User login
Pediatric asthma is the most commonly encountered childhood chronic disease, occurring in approximately 13.5% of children.1 Due to the interplay between patient, family physician (FP), and the environment, asthma often proves challenging to control. Although national guidelines for the treatment of asthma have not changed since 2007, significant research continues to examine the optimal means of preventing, controlling, and treating asthma in children. This review summarizes the evidence to date so that you can maximize your care for these young patients.
A stepwise approach to asthma control
The 2007 National Heart, Lung, and Blood Institute (NHLBI) guidelines provide a common pathway for the management of asthma (FIGURE).2 These guidelines emphasize stepwise treatment, based on symptom severity, which maximizes quality of life while minimizing morbidity. Treatment is escalated after careful assessment of frequency of daytime symptoms, frequency of nighttime symptoms, forced expiratory volume in one second (FEV1), and the number of exacerbations requiring systemic steroids over the past year. It’s also appropriate to de-escalate care when symptoms are controlled to minimize adverse effects.
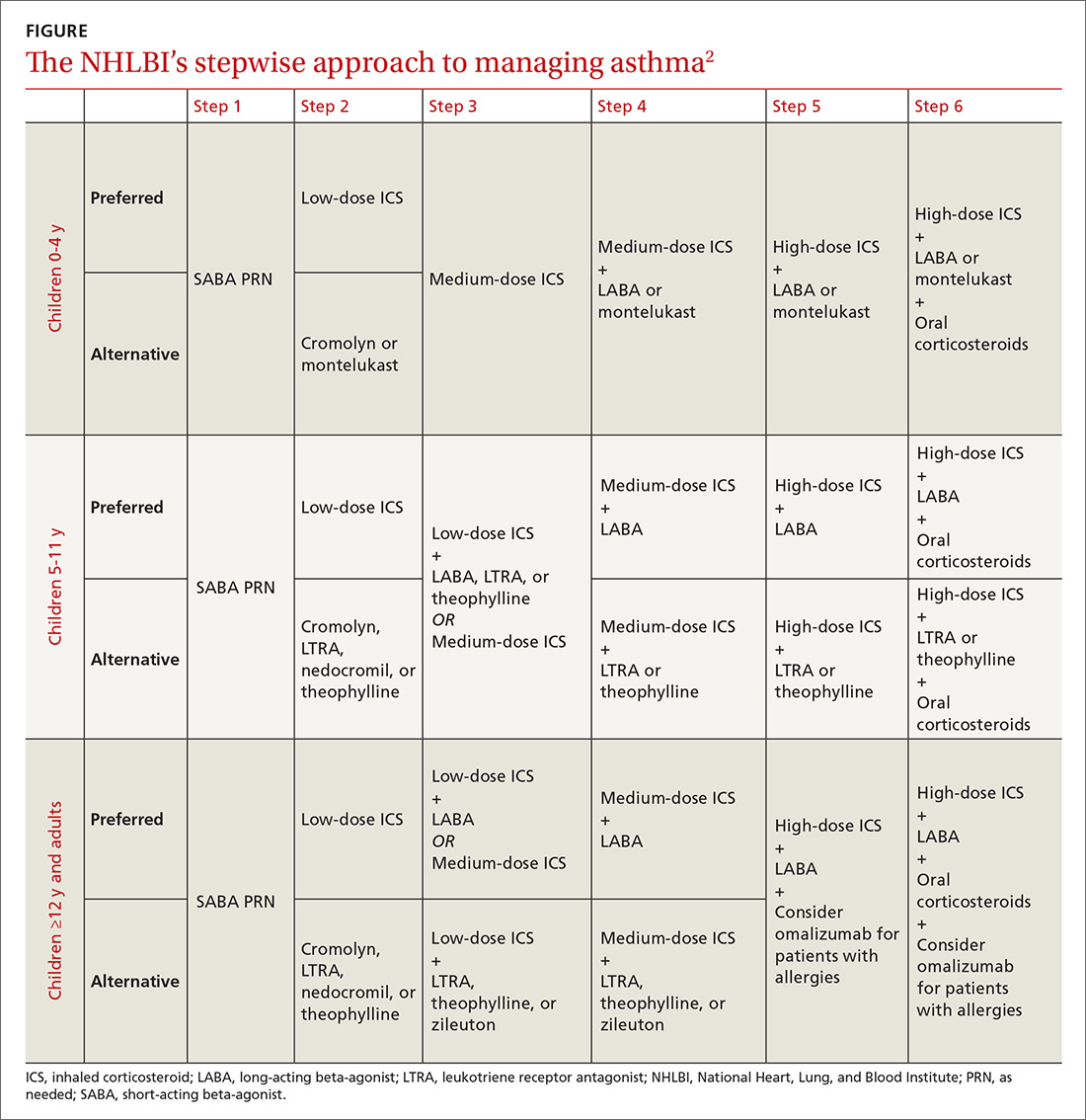
The 2017 Global Initiative for Asthma (GINA) guidelines recommend a similar stepwise approach, with generally the same progression of control medications as the NHLBI guidelines. One variation is that GINA guidelines recommend considering inhaled corticosteroids (ICS) for all asthma patients—even those with intermittent symptoms.3
The Asthma Control Test and Asthma Control Questionnaire are also available for assessment of efficacy of asthma control and can help guide FPs with the decision of when to escalate control medications. (They are available at: http://bit.ly/2o3CzGX and http://bit.ly/2p1LvAc, respectively.)
A systematic review found that both tools were effective in determining if a patient was well controlled vs not well controlled.4 Similarly, a 2012 study found that using the Asthma Control Test score was useful for assessing control and directing changes to treatment.5 In addition, using these tools consistently in a primary care setting increased the frequency of assessment without negatively impacting patient flow through a clinic.6
Short-acting beta-agonists: A mainstay for intermittent asthma
Inhaled short-acting beta-agonists (SABAs) are the mainstay of treatment for intermittent asthma as well as asthma exacerbations. Short-acting beta-agonists are effective for symptomatic relief and preventing exacerbations prior to known exposures because they dilate smooth muscle in bronchioles and relieve bronchospasm.
Albuterol, the most commonly used SABA, is a mixture of the active R-enantiomer and inactive L-enantiomer. Levalbuterol, which consists of only the active R-enantiomer, is also available and is theoretically more effective with fewer adverse effects. Studies examining the difference in efficacy between albuterol and levalbuterol, however, have been mixed, and current guidelines do not recommend one over the other.7
Metered-dose inhalers vs nebulizers
SABAs are typically prescribed in metered-dose inhalers (MDIs), dry powder inhalers, or nebulizers. A meta-analysis comparing the efficacy of nebulizers to MDIs with spacers in both the outpatient and emergency department (ED) settings indicated that MDIs work at least as well as nebulizers and may also reduce length of ED stay.8 This trend appears consistent even with children younger than 24 months.9
If you are prescribing an MDI, be sure to routinely prescribe spacers to help ensure the medication is properly administered. Various types of spacers are available; some consist of an extension of the mouthpiece, while others serve as a chamber with a one-way valve to help improve the ability of the child to inhale the medication. Spacers that do not have antistatic coating should be gently washed with water and detergent.
