User login
In the United States, unhealthy alcohol use affects medical care on several levels. The prevalence of alcohol problems is 7%20% or higher among outpatients,1 30%40% among emergency room patients, and 50% among patients with trauma.13 In 2006, approximately 430,000 hospital discharges in the United States were for persons with a principal (first‐listed) alcohol‐related diagnosis, and 1.7 million discharges listed at least one alcohol‐related diagnosis, representing 1.3% and 5.3% of all hospital discharges, respectively.4 Alcoholic psychosis (34.5%) and alcohol dependence syndrome (29.5%) together accounted for the majority of principal alcohol‐related diagnoses.4 Additionally, many patients hospitalized for other indications are susceptible to withdrawal symptoms due to physiological habituation to alcohol. Abrupt cessation of alcohol intake causes habituated drinkers to experience symptoms of alcohol withdrawal syndrome (AWS), which significantly increases intensity and cost of care. Trauma patients who develop AWS were found to have increased morbidity, more intensive care and ventilator days, and higher hospital costs than trauma patients without AWS.5 Unfortunately, attempts to develop predictive models to accurately forecast the likelihood of developing severe AWS in an individual case have been modestly successful at best.68
Regimens used to treat AWS have evolved over time, taking advantage of advances in the understanding of addiction neurophysiology. There is no specific ethanol receptor.9 Much of alcohol's acute effects on the central nervous system are mediated by its stimulation of the gamma‐aminobutyric acid (GABA) system, which is neuroinhibitory.10 Chronic alcohol use leads to habituation partly by inducing configuration changes of GABA‐A receptor subunits. This renders the GABA‐A receptor less sensitive to alcohol, barbiturates, and benzodiazepines.11 Although both GABA‐A and GABA‐B receptor activation cause increased GABA neuronal output, the GABA‐A receptor is rendered relatively less sensitive by chronic exposure to alcohol. Baclofen is a pure GABA‐B receptor agonist,12 and its GABA‐B stimulatory effect is maintained even in habituated alcoholics.13, 14 The absence of cross‐tolerance between baclofen and ethanol suggests that low doses of baclofen may be helpful in the management of AWS.
Currently, AWS is usually managed with benzodiazepines, using variable dosing depending on the severity of withdrawal symptoms. Such symptom‐triggered treatment is generally preferred over fixed‐dose regimens,15 in part because when using this method, many cases of AWS can be managed with less medication. Benzodiazepine regimens using high doses have been found to be associated with substantial morbidity and prolonged hospitalizations.16, 17
In a series of small studies, Addolorato's research team has reported decreases in AWS symptoms in association with the use of low doses of baclofen in an outpatient population,18 and has found baclofen to be associated with reduced alcohol craving in the long‐term management of alcohol dependence.11, 19, 20 Addolorato and colleagues' studies of baclofen in relieving AWS symptoms prompted our group to apply the use of baclofen in a larger group of inpatients with AWS.11, 18, 21 We conducted this study to improve understanding of the role of baclofen in the management of acute AWS in an inpatient population of subjects at risk for AWS, drawn from general hospital admissions.
Our primary null hypothesis was that Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA‐Ar) scores in acutely withdrawing alcoholic patients are equal in baclofen‐treated and placebo‐treated subject groups. Our secondary null hypothesis was that benzodiazepine doses used to treat acutely withdrawing alcoholic patients are equal in the baclofen‐treated and placebo‐treated groups.
METHODS
The protocol for this study was approved by the Essentia Health Institutional Review Board, Duluth, Minnesota.
This was a randomized, placebo‐controlled, double‐blind trial. Subjects were recruited from among patients who were admitted to 1 of 2 regional general hospitals in Duluth, Minnesota (St. Mary's Medical Center or Miller‐Dwan Medical Center) and who were identified by clinical staff as being at risk for AWS. Potential subjects were not required to have an alcohol‐related condition as their primary reason for admission, but were required to have a history of AWS or of alcohol use suggestive of significant risk for AWS, and to be able to provide informed consent, as described below.
Patients were not eligible for enrollment in the study if they had other active drug dependence in addition to alcohol; were using baclofen at the time of study enrollment; were using benzodiazepines chronically at the time of study enrollment; had a known baclofen or benzodiazepine sensitivity; were unable to take oral medications; were pregnant or breast‐feeding; had a serum creatinine level 2.0; had a history of non‐alcohol withdrawal seizures; required intravenous benzodiazepines to control their AWS; or were unable to complete the consenting procedures.
Consenting and Enrollment Procedures
Patients who were identified by clinical staff as being at high risk for AWS were approached for possible enrollment in the study. Potential subjects who met other inclusion criteria were screened to assess their mental status with the Mini‐Mental Status Exam (MMSE), using methods developed for subjects with cognitive impairment.22 Potential subjects who scored 24 of a possible score of 30 or higher on the exam were considered capable of providing informed consent. Potential subjects who scored between 20 and 23 were considered to be capable of providing consent if they were able to answer 4 questions about the study (why the study is being done, what will be required of them if they participate, how long they will be in the study, and how it will be determined if they will receive the investigational drug or the placebo).
Patients who met inclusion criteria were enrolled in the study. Subjects were randomized only if they developed signs of AWS sufficient to meet Diagnostic and Statistical Manual of Mental Disorders IV (DSMIV) criteria for AWS diagnosis, and reached at least a score of 11 (of a possible 67) on the CIWA‐Ar. All subjects received symptom‐triggered benzodiazepine treatment, and also were randomized to receive either baclofen (10 mg) or placebo every 8 hours (q8h) orally as inpatients for 72 hours or until discharge, whichever was shorter. Lorazepam was selected for the symptom‐triggered benzodiazepine treatment, as it has been used for managing AWS in the participating hospitals for several years. The initial research protocol called for 5 days of participation (15 doses of study drug), but we found that many subjects were discharged before the 5 days had elapsed after enrollment, and that compliance with follow‐up outpatient visits was poor. Accordingly, the protocol was amended to shorten the treatment period to 72 hours of participation (9 doses) or until discharge if prior to 72 hours, with the minimum observation period set to 72 hours.
Data Collection
Baseline data were collected at the time of enrollment, both from the patient and the medical record. Demographic data (age, gender, race) were obtained, as well as data on alcohol history, including approximate duration and intensity of alcohol use, and prior experience with AWS; data on comorbid conditions and medical history; and history of beta‐blocker use. During the period of observation following randomization, data were obtained on CIWA‐Ar scores; benzodiazepine doses administered; and adverse events, including use of sedatives in addition to benzodiazepines, use of restraints, use of intensive care, and clinical complications during the AWS course.
Study Procedures
The research pharmacy provided study medications (baclofen or placebo in identical form) for enrolled subjects. Subjects and study personnel were blinded to treatment group (baclofen vs placebo).
Nurses on inpatient units were provided with training in CIWA‐Ar assessment. All subjects were monitored for CIWA‐Ar scores at the time of study enrollment and for at least the next 72 hours. In monitoring the subjects, the nurses used the CIWA‐Ar protocol, in which subjects were assessed and potentially dosed with lorazepam hourly if their scores were 11 or higher. If the CIWA‐Ar score was less than 11, the subjects were assessed every 4 hours and at study discharge. CIWA‐AR results were reported as averaged over 8‐hour periods starting at study enrollment.
Data Analysis
Demographic and baseline variables with ordinal and continuous measurements, such as age, MMSE total score, and drinks per day were evaluated using group t test analysis, with two‐tailed significance estimates. Variables with prevalence reported were evaluated using the chi‐square test of significance. In accordance with the protocol, data from patients who had CIWA‐Ar assessments for at least 72 hours following randomization were included in the final study analyses. Repeated measures analysis of variance were conducted for the 2 treatment groups (baclofen and placebo), to evaluate mean CIWA‐Ar scores and mean lorazepam doses within each 8‐hour interval, as well as cumulative lorazepam dose. No covariates were included in the models. The last‐observation‐carried‐forward approach was used for those subjects who were missing CIWA‐Ar scores between baseline and their last CIWA‐Ar score. Doses for postdischarge patients without lorazepam prescriptions were set to 0 mg/8 hr. Cumulative lorazepam dose at 72 hours was also analyzed by defining the upper 25th percentile of existing doses (the upper 8 of 31) as high dose. This high‐dose lorazepam treatment level was determined to include all study participants receiving 20 mg or more of lorazepam during the first 72 hours. Fisher's exact test (two‐tailed) was then used to assess the difference in treatment group (baclofen vs placebo) for high‐dose lorazepam treatment.
