User login
Medicare update: What the latest changes will mean for you
Medicare Part D and the more recent changes in physician payments beginning in January will of course have a financial impact on your practice in the upcoming months. Knowing what you can expect will help you to navigate the road ahead.
A 5% increase in RVU valuation
Last year the Relative Value Update Committee, an American Medical Association (AMA) convened panel that advises CMS, recommended changes in work RVUs (relative value units) that increased the value of some evaluation and management (E&M) codes—particularly 99213 and 99214. Because Medicare needs to maintain budget neutrality, this change prompted a decrease in the value of a number of procedural work RVU codes.
The net effect for a typical family physician is an average increase of 5% in RVU valuation, although the exact amount will vary in individual practices based on the distribution of the codes. (To calculate the impact that these changes may have on your anticipated revenue, check out the handy tool provided by the American Academy of Family Physicians (AAFP). On the first page, there is a spreadsheet showing the change in RVU values from 2006 to 2007 for a number of codes; on the second page there is a worksheet to calculate changes in your anticipated revenue.1) Because many private insurers base their physician reimbursement system on Medicare RVU values, your practice may get an added benefit from these changes in your private payer collections.
A conversion factor that was poised to drop
The good news on the RVU front could have been negated by the highly publicized scheduled decrease in the overall Medicare physician fee schedule. (Actual Medicare payments are determined by multiplying the total RVU value of a code by a conversion factor [$37.895 in 2006], with some further adjustments to reflect geographic differences in expenses and efforts to maintain budget neutrality.) The conversion factor was scheduled to decrease by 5% in January, and only a last-minute intervention by Congress prevented this, leaving the 2007 conversion rate unchanged from 2006.
While this legislation will be a help to family physicians’ bottom lines in 2007, it doesn’t put an end to the annual struggles of organized medicine to forestall future Medicare payment decreases. These decreases are a result of prior legislation mandating the use of the sustainable growth rate formula (SGR) which relies on the change in the national gross domestic product to establish a yearly target for growth in the volume of Medicare payments to providers. When those payments exceed the SGR target, as it has in recent years, payments must be cut in the following year to recoup the excess spending.
Furthermore, when Congress blocks these payment cuts (as it has in the past few years) without changing the underlying law, this SGR “debt” just grows larger. This is why physician payments are projected to decrease up to 5% a year for up to 9 years.
Change may be in the making, though. Fixing the SGR payment rule remains a high priority for the AAFP, American Medical Association, and other medical organizations.
Pay-for-performance program buys physicians some time
Health care legislation, as we know, is the product of many trade-offs. Case in point: part of the deal to enact legislation that saved physicians from the 5% cut in Medicare payments was the establishment, for the first time, of a formal pay-for-performance (or more accurately, a pay-for-reporting) program starting this summer. The specifics of the program have yet to be established, but the general thrust is that Medicare will pay physicians up to a 1.5% bonus if they report data on the quality of their care using measures specified by the government.
The AAFP is relatively happy with this measure because it will start by rewarding the reporting on a small number of measures, and it will use measures developed and endorsed by national organizations such as the Ambulatory Care Quality Alliance of which the AAFP is a cofounder. AAFP’s position, however, could change as program details emerge.2
Whether the work involved in providing this data will be worth the small increase in payments is unclear. Nevertheless, it’s likely that in time, it will become increasingly difficult for physicians to avoid addressing quality indicator reporting and, eventually, being judged on the achievement of certain outcomes.
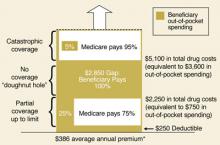
Patient satisfaction climbs with Medicare Part D
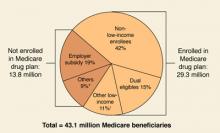
Back for its second year, the Medicare Part D program continues to feature stand-alone prescription drug plans (PDPs) for medications only and Medicare Advantage (MA) managed care plans offering drug benefits coupled with the full array of the usual Medicare benefits. Early last year, there was a great deal of concern that enrollment in the Part D program would lag, but by June, approximately 90% of the 43 million Medicare Part D eligible beneficiaries had direct drug coverage through either a Medicare PDP (16.5 million), an MA plan (6 million), or through a credible alternative plan, eg, a Medigap policy, retiree health plan, or VA plan (15.8 million).3 (For more on prescription drug coverage among Medicare beneficiaries, go to the Kaiser Family Foundation Medicare Fact Sheet.) By late 2006, 56% of seniors enrolled in a Medicare Part D plan were expressing satisfaction with the program.4
Fewer choices in the future?
Last year, 10 companies out of 266 accounted for 66% of the enrollment in Part D plans with United Healthcare and Humana dominating the marketplace.5 Companies with low numbers of enrollees may eventually lose the right to participate in the Part D program since they can’t spread the risk of medication usage across a large enough population. Also, it’s likely that about 75% of beneficiaries in a PDP will have higher premiums in 2007, although many by only a few dollars per month.5
Will the government begin direct negotiations?
Democrats want the federal government to negotiate directly with drug companies on the price of Part D medications—something the Republicans didn’t allow in the original legislation. Now that Democrats are in control of the House and Senate, this issue will likely be revisited. In addition, because more beneficiaries will have coverage for all of 2007—as opposed to just part of 2006—it’s likely that more of them will reach the “doughnut hole” during the year. If that happens, Congress is likely to hear more complaints about the inadequacy of the program’s handling of drug costs.
The MA program may also become a political hot button. In the legislation authorizing the Part D program, the Republican-led Congress significantly increased payments to MA programs in an effort to attract more enrollees.
A Commonwealth Fund study released in November 2006, confirmed this by showing that payments for each of 5.6 million enrollees in an MA plan in 2005 averaged $922 or 12.4% more than costs for beneficiaries in the traditional Medicare fee-for-service program for a total of $5.2 billion. The Commonwealth study authors noted that these extra payments undermine the original intent of the legislation which was to have an MA program provide a more efficient alternative to the traditional Medicare program.6 This is another part of the original bill that Democrats argued against, and may be another area they choose to address in the new legislative session. With the shift in control over the House and Senate, only time will tell how Medicare Part D will evolve in the months ahead.
A poll taken done by the Kaiser Family Foundation and Harvard School of Public Health soon after the November elections showed that majorities of Democrats (92%), independents (85%), and Republicans (74%) supported the government negotiating prices for prescription drugs under Medicare and a majority of all polled (79%) supported allowing the purchase of drugs from Canada. Also, more than half supported federal funding of stem cell research.
The top health priorities were expanding coverage for the uninsured (35%) and reducing health care costs (30%). While health care and the economy were the leading domestic priorities for those polled (about 15% each), they both trailed far behind the war in Iraq (46%).
SOURCE: The Public’s Health Care Agenda for the New Congress and Presidential Campaign [Kaiser Family Foundation Web site]. December 2006. Available at: www.kff.org/kaiserpolls/pomr120806pkg.cfm. Accessed on March 20, 2007.
Correspondence
Eric Henley, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107. [email protected]
1. Common E/M code payment changes 2006–2007 Available at: www.aafp.org/online/etc/medialib/aafp_org/documents/prac_mgt/codingresources/emimpacttool.par.0001.File.tmp/EM%20Impact%20Tool.xls. Accessed on March 20, 2007.
2. Champlin L. Eleventh-hour vote avoids medicare cut. AAFP News Now, December 11, 2006. Available at: www.aafp.org/online/en/home/publications/news/news-now/government-medicine/20061211nomedicarecut.html. Accessed March 20, 2007.
3. The Medicare prescription Drug Benefit fact sheet [Kaiser Family Foundation Web site]. November 2006. Available at: www.kff.org/medicare/upload/7044-05.pdf. Accessed on March 20, 2007.
4. The Kaiser Family Foundation/Harvard School of Public Health Seniors and the Medicare Prescription Drug Benefit. Available at: www.kff.org/kaiserpolls/upload/7604.pdf. Accessed on March 26, 2007.
5. Hoadley J, Hargrave E, Merrell K, Cubanski J, Neuman T. Benefit design and formularies of Medicare drug plans [Kaiser Family Foundation Web site]. Available at: www.kff.org/medicare/upload/7589.pdf. Accessed on March 20, 2007.
6. Biles B, Nicholas LH, Cooper BS, Adrion E, Guterman S. The cost of privatization: extra payments to Medicare Advantage Plans—Updated and revised. [The Commonwealth Fund Web site]. November 2006. Available at: www.cmwf.org/usr_doc/Biles_costprivatizationextrapayMAplans_970_ib.pdf. Accessed March 26, 2007.
Medicare Part D and the more recent changes in physician payments beginning in January will of course have a financial impact on your practice in the upcoming months. Knowing what you can expect will help you to navigate the road ahead.
A 5% increase in RVU valuation
Last year the Relative Value Update Committee, an American Medical Association (AMA) convened panel that advises CMS, recommended changes in work RVUs (relative value units) that increased the value of some evaluation and management (E&M) codes—particularly 99213 and 99214. Because Medicare needs to maintain budget neutrality, this change prompted a decrease in the value of a number of procedural work RVU codes.
The net effect for a typical family physician is an average increase of 5% in RVU valuation, although the exact amount will vary in individual practices based on the distribution of the codes. (To calculate the impact that these changes may have on your anticipated revenue, check out the handy tool provided by the American Academy of Family Physicians (AAFP). On the first page, there is a spreadsheet showing the change in RVU values from 2006 to 2007 for a number of codes; on the second page there is a worksheet to calculate changes in your anticipated revenue.1) Because many private insurers base their physician reimbursement system on Medicare RVU values, your practice may get an added benefit from these changes in your private payer collections.
A conversion factor that was poised to drop
The good news on the RVU front could have been negated by the highly publicized scheduled decrease in the overall Medicare physician fee schedule. (Actual Medicare payments are determined by multiplying the total RVU value of a code by a conversion factor [$37.895 in 2006], with some further adjustments to reflect geographic differences in expenses and efforts to maintain budget neutrality.) The conversion factor was scheduled to decrease by 5% in January, and only a last-minute intervention by Congress prevented this, leaving the 2007 conversion rate unchanged from 2006.
While this legislation will be a help to family physicians’ bottom lines in 2007, it doesn’t put an end to the annual struggles of organized medicine to forestall future Medicare payment decreases. These decreases are a result of prior legislation mandating the use of the sustainable growth rate formula (SGR) which relies on the change in the national gross domestic product to establish a yearly target for growth in the volume of Medicare payments to providers. When those payments exceed the SGR target, as it has in recent years, payments must be cut in the following year to recoup the excess spending.
Furthermore, when Congress blocks these payment cuts (as it has in the past few years) without changing the underlying law, this SGR “debt” just grows larger. This is why physician payments are projected to decrease up to 5% a year for up to 9 years.
Change may be in the making, though. Fixing the SGR payment rule remains a high priority for the AAFP, American Medical Association, and other medical organizations.
Pay-for-performance program buys physicians some time
Health care legislation, as we know, is the product of many trade-offs. Case in point: part of the deal to enact legislation that saved physicians from the 5% cut in Medicare payments was the establishment, for the first time, of a formal pay-for-performance (or more accurately, a pay-for-reporting) program starting this summer. The specifics of the program have yet to be established, but the general thrust is that Medicare will pay physicians up to a 1.5% bonus if they report data on the quality of their care using measures specified by the government.