Masks are also available for younger children and should be properly sized. The same spacer can be used for multiple medications, although they should be administered one at a time. Generally, albuterol should be administered prior to other medications to maximize distribution of subsequent inhaled medications.
Start low with inhaled corticosteroids
Treatment of persistent asthma consists of regular use of inhaled corticosteroids (ICSs; first line) with SABAs, as needed, for exacerbations. Guidelines recommend starting at a low dose and increasing the dose based on symptom control. Patients must consistently use ICSs for one to 2 weeks prior to obtaining full effect of the medication, and parents should be counseled to set appropriate expectations.2,3 ICSs are dispensed as MDIs, dry powder inhalers, and nebulizers; spacers should be considered.
Studies have shown a slight decrease in height for children on ICSs, with long-term studies indicating a persistent 1- to 2-cm decrease in adult height.10,11
Patient isn’t well controlled? Time for a long-acting beta-agonist
For patients not well controlled on low-dose ICSs, the dose can be increased or a long-acting beta-agonist (LABA) or leukotriene receptor antagonist (LTRA) can be added. A recent meta-analysis examining children not well controlled with ICSs, found that the addition of LABAs resulted in improved FEV1 and nighttime symptoms, and reduced the requirement for rescue inhalers when compared to increasing the ICS dose alone.12 In addition, there was no difference in adverse events between the 2 agents, although patients taking ICSs and LABAs had greater growth in height than those with an increased dose of ICS.12 Adding a LABA, however, did not decrease the need for systemic steroids and also did not reduce the number of exacerbations requiring hospitalizations.12
Another recent study demonstrated that the combination of ICSs and LABAs was noninferior to ICSs alone in preventing hospitalizations, intubations, and deaths.13 There are limited data on whether patients already on LABAs and ICSs should be continued on dual medications. Additionally, there is no clear method describing how to de-escalate therapy for those patients who are well controlled on ICSs and LABAs. A reasonable approach is to reduce doses of both medications and discontinue the LABA if tolerated.14,15 The US Food and Drug Administration has issued a black box warning that LABAs should not be used as a single controller medication because patients may be at increased risk of asthma-related deaths.16,17
What role for leukotriene receptor antagonists?
According to NHLBI guidelines, LTRAs can be considered as an alternative to ICSs when starting a control medication for mild persistent asthma.2 A recent meta-analysis, however, showed that there were increased rates of hospitalizations with LTRAs alone when compared to ICSs.18 The NHLBI guidelines also suggest that LTRAs can be used as adjunctive medication for those patients not well controlled on ICSs rather than increasing the ICS dose or adding a LABA.2
A 2011 clinical trial found no difference in quality of life measures between LTRAs and LABAs as adjunctive therapy at 2 months, but LABAs were more effective when patients were reassessed in 2 years.19 Similarly, the same study also found that adding LTRAs to low-dose ICSs rather than increasing the ICS dose was equivalent in the short term but not at 2 years.19
2 other adjunctive therapy options: Xanthines, cromolyn
Similar to LTRAs, xanthines can be considered as adjunctive therapy for children older than 5 years who are not well controlled on a low-dose ICS. Although xanthines decrease asthma symptoms when compared to placebo alone, they are not more effective than ICSs alone and should be considered only as adjunctive therapy.2,20 There have been few studies comparing xanthines to other adjunctive medications.21
Cromolyn is another adjunctive medication cited in the NHLBI guidelines for escalation of therapy.2 Although the medication has few adverse effects, its use is generally limited in the United States because data supporting its efficacy are lacking.
Omalizumab for allergy-related asthma exacerbations
Omalizumab, an anti-IgE antibody injected every 2 to 4 weeks, is available for children older than 6 years with moderate to severe asthma that is not responsive to ICSs and LABAs.22 The medication is effective in reducing allergy-related asthma exacerbations and hospitalizations, but data comparing it to other adjunctive medications are limited.22 Due to their significant systemic effects, the role of oral steroids as control medications is reserved for patients with severe asthma who are refractory to other medications. Children should be placed on oral steroids for the least amount of time required to achieve symptom control.
Acute exacerbation treatment: What to consider
Although there is no agreed-upon definition for an acute asthma exacerbation, the American Thoracic Society defines it as "an event characterized by a change from the patient’s previous status."23 All patients should be given an asthma action plan that clearly delineates the escalation of therapy in the event of an exacerbation, although only half of all patients report experiencing one.24 Symptom-based plans may prevent more acute care visits when compared to plans that use peak-flow measurements, although children on peak-flow plans may have fewer symptomatic days.25
Start with short-acting beta-agonists
The SABAs serve as the initial treatment of choice for management of asthma exacerbations. In young children (0-3 years), SABAs delivered by MDI with a spacer were more effective in reducing admission rates (11.3% vs 21.7%) when compared to SABAs delivered by nebulizers, resulting in a number needed to treat to prevent one admission of 10.26
In older children (3-18 years), SABAs delivered via spacer reduced ED length of stay, but did not significantly affect hospitalization rates. Additionally, SABAs administered with anticholinergics such as ipratropium bromide were more effective than SABAs alone in reducing admissions (16.9% vs 23.2%), particularly in older children with moderate to severe asthma, while also minimizing adverse effects.8,26
Corticosteroids: A mainstay in the ED
In addition to albuterol administration, corticosteroids remain the mainstay of ED management for asthma exacerbations. Administration of systemic steroids has been shown to reduce hospitalizations in children under 6 years, although, paradoxically, studies examining outpatient administration have demonstrated an increase in hospitalizations when compared to placebo.27
Dexamethasone and prednisone are the 2 most commonly used systemic steroids, and studies haven't indicated superiority of either.28,29 There is no difference in efficacy between oral and intravenous steroids.30 A recent clinical trial found a 2-day course of dexamethasone (0.6 mg/kg) had similar efficacy with fewer adverse effects when compared to a 5-day course of prednisone (1-2 mg/kg/day).28
Patient isn’t responding? Try IV magnesium
For patients who don't respond to corticosteroids and albuterol treatments, IV magnesium sulfate (usual dose, 25-75 mg/kg/d; maximum dose, 2000 mg/d) has been shown to improve respiratory function, but not necessarily decrease admission rates.31 Inhaled magnesium sulfate hasn't been shown to be more effective than IV administration and isn'trecommended.32
Evidence doesn’t support use of heliox
Heliox, which consists of 80% helium, is theorized to be effective in the treatment of asthma by increasing laminar flow and increasing the delivery of medications to the alveoli.32 Overall, the evidence does not support the use of heliox, which is typically restricted to patients with severe asthma exacerbations.33
Is it worth considering noninvasive positive pressure ventilation?
