User login
Researchers have found the cancer-associated pseudokinase Tribbles 2 (TRIB2) to be a potential therapeutic target in solid tumors and blood cancers, including acute myeloid leukemia (AML).
Previous research had described TRIB2 as a target of small-molecule protein kinase inhibitors originally designed to interfere with kinase domains of the epidermal growth factor receptor (EGFR) tyrosine kinase family.
Using a thermal shift assay, the team discovered TRIB2-binding compounds within the Published Kinase Inhibitor Set (PKIS). They then employed a biochemical drug repurposing approach to classify compounds that either stabilized or destabilized TRIB2 in vitro.
The researchers found that afatinib, which is already approved by the U.S. Food and Drug Administration to treat non-small cell lung cancer, led to rapid TRIB2 degradation in human AML cells.
Patrick A. Eyers, PhD, of the University of Liverpool in the U.K., and his colleagues published their findings in Science Signaling.
The team found afatinib to be relatively specific for EGFR and human epidermal growth factor receptor 2 (HER2) at nanomolar concentrations in cells.
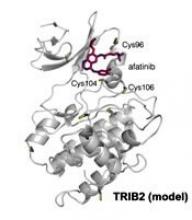
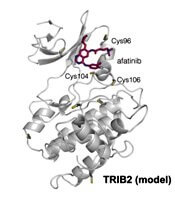
The researchers confirmed that at least two TRIB2 Cys residues interact with afatinib in vitro.
The team also discovered TRIB2 could be destabilized by neratinib and osimertinib in vitro.
“Our data prove that the cellular mechanism by which TRIB2 stability is regulated by compounds is proteasome-based,” the researchers wrote, “and we speculate that an afatinib-induced conformational change might induce TRIB2 ubiquitination.”
The researchers plan to study further TRIB2 small-molecule interactions with dynamic changes in ubiquitination status.
Furthermore, they report their work demonstrates that covalent inhibitors such as afatinib have TRIB2-degrading activity in human cells at micromolar concentrations.
The researchers determined that afatinib has similar efficacy to the TRIB2-destabilizing quinazoline neratinib at similar ranges.
The team believes their data “raise the intriguing possibility that clinical inhibitors might be used as TRIB2-degrading agents in research, and possibly clinical, contexts.”
“A long-standing goal in cancer research is drug-induced degradation of oncogenic proteins,” Dr. Eyers commented. “Our study highlights how information obtained with ‘off-target’ effects of known drugs is potentially useful because it might be exploited in the future to help eliminate a protein that is involved in a completely different type of cancer.”
The TRIB proteins play many diverse roles in cell signaling, development, and cancer. According to a paper in Developmental Dynamics, they were named after the small, round, fictional organisms from the original Star Trek television series. Their major role was to eat and reproduce.
This work was funded by two U.K. Biotechnology and Biological Sciences Research Council Doctoral Training Partnership studentships, a Tools and Resources Development Fund award, Royal Society Research Grants, North West Cancer Research grants, and funding from the National Institutes of Health.
The authors disclosed no perceived conflicts of interest, although several authors are affiliated with the Structural Genomics Consortium at the University of North Carolina at Chapel Hill, which receives direct funds from various pharmaceutical companies but remains entirely independent.
Researchers have found the cancer-associated pseudokinase Tribbles 2 (TRIB2) to be a potential therapeutic target in solid tumors and blood cancers, including acute myeloid leukemia (AML).
Previous research had described TRIB2 as a target of small-molecule protein kinase inhibitors originally designed to interfere with kinase domains of the epidermal growth factor receptor (EGFR) tyrosine kinase family.
Using a thermal shift assay, the team discovered TRIB2-binding compounds within the Published Kinase Inhibitor Set (PKIS). They then employed a biochemical drug repurposing approach to classify compounds that either stabilized or destabilized TRIB2 in vitro.
The researchers found that afatinib, which is already approved by the U.S. Food and Drug Administration to treat non-small cell lung cancer, led to rapid TRIB2 degradation in human AML cells.
Patrick A. Eyers, PhD, of the University of Liverpool in the U.K., and his colleagues published their findings in Science Signaling.
The team found afatinib to be relatively specific for EGFR and human epidermal growth factor receptor 2 (HER2) at nanomolar concentrations in cells.
The researchers confirmed that at least two TRIB2 Cys residues interact with afatinib in vitro.
The team also discovered TRIB2 could be destabilized by neratinib and osimertinib in vitro.