The AAFP is relatively happy with this measure because it will start by rewarding the reporting on a small number of measures, and it will use measures developed and endorsed by national organizations such as the Ambulatory Care Quality Alliance of which the AAFP is a cofounder. AAFP’s position, however, could change as program details emerge.2
Whether the work involved in providing this data will be worth the small increase in payments is unclear. Nevertheless, it’s likely that in time, it will become increasingly difficult for physicians to avoid addressing quality indicator reporting and, eventually, being judged on the achievement of certain outcomes.
Patient satisfaction climbs with Medicare Part D
Back for its second year, the Medicare Part D program continues to feature stand-alone prescription drug plans (PDPs) for medications only and Medicare Advantage (MA) managed care plans offering drug benefits coupled with the full array of the usual Medicare benefits. Early last year, there was a great deal of concern that enrollment in the Part D program would lag, but by June, approximately 90% of the 43 million Medicare Part D eligible beneficiaries had direct drug coverage through either a Medicare PDP (16.5 million), an MA plan (6 million), or through a credible alternative plan, eg, a Medigap policy, retiree health plan, or VA plan (15.8 million).3 (For more on prescription drug coverage among Medicare beneficiaries, go to the Kaiser Family Foundation Medicare Fact Sheet.) By late 2006, 56% of seniors enrolled in a Medicare Part D plan were expressing satisfaction with the program.4
Fewer choices in the future?
Last year, 10 companies out of 266 accounted for 66% of the enrollment in Part D plans with United Healthcare and Humana dominating the marketplace.5 Companies with low numbers of enrollees may eventually lose the right to participate in the Part D program since they can’t spread the risk of medication usage across a large enough population. Also, it’s likely that about 75% of beneficiaries in a PDP will have higher premiums in 2007, although many by only a few dollars per month.5
Will the government begin direct negotiations?
Democrats want the federal government to negotiate directly with drug companies on the price of Part D medications—something the Republicans didn’t allow in the original legislation. Now that Democrats are in control of the House and Senate, this issue will likely be revisited. In addition, because more beneficiaries will have coverage for all of 2007—as opposed to just part of 2006—it’s likely that more of them will reach the “doughnut hole” during the year. If that happens, Congress is likely to hear more complaints about the inadequacy of the program’s handling of drug costs.
The MA program may also become a political hot button. In the legislation authorizing the Part D program, the Republican-led Congress significantly increased payments to MA programs in an effort to attract more enrollees.
A Commonwealth Fund study released in November 2006, confirmed this by showing that payments for each of 5.6 million enrollees in an MA plan in 2005 averaged $922 or 12.4% more than costs for beneficiaries in the traditional Medicare fee-for-service program for a total of $5.2 billion. The Commonwealth study authors noted that these extra payments undermine the original intent of the legislation which was to have an MA program provide a more efficient alternative to the traditional Medicare program.6 This is another part of the original bill that Democrats argued against, and may be another area they choose to address in the new legislative session. With the shift in control over the House and Senate, only time will tell how Medicare Part D will evolve in the months ahead.
A poll taken done by the Kaiser Family Foundation and Harvard School of Public Health soon after the November elections showed that majorities of Democrats (92%), independents (85%), and Republicans (74%) supported the government negotiating prices for prescription drugs under Medicare and a majority of all polled (79%) supported allowing the purchase of drugs from Canada. Also, more than half supported federal funding of stem cell research.
The top health priorities were expanding coverage for the uninsured (35%) and reducing health care costs (30%). While health care and the economy were the leading domestic priorities for those polled (about 15% each), they both trailed far behind the war in Iraq (46%).
SOURCE: The Public’s Health Care Agenda for the New Congress and Presidential Campaign [Kaiser Family Foundation Web site]. December 2006. Available at: www.kff.org/kaiserpolls/pomr120806pkg.cfm. Accessed on March 20, 2007.
Correspondence
Eric Henley, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107. [email protected]
Medicare Part D and the more recent changes in physician payments beginning in January will of course have a financial impact on your practice in the upcoming months. Knowing what you can expect will help you to navigate the road ahead.
A 5% increase in RVU valuation
Last year the Relative Value Update Committee, an American Medical Association (AMA) convened panel that advises CMS, recommended changes in work RVUs (relative value units) that increased the value of some evaluation and management (E&M) codes—particularly 99213 and 99214. Because Medicare needs to maintain budget neutrality, this change prompted a decrease in the value of a number of procedural work RVU codes.
The net effect for a typical family physician is an average increase of 5% in RVU valuation, although the exact amount will vary in individual practices based on the distribution of the codes. (To calculate the impact that these changes may have on your anticipated revenue, check out the handy tool provided by the American Academy of Family Physicians (AAFP). On the first page, there is a spreadsheet showing the change in RVU values from 2006 to 2007 for a number of codes; on the second page there is a worksheet to calculate changes in your anticipated revenue.1) Because many private insurers base their physician reimbursement system on Medicare RVU values, your practice may get an added benefit from these changes in your private payer collections.
A conversion factor that was poised to drop
The good news on the RVU front could have been negated by the highly publicized scheduled decrease in the overall Medicare physician fee schedule. (Actual Medicare payments are determined by multiplying the total RVU value of a code by a conversion factor [$37.895 in 2006], with some further adjustments to reflect geographic differences in expenses and efforts to maintain budget neutrality.) The conversion factor was scheduled to decrease by 5% in January, and only a last-minute intervention by Congress prevented this, leaving the 2007 conversion rate unchanged from 2006.
While this legislation will be a help to family physicians’ bottom lines in 2007, it doesn’t put an end to the annual struggles of organized medicine to forestall future Medicare payment decreases. These decreases are a result of prior legislation mandating the use of the sustainable growth rate formula (SGR) which relies on the change in the national gross domestic product to establish a yearly target for growth in the volume of Medicare payments to providers. When those payments exceed the SGR target, as it has in recent years, payments must be cut in the following year to recoup the excess spending.
Furthermore, when Congress blocks these payment cuts (as it has in the past few years) without changing the underlying law, this SGR “debt” just grows larger. This is why physician payments are projected to decrease up to 5% a year for up to 9 years.
Change may be in the making, though. Fixing the SGR payment rule remains a high priority for the AAFP, American Medical Association, and other medical organizations.
Pay-for-performance program buys physicians some time
Health care legislation, as we know, is the product of many trade-offs. Case in point: part of the deal to enact legislation that saved physicians from the 5% cut in Medicare payments was the establishment, for the first time, of a formal pay-for-performance (or more accurately, a pay-for-reporting) program starting this summer. The specifics of the program have yet to be established, but the general thrust is that Medicare will pay physicians up to a 1.5% bonus if they report data on the quality of their care using measures specified by the government.
The AAFP is relatively happy with this measure because it will start by rewarding the reporting on a small number of measures, and it will use measures developed and endorsed by national organizations such as the Ambulatory Care Quality Alliance of which the AAFP is a cofounder. AAFP’s position, however, could change as program details emerge.2
Whether the work involved in providing this data will be worth the small increase in payments is unclear. Nevertheless, it’s likely that in time, it will become increasingly difficult for physicians to avoid addressing quality indicator reporting and, eventually, being judged on the achievement of certain outcomes.
Patient satisfaction climbs with Medicare Part D
Back for its second year, the Medicare Part D program continues to feature stand-alone prescription drug plans (PDPs) for medications only and Medicare Advantage (MA) managed care plans offering drug benefits coupled with the full array of the usual Medicare benefits. Early last year, there was a great deal of concern that enrollment in the Part D program would lag, but by June, approximately 90% of the 43 million Medicare Part D eligible beneficiaries had direct drug coverage through either a Medicare PDP (16.5 million), an MA plan (6 million), or through a credible alternative plan, eg, a Medigap policy, retiree health plan, or VA plan (15.8 million).3 (For more on prescription drug coverage among Medicare beneficiaries, go to the Kaiser Family Foundation Medicare Fact Sheet.) By late 2006, 56% of seniors enrolled in a Medicare Part D plan were expressing satisfaction with the program.4
Fewer choices in the future?
Last year, 10 companies out of 266 accounted for 66% of the enrollment in Part D plans with United Healthcare and Humana dominating the marketplace.5 Companies with low numbers of enrollees may eventually lose the right to participate in the Part D program since they can’t spread the risk of medication usage across a large enough population. Also, it’s likely that about 75% of beneficiaries in a PDP will have higher premiums in 2007, although many by only a few dollars per month.5
Will the government begin direct negotiations?
Democrats want the federal government to negotiate directly with drug companies on the price of Part D medications—something the Republicans didn’t allow in the original legislation. Now that Democrats are in control of the House and Senate, this issue will likely be revisited. In addition, because more beneficiaries will have coverage for all of 2007—as opposed to just part of 2006—it’s likely that more of them will reach the “doughnut hole” during the year. If that happens, Congress is likely to hear more complaints about the inadequacy of the program’s handling of drug costs.
The MA program may also become a political hot button. In the legislation authorizing the Part D program, the Republican-led Congress significantly increased payments to MA programs in an effort to attract more enrollees.
A Commonwealth Fund study released in November 2006, confirmed this by showing that payments for each of 5.6 million enrollees in an MA plan in 2005 averaged $922 or 12.4% more than costs for beneficiaries in the traditional Medicare fee-for-service program for a total of $5.2 billion. The Commonwealth study authors noted that these extra payments undermine the original intent of the legislation which was to have an MA program provide a more efficient alternative to the traditional Medicare program.6 This is another part of the original bill that Democrats argued against, and may be another area they choose to address in the new legislative session. With the shift in control over the House and Senate, only time will tell how Medicare Part D will evolve in the months ahead.
A poll taken done by the Kaiser Family Foundation and Harvard School of Public Health soon after the November elections showed that majorities of Democrats (92%), independents (85%), and Republicans (74%) supported the government negotiating prices for prescription drugs under Medicare and a majority of all polled (79%) supported allowing the purchase of drugs from Canada. Also, more than half supported federal funding of stem cell research.
The top health priorities were expanding coverage for the uninsured (35%) and reducing health care costs (30%). While health care and the economy were the leading domestic priorities for those polled (about 15% each), they both trailed far behind the war in Iraq (46%).
SOURCE: The Public’s Health Care Agenda for the New Congress and Presidential Campaign [Kaiser Family Foundation Web site]. December 2006. Available at: www.kff.org/kaiserpolls/pomr120806pkg.cfm. Accessed on March 20, 2007.
Correspondence
Eric Henley, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107. [email protected]
1. Common E/M code payment changes 2006–2007 Available at: www.aafp.org/online/etc/medialib/aafp_org/documents/prac_mgt/codingresources/emimpacttool.par.0001.File.tmp/EM%20Impact%20Tool.xls. Accessed on March 20, 2007.
2. Champlin L. Eleventh-hour vote avoids medicare cut. AAFP News Now, December 11, 2006. Available at: www.aafp.org/online/en/home/publications/news/news-now/government-medicine/20061211nomedicarecut.html. Accessed March 20, 2007.