RESULTS
Seventy‐nine subjects met study inclusion criteria, and provided informed consent for participation in the study. Of these, 44 subjects developed signs of AWS sufficient to meet DSMIV criteria for AWS diagnosis, and were randomized to receive either baclofen or placebo, in addition to benzodiazepine therapy. As summarized in Table 1, subjects who developed signs of AWS were similar to subjects who did not enter withdrawal, differing principally in that those who developed AWS reported more drinks per day, and more significant history of previous AWS.
| Withdrawal/Randomized | ||||
|---|---|---|---|---|
| No Withdrawal | All | Placebo | Baclofen | |
| Characteristic | (N = 35) | (N = 44) | (N = 19) | (N = 25) |
| ||||
| % Male | 82.9 | 84.1 | 94.7 | 76.0 |
| Age at admission (mean/SD) | 52.0/12.0 | 46.9/10.9 | 46.1/11.9 | 47.5/10.3 |
| MMSE total score (mean/SD) | 26.5/1.7 | 25.8/3.3 | 25.4/4.1 | 26.0/2.4 |
| Drinking history | ||||
| Age began drinking (mean/SD) | 16.7/4.2 | 16.2/4.3 | 15.5/4.5 | 16.7/4.2 |
| Years drinking (mean/SD) | 34.1/10.8 | 30.2/10.0 | 30.0/12.8 | 30.3/7.8 |
| Drinks per day (mean/SD)* | 11.2/8.8 | 16.3/9.7 | 14.4/7.8 | 18.0/11.0 |
| % Daily drinker | 65.7 | 64.1 | 57.9 | 70.0 |
| Days since last drink (mean/SD) | 1.5/0.9 | 1.3/1.3 | 1.0/0.8 | 1.6/1.5 |
| Medical history, % with history of | ||||
| Alcohol withdrawal syndrome | 60.6 | 87.5 | 87.5 | 87.5 |
| Seizures*, | 30.0 | 53.8 | 33.3 | 66.7 |
| DTs* | 48.3 | 74.3 | 80.0 | 70.0 |
| Medications, % at time of admission | ||||
| Alcohol treatment | 0.0 | 4.5 | 5.3 | 4.0 |
| Beta‐blocker | 31.4 | 25.0 | 26.3 | 24.0 |
| Sleep agent | 2.9 | 6.8 | 5.3 | 8.0 |
| Narcotic pain medication | 37.1 | 40.9 | 31.6 | 48.0 |
| Depakote | 0.0 | 4.5 | 0.0 | 8.0 |
| Benzodiazepine | 2.9 | 11.4 | 10.5 | 12.0 |
| Anti‐anxiety medication | 2.9 | 2.3 | 0.0 | 4.0 |
| Anti‐psychotic medication | 5.7 | 6.8 | 10.5 | 4.0 |
| Other psychiatric medication | 2.9 | 9.1 | 5.3 | 12.0 |
The 79 subjects who were enrolled were drawn from a population of 237 potential subjects who were screened for the study. The most common reasons that the 158 potential subjects were not enrolled were refusal (29.7%), low risk of withdrawal (19.0%), inability to provide consent (9.5%), and concurrent use of benzodiazepines (8.9%). The 15 patients who were unable to provide consent were those who scored 24 or lower on the MMSE, and did not have a surrogate decision‐maker available.
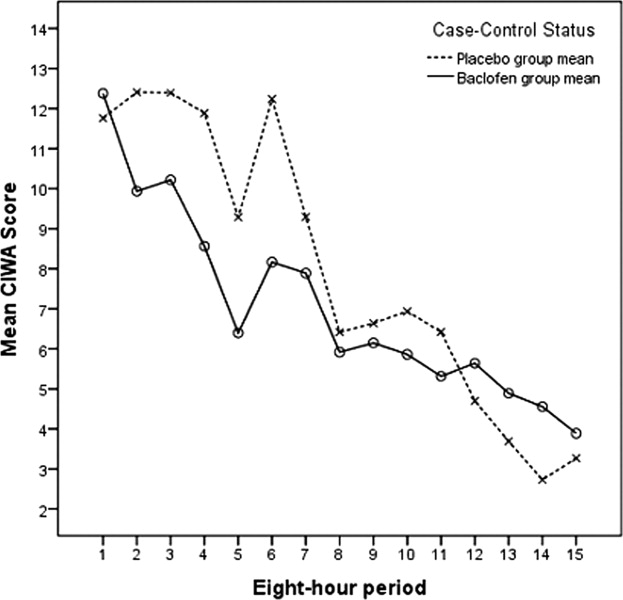
Of the 44 subjects who were randomized, 31 (18 in the baclofen group, 13 in the placebo group) completed 72 hours of CIWA‐Ar assessments, as summarized in Table 2. These assessments were completed either entirely as inpatients (24 subjects) or with inpatient assessments followed by outpatient assessments after discharge (7 subjects). Discharges prior to 72 hours occurred in 3 of the 18 subjects receiving baclofen, and in 4 of the 13 receiving placebo (odds ratio = 0.45, 95% CI = 0.082.49). Mean CIWA‐Ar scores for the 31 subjects who completed 72 hours of CIWA‐Ar assessments are presented in Figure 1.
| All | Placebo | Baclofen | |
|---|---|---|---|
| Characteristic | (N = 31) | (N = 13) | (N = 18) |
| |||
| % Male | 87.1 | 92.3 | 83.3 |
| Age at admission (mean/SD) | 47.5/10.2 | 45.7/9.3 | 48.7/10.9 |
| MMSE total score (mean/SD) | 26.3/2.2 | 26.3/1.8 | 26.3/2.6 |
| Charlson Comorbidity Score | 1.0/1.1 | 1.1/0.9 | 1.0/1.3 |
| Drinking history | |||
| Age began drinking (mean/SD) | 16.7/4.6 | 16.1/4.3 | 17.1/5.0 |
| Years drinking (mean/SD) | 29.8/8.6 | 29.5/10.0 | 30.0/7.9 |
| Drinks per day (mean/SD) | 16.0/9.9 | 12.9/8.4 | 18.6/10.5 |
| % Daily drinker | 58.1 | 53.8 | 78.6 |
| Days since last drink (mean/SD) | 1.2/0.9 | 1.1/0.8 | 1.3/1.1 |
| Current hospitalization, primary diagnosis | |||
| Alcohol withdrawal syndrome/alcoholism | 48.4 | 46.2 | 50.0 |
| Probably related to alcoholism* | 35.5 | 38.5 | 33.3 |
| Other | 16.1 | 15.4 | 16.7 |
| Medical history, % with history of | |||
| Alcohol withdrawal syndrome | 89.7 | 83.3 | 94.1 |
| Seizures | 60.7 | 45.5 | 70.6 |
| DTs | 79.2 | 80.0 | 78.6 |
| Medications, % at time of admission | |||
| Alcohol treatment | 6.5 | 7.7 | 5.6 |
| Beta‐blocker | 29.1 | 23.1 | 33.3 |
| Sleep agent | 6.5 | 7.7 | 5.6 |
| Narcotic pain medication | 45.2 | 30.8 | 55.6 |
| Depakote | 6.5 | 0.0 | 11.1 |
| Benzodiazepine | 9.7 | 15.4 | 5.6 |
| Anti‐anxiety medication | 3.2 | 0.0 | 5.6 |
| Anti‐psychotic medication | 9.7 | 15.4 | 5.6 |
| Other psychiatric medication | 6.5 | 7.7 | 5.6 |
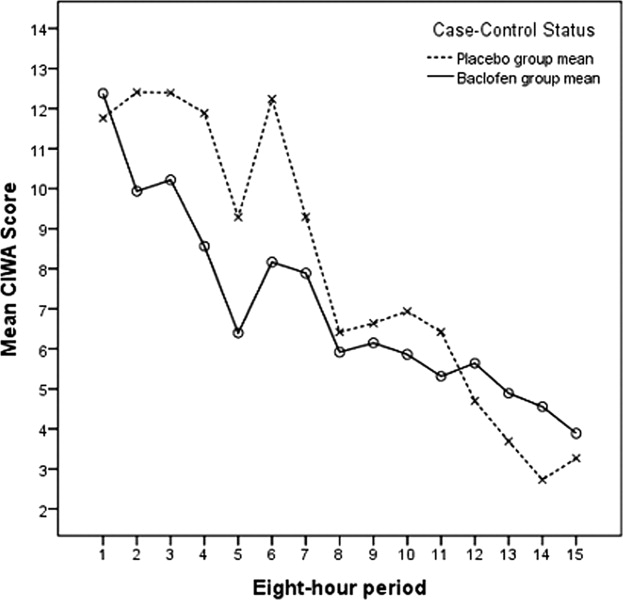
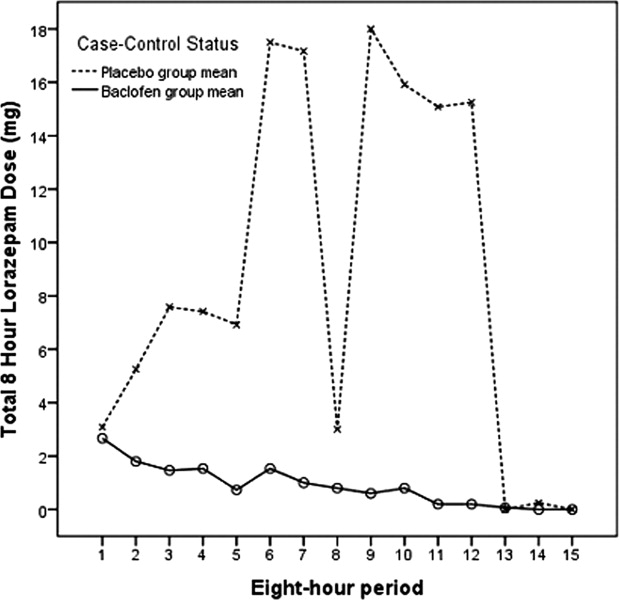
Figures 2 and 3 summarize the mean lorazepam doses in each 8‐hour period, and the cumulative lorazepam doses for the subjects in the 2 arms of the study, respectively. The cumulative dose of lorazepam administered to the 31 subjects ranged from 0 to 1035 mg in the 72 hours following randomization, with a range of 1 to 1035 mg in the placebo group and 0 to 39 mg in the baclofen group. The 8 subjects who received the highest doses of lorazepam (20 mg or more) included 1 of the 18 subjects who received baclofen and 7 of the 13 subjects who received placebo (P = 0.004). Only 4 subjects required >50 mg of lorazepam over the 72 hours; all 4 of these were patients in the placebo group (P = 0.023).
