User login
- The new NCEP III provides revised guidelines for the treatment of hyperlipidemia.
- Combining traditional risk factor assessment with the calculated 10-year risk of coronary artery disease allows for optimal patient-centered counseling.
- Statins are normally the first-line therapy for hyperlipidemia.
In 1995 and 1996, US adults made more than 18 million office visits for the evaluation and treatment of hyperlipidemia, including 3.4% of all visits to family physicians. Among visits to family physicians, 4.1% included measurement of cholesterol levels.1 Overall, mean cholesterol levels decreased from 220 in 1960–1962 to 203 in 1988–1994. During the same time period, the proportion of adults with elevated total cholesterol levels (> 240) decreased from 32% to 19%.2 Despite this progress, the availability of more effective drugs, guidelines advocating increasingly aggressive treatment, and populationwide goals established in Healthy People 2010 will continue to increase the number of patients seen by family physicians for screening, diagnosis, and treatment of hyperlipidemia.3
When to treat
The National Cholesterol Education Program (NCEP), a program within the National Institute of Health’s Heart, Lung, and Blood Institute, published a guideline in 1993 for screening and treating hyperlipidemia. Physicians have since become familiar with the NCEP concept of basing treatment decisions on assessment of patient risk factors (smoking, age, diabetes, hypertension, family history of early coronary artery disease [CAD]) and application of algorithms linked to desired low-density lipoprotein (LDL) cholesterol levels. The advantage of this strategy is its simplicity. Physicians assess whether the NCEP risk factors are present and then work with their patients to achieve the desired LDL level through lifestyle modification, drug therapy, or both.
Unfortunately, the NCEP guideline did not assess the individual’s actual risk of CAD. In its recently released Third Report, the NCEP has recognized the value of this strategy by incorporating the Framingham tables to calculate the 10-year risk of developing clinical CAD based on a patient’s individual risk factors, including cholesterol levels (Table 1).4 This new NCEP III guideline recommends traditional risk factor counting coupled, in certain situations, with the 10-year risk derived from the Framingham scoring system.
TABLE 1
FRAMINGHAM TABLES FOR CALCULATING CORONARY ARTERY DISEASE RISK
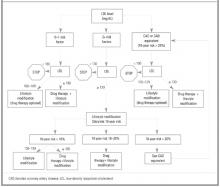
Therapy is based on the individual patient’s risk category and LDL levels (Figure). Patients whose 10-year risk is greater than 20% or those who have CAD-equivalent conditions (ie, diabetes, peripheral arterial disease, abdominal aortic aneurysm, symptomatic carotid artery disease) are considered to have a risk equivalent to that of patients with known CAD; all have an LDL goal of 100 or less.
For those with a 10-year CAD risk less than 20%, the number of positive risk factors determines the LDL goal. This new method allows physicians to communicate with their patients more clearly about individual risk and enhances shared decision making. While the NCEP III report is based on extensive literature review, the recommendations of its expert panel are not characterized according to the strength of the supporting evidence, as is done by the US Preventive Services Task Force.
Figure
TREATMENT STRATEGY BASED ON LDL LEVEL AND RISK CATEGORY
Explaining treatment benefits
The NCEP III report does not make explicit the effect of the treatment on the patient; that is, how much the proposed treatment will reduce the risk of CAD. This determination depends in part on whether the patient being treated has known CAD or a CAD-equivalent condition (secondary prevention) versus no known CAD (primary prevention). The benefits of treatment have been most clearly quantified for drug treatment and are most easily evaluated using the number needed to treat (NNT). The NNT refers to the number of patients who would have to be treated for 5 years to prevent 1 CAD event. Physicians may use the NNTs to assist patients in determining their preferences for treatment, bearing in mind that the NNT refers to an outcome for a population, such as men with high cholesterol levels. For a given individual, their risk of an adverse outcome is all or none. Nonetheless, patients may find the NNT a useful way to assess their personal values in making treatment decisions.
Treatment
Lifestyle modification
Diet modification is the cornerstone of therapy for mild to moderate hyperlipidemia. Modifying the diet is also recommended along with pharmacologic therapy in people at higher risk of CAD. NCEP III recommends a diet for “therapeutic lifestyle changes” that includes < 200 mg cholesterol per day, < 7% saturated fat, 25% to 35% total fat, 50% to 60% carbohydrates, and 15% protein of total calories.4
Although diet therapy has shown a modest redution in total cholesterol in clinical trials, no clear evidence shows that a diet low in saturated fat and cholesterol will reduce cardiovascular morbidity and mortality.5,6 Many people find it difficult to change their dietary habits and to maintain healthier ones. Systematic reviews of observational studies have found that increased consumption of fruits and vegetables is associated with lower incidence of heart attack and stroke. However, the potential for bias and confounding factors in such studies makes them less convincing than randomized controlled trials (RCTs).5
The Ornish program, in which CAD or CAD-equivalent patients pursue intense lifestyle modification for up to 3 years, has shown that revascularization procedures can be avoided. Treatment groups that ate a very-low-fat diet, received intervention on stress management, and followed a prescribed exercise program showed similar improvement in angina symptoms versus the revascularization group. Another trial showed regression of atherosclerotic plaque on angiograms.10-12
Other nonpharmacologic options include plant stanols (2 grams/day) and soluble fiber (10 to 25 grams/day) to reduce LDL-cholesterol. Plant stanols have a structure similar to that of cholesterol and interfere with cholesterol absorption when eaten along with a typical diet, resulting in reduction of blood cholesterol levels. Plant stanols and sterols can be found in certain margarines and salad oils and can be taken with each meal as substitutes for other sources of dietary fat.7-9 No RCTs have shown that these substances reduce cardiovascular events or overall mortality.
Herbal products and dietary supplements
A survey found that as many as 50% of respondents with elevated cholesterol levels would prefer an over-the-counter product such as garlic, yeast, or soy products.13 Studies of products promoted for lipid-lowering effects were found to have a modest effect on lipid levels13-18 (Table 2); however, no RCTs were found that assessed patient-oriented outcomes. Because herbal products and supplements have modest effects on lipid levels and because long-term safety data are lacking, such products should be used with caution for treatment of hyperlipidemia.
TABLE 2
PHARMACOLOGIC AND NONPHARMACOLOGIC INTERVENTIONS
| Strength of Recommendation* | Treatment | Type of Benefit | Cost Per Month ($) | Comments |
|---|---|---|---|---|
| A | Statins | OM, CVM | 40–110 | Well tolerated |
| B | Fibric acids | CVE | 60–70 | All male subjects in both primary and secondary trials |
| B | Niacin | CVE | 10–80 | Watch for adverse reactions (flushing, elevated glucose, liver function tests) |
| B | Bile acid resin | CVE | 40–60 | Ideal agent for patients with severe liver disease; watch for drug interactions |
| B | Lifestyle modification | Lipid | Varies | No strong evidence from randomized clinical trials on primary prevention of major coronary events or mortality |
| B | Soy products | Lipid | 20 | FDA has approved labeling soy products for cholesterol reduction |
| B | Red yeast | Lipid | 20–30 | Active ingredient is lovastatin; should be treated as lovastatin |
| B | Plant stanols | Lipid | 20–30 | Substitute for other source of fat calories; must be taken with each meal |
| C | Fish oils | Lipid | 5–10 | Use with caution because of high caloric value and cholesterol content in products; may increase cholesterol level with long-term use |
| C | Garlic | Lipid | 10–20 | Conflicting results with clinical trials |
| C | Green tea | Lipid | 15 | Epidemiologic study data |
| * Criteria correspond to US Preventive Services Task Force categories (A = strong evidence to support recommendation, B = fair evidence to support recommendation, C = insufficient evidence to recommend for or against). CVE denotes reduction in cardiovascular events; CVM, reduction in cardiovascular mortality; lipid, reduction in lipid levels only; OM, reduction in overall mortality. | ||||
Pharmacologic treatment
Clinical trials of hyperlipidemia therapy should address outcomes that matter most to patients, such as morbidity, mortality, quality of life, and cost, rather than stressing disease-oriented evidence, such as the ability to reduce cholesterol levels. For this review we identified major long-term RCTs that included significant coronary events or mortality as the primary outcomes. Table 3 summarizes the results of primary and secondary prevention studies.