3. The Medicare prescription Drug Benefit fact sheet [Kaiser Family Foundation Web site]. November 2006. Available at: www.kff.org/medicare/upload/7044-05.pdf. Accessed on March 20, 2007.
4. The Kaiser Family Foundation/Harvard School of Public Health Seniors and the Medicare Prescription Drug Benefit. Available at: www.kff.org/kaiserpolls/upload/7604.pdf. Accessed on March 26, 2007.
5. Hoadley J, Hargrave E, Merrell K, Cubanski J, Neuman T. Benefit design and formularies of Medicare drug plans [Kaiser Family Foundation Web site]. Available at: www.kff.org/medicare/upload/7589.pdf. Accessed on March 20, 2007.
6. Biles B, Nicholas LH, Cooper BS, Adrion E, Guterman S. The cost of privatization: extra payments to Medicare Advantage Plans—Updated and revised. [The Commonwealth Fund Web site]. November 2006. Available at: www.cmwf.org/usr_doc/Biles_costprivatizationextrapayMAplans_970_ib.pdf. Accessed March 26, 2007.
1. Common E/M code payment changes 2006–2007 Available at: www.aafp.org/online/etc/medialib/aafp_org/documents/prac_mgt/codingresources/emimpacttool.par.0001.File.tmp/EM%20Impact%20Tool.xls. Accessed on March 20, 2007.
2. Champlin L. Eleventh-hour vote avoids medicare cut. AAFP News Now, December 11, 2006. Available at: www.aafp.org/online/en/home/publications/news/news-now/government-medicine/20061211nomedicarecut.html. Accessed March 20, 2007.
3. The Medicare prescription Drug Benefit fact sheet [Kaiser Family Foundation Web site]. November 2006. Available at: www.kff.org/medicare/upload/7044-05.pdf. Accessed on March 20, 2007.
4. The Kaiser Family Foundation/Harvard School of Public Health Seniors and the Medicare Prescription Drug Benefit. Available at: www.kff.org/kaiserpolls/upload/7604.pdf. Accessed on March 26, 2007.
5. Hoadley J, Hargrave E, Merrell K, Cubanski J, Neuman T. Benefit design and formularies of Medicare drug plans [Kaiser Family Foundation Web site]. Available at: www.kff.org/medicare/upload/7589.pdf. Accessed on March 20, 2007.
6. Biles B, Nicholas LH, Cooper BS, Adrion E, Guterman S. The cost of privatization: extra payments to Medicare Advantage Plans—Updated and revised. [The Commonwealth Fund Web site]. November 2006. Available at: www.cmwf.org/usr_doc/Biles_costprivatizationextrapayMAplans_970_ib.pdf. Accessed March 26, 2007.
What hope is there for meaningful tort reform to stop another malpractice crisis?
A handful of papers published in the past few years have looked at different aspects of the current malpractice situation and have yielded some revelations (see page 775 in this issue) or data banks working on safety issues.
- Though it does a reasonable job at separating valid from invalid claims and compensating them accordingly, it often takes a tremendously long time to accomplish this and still has a 10% to 16% rate of false positive (payment with no error) and false negative (no payment with error) outcomes.
- The system is not overwhelmed with frivolous claims. Still, it costs a lot of money to manage, and less than half of this money goes to claimants.
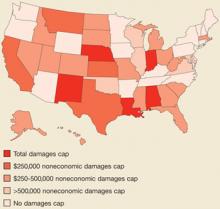
- Hard caps on total damages or noneconomic damages, unlike other state tort reforms (Figure), appear to reduce claims payments, physician premiums, and total health costs,7 while increasing physician supply.
- Defensive medicine exists, though putting a valid dollar amount on its costs is difficult.
- Anecdotal evidence suggests that physicians are leaving practice or limiting their practice (eg, family physicians discontinuing deliveries) as a result of malpractice costs.
FIGURE
Tort reforms commonly adopted by states
AMA’s proposal for change
Malpractice reform has been at or near the top of the AMA’s political agenda for the past 4 or 5 years, with strong lobbying efforts at the national level as well as support for state chapter efforts. The AMA’s proposal is based on California’s liability reform law known as MICRA that was passed over 30 years ago and has been associated with significantly lower premium growth since then compared with the rest of the US.8 Key provisions:
- Unlimited economic damages (medical expenses, future earnings)
- Limits on noneconomic damages (pain and suffering)
- Punitive damages, if available, up to $250,000 or 2 times economic damages, whichever is greater
- Allocation of damage awards in proportion to fault
- Sliding scale for attorney contingency fees.
Dubious premises. The AMA literature on malpractice includes valid information on the costs of the tort system, the rise in claims payouts, and effects on physician premiums. But it also suggests that meritless lawsuits are increasing. This is untrue. And its implication that physicians are increasingly leaving practice is anecdotal. There is no good research on the extent of this problem.8
Too narrow a focus. More important, the AMA plan is focused on physician premium costs while ignoring the unfairness of the system (eg, time to resolve claims, lack of payment for many patients with legitimate claims) and the vast number of medical errors for which claims are never filed.
The MEDIC proposal
Senators Hillary Clinton (D-NY) and Barack Obama (D-IL) have proposed federal legislation to address the malpractice crisis. Their bill would create an Office of Patient Safety in the Department of Health and Human Services, and would establish the National Medical Error Disclosure and Compensation (MEDIC) program within that office.9
Apologies would not be actionable in court. The MEDIC program would provide grants to physicians, hospitals, and health systems for the creation of programs to disclose medical errors to patients and negotiate fair compensation. The law would preserve confidentiality so that any apology offered by a health care provider as part of those negotiations would be kept confidential and could not be used in a trial. Any savings achieved from lower administrative and legal costs would be used to reduce physician malpractice premiums and toward patient safety initiatives.
Federal mandating of caps unlikely, however. At the federal level, Democrats have firmly opposed mandating caps on malpractice claims settlements. They argue that caps are unfair to patients who have been victims of medical errors. Others say this opposition reflects financial contributions from trial lawyers. It seems time to get past this conflict. Without dramatic changes in the composition of the Senate, which seems unlikely, there is little or no chance that caps will pass at the national level. At the same time, physician groups have been successful at achieving caps in a number of states (total of 26 at last count).
Signs this program could succeed. The MEDIC proposal is an attempt to find another way out of the malpractice impasse in the Senate by linking the patient safety and tort reform issues. It is primarily based on a growing movement to have physicians more directly acknowledge medical errors to patients,10 and in some cases, link these apologies to immediate financial negotiations to settle any potential claim of injury. The University of Michigan is the best known academic institution pursuing these strategies, and they report a significant decrease in the number of claims and annual litigation costs. The Lexington, Kentucky, VA Hospital has a similar program that has reduced liability costs compared with other VA hospitals.
The MEDIC proposal is attractive in its attempt to tie doctor-patient communication, patient safety, and liability together. And the anecdotal reports of success with isolated programs of its type are encouraging. It also moves the argument at a national level away from a fight about caps, which puts many physicians in the uncomfortable position of opposing Democrats who support many of their other positions—eg, Title VII funding, preservation of the traditional Medicare program, expansion of the Medicare Part D program, and better funding of public health programs. Nonetheless, there is a big row to hoe in making physicians more comfortable with acknowledging their errors, convincing them this would not be held against them in court, and assuring both physicians and hospitals that such efforts will actually lead to lower malpractice costs.
Thorpe analyzed data from 1995–2001 collected by the National Association of Insurance Commissioners to see the relationship between state tort reforms and premium levels. Premiums in states with caps on awards were 17% lower than states without caps. There was no association between premium levels and other reforms, such as caps on attorney fees or collateral offset rules (decreasing awards by the amount the plaintiff receives from other sources). Some association was noted between decreased competition among insurers and higher premiums.1
Rodwin et al used data from AMA surveys of self-employed physicians (physicians in groups or solo practice who are not employees) from 1970 to 2000. They found that while premiums increased from 1970 to 1986 and from 1996 to 2000, they had only a small effect on physician income. Premiums made up a small percentage of total practice costs and had a negligible effect on practice income, arguing against a malpractice crisis. However, this study lacked more recent data on premium increases and practice expenses and did not take into account differences among states that might be due to tort reforms such as the institution of caps on awards.2
Studdert et al reviewed 1452 closed claims in 4 categories (obstetrics, surgery, missed or delayed diagnosis, and medication) from 5 liability insurers representing 4 regions of the US, and used objective criteria and independent reviewers to classify the merits of the claims. They found that 3% of the claims had no verifiable medical injury and 37% did not involve errors. About 73% of the claims not associated with errors or injuries resulted in no compensation, while 73% of those with errors did result in compensation. Further payment for claims not involving errors were lower than those that did involve errors. Looked at another way, of the 1452 claims reviewed, about 10% received payment but had no identifiable error, while about 17% had an identifiable error but no payment was made.3
This study demonstrated that: 1) the cost of defending claims involving no error was substantial but still only amounted to about 13% of direct system costs, meaning that contesting and paying for claims caused by errors accounts for most of the costs of the liability system, and 2) the malpractice system works reasonably well at separating claims without merit from those with merit.
Nonetheless, the study also demonstrated the unfairness of a system in which 1 in 6 valid claims received no payment (this in addition to the vast number of negligent injuries that never even lead to a claim as discussed in the 1999 IOM report). Then there is the frustration with a system wherein the average time between injury and claim resolution is 5 years and 54% of the payments are absorbed by defense costs and contingency fees. That 80% of expenses were incurred in resolving claims with errors suggests that steps to decrease frivolous litigation (claims without merit) will not lead to substantial savings and that steps to streamlining the system of handling claims will be more useful.
Blake et al looked at state-specific data from the National Practitioner Data Bank, which collects reports of all malpractice payments in the US on behalf of physicians, dentists, and nurses. They looked at the relationship between payments, physician premiums, and various state tort reforms. They found that mean payments were 26% lower in states with total damage caps ($196,000 vs $265,000) and 22% less in states with noneconomic (pain and suffering) damage caps ($212,000 vs $279,000). In addition, total damage caps were associated with lower mean annual premiums and hard, but not soft (caps with exceptions) noneconomic caps were associated with premium reductions. No other state tort reforms measured showed a significant association with payments or premiums.4
Studdert et al surveyed Pennsylvania physicians in high-risk specialties (obstetrics/gynecology, ortho, ER, surgery, neurosurgery) to ascertain self-report of defensive medicine practice. Almost all respondents reported practicing defensive medicine, the most common form (92%) being unnecessary ordering of tests and imaging studies and referring for consultation. In addition, 42% said they had restricted their practice by either decreasing the performance of more risky procedures (eg, trauma surgery) or avoiding complex cases or patients perceived as more likely to sue. Defensive medicine was highly correlated with physicians’ lack of confidence in their liability insurance or its cost.5
Kessler et al looked at physician supply from 1985–2001 and its correlates to state tort reforms during that time. Three years after States that adopted direct reforms (mainly caps on damage awards) showed an average physician growth rate within 3 years that was 3.3% greater than states not adopting such reforms. The authors controlled for a variety of factors that can influence physician supply including population growth and other state-level characteristics.6
Common Good proposal
Another approach is advocated by Common Good, a bipartisan legal reform coalition. This organization has funding from the RWJ Foundation to work with researchers from the Harvard School of Public Health to investigate the creation of special health courts to hear malpractice cases.11 Their ideas are incorporated into the Fair and Reliable Medical Justice Act introduced as S.1337 by Senators Mike Enzi (R-WY) Max Baucus (D-MT).11
How it would work. Health courts would have full-time judges, neutral medical experts, faster proceedings with legal fees held to 20%, and rulings that could be appealed to a new Medical Appellate Court. Like other administrative courts that handle tax disputes, workmen’s comp, and vaccine injury, there would be no juries. Judges would issue written rulings and establish legal precedents. Once a mistake was verified, recovery would be automatic. Patients would be reimbursed for all their medical expenses and lost income plus a fixed sum that would be determined from an expert derived schedule addressing specific types of injuries.