If patients don't improve with medical treatment, noninvasive positive pressure ventilation can be considered. A recent meta-analysis suggests that there is no definitive benefit or harm from this treatment, although several studies have indicated a decrease in symptom severity.34
Intubation should be considered for hypoxemia unresponsive to medications, or in cases of exhaustion, worsening mental status, or respiratory acidosis unresponsive to medication. Ventilation should allow for a permissive respiratory acidosis (pH, 7.2), while maintaining adequate oxygenation.35
Reducing the burden of asthma
Due to the complex task of reducing triggers and providing effective controller medications, working with parents and children is integral to improving the quality of life for patients with asthma. Although there is an obvious genetic predisposition, family physicians can help reduce the risk of developing asthma by encouraging healthy behaviors at home before the child is born. In the prenatal period, this includes avoiding tobacco-smoke exposure, lessening maternal obesity, decreasing maternal antibiotic and acetaminophen use, and curtailing stress.
Evidence suggests that after birth, breastfeeding and reducing childhood obesity can help lower the risk of asthma.36 Atopic disease, in general, can be reduced by breastfeeding until at least 4 months, as well as encouraging a varied diet that does not restrict potential allergens during pregnancy or lactation, and introducing foods (including potential allergens) after the age of 4 months.
The risk of atopic disease can also be lowered by lessening potential triggers at home. These include restricting exposure to cats (but not dogs), reducing home mold by decreasing humidity and ensuring adequate ventilation, avoiding volatile organic compounds, such as chlorine, and curtailing exposure to vehicle emissions. Although often marketed to be effective in reducing allergies, dust-mite covers and soy-based formulas don't prevent or minimize allergies and are often costly.37,38 In addition, there is no evidence that vaccinations areassociated with allergies.39
CORRESPONDENCE
Douglas M. Maurer, 9040A Jackson Ave, Joint-Base Lewis-McChord, WA 98431; [email protected].
1. US Department of Health and Human Services. Centers for Disease Control and Prevention. Asthma and Schools. Available at: www.cdc.gov/healthyschools/asthma/index.htm. Updated June 17, 2015. Accessed September 28, 2016.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007. Report No.:07-4051.
3. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2017. Available at: www.ginasthma.org. Accessed April 11, 2017.
4. Jia CE, Zhang HP, Lv Y, et al. The asthma control test and asthma control questionnaire for assessing asthma control: systematic review and meta-analysis. J Allergy Clin Immunol. 2013;131:695-703.
5. Ko FW, Hui DS, Leung TS, et al. Evaluation of the asthma control test: a reliable determinant of disease stability and a predictor of future exacerbations. Respirology. 2012;17: 370-378.
6. Sudhanthar S, Thakur K, Sigal Y, et al. Improving asthma severity and control screening in a primary care pediatric practice. BMJ Qual Improv Rep. 2016;5
7. Wilkinson M, Bulloch B, Garcia-Filion P, et al. Efficacy of racemic albuterol versus levalbuterol used as a continuous nebulization for the treatment of acute asthma exacerbations: a randomized, double-blind, clinical trial. J Asthma. 2011;48:188-193.
8. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;19.
9. Delgado A, Chou KJ, Silver EJ, et al. Nebulizers vs metered-dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med. 2003;157:76-80.
10. Kelly HW, Sternberg AL, Lescher R, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
11. Loke YK, Blanco P, Thavarajh M, et al. Impact of inhaled corticosteroids on growth in children with asthma: systematic review and meta-analysis. PLoS ONE. 2015;10:e0133428.
12. Chauhan BF, Chartrand C, Ni Chroinin M, et al. Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev. 2015;24:CD007949.
13. Stempel DA, Szefler SJ, Pedersen S, et al; VESTRI Investigators. Safety of adding salmeterol to fluticasone propionate in children with asthma. N Engl J Med. 2016;375:840-849.
14. Blair MM. PL Detail-Document, Safety of long-acting beta-agonists in asthma. Pharmacist’s Letter/Prescriber’s Letter. November 2012.
15. Kew KM, Beggs S, Ahmad S. Stopping long-acting beta2-agonists (LABA) for children with asthma well controlled on LABA and inhaled corticosteroids. Cochrane Database Syst Rev. 2015;5:CD011316.
16. Nelson HS, Weiss ST, Bleecker ER, et al; SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
17. US Food and Drug Administration. FDA Drug Safety Communication: new safety requirements for long-acting inhaled asthma medications called long-acting beta-agonists (LABAs). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm200776.htm. Accessed December 23, 2016.
18. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;5:CD002314.
19. Price D, Musgrave SD, Shepstone L, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med. 2011;364:1695-1707.
20. Seddon P, Bara A, Ducharme FM, et al. Oral xanthines as maintenance treatment for asthma in children. Cochrane Database Syst Rev. 2006;1:CD002885.
21. van der Mark LB, Lyklema PHE, Geskus RB, et al. A systematic review with attempted network meta-analysis of asthma therapy recommended for five to eighteen year olds in GINA steps three and four. BMC Pulm Med. 2012;12:63.
22. Normansell R, Walker S, Milan SJ. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559.
23. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society Statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59-99.
24. Simon AE, Akinbami LJ. Asthma action plan receipt among children with asthma 2-17 years of age, United States, 2002-2013. J Pediatr. 2016;171:283-289.
25. Bhogal SK, Zemek R, Ducharme FM. Written action plans for asthma in children. Cochrane Database Syst Rev. 2006;3: CD005306.
26. Pollock M, Sinha IP, Hartling L, et al. Inhaled short-acting bronchodilators for managing emergency childhood asthma: an overview of reviews. Allergy. 2017;72:183-200.
27. Castro-Rodriguez JA, Beckhaus AA, Forno E. Efficacy of oral corticosteroids in the treatment of acute wheezing episodes in asthmatic preschoolers: systematic review with meta-analysis. Pediatr Pulmonol. 2016;51:868-876.
28. Keeney GE, Gray MP, Morrison AK, et al. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014;133:493-499.
29. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;5:CD011801.
30. Rowe BH, Keller JL, Oxman AD. Effectiveness of steroid therapy in acute exacerbations of asthma: a meta-analysis. Am J Emerg Med. 1992;10:301-310.
31. Rowe BH, Bretzlaff J, Bourdon C, et al. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;1:CD001490.
32. Powell C, Dwan K, Milan SJ, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. 2012;12:CD003898.
33. Rodrigo GJ, Pollack CV, Rodrigo C, et al. Heliox for non-intubated acute asthma patients. Cochrane Database Syst Rev. 2006;4:CD002884.
34. Korang SK, Feinberg J, Wetterslev J, et al. Non-invasive positive pressure ventilation for acute asthma in children. Cochrane Database System Rev. 2016;9:CD012067.
35. Kline-Krammes S, Patel NH, Robinson S. Childhood asthma: a guide for pediatric emergency medicine providers. Emerg Med Clin North Am. 2013;31:705-732.
36. Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, et al. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. 2016;4:1111-1122.
37. Gøtzsche P, Johansen HK. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2008;2:1465-1858.
38. Osborn DA, Sinn JKH. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database of Syst Rev. 2006;4:1465-1858.
39. Schäfer T, Bauer CP, Beyer K, et al. S3-Guideline on allergy prevention: 2014 update: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German society for Pediatric and Adolescent Medicine (DGKJ). Allegro J Int. 2014;23:186-199.