TABLE 3
PHARMACOLOGIC INTERVENTION
| Reduction in Risk | ||||
|---|---|---|---|---|
| Intervention | Major Coronary Events | All-Cause Mortality | Comments | |
| ARR (%) | NNT | NNT | ||
| Primary Prevention | ||||
| Statins | 2.0–2.3 | 44–49 | NS | Studies on normal and hypercholesterolemic patients. Mean age was 47–58 years; all patients were men except for 1 statin study that included a small number of women |
| Gemfibrozil | 1.4 | 71 | NS | |
| Cholestyramine | 1.7 | 59 | NS | |
| Secondary Prevention | ||||
| Statins | 3–3.6 | 28–33 | 24–28 | Mean age was 55–64 years. Participants were male except for the 3 statin studies and the benzafibrate study that enrolled a small number of women. Cholesterol eligibility criteria varied among the studies and included patients with normal or elevated total and LDL levels or low HDL levels |
| Gemfibrozil | 4.4 | 23 | NS | |
| Benzafibrate | 1.4 | 71 | NS | |
| Niacin | 6.2 | 17 | NS | |
| ARR denotes absolute risk reduction in percent; NNT, number of needed to treat for 5 years to prevent 1 adverse outcome; NS, not significant. | ||||
Primary prevention
Primary prevention studies have investigated the treatment of middle-aged men with hyperlipidemia and of men and women with average cholesterol levels.19-23 Results showed similar positive outcomes on reducing coronary events in all groups (Table 3). A systematic review and a meta-analysis of primary prevention studies also demonstrated that drug therapy reduced cholesterol levels and resulted in statistically significant lowering of cardiovascular events in the treated group compared with placebo without any significant reduction in overall mortality.5,24 Absolute risk reductions ranged from 1.4% to 2.3%. In other words, the number of patients that would have to be treated for 5 years to prevent a single major coronary event was 44 to 49 for the statins, 71 for gemfibrozil, and 59 for cholestyramine.
Secondary prevention
In secondary prevention trials, RCTs have demonstrated a strong, consistent relationship between cholesterol lowering and the reduction of risk for a coronary event Table 3.25-30 Patients with preexisting CAD and elevated or average lipid levels benefit from medical therapy. The relative risk of cardiovascular events was reduced by an average of 30% in the active treatment groups.
In these trials, the NNT for 5 years to prevent 1 coronary heart event or nonfatal myocardial infarction (MI) was 28 to 33 for statins, 23 for gemfibrozil, 71 for bezafibrate, and 17 for niacin. There was also a significant risk reduction for all-cause mortality in the statin trials.27,28 These data support the recommendations from NCEP III to treat patients with preexisting CAD aggressively. People with diabetes should receive similar treatment because they are more prone to the development of new CAD within 10 years.4 In addition, subgroup analyses of diabetics treated with statins in primary prevention trials demonstrated a decreased risk of cardiovascular events.26,29
While cholesterol-modifying agents include 4 different classes—statins, fibric acid derivatives, bile acid resins, and nicotinic acid—studies cited in this paper predominantly involved statins and fibric acids. In systematic reviews of both primary and secondary prevention trials, statins were the most effective agents for both cholesterol lowering and cardiovascular risk reduction.5 We found no RCTs that directly compared outcomes between cholesterol-lowering medications. Although women represented a small number of participants in these trials, a meta-analysis showed that statin therapy decreased their risk of heart disease, with an NNT of 31 for reduction of major coronary events.31 No evidence was found to support the effectiveness of hyperlipidemia therapy for people aged more than 75 years. For people aged 65 to 75 years, there is evidence to support drug therapy for secondary prevention but not for primary prevention.
Statins are well tolerated; the most common adverse reactions are gastrointestinal related and occur in approximately 3% of patients. The more serious but uncommon events associated with the use of statins are hepatitis and myopathy. Asymptomatic increases in hepatic transaminases to more than 3 times the upper normal limit occur in approximately 1% of patients.32 Therapy can be discontinued for 1 to 2 weeks; enzyme levels should return to normal if the elevations are medication related. It is not necessary to stop therapy when enzymes are elevated at less than 3 times the upper normal limit.
General guidelines on liver monitoring call for performing a baseline liver function test and repeating it 6 weeks later.33 Once a stable dose has been established, the manufacturer recommends periodic testing; however, no clear evidence supports a specific interval. Clinicians may choose to individualize decisions on testing frequency based on factors such as potential drug interactions (statins with fibric acids or niacin) or the presence of conditions that increase the risk of liver disease.34
Myopathy, defined as generalized muscle aches and pain with a serum creatine kinase level greater than 1000 U/L, occurs rarely (< 0.1%) but may be more likely to occur when statins are used concomitantly with medications such as fibric acid, antifungals, erythromycin, and cyclosporine.31,35 The best preventive strategy is to educate patients about early recognition of the signs and symptoms of myopathy. Because most statins are metabolized by the cytochrome P450-3A4, any medications that inhibit this enzyme can increase statin serum levels and increase the risk of hepatotoxicity and myopathy.
The NCEP III recommends the use of statins as firstline therapy. A standard dose of a statin decreases LDL levels by 20% to 50%, increases HDL levels by 5% to 10%, and reduces triglyceride levels by 10% to 20%. Atorvastatin and simvastatin can produce the highest reductions in LDL levels: up to 50%. Only pravastatin, simvastatin, and lovastatin have been involved in longterm RCTs of primary and secondary prevention. Atorvastatin had positive benefits in a short-term secondary prevention trial.37 Unfortunately, the only head-to-head comparisons of statins have looked at disease-oriented outcomes such as lipid levels.37 Statins are patient friendly. They require a daily evening dose because cholesterol synthesis is more active during the night. Atorvastatin can be given at any time of day because of its long half-life.
Gemfibrozil, a fibric acid, is often used to treat hypertriglyceridemia and as an adjunctive agent to statin therapy. It decreases triglycerides by 40% to 50% but has minimal effects on the rest of the lipid panel. Adverse effects are generally mild. Liver function monitoring is recommended. The usual dosage regimen for fibric acids is 2 times a day and should be adjusted for renal function.
Niacin can increase HDL by 30% and decrease triglycerides by 30% and LDL by 20%. Major adverse reactions include flushing, gastrointestinal symptoms, elevation of liver function tests, uric acid, and serum glucose levels. The new longer-acting formulation has been associated with less flushing. Another class, the bile acid resins, including cholestyramine and colestipol, may play an adjunctive role in therapy. Their effect on the lipid panel is mild compared with those of the other class and they can increase triglyceride levels. Many patients find the gritty taste of the granular formulation unpalatable. The bile acid resins have a favorable safety profile. Most adverse events occur locally in the gut.
Conclusions
The emergence of statins as a safe and effective, although costly, therapy for hyperlipidemia and the development of clinical guidelines advocating their increased use will place family physicians under added pressure to screen for and treat hyperlipidemia. While the general value of lifestyle changes is recognized in national recommendations, more effective ways for physicians to implement them successfully in ambulatory settings are needed.
An optimal evidence-based approach to hyperlipidemia uses the new NCEP III guideline, which combines traditional risk factor assessment with assessment for CAD using the Framingham tables to determine LDL goals and appropriate treatment modalities. Statins are first-line agents for patients who are candidates for drug therapy. Discussions between clinicians and patients of the NNTs for primary and secondary prevention will help foster patient-centered discussions on the role of medical, economic, and quality-of-life issues in the decision-making process.