Strengths and weaknesses. This proposal has the support of a wide array of national medical and legal leaders but not any medical associations. It attempts to address some of the most egregious parts of the current system—the time it takes to get claims resolved, the many errors that go uncompensated, and the diversion of so many dollars to overhead and legal fees rather than to patients. On the other hand, the plan does not provide firm caps, which may make the AMA and other professional associations skeptical. And the proposed health courts would rely on select medical experts and judges, a system likely to be strongly opposed by trial lawyers and some consumer groups. It also does not directly address the prevention of patient errors.
Those who fail to learn from history…
The current malpractice crisis may be abating, leaving physicians with higher malpractice premiums but some state tort reforms. History, however, suggests that the insurance cycle will eventually lead to another crisis. There may be a window of opportunity now to come up with a completely different system to address the goals and problems with our current system, but it is likely to be a small window. Capitalizing on it will require a willingness for both sides in the current stand-off to get past their own self-interests in order to come up with something better for all.
CORRESPONDENCE
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Thorpe K. The Medical malpractice “crisis”: Recent trends and the impact of state tort reforms. Health Affairs 2004 January 21;web-only.
2. Rodwin M, Chang H, Clausen J. Malpractice premiums and physicians’ income: Perceptions of a crisis conflict with empirical evidence. Health Affairs 2006;525:750-758.
3. Studdert D, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med 2006;354:2024-2033.
4. Guirguis-Blake J, Fryer GE, Phillips RL, Jr, Szabat R, Green LA. The US medical liability system: Evidence for legislative reform. Ann Fam Med 2006;4:240-246.
5. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA 2005;293:2609-2617.
6. Kessler DP, Sage WM, Becker DJ, et al. Impact of malpractice reforms on the supply of physician services. JAMA 2005;293:2618-2625.
7. Hellinger F, Encinosa W. The impact of state laws limiting malpractice damage awards on health care expenditures. AJPH 2006;96:1375-1381.
8. American Medical Association. Medical liability talking points. Available at: www.ama-assn.org/ama1/pub/upload/mm/399/mlr_fastfacts.pdf. Accessed on August 15, 2006.
9. Clinton HR, Obama B. Making patient safety the centerpiece of medical liability reform. N Engl J Med 2006;354:2205-2208.
10. O’Reilly K. Harvard adopts a disclosure and apology policy. AMA News, June 12, 2006.
11. Common Good. What are health courts? Available at: cgood.org/f-healthcourtsinfo.html. Accessed on August 15, 2006.
A handful of papers published in the past few years have looked at different aspects of the current malpractice situation and have yielded some revelations (see page 775 in this issue) or data banks working on safety issues.
- Though it does a reasonable job at separating valid from invalid claims and compensating them accordingly, it often takes a tremendously long time to accomplish this and still has a 10% to 16% rate of false positive (payment with no error) and false negative (no payment with error) outcomes.
- The system is not overwhelmed with frivolous claims. Still, it costs a lot of money to manage, and less than half of this money goes to claimants.
- Hard caps on total damages or noneconomic damages, unlike other state tort reforms (Figure), appear to reduce claims payments, physician premiums, and total health costs,7 while increasing physician supply.
- Defensive medicine exists, though putting a valid dollar amount on its costs is difficult.
- Anecdotal evidence suggests that physicians are leaving practice or limiting their practice (eg, family physicians discontinuing deliveries) as a result of malpractice costs.
FIGURE
Tort reforms commonly adopted by states
AMA’s proposal for change
Malpractice reform has been at or near the top of the AMA’s political agenda for the past 4 or 5 years, with strong lobbying efforts at the national level as well as support for state chapter efforts. The AMA’s proposal is based on California’s liability reform law known as MICRA that was passed over 30 years ago and has been associated with significantly lower premium growth since then compared with the rest of the US.8 Key provisions:
- Unlimited economic damages (medical expenses, future earnings)
- Limits on noneconomic damages (pain and suffering)
- Punitive damages, if available, up to $250,000 or 2 times economic damages, whichever is greater
- Allocation of damage awards in proportion to fault
- Sliding scale for attorney contingency fees.
Dubious premises. The AMA literature on malpractice includes valid information on the costs of the tort system, the rise in claims payouts, and effects on physician premiums. But it also suggests that meritless lawsuits are increasing. This is untrue. And its implication that physicians are increasingly leaving practice is anecdotal. There is no good research on the extent of this problem.8
Too narrow a focus. More important, the AMA plan is focused on physician premium costs while ignoring the unfairness of the system (eg, time to resolve claims, lack of payment for many patients with legitimate claims) and the vast number of medical errors for which claims are never filed.
The MEDIC proposal
Senators Hillary Clinton (D-NY) and Barack Obama (D-IL) have proposed federal legislation to address the malpractice crisis. Their bill would create an Office of Patient Safety in the Department of Health and Human Services, and would establish the National Medical Error Disclosure and Compensation (MEDIC) program within that office.9
Apologies would not be actionable in court. The MEDIC program would provide grants to physicians, hospitals, and health systems for the creation of programs to disclose medical errors to patients and negotiate fair compensation. The law would preserve confidentiality so that any apology offered by a health care provider as part of those negotiations would be kept confidential and could not be used in a trial. Any savings achieved from lower administrative and legal costs would be used to reduce physician malpractice premiums and toward patient safety initiatives.
Federal mandating of caps unlikely, however. At the federal level, Democrats have firmly opposed mandating caps on malpractice claims settlements. They argue that caps are unfair to patients who have been victims of medical errors. Others say this opposition reflects financial contributions from trial lawyers. It seems time to get past this conflict. Without dramatic changes in the composition of the Senate, which seems unlikely, there is little or no chance that caps will pass at the national level. At the same time, physician groups have been successful at achieving caps in a number of states (total of 26 at last count).
Signs this program could succeed. The MEDIC proposal is an attempt to find another way out of the malpractice impasse in the Senate by linking the patient safety and tort reform issues. It is primarily based on a growing movement to have physicians more directly acknowledge medical errors to patients,10 and in some cases, link these apologies to immediate financial negotiations to settle any potential claim of injury. The University of Michigan is the best known academic institution pursuing these strategies, and they report a significant decrease in the number of claims and annual litigation costs. The Lexington, Kentucky, VA Hospital has a similar program that has reduced liability costs compared with other VA hospitals.
The MEDIC proposal is attractive in its attempt to tie doctor-patient communication, patient safety, and liability together. And the anecdotal reports of success with isolated programs of its type are encouraging. It also moves the argument at a national level away from a fight about caps, which puts many physicians in the uncomfortable position of opposing Democrats who support many of their other positions—eg, Title VII funding, preservation of the traditional Medicare program, expansion of the Medicare Part D program, and better funding of public health programs. Nonetheless, there is a big row to hoe in making physicians more comfortable with acknowledging their errors, convincing them this would not be held against them in court, and assuring both physicians and hospitals that such efforts will actually lead to lower malpractice costs.
Thorpe analyzed data from 1995–2001 collected by the National Association of Insurance Commissioners to see the relationship between state tort reforms and premium levels. Premiums in states with caps on awards were 17% lower than states without caps. There was no association between premium levels and other reforms, such as caps on attorney fees or collateral offset rules (decreasing awards by the amount the plaintiff receives from other sources). Some association was noted between decreased competition among insurers and higher premiums.1
Rodwin et al used data from AMA surveys of self-employed physicians (physicians in groups or solo practice who are not employees) from 1970 to 2000. They found that while premiums increased from 1970 to 1986 and from 1996 to 2000, they had only a small effect on physician income. Premiums made up a small percentage of total practice costs and had a negligible effect on practice income, arguing against a malpractice crisis. However, this study lacked more recent data on premium increases and practice expenses and did not take into account differences among states that might be due to tort reforms such as the institution of caps on awards.2
Studdert et al reviewed 1452 closed claims in 4 categories (obstetrics, surgery, missed or delayed diagnosis, and medication) from 5 liability insurers representing 4 regions of the US, and used objective criteria and independent reviewers to classify the merits of the claims. They found that 3% of the claims had no verifiable medical injury and 37% did not involve errors. About 73% of the claims not associated with errors or injuries resulted in no compensation, while 73% of those with errors did result in compensation. Further payment for claims not involving errors were lower than those that did involve errors. Looked at another way, of the 1452 claims reviewed, about 10% received payment but had no identifiable error, while about 17% had an identifiable error but no payment was made.3
This study demonstrated that: 1) the cost of defending claims involving no error was substantial but still only amounted to about 13% of direct system costs, meaning that contesting and paying for claims caused by errors accounts for most of the costs of the liability system, and 2) the malpractice system works reasonably well at separating claims without merit from those with merit.
Nonetheless, the study also demonstrated the unfairness of a system in which 1 in 6 valid claims received no payment (this in addition to the vast number of negligent injuries that never even lead to a claim as discussed in the 1999 IOM report). Then there is the frustration with a system wherein the average time between injury and claim resolution is 5 years and 54% of the payments are absorbed by defense costs and contingency fees. That 80% of expenses were incurred in resolving claims with errors suggests that steps to decrease frivolous litigation (claims without merit) will not lead to substantial savings and that steps to streamlining the system of handling claims will be more useful.
