User login
In North America and Europe, penicillin allergy is the most common drug-allergy label.1 Carrying a penicillin-allergy label, which has recently gained more attention in health care systems, leads to suboptimal outcomes, increased use of broad-spectrum antibiotics, increased risk of adverse reactions, and increased cost of care.2,3 Despite the high rate of reported reactions, clinically significant immunoglobulin E (IgE)-mediated and T cell–mediated hypersensitivity reactions to penicillins are uncommon.2
Through the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, the American Academy of Allergy, Asthma, and Immunology has issued a recommendation: “Don’t overuse non-beta lactam antibiotics in patients with a history of penicillin allergy without an appropriate evaluation.”4 The primary care physician (PCP) plays a critical role in the appropriate evaluation and accurate initial labeling of penicillin allergy. Furthermore, the PCP plays an integral part, in conjunction with the allergist, in removing the “penicillin allergy” label from a patient’s chart when feasible.
The history of penicillin and prevalence of allergy
History. Penicillin, the first antibiotic, was discovered in 1928 by physician and microbiologist Alexander Fleming when he observed that a mold of the Penicillium genus inhibited growth of gram-positive pathogens.5 Along with pharmacologist Howard Florey and chemist Ernst Chain, both of whom assisted in the large-scale isolation and production of the antibiotic, Fleming won the Nobel Prize in Physiology or Medicine in 1945 for this discovery.5
Antibiotics transformed the practice of medicine across a spectrum, including safer childbirth, surgical procedures, and transplantation.6 Penicillin remains first-line therapy for many infections, such as streptococcal pharyngitis,7 and is the only recommended medication for treating syphilis during pregnancy.8 Continued effectiveness of penicillin in these cases allows broad-spectrum antibiotics to be reserved for more severe infections. Regrettably, incorrect antibiotic allergy labeling poses a significant risk to the patient and health care system.
Epidemiology. As with all medications, the potential for anaphylaxis exists after administration of penicillin. Because its use is widespread, penicillin is the most common cause of drug-induced anaphylaxis. However, the incidence of penicillin-induced anaphylaxis is low9: A 1968 World Health Organization report stated that the rate of penicillin anaphylaxis was between 0.015% and 0.04%.10 A more recent study reported an incidence of 1 in 207,191 patients after an oral dose and 1 in 95,298 after a parenteral dose.11 The most common reactions to penicillins are urticaria and delayed maculopapular rash.8
In the United States, the prevalence of reported penicillin allergy is approximately 10% (estimated range, 8% to 12%)3,12-15; among hospitalized patients, that prevalence is estimated to be as high as 15%.13,15 However, the prevalence of confirmed penicillin allergy is low and has decreased over time—demonstrated in a longitudinal study in which the rate of a positive skin test fell from 15% in 1995 to 0.8% in 2013.16,17
Studies have confirmed that as many as 90% of patients who report penicillin allergy are, in fact, able to tolerate penicillins.14,18-20 This finding might be a consequence of initial mislabeling of penicillin allergy; often, adverse reactions are documented as “allergy” when no risk of anaphylaxis exists. Furthermore, patients can outgrow IgE-mediated penicillin allergy because the presence of penicillin IgE antibodies wanes over time.14,15
Continue to: Consequences of mislabeling
Consequences of mislabeling
Clinical consequences. A multitude of clinical consequences result from carrying a “penicillin allergy” label.
Use of broad-spectrum antibiotics leads to increased risk of Clostridium difficile infection and to development of resistant bacteria, such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus.2,15
Alternative antibiotics used in the setting of a “penicillin allergy” label might be less efficacious and result in suboptimal outcomes. For example, vancomycin is less effective against methicillin-sensitive S aureus bacteremia than nafcillin or cefazolin.2,21 Beta-lactam antibiotics—in particular, cefazolin—are often first-line for perioperative prophylaxis; patients with reported penicillin allergy often receive a less-optimal alternative, such as clindamycin, vancomycin, or gentamicin.22 These patients are at increased risk of surgical site infection.2,22
In addition, using penicillin alternatives can result in greater risk of drug reactions and adverse effects.2
Increased health care costs. Primarily through observational studies, penicillin allergy has been associated with higher health care costs.23 Patients with reported penicillin allergy had, on average, a longer inpatient stay than patients without penicillin allergy, at a 3-year total estimated additional cost of $64.6 million.24 Inpatients with a listed penicillin allergy had direct drug costs ranging from “no difference” to $609 per patient more than patients without a listed penicillin allergy. Outpatient prescription costs were $14 to $193 higher per patient for patients with a listed penicillin allergy.23
Continue to: Considerations in special populations
Considerations in special populations. Evaluating penicillin allergy during routine care is key to decreasing the necessity for urgent penicillin evaluation and possible desensitization at the time of serious infection. Certain patient populations pose specific challenges:
- Pregnant patients. Unverified penicillin allergy during pregnancy is associated with an increased rate of cesarean section and longer postpartum hospitalization.25 Additionally, group B streptococcus-positive women have increased exposure to alternative antibiotics and an increased incidence of adverse drug reactions.25
- Elderly patients. Drug allergy increases with aging.1 Elderly patients in a long-term care facility are more likely to experience adverse drug effects or drug–drug interactions from the use of penicillin alternatives, such as clindamycin, vancomycin, and fluoroquinolones.2
- Oncology patients often require antibiotic prophylaxis as well as treatment for illnesses, such as neutropenic fever, for which beta-lactam antibiotics are often used as initial treatment.2,26
- Other important populations that present specific challenges include hospitalized patients, pediatric patients, and patients with a sexually transmitted infection.2
Active management of a penicillin-allergy label
Greater recognition of the consequences of penicillin allergy in recent years has led to efforts by hospitals and other health care organizations to develop processes by which patients can be successfully de-labeled as part of antibiotic stewardship programs9 and other initiatives. Ideally, every patient who has a “penicillin allergy” label would be referred to an allergist for evaluation; however, the number of allergy specialists is limited, and access to such specialists might be restricted in some areas, making this approach impracticable. Active management of penicillin allergy requires strategies to both test and de-label patients, as well as proactive approaches to prevent incorrect labeling. These proactive approaches require involvement of all members of the health care team—especially PCPs.
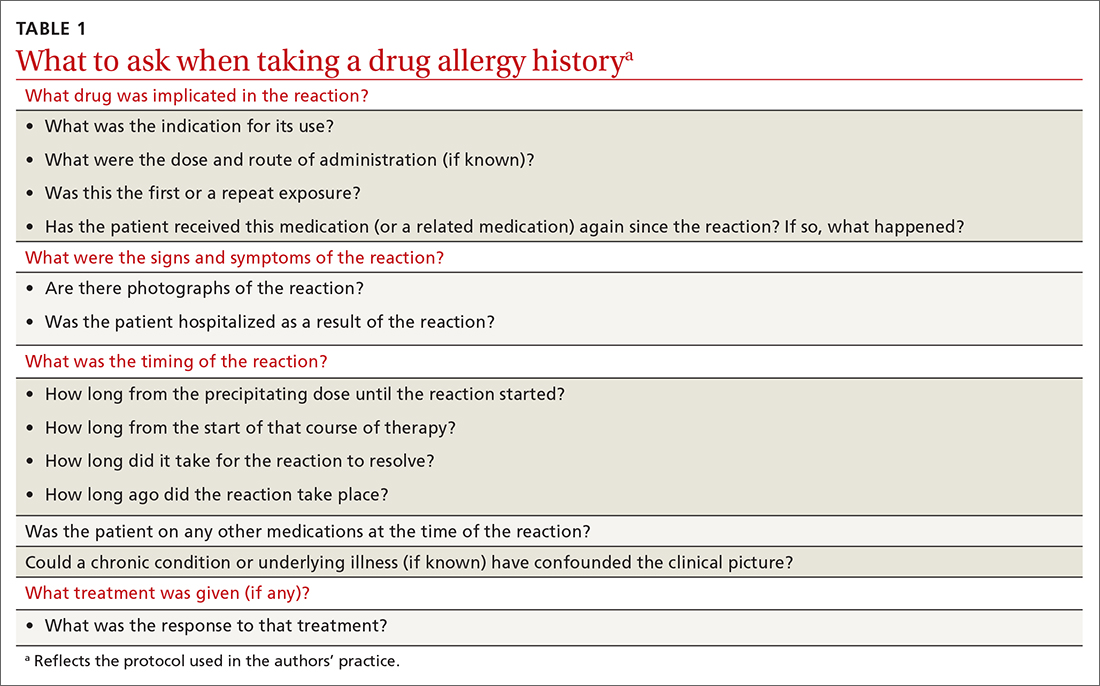
Preventing incorrect labeling. PCPs are the most likely to initially label a patient as allergic to penicillin.27 Most physicians rely on a reported history of allergy alone when selecting medication12; once a patient has been labeled “penicillin allergic,” they often retain that mislabel through adulthood.27,28 A qualitative study of PCPs’ views on prescribing penicillin found that many were aware that documented allergies were incorrect but were uncomfortable using their clinical judgment to prescribe a penicillin or change the record, for fear of a future anaphylactic reaction.29 The first step in the case of any reported reaction should be for you to elicit an accurate drug allergy history (TABLE 1).