1. Schappert SM, Nelson C. National Ambulatory Medical Care Survey: 1995–1996 summary. National Center for Health Statistics. Vital Health Stat 1999;13(142).-
2. National Center for Health Statistics. Health, United States, 1999, with health and aging chartbook. Hyattsville, Md: 1999.
3. US Department of Health and Human Services. Tracking Healthy People 2010. Washington, DC: US Government Printing Office. November 2000. Available at: http://www.cdc.gov/hchs/hphome.htm.
4. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive Summary. Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3xsum.pdf Accessed April 16, 2001.
5. Clinical evidence London, England: BMJ Publishing Group; June 2001. Available at: www.clinicalevidence.org.
6. Henkin Y, Shai I, Zuk R, et al. Dietary treatment of hyperlipidemia: Do dietitians do it better? A randomized, controlled trial. Am J Med 2000;109:549-55.
7. Ornish D. Avoiding revascularization with lifestyle changes: the multicenter lifestyle demonstration project. Am J Cardiol 1998;82:72T-76T.
8. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998;280:2001-7.
9. Gould KL, Ornish D, Scherwitz L, et al. Changes in myocardial perfusion abnormalities by positron emission tomography after long-term, intense risk factor modification. JAMA 1995;274:894-901.
10. Mensink RP, Plat J. Efficacy of dietary plant stanols. In: New developments in the dietary management of high cholesterol. New York: McGraw-Hill; 1998;27-31.
11. Blair SN, Capuzzi DM, Gottlieb SO, et al. Incremental reduction of serum total cholesterol and LDL with the addition of plant stanol ester-containing spread to statin therapy. Am J Cardiol 2000;86:46-52.
12. Miettinen TA, Puska P, Gylling H, et al. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesteremic population. N Engl J Med 1995;333:1308-12.
13. Caron MF, White CM. Evaluation of the antihyperlipidemic properties of dietary supplements. Pharmacotherapy 2001;21:481-7.
14. Harris WS. Nonpharmacologic treatment of hypertriglyceridemia: focus on fish oils. Clin Cardiol 1999;22(suppl 2):II40-3.
15. Stevinson C, Pittler MH, Ernst E. Garlic for treating hyperlipidemia. Ann Intern Med 2000;133:420-9.
16. EBM Reviews. Database of abstracts of reviews of effectiveness [database online]. Psyllium-enriched cereals lower blood total cholesterol and LDL cholesterol, but not HDL cholesterol, in hypercholesterolemic adults: results of a meta-analysis. July 2001; v1, accession no. 00125498-100000000-00737. Available at: http://www.ovid.com/products/databases. Accessed Oct. 29, 2001.
17. EBM Reviews. ACP Journal Club [database online]. Soy protein intake decreases total and LDL cholesterol and triglyceride levels. March/April 1996; 124:41, accession no. 00021607-199603000-00013. Available at: http://www.ovid.com/products/databases. Accessed April 4, 2001.
18. Jellin JM, Batz F, Hitchens K. Natural Medicines Comprehensive Database, 3rd ed. Stockton, Calif: Therapeutic Research Faculty; 2000.
19. Frick MH, Elo O, Haapa K, et al. Helsinki heart study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. N Engl J Med 1987;317:1237-45.
20. The lipid research clinics coronary primary prevention trial results I. Reduction in incidence of coronary heart disease. JAMA 1984;251:351-64.
21. The lipid research clinics coronary primary prevention trial results II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984;251:365-74.
22. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hyperlipidemia. N Engl J Med 1995;333:1301-6.
23. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. JAMA 1998;279:1615-22.
24. Pignone M, Phillips C, Mulrow C. Use of lipid lowering drugs for primary prevention of coronary heart disease: meta-analysis of randomized trials. BMJ 2000;321:983-5.
25. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) Study. Circulation 2000;102:21-7.
26. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of HDL-cholesterol. N Engl J Med 1999;341:410-8.
27. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383-9.
28. Preventio of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease. N Engl J Med 1998;339:1349-57.
29. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-9.
30. Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in coronary drug project patients: long-term benefit with niacin. J Am Coll Cardiol 1986;8:1245-55.
31. LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease; a meta-analysis of randomized controlled trials. JAMA 1999;24:2340-6.
32. Hsu I, Spinler SA, Johnson N. Comparative evaluation of the safety and efficacy of HMG-CoA reductase inhibitor monotherapy in the treatment of primary hyperlipidemia. Ann Pharmacother 1995;29:743-59.
33. Tice SA, Parry D. Medications that require hepatic monitoring. Hosp Pharm 2001;36:456-64.
34. Weismantel D. What lab monitoring is appropriate to detect adverse drug reactions in patients on cholesterol-lowering agents? J Fam Pract 2001;50:927.-
35. American College of Clinical Pharmacy. PSAP: pharmacotherapy self-assessment program, 4th ed. Kansas City, Mo: ACCP; 2001;66-7.
36. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. JAMA 2001;285:1711-8.
37. Jones P, Kafonek S, Laurora I, et al. Comparative dose efficacy study of atorvastatin vs. simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hyperlipidemia (the CURVES study). Am J Cardiol 1998;81:582-7.
- The new NCEP III provides revised guidelines for the treatment of hyperlipidemia.
- Combining traditional risk factor assessment with the calculated 10-year risk of coronary artery disease allows for optimal patient-centered counseling.
- Statins are normally the first-line therapy for hyperlipidemia.
In 1995 and 1996, US adults made more than 18 million office visits for the evaluation and treatment of hyperlipidemia, including 3.4% of all visits to family physicians. Among visits to family physicians, 4.1% included measurement of cholesterol levels.1 Overall, mean cholesterol levels decreased from 220 in 1960–1962 to 203 in 1988–1994. During the same time period, the proportion of adults with elevated total cholesterol levels (> 240) decreased from 32% to 19%.2 Despite this progress, the availability of more effective drugs, guidelines advocating increasingly aggressive treatment, and populationwide goals established in Healthy People 2010 will continue to increase the number of patients seen by family physicians for screening, diagnosis, and treatment of hyperlipidemia.3
When to treat
The National Cholesterol Education Program (NCEP), a program within the National Institute of Health’s Heart, Lung, and Blood Institute, published a guideline in 1993 for screening and treating hyperlipidemia. Physicians have since become familiar with the NCEP concept of basing treatment decisions on assessment of patient risk factors (smoking, age, diabetes, hypertension, family history of early coronary artery disease [CAD]) and application of algorithms linked to desired low-density lipoprotein (LDL) cholesterol levels. The advantage of this strategy is its simplicity. Physicians assess whether the NCEP risk factors are present and then work with their patients to achieve the desired LDL level through lifestyle modification, drug therapy, or both.
Unfortunately, the NCEP guideline did not assess the individual’s actual risk of CAD. In its recently released Third Report, the NCEP has recognized the value of this strategy by incorporating the Framingham tables to calculate the 10-year risk of developing clinical CAD based on a patient’s individual risk factors, including cholesterol levels (Table 1).4 This new NCEP III guideline recommends traditional risk factor counting coupled, in certain situations, with the 10-year risk derived from the Framingham scoring system.
TABLE 1
FRAMINGHAM TABLES FOR CALCULATING CORONARY ARTERY DISEASE RISK
Therapy is based on the individual patient’s risk category and LDL levels (Figure). Patients whose 10-year risk is greater than 20% or those who have CAD-equivalent conditions (ie, diabetes, peripheral arterial disease, abdominal aortic aneurysm, symptomatic carotid artery disease) are considered to have a risk equivalent to that of patients with known CAD; all have an LDL goal of 100 or less.