Pediatric asthma is the most commonly encountered childhood chronic disease, occurring in approximately 13.5% of children.1 Due to the interplay between patient, family physician (FP), and the environment, asthma often proves challenging to control. Although national guidelines for the treatment of asthma have not changed since 2007, significant research continues to examine the optimal means of preventing, controlling, and treating asthma in children. This review summarizes the evidence to date so that you can maximize your care for these young patients.
A stepwise approach to asthma control
The 2007 National Heart, Lung, and Blood Institute (NHLBI) guidelines provide a common pathway for the management of asthma (FIGURE).2 These guidelines emphasize stepwise treatment, based on symptom severity, which maximizes quality of life while minimizing morbidity. Treatment is escalated after careful assessment of frequency of daytime symptoms, frequency of nighttime symptoms, forced expiratory volume in one second (FEV1), and the number of exacerbations requiring systemic steroids over the past year. It’s also appropriate to de-escalate care when symptoms are controlled to minimize adverse effects.

The 2017 Global Initiative for Asthma (GINA) guidelines recommend a similar stepwise approach, with generally the same progression of control medications as the NHLBI guidelines. One variation is that GINA guidelines recommend considering inhaled corticosteroids (ICS) for all asthma patients—even those with intermittent symptoms.3
The Asthma Control Test and Asthma Control Questionnaire are also available for assessment of efficacy of asthma control and can help guide FPs with the decision of when to escalate control medications. (They are available at: http://bit.ly/2o3CzGX and http://bit.ly/2p1LvAc, respectively.)
A systematic review found that both tools were effective in determining if a patient was well controlled vs not well controlled.4 Similarly, a 2012 study found that using the Asthma Control Test score was useful for assessing control and directing changes to treatment.5 In addition, using these tools consistently in a primary care setting increased the frequency of assessment without negatively impacting patient flow through a clinic.6
Short-acting beta-agonists: A mainstay for intermittent asthma
Inhaled short-acting beta-agonists (SABAs) are the mainstay of treatment for intermittent asthma as well as asthma exacerbations. Short-acting beta-agonists are effective for symptomatic relief and preventing exacerbations prior to known exposures because they dilate smooth muscle in bronchioles and relieve bronchospasm.
Albuterol, the most commonly used SABA, is a mixture of the active R-enantiomer and inactive L-enantiomer. Levalbuterol, which consists of only the active R-enantiomer, is also available and is theoretically more effective with fewer adverse effects. Studies examining the difference in efficacy between albuterol and levalbuterol, however, have been mixed, and current guidelines do not recommend one over the other.7
Metered-dose inhalers vs nebulizers
SABAs are typically prescribed in metered-dose inhalers (MDIs), dry powder inhalers, or nebulizers. A meta-analysis comparing the efficacy of nebulizers to MDIs with spacers in both the outpatient and emergency department (ED) settings indicated that MDIs work at least as well as nebulizers and may also reduce length of ED stay.8 This trend appears consistent even with children younger than 24 months.9
If you are prescribing an MDI, be sure to routinely prescribe spacers to help ensure the medication is properly administered. Various types of spacers are available; some consist of an extension of the mouthpiece, while others serve as a chamber with a one-way valve to help improve the ability of the child to inhale the medication. Spacers that do not have antistatic coating should be gently washed with water and detergent.
Masks are also available for younger children and should be properly sized. The same spacer can be used for multiple medications, although they should be administered one at a time. Generally, albuterol should be administered prior to other medications to maximize distribution of subsequent inhaled medications.
Start low with inhaled corticosteroids
Treatment of persistent asthma consists of regular use of inhaled corticosteroids (ICSs; first line) with SABAs, as needed, for exacerbations. Guidelines recommend starting at a low dose and increasing the dose based on symptom control. Patients must consistently use ICSs for one to 2 weeks prior to obtaining full effect of the medication, and parents should be counseled to set appropriate expectations.2,3 ICSs are dispensed as MDIs, dry powder inhalers, and nebulizers; spacers should be considered.
Studies have shown a slight decrease in height for children on ICSs, with long-term studies indicating a persistent 1- to 2-cm decrease in adult height.10,11
Patient isn’t well controlled? Time for a long-acting beta-agonist
For patients not well controlled on low-dose ICSs, the dose can be increased or a long-acting beta-agonist (LABA) or leukotriene receptor antagonist (LTRA) can be added. A recent meta-analysis examining children not well controlled with ICSs, found that the addition of LABAs resulted in improved FEV1 and nighttime symptoms, and reduced the requirement for rescue inhalers when compared to increasing the ICS dose alone.12 In addition, there was no difference in adverse events between the 2 agents, although patients taking ICSs and LABAs had greater growth in height than those with an increased dose of ICS.12 Adding a LABA, however, did not decrease the need for systemic steroids and also did not reduce the number of exacerbations requiring hospitalizations.12
Another recent study demonstrated that the combination of ICSs and LABAs was noninferior to ICSs alone in preventing hospitalizations, intubations, and deaths.13 There are limited data on whether patients already on LABAs and ICSs should be continued on dual medications. Additionally, there is no clear method describing how to de-escalate therapy for those patients who are well controlled on ICSs and LABAs. A reasonable approach is to reduce doses of both medications and discontinue the LABA if tolerated.14,15 The US Food and Drug Administration has issued a black box warning that LABAs should not be used as a single controller medication because patients may be at increased risk of asthma-related deaths.16,17
What role for leukotriene receptor antagonists?
According to NHLBI guidelines, LTRAs can be considered as an alternative to ICSs when starting a control medication for mild persistent asthma.2 A recent meta-analysis, however, showed that there were increased rates of hospitalizations with LTRAs alone when compared to ICSs.18 The NHLBI guidelines also suggest that LTRAs can be used as adjunctive medication for those patients not well controlled on ICSs rather than increasing the ICS dose or adding a LABA.2
A 2011 clinical trial found no difference in quality of life measures between LTRAs and LABAs as adjunctive therapy at 2 months, but LABAs were more effective when patients were reassessed in 2 years.19 Similarly, the same study also found that adding LTRAs to low-dose ICSs rather than increasing the ICS dose was equivalent in the short term but not at 2 years.19
2 other adjunctive therapy options: Xanthines, cromolyn
Similar to LTRAs, xanthines can be considered as adjunctive therapy for children older than 5 years who are not well controlled on a low-dose ICS. Although xanthines decrease asthma symptoms when compared to placebo alone, they are not more effective than ICSs alone and should be considered only as adjunctive therapy.2,20 There have been few studies comparing xanthines to other adjunctive medications.21
Cromolyn is another adjunctive medication cited in the NHLBI guidelines for escalation of therapy.2 Although the medication has few adverse effects, its use is generally limited in the United States because data supporting its efficacy are lacking.