As with other drug reactions, you should consider the context surrounding the reaction to a penicillin. Take care to review signs and symptoms of the reaction to look for clues that make a true allergic reaction more, or less, likely.
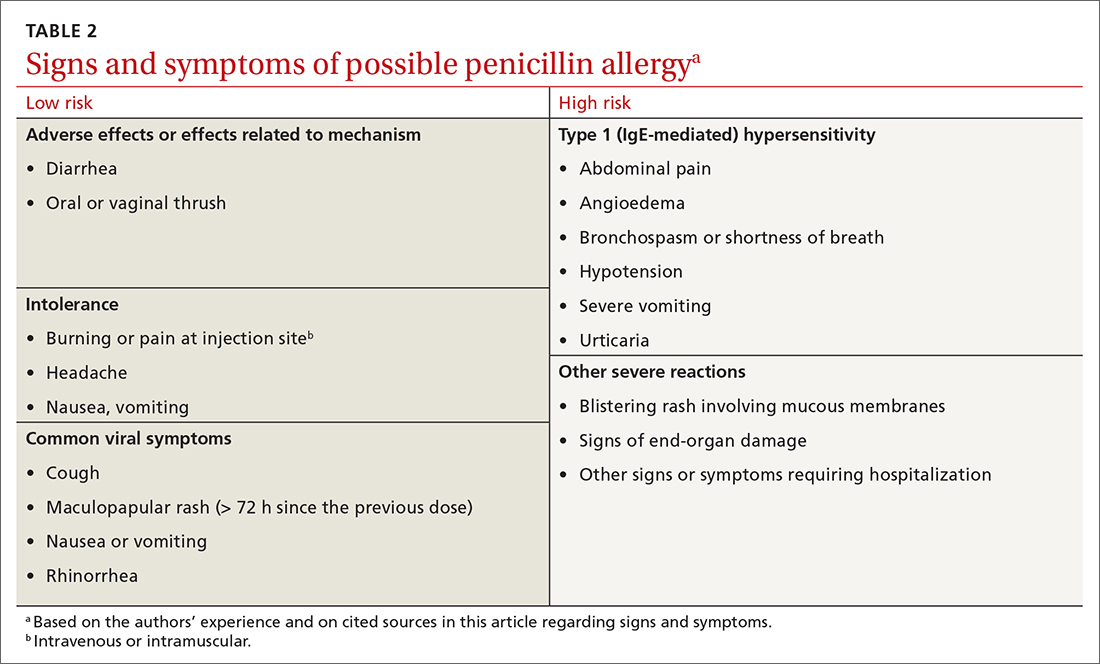
Symptoms can generally be divided into low-risk and high-risk categories27 (TABLE 2). An example of a commonly reported low-risk symptom is diarrhea that develops after several doses of a penicillin. In the absence of other symptoms, this finding is most likely due to elimination of normal gut flora,30 not to an allergic reaction to the medication. Symptoms of intolerance to the medication, such as headache and nausea, are also low risk.27,31 In contrast, immediate onset of abdominal pain after a dose of penicillin and lip or throat swelling are considered high risk.
Continue to: Patients presenting with urticaria...
Patients presenting with urticaria or maculopapular rash after taking penicillin are particularly challenging.30 A study of patients in a primary care pediatrics practice found that 7.4% of children receiving a prescription for a penicillin reported a rash.32 Here, timing of onset of symptoms provides some clarity about the likelihood of true allergy. Rashes that manifest during the first hours after exposure are more likely to be IgE mediated, particularly when accompanied by other systemic symptoms; they should be considered high risk. Delayed-onset rashes (> 72 hours after exposure) are usually non-IgE mediated and therefore are generally lower risk,8,30,33 except when associated with certain features, such as mucosal involvement and skin peeling.
Despite acknowledging viral exanthems in the differential, many physicians still label patients presenting with any rash as “allergic.”28 Take care to look for other potential causes of a rash; for example, patients taking amoxicillin who have concurrent Epstein-Barr virus infection frequently develop a maculopapular rash.34 Caubet and colleagues found that 56% of pediatric patients with a history of nonimmediate rash and a negative oral challenge to amoxicillin tested positive for viral infection.28
A family history of penicillin allergy alone should not preclude the use of penicillin.8,27,31 Similarly, if a patient has already received and tolerated a subsequent course of the same penicillin derivative after the initial reaction, the “penicillin allergy” label can be removed. If the reaction history is unknown, refer the patient to an allergist for further evaluation.
Accurate charting is key. With most hospital systems and physician practices now documenting in an electronic health record, there exists the ability to document, in great detail, patients’ reactions to medications. Previous studies have found, however, that such documentation is often done poorly, or not done at all. One such study found that (1) > 20% of patients with a “penicillin allergy” label did not have reaction details listed and (2) when reactions were listed, many were incorrectly labeled as “allergy,” not “intolerance.”35
Many electronic health record systems lump drug allergies, adverse effects, and food and environmental allergies into a single section, leading to a lack of distinction between adverse reactions and true allergy.31 Although many PCPs report that it is easy to change a patient’s allergy label in the record,29 more often, a nurse, resident, or consultant actually documents the reaction.35
Continue to: Documentation at the time of the reaction...
Documentation at the time of the reaction, within the encounter note and the allergy tab, is essential, so that other physicians caring for the patient, in the future, will be knowledgeable about the details of the reaction. Make it your responsibility to accurately document penicillin allergy in patients’ charts, including removing the “penicillin allergy” label from the chart of patients whose history is inconsistent with allergy, who have tolerated subsequent courses of the same penicillin derivative, or who have passed testing in an allergist’s office. In a study of 639 patients who tested negative for penicillin allergy, 51% still had a “penicillin allergy” label in their chart more than 4 years later.36
Penicillin allergy evaluation. When a patient cannot be cleared of a “penicillin allergy” label by history alone, and in the absence of severe features such as mucous membrane involvement, they should be further evaluated through objective testing for potential IgE-mediated allergy. This assessment includes penicillin skin testing or an oral challenge, or both.