For those with a 10-year CAD risk less than 20%, the number of positive risk factors determines the LDL goal. This new method allows physicians to communicate with their patients more clearly about individual risk and enhances shared decision making. While the NCEP III report is based on extensive literature review, the recommendations of its expert panel are not characterized according to the strength of the supporting evidence, as is done by the US Preventive Services Task Force.
Figure
TREATMENT STRATEGY BASED ON LDL LEVEL AND RISK CATEGORY
Explaining treatment benefits
The NCEP III report does not make explicit the effect of the treatment on the patient; that is, how much the proposed treatment will reduce the risk of CAD. This determination depends in part on whether the patient being treated has known CAD or a CAD-equivalent condition (secondary prevention) versus no known CAD (primary prevention). The benefits of treatment have been most clearly quantified for drug treatment and are most easily evaluated using the number needed to treat (NNT). The NNT refers to the number of patients who would have to be treated for 5 years to prevent 1 CAD event. Physicians may use the NNTs to assist patients in determining their preferences for treatment, bearing in mind that the NNT refers to an outcome for a population, such as men with high cholesterol levels. For a given individual, their risk of an adverse outcome is all or none. Nonetheless, patients may find the NNT a useful way to assess their personal values in making treatment decisions.
Treatment
Lifestyle modification
Diet modification is the cornerstone of therapy for mild to moderate hyperlipidemia. Modifying the diet is also recommended along with pharmacologic therapy in people at higher risk of CAD. NCEP III recommends a diet for “therapeutic lifestyle changes” that includes < 200 mg cholesterol per day, < 7% saturated fat, 25% to 35% total fat, 50% to 60% carbohydrates, and 15% protein of total calories.4
Although diet therapy has shown a modest redution in total cholesterol in clinical trials, no clear evidence shows that a diet low in saturated fat and cholesterol will reduce cardiovascular morbidity and mortality.5,6 Many people find it difficult to change their dietary habits and to maintain healthier ones. Systematic reviews of observational studies have found that increased consumption of fruits and vegetables is associated with lower incidence of heart attack and stroke. However, the potential for bias and confounding factors in such studies makes them less convincing than randomized controlled trials (RCTs).5
The Ornish program, in which CAD or CAD-equivalent patients pursue intense lifestyle modification for up to 3 years, has shown that revascularization procedures can be avoided. Treatment groups that ate a very-low-fat diet, received intervention on stress management, and followed a prescribed exercise program showed similar improvement in angina symptoms versus the revascularization group. Another trial showed regression of atherosclerotic plaque on angiograms.10-12
Other nonpharmacologic options include plant stanols (2 grams/day) and soluble fiber (10 to 25 grams/day) to reduce LDL-cholesterol. Plant stanols have a structure similar to that of cholesterol and interfere with cholesterol absorption when eaten along with a typical diet, resulting in reduction of blood cholesterol levels. Plant stanols and sterols can be found in certain margarines and salad oils and can be taken with each meal as substitutes for other sources of dietary fat.7-9 No RCTs have shown that these substances reduce cardiovascular events or overall mortality.
Herbal products and dietary supplements
A survey found that as many as 50% of respondents with elevated cholesterol levels would prefer an over-the-counter product such as garlic, yeast, or soy products.13 Studies of products promoted for lipid-lowering effects were found to have a modest effect on lipid levels13-18 (Table 2); however, no RCTs were found that assessed patient-oriented outcomes. Because herbal products and supplements have modest effects on lipid levels and because long-term safety data are lacking, such products should be used with caution for treatment of hyperlipidemia.
TABLE 2
PHARMACOLOGIC AND NONPHARMACOLOGIC INTERVENTIONS
| Strength of Recommendation* | Treatment | Type of Benefit | Cost Per Month ($) | Comments |
|---|---|---|---|---|
| A | Statins | OM, CVM | 40–110 | Well tolerated |
| B | Fibric acids | CVE | 60–70 | All male subjects in both primary and secondary trials |
| B | Niacin | CVE | 10–80 | Watch for adverse reactions (flushing, elevated glucose, liver function tests) |
| B | Bile acid resin | CVE | 40–60 | Ideal agent for patients with severe liver disease; watch for drug interactions |
| B | Lifestyle modification | Lipid | Varies | No strong evidence from randomized clinical trials on primary prevention of major coronary events or mortality |
| B | Soy products | Lipid | 20 | FDA has approved labeling soy products for cholesterol reduction |
| B | Red yeast | Lipid | 20–30 | Active ingredient is lovastatin; should be treated as lovastatin |
| B | Plant stanols | Lipid | 20–30 | Substitute for other source of fat calories; must be taken with each meal |
| C | Fish oils | Lipid | 5–10 | Use with caution because of high caloric value and cholesterol content in products; may increase cholesterol level with long-term use |
| C | Garlic | Lipid | 10–20 | Conflicting results with clinical trials |
| C | Green tea | Lipid | 15 | Epidemiologic study data |
| * Criteria correspond to US Preventive Services Task Force categories (A = strong evidence to support recommendation, B = fair evidence to support recommendation, C = insufficient evidence to recommend for or against). CVE denotes reduction in cardiovascular events; CVM, reduction in cardiovascular mortality; lipid, reduction in lipid levels only; OM, reduction in overall mortality. | ||||
Pharmacologic treatment
Clinical trials of hyperlipidemia therapy should address outcomes that matter most to patients, such as morbidity, mortality, quality of life, and cost, rather than stressing disease-oriented evidence, such as the ability to reduce cholesterol levels. For this review we identified major long-term RCTs that included significant coronary events or mortality as the primary outcomes. Table 3 summarizes the results of primary and secondary prevention studies.
TABLE 3
PHARMACOLOGIC INTERVENTION
| Reduction in Risk | ||||
|---|---|---|---|---|
| Intervention | Major Coronary Events | All-Cause Mortality | Comments | |
| ARR (%) | NNT | NNT | ||
| Primary Prevention | ||||
| Statins | 2.0–2.3 | 44–49 | NS | Studies on normal and hypercholesterolemic patients. Mean age was 47–58 years; all patients were men except for 1 statin study that included a small number of women |
| Gemfibrozil | 1.4 | 71 | NS | |
| Cholestyramine | 1.7 | 59 | NS | |
| Secondary Prevention | ||||
| Statins | 3–3.6 | 28–33 | 24–28 | Mean age was 55–64 years. Participants were male except for the 3 statin studies and the benzafibrate study that enrolled a small number of women. Cholesterol eligibility criteria varied among the studies and included patients with normal or elevated total and LDL levels or low HDL levels |
| Gemfibrozil | 4.4 | 23 | NS | |
| Benzafibrate | 1.4 | 71 | NS | |
| Niacin | 6.2 | 17 | NS | |
| ARR denotes absolute risk reduction in percent; NNT, number of needed to treat for 5 years to prevent 1 adverse outcome; NS, not significant. | ||||
Primary prevention
Primary prevention studies have investigated the treatment of middle-aged men with hyperlipidemia and of men and women with average cholesterol levels.19-23 Results showed similar positive outcomes on reducing coronary events in all groups (Table 3). A systematic review and a meta-analysis of primary prevention studies also demonstrated that drug therapy reduced cholesterol levels and resulted in statistically significant lowering of cardiovascular events in the treated group compared with placebo without any significant reduction in overall mortality.5,24 Absolute risk reductions ranged from 1.4% to 2.3%. In other words, the number of patients that would have to be treated for 5 years to prevent a single major coronary event was 44 to 49 for the statins, 71 for gemfibrozil, and 59 for cholestyramine.
Secondary prevention
In secondary prevention trials, RCTs have demonstrated a strong, consistent relationship between cholesterol lowering and the reduction of risk for a coronary event Table 3.25-30 Patients with preexisting CAD and elevated or average lipid levels benefit from medical therapy. The relative risk of cardiovascular events was reduced by an average of 30% in the active treatment groups.