Omalizumab for allergy-related asthma exacerbations
Omalizumab, an anti-IgE antibody injected every 2 to 4 weeks, is available for children older than 6 years with moderate to severe asthma that is not responsive to ICSs and LABAs.22 The medication is effective in reducing allergy-related asthma exacerbations and hospitalizations, but data comparing it to other adjunctive medications are limited.22 Due to their significant systemic effects, the role of oral steroids as control medications is reserved for patients with severe asthma who are refractory to other medications. Children should be placed on oral steroids for the least amount of time required to achieve symptom control.
Acute exacerbation treatment: What to consider
Although there is no agreed-upon definition for an acute asthma exacerbation, the American Thoracic Society defines it as "an event characterized by a change from the patient’s previous status."23 All patients should be given an asthma action plan that clearly delineates the escalation of therapy in the event of an exacerbation, although only half of all patients report experiencing one.24 Symptom-based plans may prevent more acute care visits when compared to plans that use peak-flow measurements, although children on peak-flow plans may have fewer symptomatic days.25
Start with short-acting beta-agonists
The SABAs serve as the initial treatment of choice for management of asthma exacerbations. In young children (0-3 years), SABAs delivered by MDI with a spacer were more effective in reducing admission rates (11.3% vs 21.7%) when compared to SABAs delivered by nebulizers, resulting in a number needed to treat to prevent one admission of 10.26
In older children (3-18 years), SABAs delivered via spacer reduced ED length of stay, but did not significantly affect hospitalization rates. Additionally, SABAs administered with anticholinergics such as ipratropium bromide were more effective than SABAs alone in reducing admissions (16.9% vs 23.2%), particularly in older children with moderate to severe asthma, while also minimizing adverse effects.8,26
Corticosteroids: A mainstay in the ED
In addition to albuterol administration, corticosteroids remain the mainstay of ED management for asthma exacerbations. Administration of systemic steroids has been shown to reduce hospitalizations in children under 6 years, although, paradoxically, studies examining outpatient administration have demonstrated an increase in hospitalizations when compared to placebo.27
Dexamethasone and prednisone are the 2 most commonly used systemic steroids, and studies haven't indicated superiority of either.28,29 There is no difference in efficacy between oral and intravenous steroids.30 A recent clinical trial found a 2-day course of dexamethasone (0.6 mg/kg) had similar efficacy with fewer adverse effects when compared to a 5-day course of prednisone (1-2 mg/kg/day).28
Patient isn’t responding? Try IV magnesium
For patients who don't respond to corticosteroids and albuterol treatments, IV magnesium sulfate (usual dose, 25-75 mg/kg/d; maximum dose, 2000 mg/d) has been shown to improve respiratory function, but not necessarily decrease admission rates.31 Inhaled magnesium sulfate hasn't been shown to be more effective than IV administration and isn'trecommended.32
Evidence doesn’t support use of heliox
Heliox, which consists of 80% helium, is theorized to be effective in the treatment of asthma by increasing laminar flow and increasing the delivery of medications to the alveoli.32 Overall, the evidence does not support the use of heliox, which is typically restricted to patients with severe asthma exacerbations.33
Is it worth considering noninvasive positive pressure ventilation?
If patients don't improve with medical treatment, noninvasive positive pressure ventilation can be considered. A recent meta-analysis suggests that there is no definitive benefit or harm from this treatment, although several studies have indicated a decrease in symptom severity.34
Intubation should be considered for hypoxemia unresponsive to medications, or in cases of exhaustion, worsening mental status, or respiratory acidosis unresponsive to medication. Ventilation should allow for a permissive respiratory acidosis (pH, 7.2), while maintaining adequate oxygenation.35
Reducing the burden of asthma
Due to the complex task of reducing triggers and providing effective controller medications, working with parents and children is integral to improving the quality of life for patients with asthma. Although there is an obvious genetic predisposition, family physicians can help reduce the risk of developing asthma by encouraging healthy behaviors at home before the child is born. In the prenatal period, this includes avoiding tobacco-smoke exposure, lessening maternal obesity, decreasing maternal antibiotic and acetaminophen use, and curtailing stress.
Evidence suggests that after birth, breastfeeding and reducing childhood obesity can help lower the risk of asthma.36 Atopic disease, in general, can be reduced by breastfeeding until at least 4 months, as well as encouraging a varied diet that does not restrict potential allergens during pregnancy or lactation, and introducing foods (including potential allergens) after the age of 4 months.
The risk of atopic disease can also be lowered by lessening potential triggers at home. These include restricting exposure to cats (but not dogs), reducing home mold by decreasing humidity and ensuring adequate ventilation, avoiding volatile organic compounds, such as chlorine, and curtailing exposure to vehicle emissions. Although often marketed to be effective in reducing allergies, dust-mite covers and soy-based formulas don't prevent or minimize allergies and are often costly.37,38 In addition, there is no evidence that vaccinations areassociated with allergies.39
CORRESPONDENCE
Douglas M. Maurer, 9040A Jackson Ave, Joint-Base Lewis-McChord, WA 98431; [email protected].
Pediatric asthma is the most commonly encountered childhood chronic disease, occurring in approximately 13.5% of children.1 Due to the interplay between patient, family physician (FP), and the environment, asthma often proves challenging to control. Although national guidelines for the treatment of asthma have not changed since 2007, significant research continues to examine the optimal means of preventing, controlling, and treating asthma in children. This review summarizes the evidence to date so that you can maximize your care for these young patients.
A stepwise approach to asthma control
The 2007 National Heart, Lung, and Blood Institute (NHLBI) guidelines provide a common pathway for the management of asthma (FIGURE).2 These guidelines emphasize stepwise treatment, based on symptom severity, which maximizes quality of life while minimizing morbidity. Treatment is escalated after careful assessment of frequency of daytime symptoms, frequency of nighttime symptoms, forced expiratory volume in one second (FEV1), and the number of exacerbations requiring systemic steroids over the past year. It’s also appropriate to de-escalate care when symptoms are controlled to minimize adverse effects.

The 2017 Global Initiative for Asthma (GINA) guidelines recommend a similar stepwise approach, with generally the same progression of control medications as the NHLBI guidelines. One variation is that GINA guidelines recommend considering inhaled corticosteroids (ICS) for all asthma patients—even those with intermittent symptoms.3
The Asthma Control Test and Asthma Control Questionnaire are also available for assessment of efficacy of asthma control and can help guide FPs with the decision of when to escalate control medications. (They are available at: http://bit.ly/2o3CzGX and http://bit.ly/2p1LvAc, respectively.)
A systematic review found that both tools were effective in determining if a patient was well controlled vs not well controlled.4 Similarly, a 2012 study found that using the Asthma Control Test score was useful for assessing control and directing changes to treatment.5 In addition, using these tools consistently in a primary care setting increased the frequency of assessment without negatively impacting patient flow through a clinic.6
Short-acting beta-agonists: A mainstay for intermittent asthma
Inhaled short-acting beta-agonists (SABAs) are the mainstay of treatment for intermittent asthma as well as asthma exacerbations. Short-acting beta-agonists are effective for symptomatic relief and preventing exacerbations prior to known exposures because they dilate smooth muscle in bronchioles and relieve bronchospasm.