Blake et al looked at state-specific data from the National Practitioner Data Bank, which collects reports of all malpractice payments in the US on behalf of physicians, dentists, and nurses. They looked at the relationship between payments, physician premiums, and various state tort reforms. They found that mean payments were 26% lower in states with total damage caps ($196,000 vs $265,000) and 22% less in states with noneconomic (pain and suffering) damage caps ($212,000 vs $279,000). In addition, total damage caps were associated with lower mean annual premiums and hard, but not soft (caps with exceptions) noneconomic caps were associated with premium reductions. No other state tort reforms measured showed a significant association with payments or premiums.4
Studdert et al surveyed Pennsylvania physicians in high-risk specialties (obstetrics/gynecology, ortho, ER, surgery, neurosurgery) to ascertain self-report of defensive medicine practice. Almost all respondents reported practicing defensive medicine, the most common form (92%) being unnecessary ordering of tests and imaging studies and referring for consultation. In addition, 42% said they had restricted their practice by either decreasing the performance of more risky procedures (eg, trauma surgery) or avoiding complex cases or patients perceived as more likely to sue. Defensive medicine was highly correlated with physicians’ lack of confidence in their liability insurance or its cost.5
Kessler et al looked at physician supply from 1985–2001 and its correlates to state tort reforms during that time. Three years after States that adopted direct reforms (mainly caps on damage awards) showed an average physician growth rate within 3 years that was 3.3% greater than states not adopting such reforms. The authors controlled for a variety of factors that can influence physician supply including population growth and other state-level characteristics.6
Common Good proposal
Another approach is advocated by Common Good, a bipartisan legal reform coalition. This organization has funding from the RWJ Foundation to work with researchers from the Harvard School of Public Health to investigate the creation of special health courts to hear malpractice cases.11 Their ideas are incorporated into the Fair and Reliable Medical Justice Act introduced as S.1337 by Senators Mike Enzi (R-WY) Max Baucus (D-MT).11
How it would work. Health courts would have full-time judges, neutral medical experts, faster proceedings with legal fees held to 20%, and rulings that could be appealed to a new Medical Appellate Court. Like other administrative courts that handle tax disputes, workmen’s comp, and vaccine injury, there would be no juries. Judges would issue written rulings and establish legal precedents. Once a mistake was verified, recovery would be automatic. Patients would be reimbursed for all their medical expenses and lost income plus a fixed sum that would be determined from an expert derived schedule addressing specific types of injuries.
Strengths and weaknesses. This proposal has the support of a wide array of national medical and legal leaders but not any medical associations. It attempts to address some of the most egregious parts of the current system—the time it takes to get claims resolved, the many errors that go uncompensated, and the diversion of so many dollars to overhead and legal fees rather than to patients. On the other hand, the plan does not provide firm caps, which may make the AMA and other professional associations skeptical. And the proposed health courts would rely on select medical experts and judges, a system likely to be strongly opposed by trial lawyers and some consumer groups. It also does not directly address the prevention of patient errors.
Those who fail to learn from history…
The current malpractice crisis may be abating, leaving physicians with higher malpractice premiums but some state tort reforms. History, however, suggests that the insurance cycle will eventually lead to another crisis. There may be a window of opportunity now to come up with a completely different system to address the goals and problems with our current system, but it is likely to be a small window. Capitalizing on it will require a willingness for both sides in the current stand-off to get past their own self-interests in order to come up with something better for all.
CORRESPONDENCE
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
A handful of papers published in the past few years have looked at different aspects of the current malpractice situation and have yielded some revelations (see page 775 in this issue) or data banks working on safety issues.
- Though it does a reasonable job at separating valid from invalid claims and compensating them accordingly, it often takes a tremendously long time to accomplish this and still has a 10% to 16% rate of false positive (payment with no error) and false negative (no payment with error) outcomes.
- The system is not overwhelmed with frivolous claims. Still, it costs a lot of money to manage, and less than half of this money goes to claimants.
- Hard caps on total damages or noneconomic damages, unlike other state tort reforms (Figure), appear to reduce claims payments, physician premiums, and total health costs,7 while increasing physician supply.
- Defensive medicine exists, though putting a valid dollar amount on its costs is difficult.
- Anecdotal evidence suggests that physicians are leaving practice or limiting their practice (eg, family physicians discontinuing deliveries) as a result of malpractice costs.
FIGURE
Tort reforms commonly adopted by states
AMA’s proposal for change
Malpractice reform has been at or near the top of the AMA’s political agenda for the past 4 or 5 years, with strong lobbying efforts at the national level as well as support for state chapter efforts. The AMA’s proposal is based on California’s liability reform law known as MICRA that was passed over 30 years ago and has been associated with significantly lower premium growth since then compared with the rest of the US.8 Key provisions:
- Unlimited economic damages (medical expenses, future earnings)
- Limits on noneconomic damages (pain and suffering)
- Punitive damages, if available, up to $250,000 or 2 times economic damages, whichever is greater
- Allocation of damage awards in proportion to fault
- Sliding scale for attorney contingency fees.
Dubious premises. The AMA literature on malpractice includes valid information on the costs of the tort system, the rise in claims payouts, and effects on physician premiums. But it also suggests that meritless lawsuits are increasing. This is untrue. And its implication that physicians are increasingly leaving practice is anecdotal. There is no good research on the extent of this problem.8
Too narrow a focus. More important, the AMA plan is focused on physician premium costs while ignoring the unfairness of the system (eg, time to resolve claims, lack of payment for many patients with legitimate claims) and the vast number of medical errors for which claims are never filed.
The MEDIC proposal
Senators Hillary Clinton (D-NY) and Barack Obama (D-IL) have proposed federal legislation to address the malpractice crisis. Their bill would create an Office of Patient Safety in the Department of Health and Human Services, and would establish the National Medical Error Disclosure and Compensation (MEDIC) program within that office.9
Apologies would not be actionable in court. The MEDIC program would provide grants to physicians, hospitals, and health systems for the creation of programs to disclose medical errors to patients and negotiate fair compensation. The law would preserve confidentiality so that any apology offered by a health care provider as part of those negotiations would be kept confidential and could not be used in a trial. Any savings achieved from lower administrative and legal costs would be used to reduce physician malpractice premiums and toward patient safety initiatives.
Federal mandating of caps unlikely, however. At the federal level, Democrats have firmly opposed mandating caps on malpractice claims settlements. They argue that caps are unfair to patients who have been victims of medical errors. Others say this opposition reflects financial contributions from trial lawyers. It seems time to get past this conflict. Without dramatic changes in the composition of the Senate, which seems unlikely, there is little or no chance that caps will pass at the national level. At the same time, physician groups have been successful at achieving caps in a number of states (total of 26 at last count).
Signs this program could succeed. The MEDIC proposal is an attempt to find another way out of the malpractice impasse in the Senate by linking the patient safety and tort reform issues. It is primarily based on a growing movement to have physicians more directly acknowledge medical errors to patients,10 and in some cases, link these apologies to immediate financial negotiations to settle any potential claim of injury. The University of Michigan is the best known academic institution pursuing these strategies, and they report a significant decrease in the number of claims and annual litigation costs. The Lexington, Kentucky, VA Hospital has a similar program that has reduced liability costs compared with other VA hospitals.
The MEDIC proposal is attractive in its attempt to tie doctor-patient communication, patient safety, and liability together. And the anecdotal reports of success with isolated programs of its type are encouraging. It also moves the argument at a national level away from a fight about caps, which puts many physicians in the uncomfortable position of opposing Democrats who support many of their other positions—eg, Title VII funding, preservation of the traditional Medicare program, expansion of the Medicare Part D program, and better funding of public health programs. Nonetheless, there is a big row to hoe in making physicians more comfortable with acknowledging their errors, convincing them this would not be held against them in court, and assuring both physicians and hospitals that such efforts will actually lead to lower malpractice costs.
Thorpe analyzed data from 1995–2001 collected by the National Association of Insurance Commissioners to see the relationship between state tort reforms and premium levels. Premiums in states with caps on awards were 17% lower than states without caps. There was no association between premium levels and other reforms, such as caps on attorney fees or collateral offset rules (decreasing awards by the amount the plaintiff receives from other sources). Some association was noted between decreased competition among insurers and higher premiums.1
Rodwin et al used data from AMA surveys of self-employed physicians (physicians in groups or solo practice who are not employees) from 1970 to 2000. They found that while premiums increased from 1970 to 1986 and from 1996 to 2000, they had only a small effect on physician income. Premiums made up a small percentage of total practice costs and had a negligible effect on practice income, arguing against a malpractice crisis. However, this study lacked more recent data on premium increases and practice expenses and did not take into account differences among states that might be due to tort reforms such as the institution of caps on awards.2
Studdert et al reviewed 1452 closed claims in 4 categories (obstetrics, surgery, missed or delayed diagnosis, and medication) from 5 liability insurers representing 4 regions of the US, and used objective criteria and independent reviewers to classify the merits of the claims. They found that 3% of the claims had no verifiable medical injury and 37% did not involve errors. About 73% of the claims not associated with errors or injuries resulted in no compensation, while 73% of those with errors did result in compensation. Further payment for claims not involving errors were lower than those that did involve errors. Looked at another way, of the 1452 claims reviewed, about 10% received payment but had no identifiable error, while about 17% had an identifiable error but no payment was made.3
This study demonstrated that: 1) the cost of defending claims involving no error was substantial but still only amounted to about 13% of direct system costs, meaning that contesting and paying for claims caused by errors accounts for most of the costs of the liability system, and 2) the malpractice system works reasonably well at separating claims without merit from those with merit.
Nonetheless, the study also demonstrated the unfairness of a system in which 1 in 6 valid claims received no payment (this in addition to the vast number of negligent injuries that never even lead to a claim as discussed in the 1999 IOM report). Then there is the frustration with a system wherein the average time between injury and claim resolution is 5 years and 54% of the payments are absorbed by defense costs and contingency fees. That 80% of expenses were incurred in resolving claims with errors suggests that steps to decrease frivolous litigation (claims without merit) will not lead to substantial savings and that steps to streamlining the system of handling claims will be more useful.
Blake et al looked at state-specific data from the National Practitioner Data Bank, which collects reports of all malpractice payments in the US on behalf of physicians, dentists, and nurses. They looked at the relationship between payments, physician premiums, and various state tort reforms. They found that mean payments were 26% lower in states with total damage caps ($196,000 vs $265,000) and 22% less in states with noneconomic (pain and suffering) damage caps ($212,000 vs $279,000). In addition, total damage caps were associated with lower mean annual premiums and hard, but not soft (caps with exceptions) noneconomic caps were associated with premium reductions. No other state tort reforms measured showed a significant association with payments or premiums.4
Studdert et al surveyed Pennsylvania physicians in high-risk specialties (obstetrics/gynecology, ortho, ER, surgery, neurosurgery) to ascertain self-report of defensive medicine practice. Almost all respondents reported practicing defensive medicine, the most common form (92%) being unnecessary ordering of tests and imaging studies and referring for consultation. In addition, 42% said they had restricted their practice by either decreasing the performance of more risky procedures (eg, trauma surgery) or avoiding complex cases or patients perceived as more likely to sue. Defensive medicine was highly correlated with physicians’ lack of confidence in their liability insurance or its cost.5
Kessler et al looked at physician supply from 1985–2001 and its correlates to state tort reforms during that time. Three years after States that adopted direct reforms (mainly caps on damage awards) showed an average physician growth rate within 3 years that was 3.3% greater than states not adopting such reforms. The authors controlled for a variety of factors that can influence physician supply including population growth and other state-level characteristics.6
Common Good proposal
Another approach is advocated by Common Good, a bipartisan legal reform coalition. This organization has funding from the RWJ Foundation to work with researchers from the Harvard School of Public Health to investigate the creation of special health courts to hear malpractice cases.11 Their ideas are incorporated into the Fair and Reliable Medical Justice Act introduced as S.1337 by Senators Mike Enzi (R-WY) Max Baucus (D-MT).11
How it would work. Health courts would have full-time judges, neutral medical experts, faster proceedings with legal fees held to 20%, and rulings that could be appealed to a new Medical Appellate Court. Like other administrative courts that handle tax disputes, workmen’s comp, and vaccine injury, there would be no juries. Judges would issue written rulings and establish legal precedents. Once a mistake was verified, recovery would be automatic. Patients would be reimbursed for all their medical expenses and lost income plus a fixed sum that would be determined from an expert derived schedule addressing specific types of injuries.