“Our data prove that the cellular mechanism by which TRIB2 stability is regulated by compounds is proteasome-based,” the researchers wrote, “and we speculate that an afatinib-induced conformational change might induce TRIB2 ubiquitination.”
The researchers plan to study further TRIB2 small-molecule interactions with dynamic changes in ubiquitination status.
Furthermore, they report their work demonstrates that covalent inhibitors such as afatinib have TRIB2-degrading activity in human cells at micromolar concentrations.
The researchers determined that afatinib has similar efficacy to the TRIB2-destabilizing quinazoline neratinib at similar ranges.
The team believes their data “raise the intriguing possibility that clinical inhibitors might be used as TRIB2-degrading agents in research, and possibly clinical, contexts.”
“A long-standing goal in cancer research is drug-induced degradation of oncogenic proteins,” Dr. Eyers commented. “Our study highlights how information obtained with ‘off-target’ effects of known drugs is potentially useful because it might be exploited in the future to help eliminate a protein that is involved in a completely different type of cancer.”
The TRIB proteins play many diverse roles in cell signaling, development, and cancer. According to a paper in Developmental Dynamics, they were named after the small, round, fictional organisms from the original Star Trek television series. Their major role was to eat and reproduce.
This work was funded by two U.K. Biotechnology and Biological Sciences Research Council Doctoral Training Partnership studentships, a Tools and Resources Development Fund award, Royal Society Research Grants, North West Cancer Research grants, and funding from the National Institutes of Health.
The authors disclosed no perceived conflicts of interest, although several authors are affiliated with the Structural Genomics Consortium at the University of North Carolina at Chapel Hill, which receives direct funds from various pharmaceutical companies but remains entirely independent.
Researchers have found the cancer-associated pseudokinase Tribbles 2 (TRIB2) to be a potential therapeutic target in solid tumors and blood cancers, including acute myeloid leukemia (AML).
Previous research had described TRIB2 as a target of small-molecule protein kinase inhibitors originally designed to interfere with kinase domains of the epidermal growth factor receptor (EGFR) tyrosine kinase family.
Using a thermal shift assay, the team discovered TRIB2-binding compounds within the Published Kinase Inhibitor Set (PKIS). They then employed a biochemical drug repurposing approach to classify compounds that either stabilized or destabilized TRIB2 in vitro.
The researchers found that afatinib, which is already approved by the U.S. Food and Drug Administration to treat non-small cell lung cancer, led to rapid TRIB2 degradation in human AML cells.
Patrick A. Eyers, PhD, of the University of Liverpool in the U.K., and his colleagues published their findings in Science Signaling.
The team found afatinib to be relatively specific for EGFR and human epidermal growth factor receptor 2 (HER2) at nanomolar concentrations in cells.
The researchers confirmed that at least two TRIB2 Cys residues interact with afatinib in vitro.
The team also discovered TRIB2 could be destabilized by neratinib and osimertinib in vitro.
“Our data prove that the cellular mechanism by which TRIB2 stability is regulated by compounds is proteasome-based,” the researchers wrote, “and we speculate that an afatinib-induced conformational change might induce TRIB2 ubiquitination.”
The researchers plan to study further TRIB2 small-molecule interactions with dynamic changes in ubiquitination status.
Furthermore, they report their work demonstrates that covalent inhibitors such as afatinib have TRIB2-degrading activity in human cells at micromolar concentrations.
The researchers determined that afatinib has similar efficacy to the TRIB2-destabilizing quinazoline neratinib at similar ranges.
The team believes their data “raise the intriguing possibility that clinical inhibitors might be used as TRIB2-degrading agents in research, and possibly clinical, contexts.”
“A long-standing goal in cancer research is drug-induced degradation of oncogenic proteins,” Dr. Eyers commented. “Our study highlights how information obtained with ‘off-target’ effects of known drugs is potentially useful because it might be exploited in the future to help eliminate a protein that is involved in a completely different type of cancer.”
The TRIB proteins play many diverse roles in cell signaling, development, and cancer. According to a paper in Developmental Dynamics, they were named after the small, round, fictional organisms from the original Star Trek television series. Their major role was to eat and reproduce.
This work was funded by two U.K. Biotechnology and Biological Sciences Research Council Doctoral Training Partnership studentships, a Tools and Resources Development Fund award, Royal Society Research Grants, North West Cancer Research grants, and funding from the National Institutes of Health.
The authors disclosed no perceived conflicts of interest, although several authors are affiliated with the Structural Genomics Consortium at the University of North Carolina at Chapel Hill, which receives direct funds from various pharmaceutical companies but remains entirely independent.