Albuterol, the most commonly used SABA, is a mixture of the active R-enantiomer and inactive L-enantiomer. Levalbuterol, which consists of only the active R-enantiomer, is also available and is theoretically more effective with fewer adverse effects. Studies examining the difference in efficacy between albuterol and levalbuterol, however, have been mixed, and current guidelines do not recommend one over the other.7
Metered-dose inhalers vs nebulizers
SABAs are typically prescribed in metered-dose inhalers (MDIs), dry powder inhalers, or nebulizers. A meta-analysis comparing the efficacy of nebulizers to MDIs with spacers in both the outpatient and emergency department (ED) settings indicated that MDIs work at least as well as nebulizers and may also reduce length of ED stay.8 This trend appears consistent even with children younger than 24 months.9
If you are prescribing an MDI, be sure to routinely prescribe spacers to help ensure the medication is properly administered. Various types of spacers are available; some consist of an extension of the mouthpiece, while others serve as a chamber with a one-way valve to help improve the ability of the child to inhale the medication. Spacers that do not have antistatic coating should be gently washed with water and detergent.
Masks are also available for younger children and should be properly sized. The same spacer can be used for multiple medications, although they should be administered one at a time. Generally, albuterol should be administered prior to other medications to maximize distribution of subsequent inhaled medications.
Start low with inhaled corticosteroids
Treatment of persistent asthma consists of regular use of inhaled corticosteroids (ICSs; first line) with SABAs, as needed, for exacerbations. Guidelines recommend starting at a low dose and increasing the dose based on symptom control. Patients must consistently use ICSs for one to 2 weeks prior to obtaining full effect of the medication, and parents should be counseled to set appropriate expectations.2,3 ICSs are dispensed as MDIs, dry powder inhalers, and nebulizers; spacers should be considered.
Studies have shown a slight decrease in height for children on ICSs, with long-term studies indicating a persistent 1- to 2-cm decrease in adult height.10,11
Patient isn’t well controlled? Time for a long-acting beta-agonist
For patients not well controlled on low-dose ICSs, the dose can be increased or a long-acting beta-agonist (LABA) or leukotriene receptor antagonist (LTRA) can be added. A recent meta-analysis examining children not well controlled with ICSs, found that the addition of LABAs resulted in improved FEV1 and nighttime symptoms, and reduced the requirement for rescue inhalers when compared to increasing the ICS dose alone.12 In addition, there was no difference in adverse events between the 2 agents, although patients taking ICSs and LABAs had greater growth in height than those with an increased dose of ICS.12 Adding a LABA, however, did not decrease the need for systemic steroids and also did not reduce the number of exacerbations requiring hospitalizations.12
Another recent study demonstrated that the combination of ICSs and LABAs was noninferior to ICSs alone in preventing hospitalizations, intubations, and deaths.13 There are limited data on whether patients already on LABAs and ICSs should be continued on dual medications. Additionally, there is no clear method describing how to de-escalate therapy for those patients who are well controlled on ICSs and LABAs. A reasonable approach is to reduce doses of both medications and discontinue the LABA if tolerated.14,15 The US Food and Drug Administration has issued a black box warning that LABAs should not be used as a single controller medication because patients may be at increased risk of asthma-related deaths.16,17
What role for leukotriene receptor antagonists?
According to NHLBI guidelines, LTRAs can be considered as an alternative to ICSs when starting a control medication for mild persistent asthma.2 A recent meta-analysis, however, showed that there were increased rates of hospitalizations with LTRAs alone when compared to ICSs.18 The NHLBI guidelines also suggest that LTRAs can be used as adjunctive medication for those patients not well controlled on ICSs rather than increasing the ICS dose or adding a LABA.2
A 2011 clinical trial found no difference in quality of life measures between LTRAs and LABAs as adjunctive therapy at 2 months, but LABAs were more effective when patients were reassessed in 2 years.19 Similarly, the same study also found that adding LTRAs to low-dose ICSs rather than increasing the ICS dose was equivalent in the short term but not at 2 years.19
2 other adjunctive therapy options: Xanthines, cromolyn
Similar to LTRAs, xanthines can be considered as adjunctive therapy for children older than 5 years who are not well controlled on a low-dose ICS. Although xanthines decrease asthma symptoms when compared to placebo alone, they are not more effective than ICSs alone and should be considered only as adjunctive therapy.2,20 There have been few studies comparing xanthines to other adjunctive medications.21
Cromolyn is another adjunctive medication cited in the NHLBI guidelines for escalation of therapy.2 Although the medication has few adverse effects, its use is generally limited in the United States because data supporting its efficacy are lacking.
Omalizumab for allergy-related asthma exacerbations
Omalizumab, an anti-IgE antibody injected every 2 to 4 weeks, is available for children older than 6 years with moderate to severe asthma that is not responsive to ICSs and LABAs.22 The medication is effective in reducing allergy-related asthma exacerbations and hospitalizations, but data comparing it to other adjunctive medications are limited.22 Due to their significant systemic effects, the role of oral steroids as control medications is reserved for patients with severe asthma who are refractory to other medications. Children should be placed on oral steroids for the least amount of time required to achieve symptom control.
Acute exacerbation treatment: What to consider
Although there is no agreed-upon definition for an acute asthma exacerbation, the American Thoracic Society defines it as "an event characterized by a change from the patient’s previous status."23 All patients should be given an asthma action plan that clearly delineates the escalation of therapy in the event of an exacerbation, although only half of all patients report experiencing one.24 Symptom-based plans may prevent more acute care visits when compared to plans that use peak-flow measurements, although children on peak-flow plans may have fewer symptomatic days.25
Start with short-acting beta-agonists
The SABAs serve as the initial treatment of choice for management of asthma exacerbations. In young children (0-3 years), SABAs delivered by MDI with a spacer were more effective in reducing admission rates (11.3% vs 21.7%) when compared to SABAs delivered by nebulizers, resulting in a number needed to treat to prevent one admission of 10.26
In older children (3-18 years), SABAs delivered via spacer reduced ED length of stay, but did not significantly affect hospitalization rates. Additionally, SABAs administered with anticholinergics such as ipratropium bromide were more effective than SABAs alone in reducing admissions (16.9% vs 23.2%), particularly in older children with moderate to severe asthma, while also minimizing adverse effects.8,26
Corticosteroids: A mainstay in the ED
In addition to albuterol administration, corticosteroids remain the mainstay of ED management for asthma exacerbations. Administration of systemic steroids has been shown to reduce hospitalizations in children under 6 years, although, paradoxically, studies examining outpatient administration have demonstrated an increase in hospitalizations when compared to placebo.27
Dexamethasone and prednisone are the 2 most commonly used systemic steroids, and studies haven't indicated superiority of either.28,29 There is no difference in efficacy between oral and intravenous steroids.30 A recent clinical trial found a 2-day course of dexamethasone (0.6 mg/kg) had similar efficacy with fewer adverse effects when compared to a 5-day course of prednisone (1-2 mg/kg/day).28
Patient isn’t responding? Try IV magnesium
For patients who don't respond to corticosteroids and albuterol treatments, IV magnesium sulfate (usual dose, 25-75 mg/kg/d; maximum dose, 2000 mg/d) has been shown to improve respiratory function, but not necessarily decrease admission rates.31 Inhaled magnesium sulfate hasn't been shown to be more effective than IV administration and isn'trecommended.32
Evidence doesn’t support use of heliox
Heliox, which consists of 80% helium, is theorized to be effective in the treatment of asthma by increasing laminar flow and increasing the delivery of medications to the alveoli.32 Overall, the evidence does not support the use of heliox, which is typically restricted to patients with severe asthma exacerbations.33
Is it worth considering noninvasive positive pressure ventilation?