Subjects who received baclofen and subjects who received placebo did not differ significantly in regard to the use of sedatives other than benzodiazepines, the use of restraints, the use of intensive care, or other clinical complications. On the basis this analysis, the study's primary null hypothesis was not rejected (the CIWA‐Ar scores in the baclofen and placebo groups were not different), but the secondary null hypothesis was rejected (baclofen was associated with lower likelihood of the use of high doses of benzodiazepines).
DISCUSSION
Benzodiazepines are effective drugs in the treatment of alcohol withdrawal syndrome. They remain the gold standard of treatment. The most effective method of administering benzodiazepines to acutely withdrawing patients has been shown to be variable dosing, based on withdrawal symptoms.
Our small study of acutely withdrawing inpatients confirmed that benzodiazepines, administered at frequencies and doses dependent on AWS symptoms, work well to control CIWA‐Ar scores over a relatively short time span. Our study also demonstrated that the addition of the GABA‐B agonist baclofen orally, at a fixed dose of 30 mg daily, will allow the same level of AWS symptom control, while reducing the risk that high doses of benzodiazepines will be needed to achieve that control. Reducing the use of high‐dose benzodiazepines has the potential to improve patient safety. In addition, since the frequency of nursing assessments parallel CIWA‐AR scores and benzodiazepine dosing frequency, using oral baclofen in this setting has the potential to decrease the nursing time required to control withdrawal symptoms.
A larger study of AWS will be needed to assess the role of baclofen in managing the frequency and severity of the complications of AWS, such as prolonged sedation, intensive care admission, and ventilator days. The current study was not powered to assess differences in the frequency of relatively rare events, and we excluded patients who required intravenous benzodiazepines for AWS symptom management. However, in light of the well‐documented risk of sedation and respiratory depression from high‐dose benzodiazepines, our findings suggest that a larger study of the role of baclofen in AWS management is warranted.
Either baclofen or benzodiazepines may have severe adverse effects in high doses. In this study, we used a low, fixed dose of baclofen (10 mg every 8 hours), a level at which severe side effects such as respiratory depression are uncommon. Our principal finding was that the use of low‐dose baclofen is associated with reduced use of high‐dose benzodiazepines in some AWS patients.
Further Research
The use of baclofen and other adjunctive treatments in the management of AWS and other alcohol dependency syndromes warrants future study. If the benzodiazepine‐sparing effects of baclofen in AWS management are confirmed in additional studies, baclofen may become an important adjunct to benzodiazepines in AWS management, particularly in settings where the use of symptom‐triggered therapy is difficult.
It is difficult to predict which suddenly abstinent alcoholics will experience severe AWS. In the current study, 44 of the 79 patients judged by experienced clinicians to be at high risk for acute AWS reached the CIWA‐Ar threshold (a score of 11 or more) to be randomized. We found that those who experienced significant withdrawal were younger, drank more heavily, and had more prior experience with severe AWS, which is consistent with earlier studies.23, 24 Nevertheless, patient history is not a reliable predictor of risk for AWS; clinicians are often obliged to watch and wait until clinical signs of AWS develop. In light of the growing evidence that baclofen alleviates many symptoms of alcohol dependence, both in patients with AWS and in those in recovery,19, 20, 2529 future research should also examine the role of baclofen in preventing AWS in at‐risk patients.
Since ethanol has effects on several neurotransmitters and receptor systems, combinations of medications that modify GABA, glutamate, and adrenergic activity in low doses may be more effective and safer in managing AWS than using high doses of a single agent. Future research should seek to identify the most effective combination of low‐dose medications in managing AWS.
Study Limitations
This study was subject to several significant limitations. With a small study population (31 subjects), the experiences of individual subjects had a strong effect on the findings (such as the dip in mean lorazepam doses for placebo subjects in Figure 2, which was due to a high‐dose subject sleeping through the observation period). However, the overall finding of the lorazepam‐sparing effect of baclofen was consistent. We excluded patients who required intravenous benzodiazepines for AWS management, and so our study did not include the most severe AWS subjects. However, all of our subjects showed signs of mild‐to‐moderate alcohol withdrawal upon enrollment (CIWA‐Ar scores of 11 or more upon randomization), and all were at risk for more severe AWS; many went on to much higher CIWA‐AR scores during the course of their AWS. Of the 44 subjects who were randomized, 13 did not complete the study; the impact that these subjects might have had on the findings is unknown. However, the subjects who did not complete the study (baclofen 54%, placebo 46%) did not differ from the remaining subjects in regard to any of the variables used in the study. More baclofen‐treated than placebo‐treated subjects were taking narcotics and/or beta‐blockers at the time of enrollment, although these differences were not statistically significant, and their impact upon our findings are unknown. This study was conducted in northeast Minnesota; the study population reflected the limited diversity of the region. Caution should be used in generalizing the findings to other populations.
CONCLUSION
These findings suggest that baclofen may have potential as an adjunct in the management of acute alcohol withdrawal.
- .Clinical practice. Unhealthy alcohol use.N Engl J Med.2005;352(6):596–607.
- ,,, et al.Patients with alcohol problems in the emergency department, part 1: improving detection. SAEM Substance Abuse Task Force. Society for Academic Emergency Medicine.Acad Emerg Med.1998;5(12):1200–1209.
- ,,.Screening for alcohol problems in primary care: a systematic review.Arch Intern Med.2000;160(13):1977–1989.
- ,.Trends in Alcohol‐Related Morbidity Among Short‐Stay Community Hospital Discharges, United States, 1979–2006. Surveillance Report #84.Arlington, VA:National Institute on Alcohol Abuse and Alcoholism; August2008.
- ,,, et al.Alcohol withdrawal syndrome: turning minor injuries into a major problem.J Trauma.2006;61(6):1441–1446.
- ,,,,,.An assessment of the potential value of elevated homocysteine in predicting alcohol‐withdrawal seizures.Epilepsia.2006;47(5):934–938.
- ,.Combining the audit questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients.Alcohol Alcohol.2005;40(6):515–519.
- .A model for predicting alcohol withdrawal delirium.Psychiatr Serv.2001;52(6):820–823.
- .[GABA‐system and alcohol: does an “ethanol receptor” exist?] [review].Zh Nevrol Psikhiatr Im S S Korsakova.2007;Suppl 1:56–62.
- ,,, et al.Identification of molecular targets associated with ethanol toxicity and implications in drug development.Curr Pharm Des.2010;16(11):1313–1355.
- ,,,,.Baclofen: a new drug for the treatment of alcohol dependence.Int J Clin Pract.2006;60(8):1003–1008.
- ,.3H‐baclofen and 3H‐GABA bind to bicuculline‐insensitive GABA B sites in rat brain.Nature.1981;290(5802):149–152.
- ,.Differential effects of GABA(A) and GABA(B) agonists on sensitization to the locomotor stimulant effects of ethanol in DBA/2 J mice.Psychopharmacology (Berl).1999;141(2):197–205.
- .Cerebral GABA receptors.Alcohol Alcohol Suppl.1994;2:181–186.
- ,,, et al.Symptom‐triggered vs fixed‐schedule doses of benzodiazepine for alcohol withdrawal.Arch Intern Med.2002;162(10):1117–1121.
- .Short‐term lorazepam infusion and concern for propylene glycol toxicity: case report and review.Pharmacother.2001;21(9):1140–1144.
- ,,,,,.The use of continuous IV sedation is associated with prolongation of mechanical ventilation.Chest.1998;114:541–548.
- ,,, et al.Rapid suppression of alcohol withdrawal syndrome by baclofen.Am J Med.2002;112(3):226–229.
- ,,,,,.Ability of baclofen in reducing alcohol craving and intake: II—preliminary clinical evidence.Alcohol Clin Exp Res.2000;24(1):67–71.
- ,,, et al.Baclofen efficacy in reducing alcohol craving and intake: a preliminary double‐blind randomized controlled study.Alcohol Alcohol.2002;37(5):504–508.
- ,,, et al.Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam.Am J Med.2006;119:276.e213–276.e218.
- ,,,,.Relationship between Alzheimer's disease severity and patient participation in decisions about their care.J Geriatr Psychiatry Neurol.2002;15(2):68–72.
- ,,.Impact of age on the severity, course, and complications of alcohol withdrawal.Arch Intern Med.1997;157(19):2234–2241.
- ,,.Independent clinical correlates of severe alcohol withdrawal.Substance Abuse.2003;24(4):197–209.
- ,,, et al.Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol‐dependent patients with liver cirrhosis: randomised, double‐blind study.Lancet.2007;370:1915–1922.
- ,,, et al.Baclofen for alcohol dependence: a preliminary open‐label study.Alcohol Clin Exp Res.2004;28(10):1517–1523.
- ,,,.[Pharmacological treatment options for prevention of alcohol relapse].Fortschr Neurol Psychiatr.2008;76(7):421–428.
- ,.Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms.Pharmacol Ther.2006;111:855–876.
- ,.Baclofen as prophylaxis and treatment for alcohol withdrawal: a retrospective chart review.J Okla State Med Assoc.2007;100(9):354–360.