Strengths and weaknesses. This proposal has the support of a wide array of national medical and legal leaders but not any medical associations. It attempts to address some of the most egregious parts of the current system—the time it takes to get claims resolved, the many errors that go uncompensated, and the diversion of so many dollars to overhead and legal fees rather than to patients. On the other hand, the plan does not provide firm caps, which may make the AMA and other professional associations skeptical. And the proposed health courts would rely on select medical experts and judges, a system likely to be strongly opposed by trial lawyers and some consumer groups. It also does not directly address the prevention of patient errors.
Those who fail to learn from history…
The current malpractice crisis may be abating, leaving physicians with higher malpractice premiums but some state tort reforms. History, however, suggests that the insurance cycle will eventually lead to another crisis. There may be a window of opportunity now to come up with a completely different system to address the goals and problems with our current system, but it is likely to be a small window. Capitalizing on it will require a willingness for both sides in the current stand-off to get past their own self-interests in order to come up with something better for all.
CORRESPONDENCE
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Thorpe K. The Medical malpractice “crisis”: Recent trends and the impact of state tort reforms. Health Affairs 2004 January 21;web-only.
2. Rodwin M, Chang H, Clausen J. Malpractice premiums and physicians’ income: Perceptions of a crisis conflict with empirical evidence. Health Affairs 2006;525:750-758.
3. Studdert D, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med 2006;354:2024-2033.
4. Guirguis-Blake J, Fryer GE, Phillips RL, Jr, Szabat R, Green LA. The US medical liability system: Evidence for legislative reform. Ann Fam Med 2006;4:240-246.
5. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA 2005;293:2609-2617.
6. Kessler DP, Sage WM, Becker DJ, et al. Impact of malpractice reforms on the supply of physician services. JAMA 2005;293:2618-2625.
7. Hellinger F, Encinosa W. The impact of state laws limiting malpractice damage awards on health care expenditures. AJPH 2006;96:1375-1381.
8. American Medical Association. Medical liability talking points. Available at: www.ama-assn.org/ama1/pub/upload/mm/399/mlr_fastfacts.pdf. Accessed on August 15, 2006.
9. Clinton HR, Obama B. Making patient safety the centerpiece of medical liability reform. N Engl J Med 2006;354:2205-2208.
10. O’Reilly K. Harvard adopts a disclosure and apology policy. AMA News, June 12, 2006.
11. Common Good. What are health courts? Available at: cgood.org/f-healthcourtsinfo.html. Accessed on August 15, 2006.
1. Thorpe K. The Medical malpractice “crisis”: Recent trends and the impact of state tort reforms. Health Affairs 2004 January 21;web-only.
2. Rodwin M, Chang H, Clausen J. Malpractice premiums and physicians’ income: Perceptions of a crisis conflict with empirical evidence. Health Affairs 2006;525:750-758.
3. Studdert D, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med 2006;354:2024-2033.
4. Guirguis-Blake J, Fryer GE, Phillips RL, Jr, Szabat R, Green LA. The US medical liability system: Evidence for legislative reform. Ann Fam Med 2006;4:240-246.
5. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA 2005;293:2609-2617.
6. Kessler DP, Sage WM, Becker DJ, et al. Impact of malpractice reforms on the supply of physician services. JAMA 2005;293:2618-2625.
7. Hellinger F, Encinosa W. The impact of state laws limiting malpractice damage awards on health care expenditures. AJPH 2006;96:1375-1381.
8. American Medical Association. Medical liability talking points. Available at: www.ama-assn.org/ama1/pub/upload/mm/399/mlr_fastfacts.pdf. Accessed on August 15, 2006.
9. Clinton HR, Obama B. Making patient safety the centerpiece of medical liability reform. N Engl J Med 2006;354:2205-2208.
10. O’Reilly K. Harvard adopts a disclosure and apology policy. AMA News, June 12, 2006.
11. Common Good. What are health courts? Available at: cgood.org/f-healthcourtsinfo.html. Accessed on August 15, 2006.
The Journal of Family Practice ©2006 Dowden Health Media
Malpractice crisis: Causes of escalating insurance premiums, and implications for you
What has led to the current malpractice crisis? There are 2 main theories.
Physicians, insurers, and hospitals generally blame lawyers and the litigation system for increasing the number of claims filed (claim frequency) and the average payout on claims (claims severity).
Attorneys and consumer groups argue that malpractice insurance goes through natural cycles in costs and charges. For the rise in premiums in the current crisis, they particularly blame decreased investment returns and poor pricing decisions by insurers.
Who’s right?
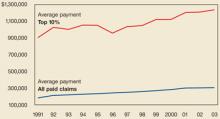
Research suggests that neither argument alone is persuasive. For instance, a study of the National Practitioner Data Bank, which collects results of all malpractice claims payments, found that claims severity did increase since 1991, but not during the current malpractice crisis period when adjusted for inflation: 52% from 1991 to 2003 but only 6% from 2000 to 2003.1 The highest growth rate has been in medium-sized awards, not the large ones you often read about. And, as always, claims severity growth varies among states (FIGURE 1).1
In contrast, when adjusted for population changes, Data Bank studies showed no significant nationwide increase in the number of paid claims from 1991 to 2003. Data from individual states also bear this out.1
Furthermore, studies of the relationship between claims’ payments and premiums have shown only a weakly positive relationship, suggesting other factors are involved. The argument that decreased investment returns have led to large price increases probably has some merit but does not explain the magnitude of the changes or the variation across states.
FIGURE 1
Amount of average paid claim, 1991-2003
So, what’s the answer for physicians?
Most likely, a combination of several changes has led to the recent crisis—increased claims’ costs, poor pricing decisions or cost projections by insurers, and decreased investment income. Rather than seeing these issues as distinct, it may be more useful to see their intrinsic relationships in leading to rapid premium increases and a malpractice crisis.
In this article, the first of 2, I discuss recent events influencing insurance premiums, which may also suggest to you avenues to explore in optimizing your own coverage. In part 2, I will address our current tort system and concrete proposals for change being pursued.
How premiums are determined
Almost all physicians carry professional liability (malpractice) insurance, either by choice or legal requirement. Coverage is usually purchased individually or by group from a commercial company or a physician-owned mutual company. Hospitals may purchase insurance or be self-insured, and their physician employees may be covered through those policies.
In theory, a tort system to resolve malpractice claims is supposed to serve as a negative incentive to physicians to practice high quality medicine. But the 1999 Institute of Medicine report on the occurrence and ramifications of medical errors, To Err is Human,2 provided evidence that the malpractice system has failed to accomplish this goal.
Unlike auto insurance, malpractice premiums are mainly determined by the class of physician (including type of work) and geography, rather than by an individual’s practice experience. Auto insurance premiums are adjusted according to the insured’s driving record. This is difficult to accomplish with malpractice insurance because claims experience is too variable over short periods of time.
Insurers take the following into account when they set premiums: 1) anticipated payouts to a class of physicians and the uncertainty of their estimate; 2) expected administrative expenses to manage the insurance; 3) future investment income; and 4) desired amount of profit. Information on past losses and expenses is used but, clearly, much of the determination involves complicated predictions.
Another characteristic that makes rate setting difficult is the length of time from the occurrence of an event to the filing of a claim to the resolution of that claim. On average, this is 4 to 5 years. The difficulty in predicting the liability for claims that have not yet been filed adds to the problem in setting premiums accurately.
How does your state manage rate setting?
Although malpractice has been a political issue at the federal level in the past few years, the reality is that, like most insurance, it is mainly regulated by the states.
States with substantial restrictions (17 states in 2004) require insurers to file rate changes and gain approval before prices can change.
States with less restrictive environments require such prior approval only if rate increases exceed a certain amount (23 in 2004) or only require notification after rates are changed (9 in 2004).1
Whether these varying types of state regulation lead to higher or lower premiums is unclear.
In addition to these strategies, states have also implemented an array of tort reforms in an attempt to address the cost of physician malpractice policies. Caps have been the most popular reform, with 26 states instituting them (FIGURE 2).3
FIGURE 2
Caps on damages by state
Recent events influencing premiums
A number of changes in the past few years have occurred in the malpractice arena
- Increased numbers of physician-owned companies and fewer commercial carriers since the malpractice crisis of the 1970s. These companies often give physicians better rates and more control, but some have been undercapitalized and not survived.
- A rise in the cost of reinsurance since September 11. Reinsurance covers costs above a certain level and limits companies’ losses in a given year. Reinsurers suffered large losses from the terrorist attacks of September 11, 2001 and, subsequently, raised their prices significantly to all liability companies.
- More hospitals self-insuring to better control rates and their risk pool leading increasing numbers of physicians to obtain their malpractice coverage through hospitals. This lowers their costs in comparison to those who practice in small groups or solo settings.
- A shift in the type of insurance from occurrence (all incidents in the policy year are covered regardless of when the claim is filed) to claims-made policies (coverage is for claims filed in the policy year regardless of when the event occurred). With claims-made insurance, physicians have to purchase costly “tail” policies to cover the possibility of incidents during the years of coverage becoming future claims.
- The growth of state-mandated funds as insurers of last resort for physicians who cannot find any coverage. These funds are financed by surcharges on hospital and physician malpractice policies, which in turn increases those costs.
- A decrease in companies’ investment yields since 2000 (from 5.2% in 2000 to4.3% in 2002), in spite of the fact that insurers’ portfolios are quite conservative as required by state law. While some companies saw their investment income drop by 50% in these years, this amount is still only a small part of insurers’ total income.
Options to explore
- Check with your state’s division of insurance website to see if there is a list of carriers, and information about their experience.
- Rather than a claims-made policy, consider getting an occurrence policy that will eliminate the worry and cost of purchasing “tail” insurance. If you work for a group practice, inquire about whether occurrence insurance is an option. If occurrence insurance is not available or seems too costly, ask your agent about what a typical tail policy would cost.
- You may want to inquire about purchasing insurance through a local hospital, which could yield cost savings.
- Don’t skimp on coverage amounts. Consider getting risk-management training to learn what you can do to minimize the risk of being sued.
A malpractice crisis, according to the AMA, occurs when “patients lose access to care as a result of a broken medical liability system… that causes physicians’ insurance premiums to skyrocket forcing them to restrict their practice.” An alternate description would be when insurers’ financial situation deteriorates resulting in higher than average increases in premiums or decrease in the supply of insurance. The existence and severity of a crisis varies from state to state (see map at right).4
Insurance becomes relatively unaffordable when premiums increase rapidly, as occurred in the second malpractice crisis in the mid-1980s and also in the current crisis, which has seen increasing premiums since 1999 and some moderation since 2004. Increases in premiums may differ across states, within states, and among specialties.