Skin testing involves skin-prick testing of major and minor determinants of penicillin; when skin-prick testing is negative, intradermal testing of major and minor determinants should follow. The negative predictive value of penicillin skin testing is high: In a prospective, multicenter investigation, researchers demonstrated that, when both the major penicillin determinant and a minor determinant mixture were used, negative predictive value was 97.9%.37
However, a minor determinant mixture is not commercially available in the United States; therefore, penicillin G is often used alone as the minor determinant. Typically, if a patient passes skin testing, a challenge dose of penicillin or amoxicillin is administered, followed by an observation period. The risk of re-sensitization after oral penicillin is thought to be low and does not preclude future use.38
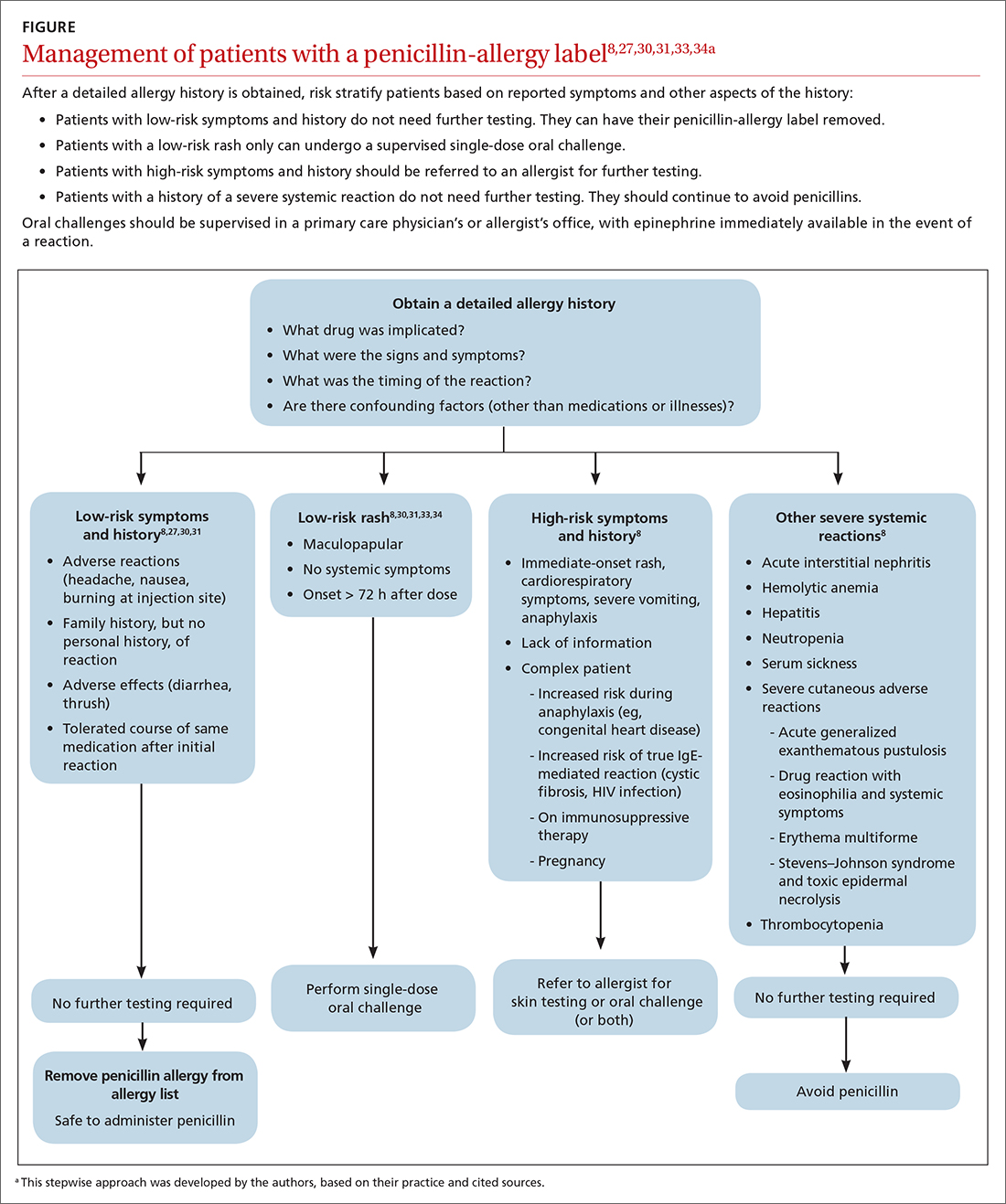
Although drug testing is most often performed in an allergist’s office, several groups have developed protocols that allow for limited testing of low-risk patients in a primary care setting.8,31 For example, several studies have demonstrated that patients presenting with low-risk skin rash can be safely tested with a supervised oral challenge alone.18,28 The FIGURE8,27,30,31,33,34 outlines our proposed workflow for risk stratification and subsequent management of patients with a “penicillin allergy” label.
Continue to: De-labeling requires a systems approach
De-labeling requires a systems approach. Given the mismatch between the large number of patients labeled “penicillin allergic” and the few allergy specialists, referral alone is not enough to solve the problem of mislabeling. Targeting specific populations for testing, such as patients presenting to an inner-city sexually transmitted infection clinic19 or preoperative patients, as is done at the Mayo Clinic,9 has been successful. Skin testing in an inpatient setting has also been shown to be safe and effective,13 allowing for protocol-driven testing under the supervision of trained pharmacists (and others), to relieve the burden on allergy specialists.9
CORRESPONDENCE
Andrew Lutzkanin, MD, 500 University Drive, PO Box 850, Hershey, PA 17033; [email protected]
1. Macy E. The clinical evaluation of penicillin allergy: what is necessary, sufficient and safe given the materials currently available? Clin Exp Allergy. 2011;41:1498-1501. doi: 10.1111/j.1365-2222.2011.03837.x
2. Shenoy ES, Macy E, Rowe T, et al. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321:188-199. doi: 10.1001/jama.2018.19283
3. Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6:1019-1027.e2. doi: 10.1016/j.jaip.2017.08.006
4. American Academy of Allergy, Asthma & Immunology: Ten things physicians and patients should question. American Board of Medicine Foundation Choosing Wisely website. 2018. Accessed July 7, 2021. www.choosingwisely.org/doctor-patient-lists/american-academy-of-allergy-asthma-immunology
5. Tan SY, Tatsumura Y. Alexander Fleming (1881-1955): discoverer of penicillin. Singapore Med J. 2015;56:366-367. doi: 10.11622/smedj.2015105
6. Marston HD, Dixon DM, Knisely JM, et al. Antimicrobial resistance. JAMA. 2016;316:1193-1204. doi: 10.1001/jama.2016.11764
7. Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2013;2013:CD000023. doi: 10.1002/14651858.CD000023.pub4
8. Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019;381:2338-2351. doi: 10.1056/NEJMra1807761
9. Khan DA. Proactive management of penicillin and other antibiotic allergies. Allergy Asthma Proc. 2020;41:82-89. doi: 10.2500/aap.2020.41.190024
10. Idsoe O, Guthe T, Willcox RR, et al. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38:159-188.
11. Chiriac AM, Macy E. Large health system databases and drug hypersensitivity. J Allergy Clin Immunol Pract. 2019;7:2125-2131. doi: 10.1016/j.jaip.2019.04.014
12. Albin S, Agarwal S. Prevalence and characteristics of reported penicillin allergy in an urban outpatient adult population. Allergy Asthma Proc. 2014;35:489-494. doi: 10.2500/aap.2014.35.3791
13. Sacco KA, Bates A, Brigham TJ, et al. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy. 2017;72:1288-1296. doi: 10.1111/all.13168
14. Khan DA, Solensky R. Drug allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S126-S137. doi: 10.1016/j.jaci.2009.10.028
15. Blumenthal KG, Shenoy ES, Varughese CA, et al. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol. 2015;115:294-300.e2. doi: 10.1016/j.anai.2015.05.011
16. Macy E, Schatz M, Lin C, et al. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13:12-18. doi: 10.7812/tpp/08-073
17. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1:258-263. doi: 10.1016/j.jaip.2013.02.002
18. Bourke J, Pavlos R, James I, et al. Improving the effectiveness of penicillin allergy de-labeling. J Allergy Clin Immunol Pract. 2015;3:365-374.e1. doi: 10.1016/j.jaip.2014.11.002
19. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993;270:2456-2463.
20. Klaustermeyer WB, Gowda VC. Penicillin skin testing: a 20-year study at the West Los Angeles Veterans Affairs Medical Center. Mil Med. 2005;170:701-704. doi: 10.7205/milmed.170.8.701.