In these trials, the NNT for 5 years to prevent 1 coronary heart event or nonfatal myocardial infarction (MI) was 28 to 33 for statins, 23 for gemfibrozil, 71 for bezafibrate, and 17 for niacin. There was also a significant risk reduction for all-cause mortality in the statin trials.27,28 These data support the recommendations from NCEP III to treat patients with preexisting CAD aggressively. People with diabetes should receive similar treatment because they are more prone to the development of new CAD within 10 years.4 In addition, subgroup analyses of diabetics treated with statins in primary prevention trials demonstrated a decreased risk of cardiovascular events.26,29
While cholesterol-modifying agents include 4 different classes—statins, fibric acid derivatives, bile acid resins, and nicotinic acid—studies cited in this paper predominantly involved statins and fibric acids. In systematic reviews of both primary and secondary prevention trials, statins were the most effective agents for both cholesterol lowering and cardiovascular risk reduction.5 We found no RCTs that directly compared outcomes between cholesterol-lowering medications. Although women represented a small number of participants in these trials, a meta-analysis showed that statin therapy decreased their risk of heart disease, with an NNT of 31 for reduction of major coronary events.31 No evidence was found to support the effectiveness of hyperlipidemia therapy for people aged more than 75 years. For people aged 65 to 75 years, there is evidence to support drug therapy for secondary prevention but not for primary prevention.
Statins are well tolerated; the most common adverse reactions are gastrointestinal related and occur in approximately 3% of patients. The more serious but uncommon events associated with the use of statins are hepatitis and myopathy. Asymptomatic increases in hepatic transaminases to more than 3 times the upper normal limit occur in approximately 1% of patients.32 Therapy can be discontinued for 1 to 2 weeks; enzyme levels should return to normal if the elevations are medication related. It is not necessary to stop therapy when enzymes are elevated at less than 3 times the upper normal limit.
General guidelines on liver monitoring call for performing a baseline liver function test and repeating it 6 weeks later.33 Once a stable dose has been established, the manufacturer recommends periodic testing; however, no clear evidence supports a specific interval. Clinicians may choose to individualize decisions on testing frequency based on factors such as potential drug interactions (statins with fibric acids or niacin) or the presence of conditions that increase the risk of liver disease.34
Myopathy, defined as generalized muscle aches and pain with a serum creatine kinase level greater than 1000 U/L, occurs rarely (< 0.1%) but may be more likely to occur when statins are used concomitantly with medications such as fibric acid, antifungals, erythromycin, and cyclosporine.31,35 The best preventive strategy is to educate patients about early recognition of the signs and symptoms of myopathy. Because most statins are metabolized by the cytochrome P450-3A4, any medications that inhibit this enzyme can increase statin serum levels and increase the risk of hepatotoxicity and myopathy.
The NCEP III recommends the use of statins as firstline therapy. A standard dose of a statin decreases LDL levels by 20% to 50%, increases HDL levels by 5% to 10%, and reduces triglyceride levels by 10% to 20%. Atorvastatin and simvastatin can produce the highest reductions in LDL levels: up to 50%. Only pravastatin, simvastatin, and lovastatin have been involved in longterm RCTs of primary and secondary prevention. Atorvastatin had positive benefits in a short-term secondary prevention trial.37 Unfortunately, the only head-to-head comparisons of statins have looked at disease-oriented outcomes such as lipid levels.37 Statins are patient friendly. They require a daily evening dose because cholesterol synthesis is more active during the night. Atorvastatin can be given at any time of day because of its long half-life.
Gemfibrozil, a fibric acid, is often used to treat hypertriglyceridemia and as an adjunctive agent to statin therapy. It decreases triglycerides by 40% to 50% but has minimal effects on the rest of the lipid panel. Adverse effects are generally mild. Liver function monitoring is recommended. The usual dosage regimen for fibric acids is 2 times a day and should be adjusted for renal function.
Niacin can increase HDL by 30% and decrease triglycerides by 30% and LDL by 20%. Major adverse reactions include flushing, gastrointestinal symptoms, elevation of liver function tests, uric acid, and serum glucose levels. The new longer-acting formulation has been associated with less flushing. Another class, the bile acid resins, including cholestyramine and colestipol, may play an adjunctive role in therapy. Their effect on the lipid panel is mild compared with those of the other class and they can increase triglyceride levels. Many patients find the gritty taste of the granular formulation unpalatable. The bile acid resins have a favorable safety profile. Most adverse events occur locally in the gut.
Conclusions
The emergence of statins as a safe and effective, although costly, therapy for hyperlipidemia and the development of clinical guidelines advocating their increased use will place family physicians under added pressure to screen for and treat hyperlipidemia. While the general value of lifestyle changes is recognized in national recommendations, more effective ways for physicians to implement them successfully in ambulatory settings are needed.
An optimal evidence-based approach to hyperlipidemia uses the new NCEP III guideline, which combines traditional risk factor assessment with assessment for CAD using the Framingham tables to determine LDL goals and appropriate treatment modalities. Statins are first-line agents for patients who are candidates for drug therapy. Discussions between clinicians and patients of the NNTs for primary and secondary prevention will help foster patient-centered discussions on the role of medical, economic, and quality-of-life issues in the decision-making process.
- The new NCEP III provides revised guidelines for the treatment of hyperlipidemia.
- Combining traditional risk factor assessment with the calculated 10-year risk of coronary artery disease allows for optimal patient-centered counseling.
- Statins are normally the first-line therapy for hyperlipidemia.
In 1995 and 1996, US adults made more than 18 million office visits for the evaluation and treatment of hyperlipidemia, including 3.4% of all visits to family physicians. Among visits to family physicians, 4.1% included measurement of cholesterol levels.1 Overall, mean cholesterol levels decreased from 220 in 1960–1962 to 203 in 1988–1994. During the same time period, the proportion of adults with elevated total cholesterol levels (> 240) decreased from 32% to 19%.2 Despite this progress, the availability of more effective drugs, guidelines advocating increasingly aggressive treatment, and populationwide goals established in Healthy People 2010 will continue to increase the number of patients seen by family physicians for screening, diagnosis, and treatment of hyperlipidemia.3
When to treat
The National Cholesterol Education Program (NCEP), a program within the National Institute of Health’s Heart, Lung, and Blood Institute, published a guideline in 1993 for screening and treating hyperlipidemia. Physicians have since become familiar with the NCEP concept of basing treatment decisions on assessment of patient risk factors (smoking, age, diabetes, hypertension, family history of early coronary artery disease [CAD]) and application of algorithms linked to desired low-density lipoprotein (LDL) cholesterol levels. The advantage of this strategy is its simplicity. Physicians assess whether the NCEP risk factors are present and then work with their patients to achieve the desired LDL level through lifestyle modification, drug therapy, or both.
Unfortunately, the NCEP guideline did not assess the individual’s actual risk of CAD. In its recently released Third Report, the NCEP has recognized the value of this strategy by incorporating the Framingham tables to calculate the 10-year risk of developing clinical CAD based on a patient’s individual risk factors, including cholesterol levels (Table 1).4 This new NCEP III guideline recommends traditional risk factor counting coupled, in certain situations, with the 10-year risk derived from the Framingham scoring system.
TABLE 1
FRAMINGHAM TABLES FOR CALCULATING CORONARY ARTERY DISEASE RISK
Therapy is based on the individual patient’s risk category and LDL levels (Figure). Patients whose 10-year risk is greater than 20% or those who have CAD-equivalent conditions (ie, diabetes, peripheral arterial disease, abdominal aortic aneurysm, symptomatic carotid artery disease) are considered to have a risk equivalent to that of patients with known CAD; all have an LDL goal of 100 or less.