In the United States, unhealthy alcohol use affects medical care on several levels. The prevalence of alcohol problems is 7%20% or higher among outpatients,1 30%40% among emergency room patients, and 50% among patients with trauma.13 In 2006, approximately 430,000 hospital discharges in the United States were for persons with a principal (first‐listed) alcohol‐related diagnosis, and 1.7 million discharges listed at least one alcohol‐related diagnosis, representing 1.3% and 5.3% of all hospital discharges, respectively.4 Alcoholic psychosis (34.5%) and alcohol dependence syndrome (29.5%) together accounted for the majority of principal alcohol‐related diagnoses.4 Additionally, many patients hospitalized for other indications are susceptible to withdrawal symptoms due to physiological habituation to alcohol. Abrupt cessation of alcohol intake causes habituated drinkers to experience symptoms of alcohol withdrawal syndrome (AWS), which significantly increases intensity and cost of care. Trauma patients who develop AWS were found to have increased morbidity, more intensive care and ventilator days, and higher hospital costs than trauma patients without AWS.5 Unfortunately, attempts to develop predictive models to accurately forecast the likelihood of developing severe AWS in an individual case have been modestly successful at best.68
Regimens used to treat AWS have evolved over time, taking advantage of advances in the understanding of addiction neurophysiology. There is no specific ethanol receptor.9 Much of alcohol's acute effects on the central nervous system are mediated by its stimulation of the gamma‐aminobutyric acid (GABA) system, which is neuroinhibitory.10 Chronic alcohol use leads to habituation partly by inducing configuration changes of GABA‐A receptor subunits. This renders the GABA‐A receptor less sensitive to alcohol, barbiturates, and benzodiazepines.11 Although both GABA‐A and GABA‐B receptor activation cause increased GABA neuronal output, the GABA‐A receptor is rendered relatively less sensitive by chronic exposure to alcohol. Baclofen is a pure GABA‐B receptor agonist,12 and its GABA‐B stimulatory effect is maintained even in habituated alcoholics.13, 14 The absence of cross‐tolerance between baclofen and ethanol suggests that low doses of baclofen may be helpful in the management of AWS.
Currently, AWS is usually managed with benzodiazepines, using variable dosing depending on the severity of withdrawal symptoms. Such symptom‐triggered treatment is generally preferred over fixed‐dose regimens,15 in part because when using this method, many cases of AWS can be managed with less medication. Benzodiazepine regimens using high doses have been found to be associated with substantial morbidity and prolonged hospitalizations.16, 17
In a series of small studies, Addolorato's research team has reported decreases in AWS symptoms in association with the use of low doses of baclofen in an outpatient population,18 and has found baclofen to be associated with reduced alcohol craving in the long‐term management of alcohol dependence.11, 19, 20 Addolorato and colleagues' studies of baclofen in relieving AWS symptoms prompted our group to apply the use of baclofen in a larger group of inpatients with AWS.11, 18, 21 We conducted this study to improve understanding of the role of baclofen in the management of acute AWS in an inpatient population of subjects at risk for AWS, drawn from general hospital admissions.
Our primary null hypothesis was that Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA‐Ar) scores in acutely withdrawing alcoholic patients are equal in baclofen‐treated and placebo‐treated subject groups. Our secondary null hypothesis was that benzodiazepine doses used to treat acutely withdrawing alcoholic patients are equal in the baclofen‐treated and placebo‐treated groups.
METHODS
The protocol for this study was approved by the Essentia Health Institutional Review Board, Duluth, Minnesota.
This was a randomized, placebo‐controlled, double‐blind trial. Subjects were recruited from among patients who were admitted to 1 of 2 regional general hospitals in Duluth, Minnesota (St. Mary's Medical Center or Miller‐Dwan Medical Center) and who were identified by clinical staff as being at risk for AWS. Potential subjects were not required to have an alcohol‐related condition as their primary reason for admission, but were required to have a history of AWS or of alcohol use suggestive of significant risk for AWS, and to be able to provide informed consent, as described below.
Patients were not eligible for enrollment in the study if they had other active drug dependence in addition to alcohol; were using baclofen at the time of study enrollment; were using benzodiazepines chronically at the time of study enrollment; had a known baclofen or benzodiazepine sensitivity; were unable to take oral medications; were pregnant or breast‐feeding; had a serum creatinine level 2.0; had a history of non‐alcohol withdrawal seizures; required intravenous benzodiazepines to control their AWS; or were unable to complete the consenting procedures.
Consenting and Enrollment Procedures
Patients who were identified by clinical staff as being at high risk for AWS were approached for possible enrollment in the study. Potential subjects who met other inclusion criteria were screened to assess their mental status with the Mini‐Mental Status Exam (MMSE), using methods developed for subjects with cognitive impairment.22 Potential subjects who scored 24 of a possible score of 30 or higher on the exam were considered capable of providing informed consent. Potential subjects who scored between 20 and 23 were considered to be capable of providing consent if they were able to answer 4 questions about the study (why the study is being done, what will be required of them if they participate, how long they will be in the study, and how it will be determined if they will receive the investigational drug or the placebo).
Patients who met inclusion criteria were enrolled in the study. Subjects were randomized only if they developed signs of AWS sufficient to meet Diagnostic and Statistical Manual of Mental Disorders IV (DSMIV) criteria for AWS diagnosis, and reached at least a score of 11 (of a possible 67) on the CIWA‐Ar. All subjects received symptom‐triggered benzodiazepine treatment, and also were randomized to receive either baclofen (10 mg) or placebo every 8 hours (q8h) orally as inpatients for 72 hours or until discharge, whichever was shorter. Lorazepam was selected for the symptom‐triggered benzodiazepine treatment, as it has been used for managing AWS in the participating hospitals for several years. The initial research protocol called for 5 days of participation (15 doses of study drug), but we found that many subjects were discharged before the 5 days had elapsed after enrollment, and that compliance with follow‐up outpatient visits was poor. Accordingly, the protocol was amended to shorten the treatment period to 72 hours of participation (9 doses) or until discharge if prior to 72 hours, with the minimum observation period set to 72 hours.
Data Collection
Baseline data were collected at the time of enrollment, both from the patient and the medical record. Demographic data (age, gender, race) were obtained, as well as data on alcohol history, including approximate duration and intensity of alcohol use, and prior experience with AWS; data on comorbid conditions and medical history; and history of beta‐blocker use. During the period of observation following randomization, data were obtained on CIWA‐Ar scores; benzodiazepine doses administered; and adverse events, including use of sedatives in addition to benzodiazepines, use of restraints, use of intensive care, and clinical complications during the AWS course.
Study Procedures
The research pharmacy provided study medications (baclofen or placebo in identical form) for enrolled subjects. Subjects and study personnel were blinded to treatment group (baclofen vs placebo).
Nurses on inpatient units were provided with training in CIWA‐Ar assessment. All subjects were monitored for CIWA‐Ar scores at the time of study enrollment and for at least the next 72 hours. In monitoring the subjects, the nurses used the CIWA‐Ar protocol, in which subjects were assessed and potentially dosed with lorazepam hourly if their scores were 11 or higher. If the CIWA‐Ar score was less than 11, the subjects were assessed every 4 hours and at study discharge. CIWA‐AR results were reported as averaged over 8‐hour periods starting at study enrollment.
Data Analysis
Demographic and baseline variables with ordinal and continuous measurements, such as age, MMSE total score, and drinks per day were evaluated using group t test analysis, with two‐tailed significance estimates. Variables with prevalence reported were evaluated using the chi‐square test of significance. In accordance with the protocol, data from patients who had CIWA‐Ar assessments for at least 72 hours following randomization were included in the final study analyses. Repeated measures analysis of variance were conducted for the 2 treatment groups (baclofen and placebo), to evaluate mean CIWA‐Ar scores and mean lorazepam doses within each 8‐hour interval, as well as cumulative lorazepam dose. No covariates were included in the models. The last‐observation‐carried‐forward approach was used for those subjects who were missing CIWA‐Ar scores between baseline and their last CIWA‐Ar score. Doses for postdischarge patients without lorazepam prescriptions were set to 0 mg/8 hr. Cumulative lorazepam dose at 72 hours was also analyzed by defining the upper 25th percentile of existing doses (the upper 8 of 31) as high dose. This high‐dose lorazepam treatment level was determined to include all study participants receiving 20 mg or more of lorazepam during the first 72 hours. Fisher's exact test (two‐tailed) was then used to assess the difference in treatment group (baclofen vs placebo) for high‐dose lorazepam treatment.
RESULTS
Seventy‐nine subjects met study inclusion criteria, and provided informed consent for participation in the study. Of these, 44 subjects developed signs of AWS sufficient to meet DSMIV criteria for AWS diagnosis, and were randomized to receive either baclofen or placebo, in addition to benzodiazepine therapy. As summarized in Table 1, subjects who developed signs of AWS were similar to subjects who did not enter withdrawal, differing principally in that those who developed AWS reported more drinks per day, and more significant history of previous AWS.
| Withdrawal/Randomized | ||||
|---|---|---|---|---|
| No Withdrawal | All | Placebo | Baclofen | |
| Characteristic | (N = 35) | (N = 44) | (N = 19) | (N = 25) |
| ||||
| % Male | 82.9 | 84.1 | 94.7 | 76.0 |
| Age at admission (mean/SD) | 52.0/12.0 | 46.9/10.9 | 46.1/11.9 | 47.5/10.3 |
| MMSE total score (mean/SD) | 26.5/1.7 | 25.8/3.3 | 25.4/4.1 | 26.0/2.4 |
| Drinking history | ||||
| Age began drinking (mean/SD) | 16.7/4.2 | 16.2/4.3 | 15.5/4.5 | 16.7/4.2 |
| Years drinking (mean/SD) | 34.1/10.8 | 30.2/10.0 | 30.0/12.8 | 30.3/7.8 |
| Drinks per day (mean/SD)* | 11.2/8.8 | 16.3/9.7 | 14.4/7.8 | 18.0/11.0 |
| % Daily drinker | 65.7 | 64.1 | 57.9 | 70.0 |
| Days since last drink (mean/SD) | 1.5/0.9 | 1.3/1.3 | 1.0/0.8 | 1.6/1.5 |
| Medical history, % with history of | ||||
| Alcohol withdrawal syndrome | 60.6 | 87.5 | 87.5 | 87.5 |
| Seizures*, | 30.0 | 53.8 | 33.3 | 66.7 |
| DTs* | 48.3 | 74.3 | 80.0 | 70.0 |
| Medications, % at time of admission | ||||
| Alcohol treatment | 0.0 | 4.5 | 5.3 | 4.0 |
| Beta‐blocker | 31.4 | 25.0 | 26.3 | 24.0 |
| Sleep agent | 2.9 | 6.8 | 5.3 | 8.0 |
| Narcotic pain medication | 37.1 | 40.9 | 31.6 | 48.0 |
| Depakote | 0.0 | 4.5 | 0.0 | 8.0 |
| Benzodiazepine | 2.9 | 11.4 | 10.5 | 12.0 |
| Anti‐anxiety medication | 2.9 | 2.3 | 0.0 | 4.0 |
| Anti‐psychotic medication | 5.7 | 6.8 | 10.5 | 4.0 |
| Other psychiatric medication | 2.9 | 9.1 | 5.3 | 12.0 |
The 79 subjects who were enrolled were drawn from a population of 237 potential subjects who were screened for the study. The most common reasons that the 158 potential subjects were not enrolled were refusal (29.7%), low risk of withdrawal (19.0%), inability to provide consent (9.5%), and concurrent use of benzodiazepines (8.9%). The 15 patients who were unable to provide consent were those who scored 24 or lower on the MMSE, and did not have a surrogate decision‐maker available.