21. McDanel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61:361-367. doi: 10.1093/cid/civ308
22. Blumenthal KG, Ryan EE, Li Y, et al. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66:329-336. doi: 10.1093/cid/cix794
23. Mattingly TJ 2nd, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6:1649-1654.e4. doi: 10.1016/j.jaip.2017.12.033
24. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133:790-796. doi: 10.1016/j.jaci.2013.09.021
25. Desai SH, Kaplan MS, Chen Q, et al. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B streptococcus infections. Perm J. 2017;21:16-80. doi: 10.7812/TPP/16-080
26. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56-e93. doi: 10.1093/cid/cir073
27. Vyles D, Mistry RD, Heffner V, et al. Reported knowledge and management of potential penicillin allergy in children. Acad Pediatr. 2019;19:684-690. doi: 10.1016/j.acap.2019.01.002
28. Caubet J-C, Kaiser L, Lemaître B, et al. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol. 2011;127:218-222. doi: 10.1016/j.jaci.2010.08.025
29. Wanat M, Anthierens S, Butler CC, et al. Patient and primary care physician perceptions of penicillin allergy testing and subsequent use of penicillin-containing antibiotics: a qualitative study. J Allergy Clin Immunol Pract. 2019;7:1888-1893.e1. doi: 10.1016/j.jaip.2019.02.036
30. Norton AE, Konvinse K, Phillips EJ, et al. Antibiotic allergy in pediatrics. Pediatrics. 2018;141: e20172497. doi: 10.1542/peds.2017-2497
31. Collins C. The low risks and high rewards of penicillin allergy delabeling: an algorithm to expedite the evaluation. J Pediatr. 2019;212:216-223. doi: 10.1016/j.jpeds.2019.05.060
32. Ibia EO, Schwartz RH, Wiedermann BL. Antibiotic rashes in children: a survey in a private practice setting. Arch Dermatol. 2000;136:849-854. doi: 10.1001/archderm.136.7.849
33. Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505. doi: 10.1001/jama.285.19.2498
34. Patel BM. Skin rash with infectious mononucleosis and ampicillin. Pediatrics. 1967;40:910-911.
35. Inglis JM, Caughey GE, Smith W, et al. Documentation of penicillin adverse drug reactions in electronic health records: inconsistent use of allergy and intolerance labels. Intern Med J. 2017;47:1292-1297. doi: 10.1111/imj.13558
36. Lachover-Roth I, Sharon S, Rosman Y, et al. Long-term follow-up after penicillin allergy delabeling in ambulatory patients. J Allergy Clin Immunol Pract. 2019;7:231-235.e1. doi: 10.1016/j.jaip.2018.04.042
37. Solensky R, Jacobs J, Lester M, et al. Penicillin allergy evaluation: a prospective, multicenter, open-label evaluation of a comprehensive penicillin skin test kit. J Allergy Clin Immunol Pract. 2019;7:1876-1885.e3. doi: 10.1016/j.jaip.2019.02.040
38. A; ; . Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273. doi: 10.1016/j.anai.2010.08.002
In North America and Europe, penicillin allergy is the most common drug-allergy label.1 Carrying a penicillin-allergy label, which has recently gained more attention in health care systems, leads to suboptimal outcomes, increased use of broad-spectrum antibiotics, increased risk of adverse reactions, and increased cost of care.2,3 Despite the high rate of reported reactions, clinically significant immunoglobulin E (IgE)-mediated and T cell–mediated hypersensitivity reactions to penicillins are uncommon.2
Through the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, the American Academy of Allergy, Asthma, and Immunology has issued a recommendation: “Don’t overuse non-beta lactam antibiotics in patients with a history of penicillin allergy without an appropriate evaluation.”4 The primary care physician (PCP) plays a critical role in the appropriate evaluation and accurate initial labeling of penicillin allergy. Furthermore, the PCP plays an integral part, in conjunction with the allergist, in removing the “penicillin allergy” label from a patient’s chart when feasible.

The history of penicillin and prevalence of allergy
History. Penicillin, the first antibiotic, was discovered in 1928 by physician and microbiologist Alexander Fleming when he observed that a mold of the Penicillium genus inhibited growth of gram-positive pathogens.5 Along with pharmacologist Howard Florey and chemist Ernst Chain, both of whom assisted in the large-scale isolation and production of the antibiotic, Fleming won the Nobel Prize in Physiology or Medicine in 1945 for this discovery.5
Antibiotics transformed the practice of medicine across a spectrum, including safer childbirth, surgical procedures, and transplantation.6 Penicillin remains first-line therapy for many infections, such as streptococcal pharyngitis,7 and is the only recommended medication for treating syphilis during pregnancy.8 Continued effectiveness of penicillin in these cases allows broad-spectrum antibiotics to be reserved for more severe infections. Regrettably, incorrect antibiotic allergy labeling poses a significant risk to the patient and health care system.
Epidemiology. As with all medications, the potential for anaphylaxis exists after administration of penicillin. Because its use is widespread, penicillin is the most common cause of drug-induced anaphylaxis. However, the incidence of penicillin-induced anaphylaxis is low9: A 1968 World Health Organization report stated that the rate of penicillin anaphylaxis was between 0.015% and 0.04%.10 A more recent study reported an incidence of 1 in 207,191 patients after an oral dose and 1 in 95,298 after a parenteral dose.11 The most common reactions to penicillins are urticaria and delayed maculopapular rash.8
In the United States, the prevalence of reported penicillin allergy is approximately 10% (estimated range, 8% to 12%)3,12-15; among hospitalized patients, that prevalence is estimated to be as high as 15%.13,15 However, the prevalence of confirmed penicillin allergy is low and has decreased over time—demonstrated in a longitudinal study in which the rate of a positive skin test fell from 15% in 1995 to 0.8% in 2013.16,17
Studies have confirmed that as many as 90% of patients who report penicillin allergy are, in fact, able to tolerate penicillins.14,18-20 This finding might be a consequence of initial mislabeling of penicillin allergy; often, adverse reactions are documented as “allergy” when no risk of anaphylaxis exists. Furthermore, patients can outgrow IgE-mediated penicillin allergy because the presence of penicillin IgE antibodies wanes over time.14,15
Continue to: Consequences of mislabeling
Consequences of mislabeling
Clinical consequences. A multitude of clinical consequences result from carrying a “penicillin allergy” label.
Use of broad-spectrum antibiotics leads to increased risk of Clostridium difficile infection and to development of resistant bacteria, such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus.2,15
Alternative antibiotics used in the setting of a “penicillin allergy” label might be less efficacious and result in suboptimal outcomes. For example, vancomycin is less effective against methicillin-sensitive S aureus bacteremia than nafcillin or cefazolin.2,21 Beta-lactam antibiotics—in particular, cefazolin—are often first-line for perioperative prophylaxis; patients with reported penicillin allergy often receive a less-optimal alternative, such as clindamycin, vancomycin, or gentamicin.22 These patients are at increased risk of surgical site infection.2,22
In addition, using penicillin alternatives can result in greater risk of drug reactions and adverse effects.2
Increased health care costs. Primarily through observational studies, penicillin allergy has been associated with higher health care costs.23 Patients with reported penicillin allergy had, on average, a longer inpatient stay than patients without penicillin allergy, at a 3-year total estimated additional cost of $64.6 million.24 Inpatients with a listed penicillin allergy had direct drug costs ranging from “no difference” to $609 per patient more than patients without a listed penicillin allergy. Outpatient prescription costs were $14 to $193 higher per patient for patients with a listed penicillin allergy.23
Continue to: Considerations in special populations
Considerations in special populations. Evaluating penicillin allergy during routine care is key to decreasing the necessity for urgent penicillin evaluation and possible desensitization at the time of serious infection. Certain patient populations pose specific challenges:
- Pregnant patients. Unverified penicillin allergy during pregnancy is associated with an increased rate of cesarean section and longer postpartum hospitalization.25 Additionally, group B streptococcus-positive women have increased exposure to alternative antibiotics and an increased incidence of adverse drug reactions.25
- Elderly patients. Drug allergy increases with aging.1 Elderly patients in a long-term care facility are more likely to experience adverse drug effects or drug–drug interactions from the use of penicillin alternatives, such as clindamycin, vancomycin, and fluoroquinolones.2
- Oncology patients often require antibiotic prophylaxis as well as treatment for illnesses, such as neutropenic fever, for which beta-lactam antibiotics are often used as initial treatment.2,26
- Other important populations that present specific challenges include hospitalized patients, pediatric patients, and patients with a sexually transmitted infection.2
Active management of a penicillin-allergy label
Greater recognition of the consequences of penicillin allergy in recent years has led to efforts by hospitals and other health care organizations to develop processes by which patients can be successfully de-labeled as part of antibiotic stewardship programs9 and other initiatives. Ideally, every patient who has a “penicillin allergy” label would be referred to an allergist for evaluation; however, the number of allergy specialists is limited, and access to such specialists might be restricted in some areas, making this approach impracticable. Active management of penicillin allergy requires strategies to both test and de-label patients, as well as proactive approaches to prevent incorrect labeling. These proactive approaches require involvement of all members of the health care team—especially PCPs.