For those with a 10-year CAD risk less than 20%, the number of positive risk factors determines the LDL goal. This new method allows physicians to communicate with their patients more clearly about individual risk and enhances shared decision making. While the NCEP III report is based on extensive literature review, the recommendations of its expert panel are not characterized according to the strength of the supporting evidence, as is done by the US Preventive Services Task Force.
Figure
TREATMENT STRATEGY BASED ON LDL LEVEL AND RISK CATEGORY
Explaining treatment benefits
The NCEP III report does not make explicit the effect of the treatment on the patient; that is, how much the proposed treatment will reduce the risk of CAD. This determination depends in part on whether the patient being treated has known CAD or a CAD-equivalent condition (secondary prevention) versus no known CAD (primary prevention). The benefits of treatment have been most clearly quantified for drug treatment and are most easily evaluated using the number needed to treat (NNT). The NNT refers to the number of patients who would have to be treated for 5 years to prevent 1 CAD event. Physicians may use the NNTs to assist patients in determining their preferences for treatment, bearing in mind that the NNT refers to an outcome for a population, such as men with high cholesterol levels. For a given individual, their risk of an adverse outcome is all or none. Nonetheless, patients may find the NNT a useful way to assess their personal values in making treatment decisions.
Treatment
Lifestyle modification
Diet modification is the cornerstone of therapy for mild to moderate hyperlipidemia. Modifying the diet is also recommended along with pharmacologic therapy in people at higher risk of CAD. NCEP III recommends a diet for “therapeutic lifestyle changes” that includes < 200 mg cholesterol per day, < 7% saturated fat, 25% to 35% total fat, 50% to 60% carbohydrates, and 15% protein of total calories.4
Although diet therapy has shown a modest redution in total cholesterol in clinical trials, no clear evidence shows that a diet low in saturated fat and cholesterol will reduce cardiovascular morbidity and mortality.5,6 Many people find it difficult to change their dietary habits and to maintain healthier ones. Systematic reviews of observational studies have found that increased consumption of fruits and vegetables is associated with lower incidence of heart attack and stroke. However, the potential for bias and confounding factors in such studies makes them less convincing than randomized controlled trials (RCTs).5
The Ornish program, in which CAD or CAD-equivalent patients pursue intense lifestyle modification for up to 3 years, has shown that revascularization procedures can be avoided. Treatment groups that ate a very-low-fat diet, received intervention on stress management, and followed a prescribed exercise program showed similar improvement in angina symptoms versus the revascularization group. Another trial showed regression of atherosclerotic plaque on angiograms.10-12
Other nonpharmacologic options include plant stanols (2 grams/day) and soluble fiber (10 to 25 grams/day) to reduce LDL-cholesterol. Plant stanols have a structure similar to that of cholesterol and interfere with cholesterol absorption when eaten along with a typical diet, resulting in reduction of blood cholesterol levels. Plant stanols and sterols can be found in certain margarines and salad oils and can be taken with each meal as substitutes for other sources of dietary fat.7-9 No RCTs have shown that these substances reduce cardiovascular events or overall mortality.
Herbal products and dietary supplements
A survey found that as many as 50% of respondents with elevated cholesterol levels would prefer an over-the-counter product such as garlic, yeast, or soy products.13 Studies of products promoted for lipid-lowering effects were found to have a modest effect on lipid levels13-18 (Table 2); however, no RCTs were found that assessed patient-oriented outcomes. Because herbal products and supplements have modest effects on lipid levels and because long-term safety data are lacking, such products should be used with caution for treatment of hyperlipidemia.
TABLE 2
PHARMACOLOGIC AND NONPHARMACOLOGIC INTERVENTIONS
| Strength of Recommendation* | Treatment | Type of Benefit | Cost Per Month ($) | Comments |
|---|---|---|---|---|
| A | Statins | OM, CVM | 40–110 | Well tolerated |
| B | Fibric acids | CVE | 60–70 | All male subjects in both primary and secondary trials |
| B | Niacin | CVE | 10–80 | Watch for adverse reactions (flushing, elevated glucose, liver function tests) |
| B | Bile acid resin | CVE | 40–60 | Ideal agent for patients with severe liver disease; watch for drug interactions |
| B | Lifestyle modification | Lipid | Varies | No strong evidence from randomized clinical trials on primary prevention of major coronary events or mortality |
| B | Soy products | Lipid | 20 | FDA has approved labeling soy products for cholesterol reduction |
| B | Red yeast | Lipid | 20–30 | Active ingredient is lovastatin; should be treated as lovastatin |
| B | Plant stanols | Lipid | 20–30 | Substitute for other source of fat calories; must be taken with each meal |
| C | Fish oils | Lipid | 5–10 | Use with caution because of high caloric value and cholesterol content in products; may increase cholesterol level with long-term use |
| C | Garlic | Lipid | 10–20 | Conflicting results with clinical trials |
| C | Green tea | Lipid | 15 | Epidemiologic study data |
| * Criteria correspond to US Preventive Services Task Force categories (A = strong evidence to support recommendation, B = fair evidence to support recommendation, C = insufficient evidence to recommend for or against). CVE denotes reduction in cardiovascular events; CVM, reduction in cardiovascular mortality; lipid, reduction in lipid levels only; OM, reduction in overall mortality. | ||||
Pharmacologic treatment
Clinical trials of hyperlipidemia therapy should address outcomes that matter most to patients, such as morbidity, mortality, quality of life, and cost, rather than stressing disease-oriented evidence, such as the ability to reduce cholesterol levels. For this review we identified major long-term RCTs that included significant coronary events or mortality as the primary outcomes. Table 3 summarizes the results of primary and secondary prevention studies.
TABLE 3
PHARMACOLOGIC INTERVENTION
| Reduction in Risk | ||||
|---|---|---|---|---|
| Intervention | Major Coronary Events | All-Cause Mortality | Comments | |
| ARR (%) | NNT | NNT | ||
| Primary Prevention | ||||
| Statins | 2.0–2.3 | 44–49 | NS | Studies on normal and hypercholesterolemic patients. Mean age was 47–58 years; all patients were men except for 1 statin study that included a small number of women |
| Gemfibrozil | 1.4 | 71 | NS | |
| Cholestyramine | 1.7 | 59 | NS | |
| Secondary Prevention | ||||
| Statins | 3–3.6 | 28–33 | 24–28 | Mean age was 55–64 years. Participants were male except for the 3 statin studies and the benzafibrate study that enrolled a small number of women. Cholesterol eligibility criteria varied among the studies and included patients with normal or elevated total and LDL levels or low HDL levels |
| Gemfibrozil | 4.4 | 23 | NS | |
| Benzafibrate | 1.4 | 71 | NS | |
| Niacin | 6.2 | 17 | NS | |
| ARR denotes absolute risk reduction in percent; NNT, number of needed to treat for 5 years to prevent 1 adverse outcome; NS, not significant. | ||||
Primary prevention
Primary prevention studies have investigated the treatment of middle-aged men with hyperlipidemia and of men and women with average cholesterol levels.19-23 Results showed similar positive outcomes on reducing coronary events in all groups (Table 3). A systematic review and a meta-analysis of primary prevention studies also demonstrated that drug therapy reduced cholesterol levels and resulted in statistically significant lowering of cardiovascular events in the treated group compared with placebo without any significant reduction in overall mortality.5,24 Absolute risk reductions ranged from 1.4% to 2.3%. In other words, the number of patients that would have to be treated for 5 years to prevent a single major coronary event was 44 to 49 for the statins, 71 for gemfibrozil, and 59 for cholestyramine.
Secondary prevention
In secondary prevention trials, RCTs have demonstrated a strong, consistent relationship between cholesterol lowering and the reduction of risk for a coronary event Table 3.25-30 Patients with preexisting CAD and elevated or average lipid levels benefit from medical therapy. The relative risk of cardiovascular events was reduced by an average of 30% in the active treatment groups.