How providers feel about premium increases depends on both the size and rapidity of the increases and by their ability to collect more for their services to pay for increases. In the current crisis, providers have had more difficulty maintaining a balance between cost increases and income as discounted fee contracts and lack of growth in Medicare and Medicaid payments have constrained growth in practice revenue.
CORRESPONDENCE
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Chandra A, Nundy S, Seabury SA. The growth of physician medical malpractice payments: evidence from the National Practitioner Data Bank. Health Aff (Millwood) 2005 Jan-Jun; Suppl Web Exclusives:W5-240-W5-249. Available at: content.healthaffairs.org/cgi/content/abstract/hlthaff.w5.240. Accessed on July 18, 2006.
2. Institute of Medicine. To Err Is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
3. Mello MM. Medical malpractice: Impact of crisis and effect of state tort reforms. The Synthesis Project Policy Brief #10, May 2006. Available at: www.rwjf.org/publications/synthesis/reports_and_briefs/charts/no10_pb_fig2.jpg. Accessed on July 18, 2006.
4. Medical liability crisis map. AMA News and Information [website]. Available at: www.amaassn.org/ama/noindex/category/11871.html. Accessed on July 18. 2006.
What has led to the current malpractice crisis? There are 2 main theories.
Physicians, insurers, and hospitals generally blame lawyers and the litigation system for increasing the number of claims filed (claim frequency) and the average payout on claims (claims severity).
Attorneys and consumer groups argue that malpractice insurance goes through natural cycles in costs and charges. For the rise in premiums in the current crisis, they particularly blame decreased investment returns and poor pricing decisions by insurers.
Who’s right?
Research suggests that neither argument alone is persuasive. For instance, a study of the National Practitioner Data Bank, which collects results of all malpractice claims payments, found that claims severity did increase since 1991, but not during the current malpractice crisis period when adjusted for inflation: 52% from 1991 to 2003 but only 6% from 2000 to 2003.1 The highest growth rate has been in medium-sized awards, not the large ones you often read about. And, as always, claims severity growth varies among states (FIGURE 1).1
In contrast, when adjusted for population changes, Data Bank studies showed no significant nationwide increase in the number of paid claims from 1991 to 2003. Data from individual states also bear this out.1
Furthermore, studies of the relationship between claims’ payments and premiums have shown only a weakly positive relationship, suggesting other factors are involved. The argument that decreased investment returns have led to large price increases probably has some merit but does not explain the magnitude of the changes or the variation across states.
FIGURE 1
Amount of average paid claim, 1991-2003
So, what’s the answer for physicians?
Most likely, a combination of several changes has led to the recent crisis—increased claims’ costs, poor pricing decisions or cost projections by insurers, and decreased investment income. Rather than seeing these issues as distinct, it may be more useful to see their intrinsic relationships in leading to rapid premium increases and a malpractice crisis.
In this article, the first of 2, I discuss recent events influencing insurance premiums, which may also suggest to you avenues to explore in optimizing your own coverage. In part 2, I will address our current tort system and concrete proposals for change being pursued.
How premiums are determined
Almost all physicians carry professional liability (malpractice) insurance, either by choice or legal requirement. Coverage is usually purchased individually or by group from a commercial company or a physician-owned mutual company. Hospitals may purchase insurance or be self-insured, and their physician employees may be covered through those policies.
In theory, a tort system to resolve malpractice claims is supposed to serve as a negative incentive to physicians to practice high quality medicine. But the 1999 Institute of Medicine report on the occurrence and ramifications of medical errors, To Err is Human,2 provided evidence that the malpractice system has failed to accomplish this goal.
Unlike auto insurance, malpractice premiums are mainly determined by the class of physician (including type of work) and geography, rather than by an individual’s practice experience. Auto insurance premiums are adjusted according to the insured’s driving record. This is difficult to accomplish with malpractice insurance because claims experience is too variable over short periods of time.
Insurers take the following into account when they set premiums: 1) anticipated payouts to a class of physicians and the uncertainty of their estimate; 2) expected administrative expenses to manage the insurance; 3) future investment income; and 4) desired amount of profit. Information on past losses and expenses is used but, clearly, much of the determination involves complicated predictions.
Another characteristic that makes rate setting difficult is the length of time from the occurrence of an event to the filing of a claim to the resolution of that claim. On average, this is 4 to 5 years. The difficulty in predicting the liability for claims that have not yet been filed adds to the problem in setting premiums accurately.
How does your state manage rate setting?
Although malpractice has been a political issue at the federal level in the past few years, the reality is that, like most insurance, it is mainly regulated by the states.
States with substantial restrictions (17 states in 2004) require insurers to file rate changes and gain approval before prices can change.
States with less restrictive environments require such prior approval only if rate increases exceed a certain amount (23 in 2004) or only require notification after rates are changed (9 in 2004).1
Whether these varying types of state regulation lead to higher or lower premiums is unclear.
In addition to these strategies, states have also implemented an array of tort reforms in an attempt to address the cost of physician malpractice policies. Caps have been the most popular reform, with 26 states instituting them (FIGURE 2).3
FIGURE 2
Caps on damages by state
Recent events influencing premiums
A number of changes in the past few years have occurred in the malpractice arena
- Increased numbers of physician-owned companies and fewer commercial carriers since the malpractice crisis of the 1970s. These companies often give physicians better rates and more control, but some have been undercapitalized and not survived.
- A rise in the cost of reinsurance since September 11. Reinsurance covers costs above a certain level and limits companies’ losses in a given year. Reinsurers suffered large losses from the terrorist attacks of September 11, 2001 and, subsequently, raised their prices significantly to all liability companies.
- More hospitals self-insuring to better control rates and their risk pool leading increasing numbers of physicians to obtain their malpractice coverage through hospitals. This lowers their costs in comparison to those who practice in small groups or solo settings.
- A shift in the type of insurance from occurrence (all incidents in the policy year are covered regardless of when the claim is filed) to claims-made policies (coverage is for claims filed in the policy year regardless of when the event occurred). With claims-made insurance, physicians have to purchase costly “tail” policies to cover the possibility of incidents during the years of coverage becoming future claims.
- The growth of state-mandated funds as insurers of last resort for physicians who cannot find any coverage. These funds are financed by surcharges on hospital and physician malpractice policies, which in turn increases those costs.
- A decrease in companies’ investment yields since 2000 (from 5.2% in 2000 to4.3% in 2002), in spite of the fact that insurers’ portfolios are quite conservative as required by state law. While some companies saw their investment income drop by 50% in these years, this amount is still only a small part of insurers’ total income.
Options to explore
- Check with your state’s division of insurance website to see if there is a list of carriers, and information about their experience.
- Rather than a claims-made policy, consider getting an occurrence policy that will eliminate the worry and cost of purchasing “tail” insurance. If you work for a group practice, inquire about whether occurrence insurance is an option. If occurrence insurance is not available or seems too costly, ask your agent about what a typical tail policy would cost.
- You may want to inquire about purchasing insurance through a local hospital, which could yield cost savings.
- Don’t skimp on coverage amounts. Consider getting risk-management training to learn what you can do to minimize the risk of being sued.
A malpractice crisis, according to the AMA, occurs when “patients lose access to care as a result of a broken medical liability system… that causes physicians’ insurance premiums to skyrocket forcing them to restrict their practice.” An alternate description would be when insurers’ financial situation deteriorates resulting in higher than average increases in premiums or decrease in the supply of insurance. The existence and severity of a crisis varies from state to state (see map at right).4
Insurance becomes relatively unaffordable when premiums increase rapidly, as occurred in the second malpractice crisis in the mid-1980s and also in the current crisis, which has seen increasing premiums since 1999 and some moderation since 2004. Increases in premiums may differ across states, within states, and among specialties.
How providers feel about premium increases depends on both the size and rapidity of the increases and by their ability to collect more for their services to pay for increases. In the current crisis, providers have had more difficulty maintaining a balance between cost increases and income as discounted fee contracts and lack of growth in Medicare and Medicaid payments have constrained growth in practice revenue.
CORRESPONDENCE
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
What has led to the current malpractice crisis? There are 2 main theories.
Physicians, insurers, and hospitals generally blame lawyers and the litigation system for increasing the number of claims filed (claim frequency) and the average payout on claims (claims severity).
Attorneys and consumer groups argue that malpractice insurance goes through natural cycles in costs and charges. For the rise in premiums in the current crisis, they particularly blame decreased investment returns and poor pricing decisions by insurers.
Who’s right?
Research suggests that neither argument alone is persuasive. For instance, a study of the National Practitioner Data Bank, which collects results of all malpractice claims payments, found that claims severity did increase since 1991, but not during the current malpractice crisis period when adjusted for inflation: 52% from 1991 to 2003 but only 6% from 2000 to 2003.1 The highest growth rate has been in medium-sized awards, not the large ones you often read about. And, as always, claims severity growth varies among states (FIGURE 1).1
In contrast, when adjusted for population changes, Data Bank studies showed no significant nationwide increase in the number of paid claims from 1991 to 2003. Data from individual states also bear this out.1
Furthermore, studies of the relationship between claims’ payments and premiums have shown only a weakly positive relationship, suggesting other factors are involved. The argument that decreased investment returns have led to large price increases probably has some merit but does not explain the magnitude of the changes or the variation across states.
FIGURE 1
Amount of average paid claim, 1991-2003
So, what’s the answer for physicians?
Most likely, a combination of several changes has led to the recent crisis—increased claims’ costs, poor pricing decisions or cost projections by insurers, and decreased investment income. Rather than seeing these issues as distinct, it may be more useful to see their intrinsic relationships in leading to rapid premium increases and a malpractice crisis.
In this article, the first of 2, I discuss recent events influencing insurance premiums, which may also suggest to you avenues to explore in optimizing your own coverage. In part 2, I will address our current tort system and concrete proposals for change being pursued.
How premiums are determined
Almost all physicians carry professional liability (malpractice) insurance, either by choice or legal requirement. Coverage is usually purchased individually or by group from a commercial company or a physician-owned mutual company. Hospitals may purchase insurance or be self-insured, and their physician employees may be covered through those policies.
In theory, a tort system to resolve malpractice claims is supposed to serve as a negative incentive to physicians to practice high quality medicine. But the 1999 Institute of Medicine report on the occurrence and ramifications of medical errors, To Err is Human,2 provided evidence that the malpractice system has failed to accomplish this goal.
Unlike auto insurance, malpractice premiums are mainly determined by the class of physician (including type of work) and geography, rather than by an individual’s practice experience. Auto insurance premiums are adjusted according to the insured’s driving record. This is difficult to accomplish with malpractice insurance because claims experience is too variable over short periods of time.
Insurers take the following into account when they set premiums: 1) anticipated payouts to a class of physicians and the uncertainty of their estimate; 2) expected administrative expenses to manage the insurance; 3) future investment income; and 4) desired amount of profit. Information on past losses and expenses is used but, clearly, much of the determination involves complicated predictions.
Another characteristic that makes rate setting difficult is the length of time from the occurrence of an event to the filing of a claim to the resolution of that claim. On average, this is 4 to 5 years. The difficulty in predicting the liability for claims that have not yet been filed adds to the problem in setting premiums accurately.