If patients don't improve with medical treatment, noninvasive positive pressure ventilation can be considered. A recent meta-analysis suggests that there is no definitive benefit or harm from this treatment, although several studies have indicated a decrease in symptom severity.34
Intubation should be considered for hypoxemia unresponsive to medications, or in cases of exhaustion, worsening mental status, or respiratory acidosis unresponsive to medication. Ventilation should allow for a permissive respiratory acidosis (pH, 7.2), while maintaining adequate oxygenation.35
Reducing the burden of asthma
Due to the complex task of reducing triggers and providing effective controller medications, working with parents and children is integral to improving the quality of life for patients with asthma. Although there is an obvious genetic predisposition, family physicians can help reduce the risk of developing asthma by encouraging healthy behaviors at home before the child is born. In the prenatal period, this includes avoiding tobacco-smoke exposure, lessening maternal obesity, decreasing maternal antibiotic and acetaminophen use, and curtailing stress.
Evidence suggests that after birth, breastfeeding and reducing childhood obesity can help lower the risk of asthma.36 Atopic disease, in general, can be reduced by breastfeeding until at least 4 months, as well as encouraging a varied diet that does not restrict potential allergens during pregnancy or lactation, and introducing foods (including potential allergens) after the age of 4 months.
The risk of atopic disease can also be lowered by lessening potential triggers at home. These include restricting exposure to cats (but not dogs), reducing home mold by decreasing humidity and ensuring adequate ventilation, avoiding volatile organic compounds, such as chlorine, and curtailing exposure to vehicle emissions. Although often marketed to be effective in reducing allergies, dust-mite covers and soy-based formulas don't prevent or minimize allergies and are often costly.37,38 In addition, there is no evidence that vaccinations areassociated with allergies.39
CORRESPONDENCE
Douglas M. Maurer, 9040A Jackson Ave, Joint-Base Lewis-McChord, WA 98431; [email protected].
1. US Department of Health and Human Services. Centers for Disease Control and Prevention. Asthma and Schools. Available at: www.cdc.gov/healthyschools/asthma/index.htm. Updated June 17, 2015. Accessed September 28, 2016.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007. Report No.:07-4051.
3. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2017. Available at: www.ginasthma.org. Accessed April 11, 2017.
4. Jia CE, Zhang HP, Lv Y, et al. The asthma control test and asthma control questionnaire for assessing asthma control: systematic review and meta-analysis. J Allergy Clin Immunol. 2013;131:695-703.
5. Ko FW, Hui DS, Leung TS, et al. Evaluation of the asthma control test: a reliable determinant of disease stability and a predictor of future exacerbations. Respirology. 2012;17: 370-378.
6. Sudhanthar S, Thakur K, Sigal Y, et al. Improving asthma severity and control screening in a primary care pediatric practice. BMJ Qual Improv Rep. 2016;5
7. Wilkinson M, Bulloch B, Garcia-Filion P, et al. Efficacy of racemic albuterol versus levalbuterol used as a continuous nebulization for the treatment of acute asthma exacerbations: a randomized, double-blind, clinical trial. J Asthma. 2011;48:188-193.
8. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;19.
9. Delgado A, Chou KJ, Silver EJ, et al. Nebulizers vs metered-dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med. 2003;157:76-80.
10. Kelly HW, Sternberg AL, Lescher R, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
11. Loke YK, Blanco P, Thavarajh M, et al. Impact of inhaled corticosteroids on growth in children with asthma: systematic review and meta-analysis. PLoS ONE. 2015;10:e0133428.
12. Chauhan BF, Chartrand C, Ni Chroinin M, et al. Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev. 2015;24:CD007949.
13. Stempel DA, Szefler SJ, Pedersen S, et al; VESTRI Investigators. Safety of adding salmeterol to fluticasone propionate in children with asthma. N Engl J Med. 2016;375:840-849.
14. Blair MM. PL Detail-Document, Safety of long-acting beta-agonists in asthma. Pharmacist’s Letter/Prescriber’s Letter. November 2012.
15. Kew KM, Beggs S, Ahmad S. Stopping long-acting beta2-agonists (LABA) for children with asthma well controlled on LABA and inhaled corticosteroids. Cochrane Database Syst Rev. 2015;5:CD011316.
16. Nelson HS, Weiss ST, Bleecker ER, et al; SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
17. US Food and Drug Administration. FDA Drug Safety Communication: new safety requirements for long-acting inhaled asthma medications called long-acting beta-agonists (LABAs). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm200776.htm. Accessed December 23, 2016.
18. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;5:CD002314.
19. Price D, Musgrave SD, Shepstone L, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med. 2011;364:1695-1707.
20. Seddon P, Bara A, Ducharme FM, et al. Oral xanthines as maintenance treatment for asthma in children. Cochrane Database Syst Rev. 2006;1:CD002885.
21. van der Mark LB, Lyklema PHE, Geskus RB, et al. A systematic review with attempted network meta-analysis of asthma therapy recommended for five to eighteen year olds in GINA steps three and four. BMC Pulm Med. 2012;12:63.
22. Normansell R, Walker S, Milan SJ. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559.
23. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society Statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59-99.
24. Simon AE, Akinbami LJ. Asthma action plan receipt among children with asthma 2-17 years of age, United States, 2002-2013. J Pediatr. 2016;171:283-289.
25. Bhogal SK, Zemek R, Ducharme FM. Written action plans for asthma in children. Cochrane Database Syst Rev. 2006;3: CD005306.
26. Pollock M, Sinha IP, Hartling L, et al. Inhaled short-acting bronchodilators for managing emergency childhood asthma: an overview of reviews. Allergy. 2017;72:183-200.
27. Castro-Rodriguez JA, Beckhaus AA, Forno E. Efficacy of oral corticosteroids in the treatment of acute wheezing episodes in asthmatic preschoolers: systematic review with meta-analysis. Pediatr Pulmonol. 2016;51:868-876.
28. Keeney GE, Gray MP, Morrison AK, et al. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014;133:493-499.
29. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;5:CD011801.
30. Rowe BH, Keller JL, Oxman AD. Effectiveness of steroid therapy in acute exacerbations of asthma: a meta-analysis. Am J Emerg Med. 1992;10:301-310.