Preventing incorrect labeling. PCPs are the most likely to initially label a patient as allergic to penicillin.27 Most physicians rely on a reported history of allergy alone when selecting medication12; once a patient has been labeled “penicillin allergic,” they often retain that mislabel through adulthood.27,28 A qualitative study of PCPs’ views on prescribing penicillin found that many were aware that documented allergies were incorrect but were uncomfortable using their clinical judgment to prescribe a penicillin or change the record, for fear of a future anaphylactic reaction.29 The first step in the case of any reported reaction should be for you to elicit an accurate drug allergy history (TABLE 1).

As with other drug reactions, you should consider the context surrounding the reaction to a penicillin. Take care to review signs and symptoms of the reaction to look for clues that make a true allergic reaction more, or less, likely.
Symptoms can generally be divided into low-risk and high-risk categories27 (TABLE 2). An example of a commonly reported low-risk symptom is diarrhea that develops after several doses of a penicillin. In the absence of other symptoms, this finding is most likely due to elimination of normal gut flora,30 not to an allergic reaction to the medication. Symptoms of intolerance to the medication, such as headache and nausea, are also low risk.27,31 In contrast, immediate onset of abdominal pain after a dose of penicillin and lip or throat swelling are considered high risk.

Continue to: Patients presenting with urticaria...
Patients presenting with urticaria or maculopapular rash after taking penicillin are particularly challenging.30 A study of patients in a primary care pediatrics practice found that 7.4% of children receiving a prescription for a penicillin reported a rash.32 Here, timing of onset of symptoms provides some clarity about the likelihood of true allergy. Rashes that manifest during the first hours after exposure are more likely to be IgE mediated, particularly when accompanied by other systemic symptoms; they should be considered high risk. Delayed-onset rashes (> 72 hours after exposure) are usually non-IgE mediated and therefore are generally lower risk,8,30,33 except when associated with certain features, such as mucosal involvement and skin peeling.
Despite acknowledging viral exanthems in the differential, many physicians still label patients presenting with any rash as “allergic.”28 Take care to look for other potential causes of a rash; for example, patients taking amoxicillin who have concurrent Epstein-Barr virus infection frequently develop a maculopapular rash.34 Caubet and colleagues found that 56% of pediatric patients with a history of nonimmediate rash and a negative oral challenge to amoxicillin tested positive for viral infection.28
A family history of penicillin allergy alone should not preclude the use of penicillin.8,27,31 Similarly, if a patient has already received and tolerated a subsequent course of the same penicillin derivative after the initial reaction, the “penicillin allergy” label can be removed. If the reaction history is unknown, refer the patient to an allergist for further evaluation.
Accurate charting is key. With most hospital systems and physician practices now documenting in an electronic health record, there exists the ability to document, in great detail, patients’ reactions to medications. Previous studies have found, however, that such documentation is often done poorly, or not done at all. One such study found that (1) > 20% of patients with a “penicillin allergy” label did not have reaction details listed and (2) when reactions were listed, many were incorrectly labeled as “allergy,” not “intolerance.”35
Many electronic health record systems lump drug allergies, adverse effects, and food and environmental allergies into a single section, leading to a lack of distinction between adverse reactions and true allergy.31 Although many PCPs report that it is easy to change a patient’s allergy label in the record,29 more often, a nurse, resident, or consultant actually documents the reaction.35
Continue to: Documentation at the time of the reaction...
Documentation at the time of the reaction, within the encounter note and the allergy tab, is essential, so that other physicians caring for the patient, in the future, will be knowledgeable about the details of the reaction. Make it your responsibility to accurately document penicillin allergy in patients’ charts, including removing the “penicillin allergy” label from the chart of patients whose history is inconsistent with allergy, who have tolerated subsequent courses of the same penicillin derivative, or who have passed testing in an allergist’s office. In a study of 639 patients who tested negative for penicillin allergy, 51% still had a “penicillin allergy” label in their chart more than 4 years later.36
Penicillin allergy evaluation. When a patient cannot be cleared of a “penicillin allergy” label by history alone, and in the absence of severe features such as mucous membrane involvement, they should be further evaluated through objective testing for potential IgE-mediated allergy. This assessment includes penicillin skin testing or an oral challenge, or both.
Skin testing involves skin-prick testing of major and minor determinants of penicillin; when skin-prick testing is negative, intradermal testing of major and minor determinants should follow. The negative predictive value of penicillin skin testing is high: In a prospective, multicenter investigation, researchers demonstrated that, when both the major penicillin determinant and a minor determinant mixture were used, negative predictive value was 97.9%.37
However, a minor determinant mixture is not commercially available in the United States; therefore, penicillin G is often used alone as the minor determinant. Typically, if a patient passes skin testing, a challenge dose of penicillin or amoxicillin is administered, followed by an observation period. The risk of re-sensitization after oral penicillin is thought to be low and does not preclude future use.38
Although drug testing is most often performed in an allergist’s office, several groups have developed protocols that allow for limited testing of low-risk patients in a primary care setting.8,31 For example, several studies have demonstrated that patients presenting with low-risk skin rash can be safely tested with a supervised oral challenge alone.18,28 The FIGURE8,27,30,31,33,34 outlines our proposed workflow for risk stratification and subsequent management of patients with a “penicillin allergy” label.

Continue to: De-labeling requires a systems approach
De-labeling requires a systems approach. Given the mismatch between the large number of patients labeled “penicillin allergic” and the few allergy specialists, referral alone is not enough to solve the problem of mislabeling. Targeting specific populations for testing, such as patients presenting to an inner-city sexually transmitted infection clinic19 or preoperative patients, as is done at the Mayo Clinic,9 has been successful. Skin testing in an inpatient setting has also been shown to be safe and effective,13 allowing for protocol-driven testing under the supervision of trained pharmacists (and others), to relieve the burden on allergy specialists.9
CORRESPONDENCE
Andrew Lutzkanin, MD, 500 University Drive, PO Box 850, Hershey, PA 17033; [email protected]
In North America and Europe, penicillin allergy is the most common drug-allergy label.1 Carrying a penicillin-allergy label, which has recently gained more attention in health care systems, leads to suboptimal outcomes, increased use of broad-spectrum antibiotics, increased risk of adverse reactions, and increased cost of care.2,3 Despite the high rate of reported reactions, clinically significant immunoglobulin E (IgE)-mediated and T cell–mediated hypersensitivity reactions to penicillins are uncommon.2
Through the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, the American Academy of Allergy, Asthma, and Immunology has issued a recommendation: “Don’t overuse non-beta lactam antibiotics in patients with a history of penicillin allergy without an appropriate evaluation.”4 The primary care physician (PCP) plays a critical role in the appropriate evaluation and accurate initial labeling of penicillin allergy. Furthermore, the PCP plays an integral part, in conjunction with the allergist, in removing the “penicillin allergy” label from a patient’s chart when feasible.

