent or recurrent hypercortisolemia after surgery. In those cases, individualized adjuvant treatments are recommended. These include repeat surgery, bilateral adrenalectomy, radiation, or medical treatments, including pituitary-directed drugs, adrenal steroidogenesis inhibitors, or glucocorticoid receptor-blocking agents. The last two groups are used for other types of CS.
Dr. Fleseriu pointed out that CS is “associated with increased metabolic, cardiovascular, psychiatric, infectious, and musculoskeletal morbidity, which are only partially reversible with successful [CS] treatment.” These comorbidities need to be addressed via individualized therapies. Moreover, long-term mortality is increased in all forms of CS. Thus, patients require lifelong follow-up to detect recurrence at an early stage and to treat comorbidities.
“It is likely that delayed diagnosis might explain the long-term consequences of CS, including increased morbidity and mortality despite remission,” she said.
Familiarity with the presenting signs and symptoms of CS and ordering recommended screening and confirmatory tests will enable appropriate management of the condition, leading to better outcomes.
Dr. Fleseriu reported receiving research grants from Sparrow Pharmaceuticals to Oregon Health and Science University as principal investigator and receiving occasional fees for scientific consulting/advisory boards from Sparrow Pharmaceuticals, Recordati Rare Diseases Inc., and Xeris Biopharma Holdings Inc.
A version of this article first appeared on Medscape.com.