Of the 44 subjects who were randomized, 31 (18 in the baclofen group, 13 in the placebo group) completed 72 hours of CIWA‐Ar assessments, as summarized in Table 2. These assessments were completed either entirely as inpatients (24 subjects) or with inpatient assessments followed by outpatient assessments after discharge (7 subjects). Discharges prior to 72 hours occurred in 3 of the 18 subjects receiving baclofen, and in 4 of the 13 receiving placebo (odds ratio = 0.45, 95% CI = 0.082.49). Mean CIWA‐Ar scores for the 31 subjects who completed 72 hours of CIWA‐Ar assessments are presented in Figure 1.
| All | Placebo | Baclofen | |
|---|---|---|---|
| Characteristic | (N = 31) | (N = 13) | (N = 18) |
| |||
| % Male | 87.1 | 92.3 | 83.3 |
| Age at admission (mean/SD) | 47.5/10.2 | 45.7/9.3 | 48.7/10.9 |
| MMSE total score (mean/SD) | 26.3/2.2 | 26.3/1.8 | 26.3/2.6 |
| Charlson Comorbidity Score | 1.0/1.1 | 1.1/0.9 | 1.0/1.3 |
| Drinking history | |||
| Age began drinking (mean/SD) | 16.7/4.6 | 16.1/4.3 | 17.1/5.0 |
| Years drinking (mean/SD) | 29.8/8.6 | 29.5/10.0 | 30.0/7.9 |
| Drinks per day (mean/SD) | 16.0/9.9 | 12.9/8.4 | 18.6/10.5 |
| % Daily drinker | 58.1 | 53.8 | 78.6 |
| Days since last drink (mean/SD) | 1.2/0.9 | 1.1/0.8 | 1.3/1.1 |
| Current hospitalization, primary diagnosis | |||
| Alcohol withdrawal syndrome/alcoholism | 48.4 | 46.2 | 50.0 |
| Probably related to alcoholism* | 35.5 | 38.5 | 33.3 |
| Other | 16.1 | 15.4 | 16.7 |
| Medical history, % with history of | |||
| Alcohol withdrawal syndrome | 89.7 | 83.3 | 94.1 |
| Seizures | 60.7 | 45.5 | 70.6 |
| DTs | 79.2 | 80.0 | 78.6 |
| Medications, % at time of admission | |||
| Alcohol treatment | 6.5 | 7.7 | 5.6 |
| Beta‐blocker | 29.1 | 23.1 | 33.3 |
| Sleep agent | 6.5 | 7.7 | 5.6 |
| Narcotic pain medication | 45.2 | 30.8 | 55.6 |
| Depakote | 6.5 | 0.0 | 11.1 |
| Benzodiazepine | 9.7 | 15.4 | 5.6 |
| Anti‐anxiety medication | 3.2 | 0.0 | 5.6 |
| Anti‐psychotic medication | 9.7 | 15.4 | 5.6 |
| Other psychiatric medication | 6.5 | 7.7 | 5.6 |

Figures 2 and 3 summarize the mean lorazepam doses in each 8‐hour period, and the cumulative lorazepam doses for the subjects in the 2 arms of the study, respectively. The cumulative dose of lorazepam administered to the 31 subjects ranged from 0 to 1035 mg in the 72 hours following randomization, with a range of 1 to 1035 mg in the placebo group and 0 to 39 mg in the baclofen group. The 8 subjects who received the highest doses of lorazepam (20 mg or more) included 1 of the 18 subjects who received baclofen and 7 of the 13 subjects who received placebo (P = 0.004). Only 4 subjects required >50 mg of lorazepam over the 72 hours; all 4 of these were patients in the placebo group (P = 0.023).


Subjects who received baclofen and subjects who received placebo did not differ significantly in regard to the use of sedatives other than benzodiazepines, the use of restraints, the use of intensive care, or other clinical complications. On the basis this analysis, the study's primary null hypothesis was not rejected (the CIWA‐Ar scores in the baclofen and placebo groups were not different), but the secondary null hypothesis was rejected (baclofen was associated with lower likelihood of the use of high doses of benzodiazepines).
DISCUSSION
Benzodiazepines are effective drugs in the treatment of alcohol withdrawal syndrome. They remain the gold standard of treatment. The most effective method of administering benzodiazepines to acutely withdrawing patients has been shown to be variable dosing, based on withdrawal symptoms.
Our small study of acutely withdrawing inpatients confirmed that benzodiazepines, administered at frequencies and doses dependent on AWS symptoms, work well to control CIWA‐Ar scores over a relatively short time span. Our study also demonstrated that the addition of the GABA‐B agonist baclofen orally, at a fixed dose of 30 mg daily, will allow the same level of AWS symptom control, while reducing the risk that high doses of benzodiazepines will be needed to achieve that control. Reducing the use of high‐dose benzodiazepines has the potential to improve patient safety. In addition, since the frequency of nursing assessments parallel CIWA‐AR scores and benzodiazepine dosing frequency, using oral baclofen in this setting has the potential to decrease the nursing time required to control withdrawal symptoms.
A larger study of AWS will be needed to assess the role of baclofen in managing the frequency and severity of the complications of AWS, such as prolonged sedation, intensive care admission, and ventilator days. The current study was not powered to assess differences in the frequency of relatively rare events, and we excluded patients who required intravenous benzodiazepines for AWS symptom management. However, in light of the well‐documented risk of sedation and respiratory depression from high‐dose benzodiazepines, our findings suggest that a larger study of the role of baclofen in AWS management is warranted.
Either baclofen or benzodiazepines may have severe adverse effects in high doses. In this study, we used a low, fixed dose of baclofen (10 mg every 8 hours), a level at which severe side effects such as respiratory depression are uncommon. Our principal finding was that the use of low‐dose baclofen is associated with reduced use of high‐dose benzodiazepines in some AWS patients.
Further Research
The use of baclofen and other adjunctive treatments in the management of AWS and other alcohol dependency syndromes warrants future study. If the benzodiazepine‐sparing effects of baclofen in AWS management are confirmed in additional studies, baclofen may become an important adjunct to benzodiazepines in AWS management, particularly in settings where the use of symptom‐triggered therapy is difficult.
It is difficult to predict which suddenly abstinent alcoholics will experience severe AWS. In the current study, 44 of the 79 patients judged by experienced clinicians to be at high risk for acute AWS reached the CIWA‐Ar threshold (a score of 11 or more) to be randomized. We found that those who experienced significant withdrawal were younger, drank more heavily, and had more prior experience with severe AWS, which is consistent with earlier studies.23, 24 Nevertheless, patient history is not a reliable predictor of risk for AWS; clinicians are often obliged to watch and wait until clinical signs of AWS develop. In light of the growing evidence that baclofen alleviates many symptoms of alcohol dependence, both in patients with AWS and in those in recovery,19, 20, 2529 future research should also examine the role of baclofen in preventing AWS in at‐risk patients.
Since ethanol has effects on several neurotransmitters and receptor systems, combinations of medications that modify GABA, glutamate, and adrenergic activity in low doses may be more effective and safer in managing AWS than using high doses of a single agent. Future research should seek to identify the most effective combination of low‐dose medications in managing AWS.
Study Limitations
This study was subject to several significant limitations. With a small study population (31 subjects), the experiences of individual subjects had a strong effect on the findings (such as the dip in mean lorazepam doses for placebo subjects in Figure 2, which was due to a high‐dose subject sleeping through the observation period). However, the overall finding of the lorazepam‐sparing effect of baclofen was consistent. We excluded patients who required intravenous benzodiazepines for AWS management, and so our study did not include the most severe AWS subjects. However, all of our subjects showed signs of mild‐to‐moderate alcohol withdrawal upon enrollment (CIWA‐Ar scores of 11 or more upon randomization), and all were at risk for more severe AWS; many went on to much higher CIWA‐AR scores during the course of their AWS. Of the 44 subjects who were randomized, 13 did not complete the study; the impact that these subjects might have had on the findings is unknown. However, the subjects who did not complete the study (baclofen 54%, placebo 46%) did not differ from the remaining subjects in regard to any of the variables used in the study. More baclofen‐treated than placebo‐treated subjects were taking narcotics and/or beta‐blockers at the time of enrollment, although these differences were not statistically significant, and their impact upon our findings are unknown. This study was conducted in northeast Minnesota; the study population reflected the limited diversity of the region. Caution should be used in generalizing the findings to other populations.