The history of penicillin and prevalence of allergy
History. Penicillin, the first antibiotic, was discovered in 1928 by physician and microbiologist Alexander Fleming when he observed that a mold of the Penicillium genus inhibited growth of gram-positive pathogens.5 Along with pharmacologist Howard Florey and chemist Ernst Chain, both of whom assisted in the large-scale isolation and production of the antibiotic, Fleming won the Nobel Prize in Physiology or Medicine in 1945 for this discovery.5
Antibiotics transformed the practice of medicine across a spectrum, including safer childbirth, surgical procedures, and transplantation.6 Penicillin remains first-line therapy for many infections, such as streptococcal pharyngitis,7 and is the only recommended medication for treating syphilis during pregnancy.8 Continued effectiveness of penicillin in these cases allows broad-spectrum antibiotics to be reserved for more severe infections. Regrettably, incorrect antibiotic allergy labeling poses a significant risk to the patient and health care system.
Epidemiology. As with all medications, the potential for anaphylaxis exists after administration of penicillin. Because its use is widespread, penicillin is the most common cause of drug-induced anaphylaxis. However, the incidence of penicillin-induced anaphylaxis is low9: A 1968 World Health Organization report stated that the rate of penicillin anaphylaxis was between 0.015% and 0.04%.10 A more recent study reported an incidence of 1 in 207,191 patients after an oral dose and 1 in 95,298 after a parenteral dose.11 The most common reactions to penicillins are urticaria and delayed maculopapular rash.8
In the United States, the prevalence of reported penicillin allergy is approximately 10% (estimated range, 8% to 12%)3,12-15; among hospitalized patients, that prevalence is estimated to be as high as 15%.13,15 However, the prevalence of confirmed penicillin allergy is low and has decreased over time—demonstrated in a longitudinal study in which the rate of a positive skin test fell from 15% in 1995 to 0.8% in 2013.16,17
Studies have confirmed that as many as 90% of patients who report penicillin allergy are, in fact, able to tolerate penicillins.14,18-20 This finding might be a consequence of initial mislabeling of penicillin allergy; often, adverse reactions are documented as “allergy” when no risk of anaphylaxis exists. Furthermore, patients can outgrow IgE-mediated penicillin allergy because the presence of penicillin IgE antibodies wanes over time.14,15
Continue to: Consequences of mislabeling
Consequences of mislabeling
Clinical consequences. A multitude of clinical consequences result from carrying a “penicillin allergy” label.
Use of broad-spectrum antibiotics leads to increased risk of Clostridium difficile infection and to development of resistant bacteria, such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus.2,15
Alternative antibiotics used in the setting of a “penicillin allergy” label might be less efficacious and result in suboptimal outcomes. For example, vancomycin is less effective against methicillin-sensitive S aureus bacteremia than nafcillin or cefazolin.2,21 Beta-lactam antibiotics—in particular, cefazolin—are often first-line for perioperative prophylaxis; patients with reported penicillin allergy often receive a less-optimal alternative, such as clindamycin, vancomycin, or gentamicin.22 These patients are at increased risk of surgical site infection.2,22
In addition, using penicillin alternatives can result in greater risk of drug reactions and adverse effects.2
Increased health care costs. Primarily through observational studies, penicillin allergy has been associated with higher health care costs.23 Patients with reported penicillin allergy had, on average, a longer inpatient stay than patients without penicillin allergy, at a 3-year total estimated additional cost of $64.6 million.24 Inpatients with a listed penicillin allergy had direct drug costs ranging from “no difference” to $609 per patient more than patients without a listed penicillin allergy. Outpatient prescription costs were $14 to $193 higher per patient for patients with a listed penicillin allergy.23
Continue to: Considerations in special populations
Considerations in special populations. Evaluating penicillin allergy during routine care is key to decreasing the necessity for urgent penicillin evaluation and possible desensitization at the time of serious infection. Certain patient populations pose specific challenges:
- Pregnant patients. Unverified penicillin allergy during pregnancy is associated with an increased rate of cesarean section and longer postpartum hospitalization.25 Additionally, group B streptococcus-positive women have increased exposure to alternative antibiotics and an increased incidence of adverse drug reactions.25
- Elderly patients. Drug allergy increases with aging.1 Elderly patients in a long-term care facility are more likely to experience adverse drug effects or drug–drug interactions from the use of penicillin alternatives, such as clindamycin, vancomycin, and fluoroquinolones.2
- Oncology patients often require antibiotic prophylaxis as well as treatment for illnesses, such as neutropenic fever, for which beta-lactam antibiotics are often used as initial treatment.2,26
- Other important populations that present specific challenges include hospitalized patients, pediatric patients, and patients with a sexually transmitted infection.2
Active management of a penicillin-allergy label
Greater recognition of the consequences of penicillin allergy in recent years has led to efforts by hospitals and other health care organizations to develop processes by which patients can be successfully de-labeled as part of antibiotic stewardship programs9 and other initiatives. Ideally, every patient who has a “penicillin allergy” label would be referred to an allergist for evaluation; however, the number of allergy specialists is limited, and access to such specialists might be restricted in some areas, making this approach impracticable. Active management of penicillin allergy requires strategies to both test and de-label patients, as well as proactive approaches to prevent incorrect labeling. These proactive approaches require involvement of all members of the health care team—especially PCPs.
Preventing incorrect labeling. PCPs are the most likely to initially label a patient as allergic to penicillin.27 Most physicians rely on a reported history of allergy alone when selecting medication12; once a patient has been labeled “penicillin allergic,” they often retain that mislabel through adulthood.27,28 A qualitative study of PCPs’ views on prescribing penicillin found that many were aware that documented allergies were incorrect but were uncomfortable using their clinical judgment to prescribe a penicillin or change the record, for fear of a future anaphylactic reaction.29 The first step in the case of any reported reaction should be for you to elicit an accurate drug allergy history (TABLE 1).

As with other drug reactions, you should consider the context surrounding the reaction to a penicillin. Take care to review signs and symptoms of the reaction to look for clues that make a true allergic reaction more, or less, likely.
Symptoms can generally be divided into low-risk and high-risk categories27 (TABLE 2). An example of a commonly reported low-risk symptom is diarrhea that develops after several doses of a penicillin. In the absence of other symptoms, this finding is most likely due to elimination of normal gut flora,30 not to an allergic reaction to the medication. Symptoms of intolerance to the medication, such as headache and nausea, are also low risk.27,31 In contrast, immediate onset of abdominal pain after a dose of penicillin and lip or throat swelling are considered high risk.

Continue to: Patients presenting with urticaria...
Patients presenting with urticaria or maculopapular rash after taking penicillin are particularly challenging.30 A study of patients in a primary care pediatrics practice found that 7.4% of children receiving a prescription for a penicillin reported a rash.32 Here, timing of onset of symptoms provides some clarity about the likelihood of true allergy. Rashes that manifest during the first hours after exposure are more likely to be IgE mediated, particularly when accompanied by other systemic symptoms; they should be considered high risk. Delayed-onset rashes (> 72 hours after exposure) are usually non-IgE mediated and therefore are generally lower risk,8,30,33 except when associated with certain features, such as mucosal involvement and skin peeling.
Despite acknowledging viral exanthems in the differential, many physicians still label patients presenting with any rash as “allergic.”28 Take care to look for other potential causes of a rash; for example, patients taking amoxicillin who have concurrent Epstein-Barr virus infection frequently develop a maculopapular rash.34 Caubet and colleagues found that 56% of pediatric patients with a history of nonimmediate rash and a negative oral challenge to amoxicillin tested positive for viral infection.28
A family history of penicillin allergy alone should not preclude the use of penicillin.8,27,31 Similarly, if a patient has already received and tolerated a subsequent course of the same penicillin derivative after the initial reaction, the “penicillin allergy” label can be removed. If the reaction history is unknown, refer the patient to an allergist for further evaluation.