31. Rowe BH, Bretzlaff J, Bourdon C, et al. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;1:CD001490.
32. Powell C, Dwan K, Milan SJ, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. 2012;12:CD003898.
33. Rodrigo GJ, Pollack CV, Rodrigo C, et al. Heliox for non-intubated acute asthma patients. Cochrane Database Syst Rev. 2006;4:CD002884.
34. Korang SK, Feinberg J, Wetterslev J, et al. Non-invasive positive pressure ventilation for acute asthma in children. Cochrane Database System Rev. 2016;9:CD012067.
35. Kline-Krammes S, Patel NH, Robinson S. Childhood asthma: a guide for pediatric emergency medicine providers. Emerg Med Clin North Am. 2013;31:705-732.
36. Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, et al. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. 2016;4:1111-1122.
37. Gøtzsche P, Johansen HK. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2008;2:1465-1858.
38. Osborn DA, Sinn JKH. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database of Syst Rev. 2006;4:1465-1858.
39. Schäfer T, Bauer CP, Beyer K, et al. S3-Guideline on allergy prevention: 2014 update: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German society for Pediatric and Adolescent Medicine (DGKJ). Allegro J Int. 2014;23:186-199.
1. US Department of Health and Human Services. Centers for Disease Control and Prevention. Asthma and Schools. Available at: www.cdc.gov/healthyschools/asthma/index.htm. Updated June 17, 2015. Accessed September 28, 2016.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007. Report No.:07-4051.
3. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2017. Available at: www.ginasthma.org. Accessed April 11, 2017.
4. Jia CE, Zhang HP, Lv Y, et al. The asthma control test and asthma control questionnaire for assessing asthma control: systematic review and meta-analysis. J Allergy Clin Immunol. 2013;131:695-703.
5. Ko FW, Hui DS, Leung TS, et al. Evaluation of the asthma control test: a reliable determinant of disease stability and a predictor of future exacerbations. Respirology. 2012;17: 370-378.
6. Sudhanthar S, Thakur K, Sigal Y, et al. Improving asthma severity and control screening in a primary care pediatric practice. BMJ Qual Improv Rep. 2016;5
7. Wilkinson M, Bulloch B, Garcia-Filion P, et al. Efficacy of racemic albuterol versus levalbuterol used as a continuous nebulization for the treatment of acute asthma exacerbations: a randomized, double-blind, clinical trial. J Asthma. 2011;48:188-193.
8. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;19.
9. Delgado A, Chou KJ, Silver EJ, et al. Nebulizers vs metered-dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med. 2003;157:76-80.
10. Kelly HW, Sternberg AL, Lescher R, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
11. Loke YK, Blanco P, Thavarajh M, et al. Impact of inhaled corticosteroids on growth in children with asthma: systematic review and meta-analysis. PLoS ONE. 2015;10:e0133428.
12. Chauhan BF, Chartrand C, Ni Chroinin M, et al. Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev. 2015;24:CD007949.
13. Stempel DA, Szefler SJ, Pedersen S, et al; VESTRI Investigators. Safety of adding salmeterol to fluticasone propionate in children with asthma. N Engl J Med. 2016;375:840-849.
14. Blair MM. PL Detail-Document, Safety of long-acting beta-agonists in asthma. Pharmacist’s Letter/Prescriber’s Letter. November 2012.
15. Kew KM, Beggs S, Ahmad S. Stopping long-acting beta2-agonists (LABA) for children with asthma well controlled on LABA and inhaled corticosteroids. Cochrane Database Syst Rev. 2015;5:CD011316.
16. Nelson HS, Weiss ST, Bleecker ER, et al; SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
17. US Food and Drug Administration. FDA Drug Safety Communication: new safety requirements for long-acting inhaled asthma medications called long-acting beta-agonists (LABAs). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm200776.htm. Accessed December 23, 2016.
18. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;5:CD002314.
19. Price D, Musgrave SD, Shepstone L, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med. 2011;364:1695-1707.
20. Seddon P, Bara A, Ducharme FM, et al. Oral xanthines as maintenance treatment for asthma in children. Cochrane Database Syst Rev. 2006;1:CD002885.
21. van der Mark LB, Lyklema PHE, Geskus RB, et al. A systematic review with attempted network meta-analysis of asthma therapy recommended for five to eighteen year olds in GINA steps three and four. BMC Pulm Med. 2012;12:63.
22. Normansell R, Walker S, Milan SJ. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559.
23. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society Statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59-99.
24. Simon AE, Akinbami LJ. Asthma action plan receipt among children with asthma 2-17 years of age, United States, 2002-2013. J Pediatr. 2016;171:283-289.
25. Bhogal SK, Zemek R, Ducharme FM. Written action plans for asthma in children. Cochrane Database Syst Rev. 2006;3: CD005306.
26. Pollock M, Sinha IP, Hartling L, et al. Inhaled short-acting bronchodilators for managing emergency childhood asthma: an overview of reviews. Allergy. 2017;72:183-200.
27. Castro-Rodriguez JA, Beckhaus AA, Forno E. Efficacy of oral corticosteroids in the treatment of acute wheezing episodes in asthmatic preschoolers: systematic review with meta-analysis. Pediatr Pulmonol. 2016;51:868-876.
28. Keeney GE, Gray MP, Morrison AK, et al. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014;133:493-499.
29. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;5:CD011801.
30. Rowe BH, Keller JL, Oxman AD. Effectiveness of steroid therapy in acute exacerbations of asthma: a meta-analysis. Am J Emerg Med. 1992;10:301-310.
31. Rowe BH, Bretzlaff J, Bourdon C, et al. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;1:CD001490.
32. Powell C, Dwan K, Milan SJ, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. 2012;12:CD003898.
33. Rodrigo GJ, Pollack CV, Rodrigo C, et al. Heliox for non-intubated acute asthma patients. Cochrane Database Syst Rev. 2006;4:CD002884.
34. Korang SK, Feinberg J, Wetterslev J, et al. Non-invasive positive pressure ventilation for acute asthma in children. Cochrane Database System Rev. 2016;9:CD012067.
35. Kline-Krammes S, Patel NH, Robinson S. Childhood asthma: a guide for pediatric emergency medicine providers. Emerg Med Clin North Am. 2013;31:705-732.
36. Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, et al. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. 2016;4:1111-1122.
37. Gøtzsche P, Johansen HK. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2008;2:1465-1858.
38. Osborn DA, Sinn JKH. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database of Syst Rev. 2006;4:1465-1858.
39. Schäfer T, Bauer CP, Beyer K, et al. S3-Guideline on allergy prevention: 2014 update: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German society for Pediatric and Adolescent Medicine (DGKJ). Allegro J Int. 2014;23:186-199.
PRACTICE RECOMMENDATIONS
› Reassure parents that metered-dose inhalers are as effective as nebulizers for asthma exacerbations. A
› Use a 2-day course of systemic steroids for asthma exacerbations rather than extended regimens. B
› Develop an asthma action plan for every patient with asthma to decrease acute care visits. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