CONCLUSION
These findings suggest that baclofen may have potential as an adjunct in the management of acute alcohol withdrawal.
In the United States, unhealthy alcohol use affects medical care on several levels. The prevalence of alcohol problems is 7%20% or higher among outpatients,1 30%40% among emergency room patients, and 50% among patients with trauma.13 In 2006, approximately 430,000 hospital discharges in the United States were for persons with a principal (first‐listed) alcohol‐related diagnosis, and 1.7 million discharges listed at least one alcohol‐related diagnosis, representing 1.3% and 5.3% of all hospital discharges, respectively.4 Alcoholic psychosis (34.5%) and alcohol dependence syndrome (29.5%) together accounted for the majority of principal alcohol‐related diagnoses.4 Additionally, many patients hospitalized for other indications are susceptible to withdrawal symptoms due to physiological habituation to alcohol. Abrupt cessation of alcohol intake causes habituated drinkers to experience symptoms of alcohol withdrawal syndrome (AWS), which significantly increases intensity and cost of care. Trauma patients who develop AWS were found to have increased morbidity, more intensive care and ventilator days, and higher hospital costs than trauma patients without AWS.5 Unfortunately, attempts to develop predictive models to accurately forecast the likelihood of developing severe AWS in an individual case have been modestly successful at best.68
Regimens used to treat AWS have evolved over time, taking advantage of advances in the understanding of addiction neurophysiology. There is no specific ethanol receptor.9 Much of alcohol's acute effects on the central nervous system are mediated by its stimulation of the gamma‐aminobutyric acid (GABA) system, which is neuroinhibitory.10 Chronic alcohol use leads to habituation partly by inducing configuration changes of GABA‐A receptor subunits. This renders the GABA‐A receptor less sensitive to alcohol, barbiturates, and benzodiazepines.11 Although both GABA‐A and GABA‐B receptor activation cause increased GABA neuronal output, the GABA‐A receptor is rendered relatively less sensitive by chronic exposure to alcohol. Baclofen is a pure GABA‐B receptor agonist,12 and its GABA‐B stimulatory effect is maintained even in habituated alcoholics.13, 14 The absence of cross‐tolerance between baclofen and ethanol suggests that low doses of baclofen may be helpful in the management of AWS.
Currently, AWS is usually managed with benzodiazepines, using variable dosing depending on the severity of withdrawal symptoms. Such symptom‐triggered treatment is generally preferred over fixed‐dose regimens,15 in part because when using this method, many cases of AWS can be managed with less medication. Benzodiazepine regimens using high doses have been found to be associated with substantial morbidity and prolonged hospitalizations.16, 17
In a series of small studies, Addolorato's research team has reported decreases in AWS symptoms in association with the use of low doses of baclofen in an outpatient population,18 and has found baclofen to be associated with reduced alcohol craving in the long‐term management of alcohol dependence.11, 19, 20 Addolorato and colleagues' studies of baclofen in relieving AWS symptoms prompted our group to apply the use of baclofen in a larger group of inpatients with AWS.11, 18, 21 We conducted this study to improve understanding of the role of baclofen in the management of acute AWS in an inpatient population of subjects at risk for AWS, drawn from general hospital admissions.
Our primary null hypothesis was that Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA‐Ar) scores in acutely withdrawing alcoholic patients are equal in baclofen‐treated and placebo‐treated subject groups. Our secondary null hypothesis was that benzodiazepine doses used to treat acutely withdrawing alcoholic patients are equal in the baclofen‐treated and placebo‐treated groups.
METHODS
The protocol for this study was approved by the Essentia Health Institutional Review Board, Duluth, Minnesota.
This was a randomized, placebo‐controlled, double‐blind trial. Subjects were recruited from among patients who were admitted to 1 of 2 regional general hospitals in Duluth, Minnesota (St. Mary's Medical Center or Miller‐Dwan Medical Center) and who were identified by clinical staff as being at risk for AWS. Potential subjects were not required to have an alcohol‐related condition as their primary reason for admission, but were required to have a history of AWS or of alcohol use suggestive of significant risk for AWS, and to be able to provide informed consent, as described below.
Patients were not eligible for enrollment in the study if they had other active drug dependence in addition to alcohol; were using baclofen at the time of study enrollment; were using benzodiazepines chronically at the time of study enrollment; had a known baclofen or benzodiazepine sensitivity; were unable to take oral medications; were pregnant or breast‐feeding; had a serum creatinine level 2.0; had a history of non‐alcohol withdrawal seizures; required intravenous benzodiazepines to control their AWS; or were unable to complete the consenting procedures.
Consenting and Enrollment Procedures
Patients who were identified by clinical staff as being at high risk for AWS were approached for possible enrollment in the study. Potential subjects who met other inclusion criteria were screened to assess their mental status with the Mini‐Mental Status Exam (MMSE), using methods developed for subjects with cognitive impairment.22 Potential subjects who scored 24 of a possible score of 30 or higher on the exam were considered capable of providing informed consent. Potential subjects who scored between 20 and 23 were considered to be capable of providing consent if they were able to answer 4 questions about the study (why the study is being done, what will be required of them if they participate, how long they will be in the study, and how it will be determined if they will receive the investigational drug or the placebo).
Patients who met inclusion criteria were enrolled in the study. Subjects were randomized only if they developed signs of AWS sufficient to meet Diagnostic and Statistical Manual of Mental Disorders IV (DSMIV) criteria for AWS diagnosis, and reached at least a score of 11 (of a possible 67) on the CIWA‐Ar. All subjects received symptom‐triggered benzodiazepine treatment, and also were randomized to receive either baclofen (10 mg) or placebo every 8 hours (q8h) orally as inpatients for 72 hours or until discharge, whichever was shorter. Lorazepam was selected for the symptom‐triggered benzodiazepine treatment, as it has been used for managing AWS in the participating hospitals for several years. The initial research protocol called for 5 days of participation (15 doses of study drug), but we found that many subjects were discharged before the 5 days had elapsed after enrollment, and that compliance with follow‐up outpatient visits was poor. Accordingly, the protocol was amended to shorten the treatment period to 72 hours of participation (9 doses) or until discharge if prior to 72 hours, with the minimum observation period set to 72 hours.
Data Collection
Baseline data were collected at the time of enrollment, both from the patient and the medical record. Demographic data (age, gender, race) were obtained, as well as data on alcohol history, including approximate duration and intensity of alcohol use, and prior experience with AWS; data on comorbid conditions and medical history; and history of beta‐blocker use. During the period of observation following randomization, data were obtained on CIWA‐Ar scores; benzodiazepine doses administered; and adverse events, including use of sedatives in addition to benzodiazepines, use of restraints, use of intensive care, and clinical complications during the AWS course.
Study Procedures
The research pharmacy provided study medications (baclofen or placebo in identical form) for enrolled subjects. Subjects and study personnel were blinded to treatment group (baclofen vs placebo).
Nurses on inpatient units were provided with training in CIWA‐Ar assessment. All subjects were monitored for CIWA‐Ar scores at the time of study enrollment and for at least the next 72 hours. In monitoring the subjects, the nurses used the CIWA‐Ar protocol, in which subjects were assessed and potentially dosed with lorazepam hourly if their scores were 11 or higher. If the CIWA‐Ar score was less than 11, the subjects were assessed every 4 hours and at study discharge. CIWA‐AR results were reported as averaged over 8‐hour periods starting at study enrollment.
Data Analysis
Demographic and baseline variables with ordinal and continuous measurements, such as age, MMSE total score, and drinks per day were evaluated using group t test analysis, with two‐tailed significance estimates. Variables with prevalence reported were evaluated using the chi‐square test of significance. In accordance with the protocol, data from patients who had CIWA‐Ar assessments for at least 72 hours following randomization were included in the final study analyses. Repeated measures analysis of variance were conducted for the 2 treatment groups (baclofen and placebo), to evaluate mean CIWA‐Ar scores and mean lorazepam doses within each 8‐hour interval, as well as cumulative lorazepam dose. No covariates were included in the models. The last‐observation‐carried‐forward approach was used for those subjects who were missing CIWA‐Ar scores between baseline and their last CIWA‐Ar score. Doses for postdischarge patients without lorazepam prescriptions were set to 0 mg/8 hr. Cumulative lorazepam dose at 72 hours was also analyzed by defining the upper 25th percentile of existing doses (the upper 8 of 31) as high dose. This high‐dose lorazepam treatment level was determined to include all study participants receiving 20 mg or more of lorazepam during the first 72 hours. Fisher's exact test (two‐tailed) was then used to assess the difference in treatment group (baclofen vs placebo) for high‐dose lorazepam treatment.