Accurate charting is key. With most hospital systems and physician practices now documenting in an electronic health record, there exists the ability to document, in great detail, patients’ reactions to medications. Previous studies have found, however, that such documentation is often done poorly, or not done at all. One such study found that (1) > 20% of patients with a “penicillin allergy” label did not have reaction details listed and (2) when reactions were listed, many were incorrectly labeled as “allergy,” not “intolerance.”35
Many electronic health record systems lump drug allergies, adverse effects, and food and environmental allergies into a single section, leading to a lack of distinction between adverse reactions and true allergy.31 Although many PCPs report that it is easy to change a patient’s allergy label in the record,29 more often, a nurse, resident, or consultant actually documents the reaction.35
Continue to: Documentation at the time of the reaction...
Documentation at the time of the reaction, within the encounter note and the allergy tab, is essential, so that other physicians caring for the patient, in the future, will be knowledgeable about the details of the reaction. Make it your responsibility to accurately document penicillin allergy in patients’ charts, including removing the “penicillin allergy” label from the chart of patients whose history is inconsistent with allergy, who have tolerated subsequent courses of the same penicillin derivative, or who have passed testing in an allergist’s office. In a study of 639 patients who tested negative for penicillin allergy, 51% still had a “penicillin allergy” label in their chart more than 4 years later.36
Penicillin allergy evaluation. When a patient cannot be cleared of a “penicillin allergy” label by history alone, and in the absence of severe features such as mucous membrane involvement, they should be further evaluated through objective testing for potential IgE-mediated allergy. This assessment includes penicillin skin testing or an oral challenge, or both.
Skin testing involves skin-prick testing of major and minor determinants of penicillin; when skin-prick testing is negative, intradermal testing of major and minor determinants should follow. The negative predictive value of penicillin skin testing is high: In a prospective, multicenter investigation, researchers demonstrated that, when both the major penicillin determinant and a minor determinant mixture were used, negative predictive value was 97.9%.37
However, a minor determinant mixture is not commercially available in the United States; therefore, penicillin G is often used alone as the minor determinant. Typically, if a patient passes skin testing, a challenge dose of penicillin or amoxicillin is administered, followed by an observation period. The risk of re-sensitization after oral penicillin is thought to be low and does not preclude future use.38
Although drug testing is most often performed in an allergist’s office, several groups have developed protocols that allow for limited testing of low-risk patients in a primary care setting.8,31 For example, several studies have demonstrated that patients presenting with low-risk skin rash can be safely tested with a supervised oral challenge alone.18,28 The FIGURE8,27,30,31,33,34 outlines our proposed workflow for risk stratification and subsequent management of patients with a “penicillin allergy” label.

Continue to: De-labeling requires a systems approach
De-labeling requires a systems approach. Given the mismatch between the large number of patients labeled “penicillin allergic” and the few allergy specialists, referral alone is not enough to solve the problem of mislabeling. Targeting specific populations for testing, such as patients presenting to an inner-city sexually transmitted infection clinic19 or preoperative patients, as is done at the Mayo Clinic,9 has been successful. Skin testing in an inpatient setting has also been shown to be safe and effective,13 allowing for protocol-driven testing under the supervision of trained pharmacists (and others), to relieve the burden on allergy specialists.9
CORRESPONDENCE
Andrew Lutzkanin, MD, 500 University Drive, PO Box 850, Hershey, PA 17033; [email protected]
1. Macy E. The clinical evaluation of penicillin allergy: what is necessary, sufficient and safe given the materials currently available? Clin Exp Allergy. 2011;41:1498-1501. doi: 10.1111/j.1365-2222.2011.03837.x
2. Shenoy ES, Macy E, Rowe T, et al. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321:188-199. doi: 10.1001/jama.2018.19283
3. Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6:1019-1027.e2. doi: 10.1016/j.jaip.2017.08.006
4. American Academy of Allergy, Asthma & Immunology: Ten things physicians and patients should question. American Board of Medicine Foundation Choosing Wisely website. 2018. Accessed July 7, 2021. www.choosingwisely.org/doctor-patient-lists/american-academy-of-allergy-asthma-immunology
5. Tan SY, Tatsumura Y. Alexander Fleming (1881-1955): discoverer of penicillin. Singapore Med J. 2015;56:366-367. doi: 10.11622/smedj.2015105
6. Marston HD, Dixon DM, Knisely JM, et al. Antimicrobial resistance. JAMA. 2016;316:1193-1204. doi: 10.1001/jama.2016.11764
7. Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2013;2013:CD000023. doi: 10.1002/14651858.CD000023.pub4
8. Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019;381:2338-2351. doi: 10.1056/NEJMra1807761
9. Khan DA. Proactive management of penicillin and other antibiotic allergies. Allergy Asthma Proc. 2020;41:82-89. doi: 10.2500/aap.2020.41.190024
10. Idsoe O, Guthe T, Willcox RR, et al. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38:159-188.
11. Chiriac AM, Macy E. Large health system databases and drug hypersensitivity. J Allergy Clin Immunol Pract. 2019;7:2125-2131. doi: 10.1016/j.jaip.2019.04.014
12. Albin S, Agarwal S. Prevalence and characteristics of reported penicillin allergy in an urban outpatient adult population. Allergy Asthma Proc. 2014;35:489-494. doi: 10.2500/aap.2014.35.3791
13. Sacco KA, Bates A, Brigham TJ, et al. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy. 2017;72:1288-1296. doi: 10.1111/all.13168
14. Khan DA, Solensky R. Drug allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S126-S137. doi: 10.1016/j.jaci.2009.10.028
15. Blumenthal KG, Shenoy ES, Varughese CA, et al. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol. 2015;115:294-300.e2. doi: 10.1016/j.anai.2015.05.011
16. Macy E, Schatz M, Lin C, et al. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13:12-18. doi: 10.7812/tpp/08-073
17. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1:258-263. doi: 10.1016/j.jaip.2013.02.002
18. Bourke J, Pavlos R, James I, et al. Improving the effectiveness of penicillin allergy de-labeling. J Allergy Clin Immunol Pract. 2015;3:365-374.e1. doi: 10.1016/j.jaip.2014.11.002
19. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993;270:2456-2463.
20. Klaustermeyer WB, Gowda VC. Penicillin skin testing: a 20-year study at the West Los Angeles Veterans Affairs Medical Center. Mil Med. 2005;170:701-704. doi: 10.7205/milmed.170.8.701.
21. McDanel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61:361-367. doi: 10.1093/cid/civ308
22. Blumenthal KG, Ryan EE, Li Y, et al. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66:329-336. doi: 10.1093/cid/cix794
23. Mattingly TJ 2nd, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6:1649-1654.e4. doi: 10.1016/j.jaip.2017.12.033
24. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133:790-796. doi: 10.1016/j.jaci.2013.09.021
25. Desai SH, Kaplan MS, Chen Q, et al. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B streptococcus infections. Perm J. 2017;21:16-80. doi: 10.7812/TPP/16-080
26. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56-e93. doi: 10.1093/cid/cir073
27. Vyles D, Mistry RD, Heffner V, et al. Reported knowledge and management of potential penicillin allergy in children. Acad Pediatr. 2019;19:684-690. doi: 10.1016/j.acap.2019.01.002
28. Caubet J-C, Kaiser L, Lemaître B, et al. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol. 2011;127:218-222. doi: 10.1016/j.jaci.2010.08.025
29. Wanat M, Anthierens S, Butler CC, et al. Patient and primary care physician perceptions of penicillin allergy testing and subsequent use of penicillin-containing antibiotics: a qualitative study. J Allergy Clin Immunol Pract. 2019;7:1888-1893.e1. doi: 10.1016/j.jaip.2019.02.036
30. Norton AE, Konvinse K, Phillips EJ, et al. Antibiotic allergy in pediatrics. Pediatrics. 2018;141: e20172497. doi: 10.1542/peds.2017-2497
31. Collins C. The low risks and high rewards of penicillin allergy delabeling: an algorithm to expedite the evaluation. J Pediatr. 2019;212:216-223. doi: 10.1016/j.jpeds.2019.05.060
32. Ibia EO, Schwartz RH, Wiedermann BL. Antibiotic rashes in children: a survey in a private practice setting. Arch Dermatol. 2000;136:849-854. doi: 10.1001/archderm.136.7.849
33. Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505. doi: 10.1001/jama.285.19.2498