In these trials, the NNT for 5 years to prevent 1 coronary heart event or nonfatal myocardial infarction (MI) was 28 to 33 for statins, 23 for gemfibrozil, 71 for bezafibrate, and 17 for niacin. There was also a significant risk reduction for all-cause mortality in the statin trials.27,28 These data support the recommendations from NCEP III to treat patients with preexisting CAD aggressively. People with diabetes should receive similar treatment because they are more prone to the development of new CAD within 10 years.4 In addition, subgroup analyses of diabetics treated with statins in primary prevention trials demonstrated a decreased risk of cardiovascular events.26,29
While cholesterol-modifying agents include 4 different classes—statins, fibric acid derivatives, bile acid resins, and nicotinic acid—studies cited in this paper predominantly involved statins and fibric acids. In systematic reviews of both primary and secondary prevention trials, statins were the most effective agents for both cholesterol lowering and cardiovascular risk reduction.5 We found no RCTs that directly compared outcomes between cholesterol-lowering medications. Although women represented a small number of participants in these trials, a meta-analysis showed that statin therapy decreased their risk of heart disease, with an NNT of 31 for reduction of major coronary events.31 No evidence was found to support the effectiveness of hyperlipidemia therapy for people aged more than 75 years. For people aged 65 to 75 years, there is evidence to support drug therapy for secondary prevention but not for primary prevention.
Statins are well tolerated; the most common adverse reactions are gastrointestinal related and occur in approximately 3% of patients. The more serious but uncommon events associated with the use of statins are hepatitis and myopathy. Asymptomatic increases in hepatic transaminases to more than 3 times the upper normal limit occur in approximately 1% of patients.32 Therapy can be discontinued for 1 to 2 weeks; enzyme levels should return to normal if the elevations are medication related. It is not necessary to stop therapy when enzymes are elevated at less than 3 times the upper normal limit.
General guidelines on liver monitoring call for performing a baseline liver function test and repeating it 6 weeks later.33 Once a stable dose has been established, the manufacturer recommends periodic testing; however, no clear evidence supports a specific interval. Clinicians may choose to individualize decisions on testing frequency based on factors such as potential drug interactions (statins with fibric acids or niacin) or the presence of conditions that increase the risk of liver disease.34
Myopathy, defined as generalized muscle aches and pain with a serum creatine kinase level greater than 1000 U/L, occurs rarely (< 0.1%) but may be more likely to occur when statins are used concomitantly with medications such as fibric acid, antifungals, erythromycin, and cyclosporine.31,35 The best preventive strategy is to educate patients about early recognition of the signs and symptoms of myopathy. Because most statins are metabolized by the cytochrome P450-3A4, any medications that inhibit this enzyme can increase statin serum levels and increase the risk of hepatotoxicity and myopathy.
The NCEP III recommends the use of statins as firstline therapy. A standard dose of a statin decreases LDL levels by 20% to 50%, increases HDL levels by 5% to 10%, and reduces triglyceride levels by 10% to 20%. Atorvastatin and simvastatin can produce the highest reductions in LDL levels: up to 50%. Only pravastatin, simvastatin, and lovastatin have been involved in longterm RCTs of primary and secondary prevention. Atorvastatin had positive benefits in a short-term secondary prevention trial.37 Unfortunately, the only head-to-head comparisons of statins have looked at disease-oriented outcomes such as lipid levels.37 Statins are patient friendly. They require a daily evening dose because cholesterol synthesis is more active during the night. Atorvastatin can be given at any time of day because of its long half-life.
Gemfibrozil, a fibric acid, is often used to treat hypertriglyceridemia and as an adjunctive agent to statin therapy. It decreases triglycerides by 40% to 50% but has minimal effects on the rest of the lipid panel. Adverse effects are generally mild. Liver function monitoring is recommended. The usual dosage regimen for fibric acids is 2 times a day and should be adjusted for renal function.
Niacin can increase HDL by 30% and decrease triglycerides by 30% and LDL by 20%. Major adverse reactions include flushing, gastrointestinal symptoms, elevation of liver function tests, uric acid, and serum glucose levels. The new longer-acting formulation has been associated with less flushing. Another class, the bile acid resins, including cholestyramine and colestipol, may play an adjunctive role in therapy. Their effect on the lipid panel is mild compared with those of the other class and they can increase triglyceride levels. Many patients find the gritty taste of the granular formulation unpalatable. The bile acid resins have a favorable safety profile. Most adverse events occur locally in the gut.
Conclusions
The emergence of statins as a safe and effective, although costly, therapy for hyperlipidemia and the development of clinical guidelines advocating their increased use will place family physicians under added pressure to screen for and treat hyperlipidemia. While the general value of lifestyle changes is recognized in national recommendations, more effective ways for physicians to implement them successfully in ambulatory settings are needed.
An optimal evidence-based approach to hyperlipidemia uses the new NCEP III guideline, which combines traditional risk factor assessment with assessment for CAD using the Framingham tables to determine LDL goals and appropriate treatment modalities. Statins are first-line agents for patients who are candidates for drug therapy. Discussions between clinicians and patients of the NNTs for primary and secondary prevention will help foster patient-centered discussions on the role of medical, economic, and quality-of-life issues in the decision-making process.
1. Schappert SM, Nelson C. National Ambulatory Medical Care Survey: 1995–1996 summary. National Center for Health Statistics. Vital Health Stat 1999;13(142).-
2. National Center for Health Statistics. Health, United States, 1999, with health and aging chartbook. Hyattsville, Md: 1999.
3. US Department of Health and Human Services. Tracking Healthy People 2010. Washington, DC: US Government Printing Office. November 2000. Available at: http://www.cdc.gov/hchs/hphome.htm.
4. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive Summary. Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3xsum.pdf Accessed April 16, 2001.
5. Clinical evidence London, England: BMJ Publishing Group; June 2001. Available at: www.clinicalevidence.org.
6. Henkin Y, Shai I, Zuk R, et al. Dietary treatment of hyperlipidemia: Do dietitians do it better? A randomized, controlled trial. Am J Med 2000;109:549-55.
7. Ornish D. Avoiding revascularization with lifestyle changes: the multicenter lifestyle demonstration project. Am J Cardiol 1998;82:72T-76T.
8. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998;280:2001-7.
9. Gould KL, Ornish D, Scherwitz L, et al. Changes in myocardial perfusion abnormalities by positron emission tomography after long-term, intense risk factor modification. JAMA 1995;274:894-901.
10. Mensink RP, Plat J. Efficacy of dietary plant stanols. In: New developments in the dietary management of high cholesterol. New York: McGraw-Hill; 1998;27-31.
11. Blair SN, Capuzzi DM, Gottlieb SO, et al. Incremental reduction of serum total cholesterol and LDL with the addition of plant stanol ester-containing spread to statin therapy. Am J Cardiol 2000;86:46-52.
12. Miettinen TA, Puska P, Gylling H, et al. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesteremic population. N Engl J Med 1995;333:1308-12.
13. Caron MF, White CM. Evaluation of the antihyperlipidemic properties of dietary supplements. Pharmacotherapy 2001;21:481-7.
14. Harris WS. Nonpharmacologic treatment of hypertriglyceridemia: focus on fish oils. Clin Cardiol 1999;22(suppl 2):II40-3.
15. Stevinson C, Pittler MH, Ernst E. Garlic for treating hyperlipidemia. Ann Intern Med 2000;133:420-9.
16. EBM Reviews. Database of abstracts of reviews of effectiveness [database online]. Psyllium-enriched cereals lower blood total cholesterol and LDL cholesterol, but not HDL cholesterol, in hypercholesterolemic adults: results of a meta-analysis. July 2001; v1, accession no. 00125498-100000000-00737. Available at: http://www.ovid.com/products/databases. Accessed Oct. 29, 2001.