RESULTS
Seventy‐nine subjects met study inclusion criteria, and provided informed consent for participation in the study. Of these, 44 subjects developed signs of AWS sufficient to meet DSMIV criteria for AWS diagnosis, and were randomized to receive either baclofen or placebo, in addition to benzodiazepine therapy. As summarized in Table 1, subjects who developed signs of AWS were similar to subjects who did not enter withdrawal, differing principally in that those who developed AWS reported more drinks per day, and more significant history of previous AWS.
| Withdrawal/Randomized | ||||
|---|---|---|---|---|
| No Withdrawal | All | Placebo | Baclofen | |
| Characteristic | (N = 35) | (N = 44) | (N = 19) | (N = 25) |
| ||||
| % Male | 82.9 | 84.1 | 94.7 | 76.0 |
| Age at admission (mean/SD) | 52.0/12.0 | 46.9/10.9 | 46.1/11.9 | 47.5/10.3 |
| MMSE total score (mean/SD) | 26.5/1.7 | 25.8/3.3 | 25.4/4.1 | 26.0/2.4 |
| Drinking history | ||||
| Age began drinking (mean/SD) | 16.7/4.2 | 16.2/4.3 | 15.5/4.5 | 16.7/4.2 |
| Years drinking (mean/SD) | 34.1/10.8 | 30.2/10.0 | 30.0/12.8 | 30.3/7.8 |
| Drinks per day (mean/SD)* | 11.2/8.8 | 16.3/9.7 | 14.4/7.8 | 18.0/11.0 |
| % Daily drinker | 65.7 | 64.1 | 57.9 | 70.0 |
| Days since last drink (mean/SD) | 1.5/0.9 | 1.3/1.3 | 1.0/0.8 | 1.6/1.5 |
| Medical history, % with history of | ||||
| Alcohol withdrawal syndrome | 60.6 | 87.5 | 87.5 | 87.5 |
| Seizures*, | 30.0 | 53.8 | 33.3 | 66.7 |
| DTs* | 48.3 | 74.3 | 80.0 | 70.0 |
| Medications, % at time of admission | ||||
| Alcohol treatment | 0.0 | 4.5 | 5.3 | 4.0 |
| Beta‐blocker | 31.4 | 25.0 | 26.3 | 24.0 |
| Sleep agent | 2.9 | 6.8 | 5.3 | 8.0 |
| Narcotic pain medication | 37.1 | 40.9 | 31.6 | 48.0 |
| Depakote | 0.0 | 4.5 | 0.0 | 8.0 |
| Benzodiazepine | 2.9 | 11.4 | 10.5 | 12.0 |
| Anti‐anxiety medication | 2.9 | 2.3 | 0.0 | 4.0 |
| Anti‐psychotic medication | 5.7 | 6.8 | 10.5 | 4.0 |
| Other psychiatric medication | 2.9 | 9.1 | 5.3 | 12.0 |
The 79 subjects who were enrolled were drawn from a population of 237 potential subjects who were screened for the study. The most common reasons that the 158 potential subjects were not enrolled were refusal (29.7%), low risk of withdrawal (19.0%), inability to provide consent (9.5%), and concurrent use of benzodiazepines (8.9%). The 15 patients who were unable to provide consent were those who scored 24 or lower on the MMSE, and did not have a surrogate decision‐maker available.
Of the 44 subjects who were randomized, 31 (18 in the baclofen group, 13 in the placebo group) completed 72 hours of CIWA‐Ar assessments, as summarized in Table 2. These assessments were completed either entirely as inpatients (24 subjects) or with inpatient assessments followed by outpatient assessments after discharge (7 subjects). Discharges prior to 72 hours occurred in 3 of the 18 subjects receiving baclofen, and in 4 of the 13 receiving placebo (odds ratio = 0.45, 95% CI = 0.082.49). Mean CIWA‐Ar scores for the 31 subjects who completed 72 hours of CIWA‐Ar assessments are presented in Figure 1.
| All | Placebo | Baclofen | |
|---|---|---|---|
| Characteristic | (N = 31) | (N = 13) | (N = 18) |
| |||
| % Male | 87.1 | 92.3 | 83.3 |
| Age at admission (mean/SD) | 47.5/10.2 | 45.7/9.3 | 48.7/10.9 |
| MMSE total score (mean/SD) | 26.3/2.2 | 26.3/1.8 | 26.3/2.6 |
| Charlson Comorbidity Score | 1.0/1.1 | 1.1/0.9 | 1.0/1.3 |
| Drinking history | |||
| Age began drinking (mean/SD) | 16.7/4.6 | 16.1/4.3 | 17.1/5.0 |
| Years drinking (mean/SD) | 29.8/8.6 | 29.5/10.0 | 30.0/7.9 |
| Drinks per day (mean/SD) | 16.0/9.9 | 12.9/8.4 | 18.6/10.5 |
| % Daily drinker | 58.1 | 53.8 | 78.6 |
| Days since last drink (mean/SD) | 1.2/0.9 | 1.1/0.8 | 1.3/1.1 |
| Current hospitalization, primary diagnosis | |||
| Alcohol withdrawal syndrome/alcoholism | 48.4 | 46.2 | 50.0 |
| Probably related to alcoholism* | 35.5 | 38.5 | 33.3 |
| Other | 16.1 | 15.4 | 16.7 |
| Medical history, % with history of | |||
| Alcohol withdrawal syndrome | 89.7 | 83.3 | 94.1 |
| Seizures | 60.7 | 45.5 | 70.6 |
| DTs | 79.2 | 80.0 | 78.6 |
| Medications, % at time of admission | |||
| Alcohol treatment | 6.5 | 7.7 | 5.6 |
| Beta‐blocker | 29.1 | 23.1 | 33.3 |
| Sleep agent | 6.5 | 7.7 | 5.6 |
| Narcotic pain medication | 45.2 | 30.8 | 55.6 |
| Depakote | 6.5 | 0.0 | 11.1 |
| Benzodiazepine | 9.7 | 15.4 | 5.6 |
| Anti‐anxiety medication | 3.2 | 0.0 | 5.6 |
| Anti‐psychotic medication | 9.7 | 15.4 | 5.6 |
| Other psychiatric medication | 6.5 | 7.7 | 5.6 |

Figures 2 and 3 summarize the mean lorazepam doses in each 8‐hour period, and the cumulative lorazepam doses for the subjects in the 2 arms of the study, respectively. The cumulative dose of lorazepam administered to the 31 subjects ranged from 0 to 1035 mg in the 72 hours following randomization, with a range of 1 to 1035 mg in the placebo group and 0 to 39 mg in the baclofen group. The 8 subjects who received the highest doses of lorazepam (20 mg or more) included 1 of the 18 subjects who received baclofen and 7 of the 13 subjects who received placebo (P = 0.004). Only 4 subjects required >50 mg of lorazepam over the 72 hours; all 4 of these were patients in the placebo group (P = 0.023).


Subjects who received baclofen and subjects who received placebo did not differ significantly in regard to the use of sedatives other than benzodiazepines, the use of restraints, the use of intensive care, or other clinical complications. On the basis this analysis, the study's primary null hypothesis was not rejected (the CIWA‐Ar scores in the baclofen and placebo groups were not different), but the secondary null hypothesis was rejected (baclofen was associated with lower likelihood of the use of high doses of benzodiazepines).
DISCUSSION
Benzodiazepines are effective drugs in the treatment of alcohol withdrawal syndrome. They remain the gold standard of treatment. The most effective method of administering benzodiazepines to acutely withdrawing patients has been shown to be variable dosing, based on withdrawal symptoms.
Our small study of acutely withdrawing inpatients confirmed that benzodiazepines, administered at frequencies and doses dependent on AWS symptoms, work well to control CIWA‐Ar scores over a relatively short time span. Our study also demonstrated that the addition of the GABA‐B agonist baclofen orally, at a fixed dose of 30 mg daily, will allow the same level of AWS symptom control, while reducing the risk that high doses of benzodiazepines will be needed to achieve that control. Reducing the use of high‐dose benzodiazepines has the potential to improve patient safety. In addition, since the frequency of nursing assessments parallel CIWA‐AR scores and benzodiazepine dosing frequency, using oral baclofen in this setting has the potential to decrease the nursing time required to control withdrawal symptoms.
A larger study of AWS will be needed to assess the role of baclofen in managing the frequency and severity of the complications of AWS, such as prolonged sedation, intensive care admission, and ventilator days. The current study was not powered to assess differences in the frequency of relatively rare events, and we excluded patients who required intravenous benzodiazepines for AWS symptom management. However, in light of the well‐documented risk of sedation and respiratory depression from high‐dose benzodiazepines, our findings suggest that a larger study of the role of baclofen in AWS management is warranted.
Either baclofen or benzodiazepines may have severe adverse effects in high doses. In this study, we used a low, fixed dose of baclofen (10 mg every 8 hours), a level at which severe side effects such as respiratory depression are uncommon. Our principal finding was that the use of low‐dose baclofen is associated with reduced use of high‐dose benzodiazepines in some AWS patients.
Further Research
The use of baclofen and other adjunctive treatments in the management of AWS and other alcohol dependency syndromes warrants future study. If the benzodiazepine‐sparing effects of baclofen in AWS management are confirmed in additional studies, baclofen may become an important adjunct to benzodiazepines in AWS management, particularly in settings where the use of symptom‐triggered therapy is difficult.
It is difficult to predict which suddenly abstinent alcoholics will experience severe AWS. In the current study, 44 of the 79 patients judged by experienced clinicians to be at high risk for acute AWS reached the CIWA‐Ar threshold (a score of 11 or more) to be randomized. We found that those who experienced significant withdrawal were younger, drank more heavily, and had more prior experience with severe AWS, which is consistent with earlier studies.23, 24 Nevertheless, patient history is not a reliable predictor of risk for AWS; clinicians are often obliged to watch and wait until clinical signs of AWS develop. In light of the growing evidence that baclofen alleviates many symptoms of alcohol dependence, both in patients with AWS and in those in recovery,19, 20, 2529 future research should also examine the role of baclofen in preventing AWS in at‐risk patients.
Since ethanol has effects on several neurotransmitters and receptor systems, combinations of medications that modify GABA, glutamate, and adrenergic activity in low doses may be more effective and safer in managing AWS than using high doses of a single agent. Future research should seek to identify the most effective combination of low‐dose medications in managing AWS.