34. Patel BM. Skin rash with infectious mononucleosis and ampicillin. Pediatrics. 1967;40:910-911.
35. Inglis JM, Caughey GE, Smith W, et al. Documentation of penicillin adverse drug reactions in electronic health records: inconsistent use of allergy and intolerance labels. Intern Med J. 2017;47:1292-1297. doi: 10.1111/imj.13558
36. Lachover-Roth I, Sharon S, Rosman Y, et al. Long-term follow-up after penicillin allergy delabeling in ambulatory patients. J Allergy Clin Immunol Pract. 2019;7:231-235.e1. doi: 10.1016/j.jaip.2018.04.042
37. Solensky R, Jacobs J, Lester M, et al. Penicillin allergy evaluation: a prospective, multicenter, open-label evaluation of a comprehensive penicillin skin test kit. J Allergy Clin Immunol Pract. 2019;7:1876-1885.e3. doi: 10.1016/j.jaip.2019.02.040
38. A; ; . Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273. doi: 10.1016/j.anai.2010.08.002
1. Macy E. The clinical evaluation of penicillin allergy: what is necessary, sufficient and safe given the materials currently available? Clin Exp Allergy. 2011;41:1498-1501. doi: 10.1111/j.1365-2222.2011.03837.x
2. Shenoy ES, Macy E, Rowe T, et al. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321:188-199. doi: 10.1001/jama.2018.19283
3. Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6:1019-1027.e2. doi: 10.1016/j.jaip.2017.08.006
4. American Academy of Allergy, Asthma & Immunology: Ten things physicians and patients should question. American Board of Medicine Foundation Choosing Wisely website. 2018. Accessed July 7, 2021. www.choosingwisely.org/doctor-patient-lists/american-academy-of-allergy-asthma-immunology
5. Tan SY, Tatsumura Y. Alexander Fleming (1881-1955): discoverer of penicillin. Singapore Med J. 2015;56:366-367. doi: 10.11622/smedj.2015105
6. Marston HD, Dixon DM, Knisely JM, et al. Antimicrobial resistance. JAMA. 2016;316:1193-1204. doi: 10.1001/jama.2016.11764
7. Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2013;2013:CD000023. doi: 10.1002/14651858.CD000023.pub4
8. Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019;381:2338-2351. doi: 10.1056/NEJMra1807761
9. Khan DA. Proactive management of penicillin and other antibiotic allergies. Allergy Asthma Proc. 2020;41:82-89. doi: 10.2500/aap.2020.41.190024
10. Idsoe O, Guthe T, Willcox RR, et al. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38:159-188.
11. Chiriac AM, Macy E. Large health system databases and drug hypersensitivity. J Allergy Clin Immunol Pract. 2019;7:2125-2131. doi: 10.1016/j.jaip.2019.04.014
12. Albin S, Agarwal S. Prevalence and characteristics of reported penicillin allergy in an urban outpatient adult population. Allergy Asthma Proc. 2014;35:489-494. doi: 10.2500/aap.2014.35.3791
13. Sacco KA, Bates A, Brigham TJ, et al. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy. 2017;72:1288-1296. doi: 10.1111/all.13168
14. Khan DA, Solensky R. Drug allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S126-S137. doi: 10.1016/j.jaci.2009.10.028
15. Blumenthal KG, Shenoy ES, Varughese CA, et al. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol. 2015;115:294-300.e2. doi: 10.1016/j.anai.2015.05.011
16. Macy E, Schatz M, Lin C, et al. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J. 2009;13:12-18. doi: 10.7812/tpp/08-073
17. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1:258-263. doi: 10.1016/j.jaip.2013.02.002
18. Bourke J, Pavlos R, James I, et al. Improving the effectiveness of penicillin allergy de-labeling. J Allergy Clin Immunol Pract. 2015;3:365-374.e1. doi: 10.1016/j.jaip.2014.11.002
19. Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993;270:2456-2463.
20. Klaustermeyer WB, Gowda VC. Penicillin skin testing: a 20-year study at the West Los Angeles Veterans Affairs Medical Center. Mil Med. 2005;170:701-704. doi: 10.7205/milmed.170.8.701.
21. McDanel JS, Perencevich EN, Diekema DJ, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61:361-367. doi: 10.1093/cid/civ308
22. Blumenthal KG, Ryan EE, Li Y, et al. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66:329-336. doi: 10.1093/cid/cix794
23. Mattingly TJ 2nd, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6:1649-1654.e4. doi: 10.1016/j.jaip.2017.12.033
24. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133:790-796. doi: 10.1016/j.jaci.2013.09.021
25. Desai SH, Kaplan MS, Chen Q, et al. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B streptococcus infections. Perm J. 2017;21:16-80. doi: 10.7812/TPP/16-080
26. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56-e93. doi: 10.1093/cid/cir073
27. Vyles D, Mistry RD, Heffner V, et al. Reported knowledge and management of potential penicillin allergy in children. Acad Pediatr. 2019;19:684-690. doi: 10.1016/j.acap.2019.01.002
28. Caubet J-C, Kaiser L, Lemaître B, et al. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol. 2011;127:218-222. doi: 10.1016/j.jaci.2010.08.025
29. Wanat M, Anthierens S, Butler CC, et al. Patient and primary care physician perceptions of penicillin allergy testing and subsequent use of penicillin-containing antibiotics: a qualitative study. J Allergy Clin Immunol Pract. 2019;7:1888-1893.e1. doi: 10.1016/j.jaip.2019.02.036
30. Norton AE, Konvinse K, Phillips EJ, et al. Antibiotic allergy in pediatrics. Pediatrics. 2018;141: e20172497. doi: 10.1542/peds.2017-2497
31. Collins C. The low risks and high rewards of penicillin allergy delabeling: an algorithm to expedite the evaluation. J Pediatr. 2019;212:216-223. doi: 10.1016/j.jpeds.2019.05.060
32. Ibia EO, Schwartz RH, Wiedermann BL. Antibiotic rashes in children: a survey in a private practice setting. Arch Dermatol. 2000;136:849-854. doi: 10.1001/archderm.136.7.849
33. Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285:2498-2505. doi: 10.1001/jama.285.19.2498
34. Patel BM. Skin rash with infectious mononucleosis and ampicillin. Pediatrics. 1967;40:910-911.
35. Inglis JM, Caughey GE, Smith W, et al. Documentation of penicillin adverse drug reactions in electronic health records: inconsistent use of allergy and intolerance labels. Intern Med J. 2017;47:1292-1297. doi: 10.1111/imj.13558
36. Lachover-Roth I, Sharon S, Rosman Y, et al. Long-term follow-up after penicillin allergy delabeling in ambulatory patients. J Allergy Clin Immunol Pract. 2019;7:231-235.e1. doi: 10.1016/j.jaip.2018.04.042
37. Solensky R, Jacobs J, Lester M, et al. Penicillin allergy evaluation: a prospective, multicenter, open-label evaluation of a comprehensive penicillin skin test kit. J Allergy Clin Immunol Pract. 2019;7:1876-1885.e3. doi: 10.1016/j.jaip.2019.02.040
38. A; ; . Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273. doi: 10.1016/j.anai.2010.08.002
PRACTICE RECOMMENDATIONS
› Obtain an accurate drug allergy history from all patients who have a listed penicillin allergy. B
› De-label penicillin allergy in patients who report symptoms of an adverse reaction (diarrhea, headache, or nausea) but who (1) do not have other systemic symptoms; (2) do have a family history, but no personal history, of a reaction; or (3) have tolerated the same penicillin derivative since the initial reaction. B
› Refer patients whose reaction history includes hives, shortness of breath, or other allergic-type signs and symptoms for potential skin testing or oral challenge, or both. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