17. EBM Reviews. ACP Journal Club [database online]. Soy protein intake decreases total and LDL cholesterol and triglyceride levels. March/April 1996; 124:41, accession no. 00021607-199603000-00013. Available at: http://www.ovid.com/products/databases. Accessed April 4, 2001.
18. Jellin JM, Batz F, Hitchens K. Natural Medicines Comprehensive Database, 3rd ed. Stockton, Calif: Therapeutic Research Faculty; 2000.
19. Frick MH, Elo O, Haapa K, et al. Helsinki heart study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. N Engl J Med 1987;317:1237-45.
20. The lipid research clinics coronary primary prevention trial results I. Reduction in incidence of coronary heart disease. JAMA 1984;251:351-64.
21. The lipid research clinics coronary primary prevention trial results II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984;251:365-74.
22. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hyperlipidemia. N Engl J Med 1995;333:1301-6.
23. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. JAMA 1998;279:1615-22.
24. Pignone M, Phillips C, Mulrow C. Use of lipid lowering drugs for primary prevention of coronary heart disease: meta-analysis of randomized trials. BMJ 2000;321:983-5.
25. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) Study. Circulation 2000;102:21-7.
26. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of HDL-cholesterol. N Engl J Med 1999;341:410-8.
27. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383-9.
28. Preventio of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease. N Engl J Med 1998;339:1349-57.
29. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-9.
30. Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in coronary drug project patients: long-term benefit with niacin. J Am Coll Cardiol 1986;8:1245-55.
31. LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease; a meta-analysis of randomized controlled trials. JAMA 1999;24:2340-6.
32. Hsu I, Spinler SA, Johnson N. Comparative evaluation of the safety and efficacy of HMG-CoA reductase inhibitor monotherapy in the treatment of primary hyperlipidemia. Ann Pharmacother 1995;29:743-59.
33. Tice SA, Parry D. Medications that require hepatic monitoring. Hosp Pharm 2001;36:456-64.
34. Weismantel D. What lab monitoring is appropriate to detect adverse drug reactions in patients on cholesterol-lowering agents? J Fam Pract 2001;50:927.-
35. American College of Clinical Pharmacy. PSAP: pharmacotherapy self-assessment program, 4th ed. Kansas City, Mo: ACCP; 2001;66-7.
36. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. JAMA 2001;285:1711-8.
37. Jones P, Kafonek S, Laurora I, et al. Comparative dose efficacy study of atorvastatin vs. simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hyperlipidemia (the CURVES study). Am J Cardiol 1998;81:582-7.
1. Schappert SM, Nelson C. National Ambulatory Medical Care Survey: 1995–1996 summary. National Center for Health Statistics. Vital Health Stat 1999;13(142).-
2. National Center for Health Statistics. Health, United States, 1999, with health and aging chartbook. Hyattsville, Md: 1999.
3. US Department of Health and Human Services. Tracking Healthy People 2010. Washington, DC: US Government Printing Office. November 2000. Available at: http://www.cdc.gov/hchs/hphome.htm.
4. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive Summary. Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3xsum.pdf Accessed April 16, 2001.
5. Clinical evidence London, England: BMJ Publishing Group; June 2001. Available at: www.clinicalevidence.org.
6. Henkin Y, Shai I, Zuk R, et al. Dietary treatment of hyperlipidemia: Do dietitians do it better? A randomized, controlled trial. Am J Med 2000;109:549-55.
7. Ornish D. Avoiding revascularization with lifestyle changes: the multicenter lifestyle demonstration project. Am J Cardiol 1998;82:72T-76T.
8. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998;280:2001-7.
9. Gould KL, Ornish D, Scherwitz L, et al. Changes in myocardial perfusion abnormalities by positron emission tomography after long-term, intense risk factor modification. JAMA 1995;274:894-901.
10. Mensink RP, Plat J. Efficacy of dietary plant stanols. In: New developments in the dietary management of high cholesterol. New York: McGraw-Hill; 1998;27-31.
11. Blair SN, Capuzzi DM, Gottlieb SO, et al. Incremental reduction of serum total cholesterol and LDL with the addition of plant stanol ester-containing spread to statin therapy. Am J Cardiol 2000;86:46-52.
12. Miettinen TA, Puska P, Gylling H, et al. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesteremic population. N Engl J Med 1995;333:1308-12.
13. Caron MF, White CM. Evaluation of the antihyperlipidemic properties of dietary supplements. Pharmacotherapy 2001;21:481-7.
14. Harris WS. Nonpharmacologic treatment of hypertriglyceridemia: focus on fish oils. Clin Cardiol 1999;22(suppl 2):II40-3.
15. Stevinson C, Pittler MH, Ernst E. Garlic for treating hyperlipidemia. Ann Intern Med 2000;133:420-9.
16. EBM Reviews. Database of abstracts of reviews of effectiveness [database online]. Psyllium-enriched cereals lower blood total cholesterol and LDL cholesterol, but not HDL cholesterol, in hypercholesterolemic adults: results of a meta-analysis. July 2001; v1, accession no. 00125498-100000000-00737. Available at: http://www.ovid.com/products/databases. Accessed Oct. 29, 2001.
17. EBM Reviews. ACP Journal Club [database online]. Soy protein intake decreases total and LDL cholesterol and triglyceride levels. March/April 1996; 124:41, accession no. 00021607-199603000-00013. Available at: http://www.ovid.com/products/databases. Accessed April 4, 2001.
18. Jellin JM, Batz F, Hitchens K. Natural Medicines Comprehensive Database, 3rd ed. Stockton, Calif: Therapeutic Research Faculty; 2000.
19. Frick MH, Elo O, Haapa K, et al. Helsinki heart study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. N Engl J Med 1987;317:1237-45.
20. The lipid research clinics coronary primary prevention trial results I. Reduction in incidence of coronary heart disease. JAMA 1984;251:351-64.
21. The lipid research clinics coronary primary prevention trial results II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984;251:365-74.
22. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hyperlipidemia. N Engl J Med 1995;333:1301-6.
23. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. JAMA 1998;279:1615-22.
24. Pignone M, Phillips C, Mulrow C. Use of lipid lowering drugs for primary prevention of coronary heart disease: meta-analysis of randomized trials. BMJ 2000;321:983-5.
25. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) Study. Circulation 2000;102:21-7.
26. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of HDL-cholesterol. N Engl J Med 1999;341:410-8.
27. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383-9.
28. Preventio of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease. N Engl J Med 1998;339:1349-57.
29. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-9.
30. Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in coronary drug project patients: long-term benefit with niacin. J Am Coll Cardiol 1986;8:1245-55.
31. LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease; a meta-analysis of randomized controlled trials. JAMA 1999;24:2340-6.
32. Hsu I, Spinler SA, Johnson N. Comparative evaluation of the safety and efficacy of HMG-CoA reductase inhibitor monotherapy in the treatment of primary hyperlipidemia. Ann Pharmacother 1995;29:743-59.
33. Tice SA, Parry D. Medications that require hepatic monitoring. Hosp Pharm 2001;36:456-64.
34. Weismantel D. What lab monitoring is appropriate to detect adverse drug reactions in patients on cholesterol-lowering agents? J Fam Pract 2001;50:927.-
35. American College of Clinical Pharmacy. PSAP: pharmacotherapy self-assessment program, 4th ed. Kansas City, Mo: ACCP; 2001;66-7.
36. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. JAMA 2001;285:1711-8.
37. Jones P, Kafonek S, Laurora I, et al. Comparative dose efficacy study of atorvastatin vs. simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hyperlipidemia (the CURVES study). Am J Cardiol 1998;81:582-7.