Study Limitations
This study was subject to several significant limitations. With a small study population (31 subjects), the experiences of individual subjects had a strong effect on the findings (such as the dip in mean lorazepam doses for placebo subjects in Figure 2, which was due to a high‐dose subject sleeping through the observation period). However, the overall finding of the lorazepam‐sparing effect of baclofen was consistent. We excluded patients who required intravenous benzodiazepines for AWS management, and so our study did not include the most severe AWS subjects. However, all of our subjects showed signs of mild‐to‐moderate alcohol withdrawal upon enrollment (CIWA‐Ar scores of 11 or more upon randomization), and all were at risk for more severe AWS; many went on to much higher CIWA‐AR scores during the course of their AWS. Of the 44 subjects who were randomized, 13 did not complete the study; the impact that these subjects might have had on the findings is unknown. However, the subjects who did not complete the study (baclofen 54%, placebo 46%) did not differ from the remaining subjects in regard to any of the variables used in the study. More baclofen‐treated than placebo‐treated subjects were taking narcotics and/or beta‐blockers at the time of enrollment, although these differences were not statistically significant, and their impact upon our findings are unknown. This study was conducted in northeast Minnesota; the study population reflected the limited diversity of the region. Caution should be used in generalizing the findings to other populations.
CONCLUSION
These findings suggest that baclofen may have potential as an adjunct in the management of acute alcohol withdrawal.
- .Clinical practice. Unhealthy alcohol use.N Engl J Med.2005;352(6):596–607.
- ,,, et al.Patients with alcohol problems in the emergency department, part 1: improving detection. SAEM Substance Abuse Task Force. Society for Academic Emergency Medicine.Acad Emerg Med.1998;5(12):1200–1209.
- ,,.Screening for alcohol problems in primary care: a systematic review.Arch Intern Med.2000;160(13):1977–1989.
- ,.Trends in Alcohol‐Related Morbidity Among Short‐Stay Community Hospital Discharges, United States, 1979–2006. Surveillance Report #84.Arlington, VA:National Institute on Alcohol Abuse and Alcoholism; August2008.
- ,,, et al.Alcohol withdrawal syndrome: turning minor injuries into a major problem.J Trauma.2006;61(6):1441–1446.
- ,,,,,.An assessment of the potential value of elevated homocysteine in predicting alcohol‐withdrawal seizures.Epilepsia.2006;47(5):934–938.
- ,.Combining the audit questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients.Alcohol Alcohol.2005;40(6):515–519.
- .A model for predicting alcohol withdrawal delirium.Psychiatr Serv.2001;52(6):820–823.
- .[GABA‐system and alcohol: does an “ethanol receptor” exist?] [review].Zh Nevrol Psikhiatr Im S S Korsakova.2007;Suppl 1:56–62.
- ,,, et al.Identification of molecular targets associated with ethanol toxicity and implications in drug development.Curr Pharm Des.2010;16(11):1313–1355.
- ,,,,.Baclofen: a new drug for the treatment of alcohol dependence.Int J Clin Pract.2006;60(8):1003–1008.
- ,.3H‐baclofen and 3H‐GABA bind to bicuculline‐insensitive GABA B sites in rat brain.Nature.1981;290(5802):149–152.
- ,.Differential effects of GABA(A) and GABA(B) agonists on sensitization to the locomotor stimulant effects of ethanol in DBA/2 J mice.Psychopharmacology (Berl).1999;141(2):197–205.
- .Cerebral GABA receptors.Alcohol Alcohol Suppl.1994;2:181–186.
- ,,, et al.Symptom‐triggered vs fixed‐schedule doses of benzodiazepine for alcohol withdrawal.Arch Intern Med.2002;162(10):1117–1121.
- .Short‐term lorazepam infusion and concern for propylene glycol toxicity: case report and review.Pharmacother.2001;21(9):1140–1144.
- ,,,,,.The use of continuous IV sedation is associated with prolongation of mechanical ventilation.Chest.1998;114:541–548.
- ,,, et al.Rapid suppression of alcohol withdrawal syndrome by baclofen.Am J Med.2002;112(3):226–229.
- ,,,,,.Ability of baclofen in reducing alcohol craving and intake: II—preliminary clinical evidence.Alcohol Clin Exp Res.2000;24(1):67–71.
- ,,, et al.Baclofen efficacy in reducing alcohol craving and intake: a preliminary double‐blind randomized controlled study.Alcohol Alcohol.2002;37(5):504–508.
- ,,, et al.Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam.Am J Med.2006;119:276.e213–276.e218.
- ,,,,.Relationship between Alzheimer's disease severity and patient participation in decisions about their care.J Geriatr Psychiatry Neurol.2002;15(2):68–72.
- ,,.Impact of age on the severity, course, and complications of alcohol withdrawal.Arch Intern Med.1997;157(19):2234–2241.
- ,,.Independent clinical correlates of severe alcohol withdrawal.Substance Abuse.2003;24(4):197–209.
- ,,, et al.Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol‐dependent patients with liver cirrhosis: randomised, double‐blind study.Lancet.2007;370:1915–1922.
- ,,, et al.Baclofen for alcohol dependence: a preliminary open‐label study.Alcohol Clin Exp Res.2004;28(10):1517–1523.
- ,,,.[Pharmacological treatment options for prevention of alcohol relapse].Fortschr Neurol Psychiatr.2008;76(7):421–428.
- ,.Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms.Pharmacol Ther.2006;111:855–876.
- ,.Baclofen as prophylaxis and treatment for alcohol withdrawal: a retrospective chart review.J Okla State Med Assoc.2007;100(9):354–360.
- .Clinical practice. Unhealthy alcohol use.N Engl J Med.2005;352(6):596–607.
- ,,, et al.Patients with alcohol problems in the emergency department, part 1: improving detection. SAEM Substance Abuse Task Force. Society for Academic Emergency Medicine.Acad Emerg Med.1998;5(12):1200–1209.
- ,,.Screening for alcohol problems in primary care: a systematic review.Arch Intern Med.2000;160(13):1977–1989.
- ,.Trends in Alcohol‐Related Morbidity Among Short‐Stay Community Hospital Discharges, United States, 1979–2006. Surveillance Report #84.Arlington, VA:National Institute on Alcohol Abuse and Alcoholism; August2008.
- ,,, et al.Alcohol withdrawal syndrome: turning minor injuries into a major problem.J Trauma.2006;61(6):1441–1446.
- ,,,,,.An assessment of the potential value of elevated homocysteine in predicting alcohol‐withdrawal seizures.Epilepsia.2006;47(5):934–938.
- ,.Combining the audit questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients.Alcohol Alcohol.2005;40(6):515–519.
- .A model for predicting alcohol withdrawal delirium.Psychiatr Serv.2001;52(6):820–823.
- .[GABA‐system and alcohol: does an “ethanol receptor” exist?] [review].Zh Nevrol Psikhiatr Im S S Korsakova.2007;Suppl 1:56–62.
- ,,, et al.Identification of molecular targets associated with ethanol toxicity and implications in drug development.Curr Pharm Des.2010;16(11):1313–1355.
- ,,,,.Baclofen: a new drug for the treatment of alcohol dependence.Int J Clin Pract.2006;60(8):1003–1008.
- ,.3H‐baclofen and 3H‐GABA bind to bicuculline‐insensitive GABA B sites in rat brain.Nature.1981;290(5802):149–152.
- ,.Differential effects of GABA(A) and GABA(B) agonists on sensitization to the locomotor stimulant effects of ethanol in DBA/2 J mice.Psychopharmacology (Berl).1999;141(2):197–205.
- .Cerebral GABA receptors.Alcohol Alcohol Suppl.1994;2:181–186.
- ,,, et al.Symptom‐triggered vs fixed‐schedule doses of benzodiazepine for alcohol withdrawal.Arch Intern Med.2002;162(10):1117–1121.
- .Short‐term lorazepam infusion and concern for propylene glycol toxicity: case report and review.Pharmacother.2001;21(9):1140–1144.
- ,,,,,.The use of continuous IV sedation is associated with prolongation of mechanical ventilation.Chest.1998;114:541–548.
- ,,, et al.Rapid suppression of alcohol withdrawal syndrome by baclofen.Am J Med.2002;112(3):226–229.
- ,,,,,.Ability of baclofen in reducing alcohol craving and intake: II—preliminary clinical evidence.Alcohol Clin Exp Res.2000;24(1):67–71.
- ,,, et al.Baclofen efficacy in reducing alcohol craving and intake: a preliminary double‐blind randomized controlled study.Alcohol Alcohol.2002;37(5):504–508.
- ,,, et al.Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam.Am J Med.2006;119:276.e213–276.e218.
- ,,,,.Relationship between Alzheimer's disease severity and patient participation in decisions about their care.J Geriatr Psychiatry Neurol.2002;15(2):68–72.
- ,,.Impact of age on the severity, course, and complications of alcohol withdrawal.Arch Intern Med.1997;157(19):2234–2241.
- ,,.Independent clinical correlates of severe alcohol withdrawal.Substance Abuse.2003;24(4):197–209.
- ,,, et al.Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol‐dependent patients with liver cirrhosis: randomised, double‐blind study.Lancet.2007;370:1915–1922.
- ,,, et al.Baclofen for alcohol dependence: a preliminary open‐label study.Alcohol Clin Exp Res.2004;28(10):1517–1523.
- ,,,.[Pharmacological treatment options for prevention of alcohol relapse].Fortschr Neurol Psychiatr.2008;76(7):421–428.
- ,.Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms.Pharmacol Ther.2006;111:855–876.
- ,.Baclofen as prophylaxis and treatment for alcohol withdrawal: a retrospective chart review.J Okla State Med Assoc.2007;100(9):354–360.
Copyright © 2011 Society of Hospital Medicine