How does your state manage rate setting?
Although malpractice has been a political issue at the federal level in the past few years, the reality is that, like most insurance, it is mainly regulated by the states.
States with substantial restrictions (17 states in 2004) require insurers to file rate changes and gain approval before prices can change.
States with less restrictive environments require such prior approval only if rate increases exceed a certain amount (23 in 2004) or only require notification after rates are changed (9 in 2004).1
Whether these varying types of state regulation lead to higher or lower premiums is unclear.
In addition to these strategies, states have also implemented an array of tort reforms in an attempt to address the cost of physician malpractice policies. Caps have been the most popular reform, with 26 states instituting them (FIGURE 2).3
FIGURE 2
Caps on damages by state
Recent events influencing premiums
A number of changes in the past few years have occurred in the malpractice arena
- Increased numbers of physician-owned companies and fewer commercial carriers since the malpractice crisis of the 1970s. These companies often give physicians better rates and more control, but some have been undercapitalized and not survived.
- A rise in the cost of reinsurance since September 11. Reinsurance covers costs above a certain level and limits companies’ losses in a given year. Reinsurers suffered large losses from the terrorist attacks of September 11, 2001 and, subsequently, raised their prices significantly to all liability companies.
- More hospitals self-insuring to better control rates and their risk pool leading increasing numbers of physicians to obtain their malpractice coverage through hospitals. This lowers their costs in comparison to those who practice in small groups or solo settings.
- A shift in the type of insurance from occurrence (all incidents in the policy year are covered regardless of when the claim is filed) to claims-made policies (coverage is for claims filed in the policy year regardless of when the event occurred). With claims-made insurance, physicians have to purchase costly “tail” policies to cover the possibility of incidents during the years of coverage becoming future claims.
- The growth of state-mandated funds as insurers of last resort for physicians who cannot find any coverage. These funds are financed by surcharges on hospital and physician malpractice policies, which in turn increases those costs.
- A decrease in companies’ investment yields since 2000 (from 5.2% in 2000 to4.3% in 2002), in spite of the fact that insurers’ portfolios are quite conservative as required by state law. While some companies saw their investment income drop by 50% in these years, this amount is still only a small part of insurers’ total income.
Options to explore
- Check with your state’s division of insurance website to see if there is a list of carriers, and information about their experience.
- Rather than a claims-made policy, consider getting an occurrence policy that will eliminate the worry and cost of purchasing “tail” insurance. If you work for a group practice, inquire about whether occurrence insurance is an option. If occurrence insurance is not available or seems too costly, ask your agent about what a typical tail policy would cost.
- You may want to inquire about purchasing insurance through a local hospital, which could yield cost savings.
- Don’t skimp on coverage amounts. Consider getting risk-management training to learn what you can do to minimize the risk of being sued.
A malpractice crisis, according to the AMA, occurs when “patients lose access to care as a result of a broken medical liability system… that causes physicians’ insurance premiums to skyrocket forcing them to restrict their practice.” An alternate description would be when insurers’ financial situation deteriorates resulting in higher than average increases in premiums or decrease in the supply of insurance. The existence and severity of a crisis varies from state to state (see map at right).4
Insurance becomes relatively unaffordable when premiums increase rapidly, as occurred in the second malpractice crisis in the mid-1980s and also in the current crisis, which has seen increasing premiums since 1999 and some moderation since 2004. Increases in premiums may differ across states, within states, and among specialties.
How providers feel about premium increases depends on both the size and rapidity of the increases and by their ability to collect more for their services to pay for increases. In the current crisis, providers have had more difficulty maintaining a balance between cost increases and income as discounted fee contracts and lack of growth in Medicare and Medicaid payments have constrained growth in practice revenue.
CORRESPONDENCE
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Chandra A, Nundy S, Seabury SA. The growth of physician medical malpractice payments: evidence from the National Practitioner Data Bank. Health Aff (Millwood) 2005 Jan-Jun; Suppl Web Exclusives:W5-240-W5-249. Available at: content.healthaffairs.org/cgi/content/abstract/hlthaff.w5.240. Accessed on July 18, 2006.
2. Institute of Medicine. To Err Is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
3. Mello MM. Medical malpractice: Impact of crisis and effect of state tort reforms. The Synthesis Project Policy Brief #10, May 2006. Available at: www.rwjf.org/publications/synthesis/reports_and_briefs/charts/no10_pb_fig2.jpg. Accessed on July 18, 2006.
4. Medical liability crisis map. AMA News and Information [website]. Available at: www.amaassn.org/ama/noindex/category/11871.html. Accessed on July 18. 2006.
1. Chandra A, Nundy S, Seabury SA. The growth of physician medical malpractice payments: evidence from the National Practitioner Data Bank. Health Aff (Millwood) 2005 Jan-Jun; Suppl Web Exclusives:W5-240-W5-249. Available at: content.healthaffairs.org/cgi/content/abstract/hlthaff.w5.240. Accessed on July 18, 2006.
2. Institute of Medicine. To Err Is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
3. Mello MM. Medical malpractice: Impact of crisis and effect of state tort reforms. The Synthesis Project Policy Brief #10, May 2006. Available at: www.rwjf.org/publications/synthesis/reports_and_briefs/charts/no10_pb_fig2.jpg. Accessed on July 18, 2006.
4. Medical liability crisis map. AMA News and Information [website]. Available at: www.amaassn.org/ama/noindex/category/11871.html. Accessed on July 18. 2006.
Trouble at the FDA: Can we fix the problems affecting you and your patients?
While often held in high esteem, the FDA has seen its reputation tarnished in recent years by adverse drug regulation experiences, apparent conflicts of interest, and problems with consistent agency leadership.
Examples of problems at the FDA
- The COX-2 inhibitor disaster that led to the withdrawal of rofecoxib (Vioxx) from the market due its association with adverse cardiovascular effects; a problem the company may have hidden from physicians and the public through manipulation of research reports.2
- The recent addition of black box warnings on selective serotonin reuptake inhibitor (SSRI) labels to warn of a potential association with suicidal behavior in adolescents after publicity that some FDA scientists were prevented from presenting this information to review panels.
- A plea bargain agreement in which Pfizer agreed to pay $430 million to resolve charges that Warner-Lambert, a company it took over, paid doctors to prescribe gabapentin (Neurontin) for off-label uses including bipolar disorder, attention deficit/hyperactivity disorder, and alcohol withdrawal seizures. Although physicians can prescribe drugs for whatever reason they choose, pharmaceutical companies are prohibited from promoting drugs for unapproved uses.3
- A lack of strong leadership with acting commissioners leading the agency 3 of the past 5 years. The last permanent commissioner resigned in September 2005 after 2 months in the position amidst accusations that he or his family had financial interests in some of the companies regulated by the agency.4
Given these and other negative events in conjunction with the dramatic increase in prescription drug use by Americans—up 60% in the past decade to 3.1 billion prescriptions in 2004 with reports of 375,000 adverse events—it’s not surprising that a recent survey showed the public wants a stronger FDA. In fact, two thirds support the creation of an independent oversight panel, 70% want the FDA to improve its gathering and reporting on possible harms of drugs and medical devices after their approval, and many believe that industry has too much influence over agency decisions.5
How the FDA performs its job matters very much to physicians, the public, and business. In recent months, outside experts and the FDA itself have proposed changes in how the agency handles new drug approvals that will affect all its stakeholders. Before reviewing those proposals, it’s worth understanding how the approval process works.
The Food and Drug Administration (FDA) is responsible for protecting the public health by assuring the safety and efficacy of human and veterinary drugs, biological products, medical devices, our nation’s food supply, cosmetics, and products that emit radiation—a list that accounts for at least 20% of consumer spending. It does this on a budget of about $1.7 billion and 10,000 employees. In comparison, the Department of Agriculture has a budget 50 times larger and a workforce 10 times larger.1
Speeding approval of new drugs
In the early 1990s, Congress responded to complaints about the FDA’s slow drug approval process, particularly regarding HIV drugs, by passing the Prescription Drug User Fee Act (PDUFA). The law mandated that pharmaceutical companies pay fees to the FDA that were then used by the agency to speed up the approval process by hiring more staff and adhering to a strict timetable for review. The PDUFA accomplished its goal; for instance, priority review drugs had their time to approval drop from a median of 14.9 months in 1993 to 6.7 months in 2003, and standard review drugs went from 27.2 months to 23.1 months during the same period.6 Ironically, in spite of the quicker approval process, in 2005, pharmaceutical companies had a record low number of FDA drug approvals—only 20 as opposed to 36 in 2004.7
As quality improvement experts say, however, you often get exactly the results your system was built for. Thus, it is not surprising that in recent years some FDA scientists have complained of increasing time pressures to perform reviews, increased pressure to approve drugs, and inability to communicate directly with the companies about the drugs they were reviewing so as to clarify study designs and data analyses.8
In addition, many observers believe that having the industry support the FDA budget (about $300 million/year) presents a potential conflict of interest for the agency, particularly in the current pro-business climate of the Bush administration.
Given these reports as well as the adverse drug safety events that have occurred recently, Congress and independent scientists are now calling for more attention to safety and less focus on the approval timetable.
How the drug approval process works
Companies obtain FDA approval for human trials after promising results from animal trials. Human trials begin with phase 1 trials, which are small studies looking at safety issues in healthy volunteers. Phase 2 studies are trials of safety and efficacy (how well the drug works) in patients with the target condition. If these prove successful, phase 3 studies are undertaken, which include at least 2 large randomized control trials of safety and efficacy. After these studies, which may involve 2000 to 5000 patients at most, the FDA can grant approval for the drug to be sold to the public.
The agency may require a company to conduct additional post-marketing studies as a condition of drug approval. These so-called phase 4 studies are often designed to identify uncommon adverse events or further investigate a drug’s effectiveness. Unfortunately, completion of many of these studies is either significantly delayed or never happens. For instance, as of September 2005, the FDA reported that 65% of the 1231 “post-marketing” studies that companies had pledged to carry out were still pending and that many of these had not even been started.9
After the drug is released, the agency may collect post-marketing data on adverse events and ask the companies to participate in this effort. But no further studies can be required of the company.
Major complaints about phase 3 studies are 1) they are relatively small and often study patients who differ in terms of demographics and extent of disease from those who end up using the medicine; 2) they are placebo-controlled trials rather than comparison trials between the new drug and the current standard therapy; and 3) the FDA increasingly uses surrogate endpoints to judge efficacy, such as tumor shrinkage for cancer drugs or LDL cholesterol decrease for lipid therapy, instead of definitive outcomes like morbidity and mortality. To the extent these surrogate endpoints strongly correlate with major outcomes, this can appropriately speed up the approval process, but the value of surrogate markers is not always clear. These characteristics of phase 3 studies often lead to approval of many “me-too” drugs without the information necessary to decide whether they are better than current standard therapy.
Deficiencies in using surrogate markers
A particular problem with surrogate markers occurs with the review of higher-priority drugs. For these, the FDA may grant provisional approval on the basis of a surrogate measure of clinical benefit shown in a single, uncontrolled trial as long as the treatment addresses an unmet need for a serious medical illness. In return, the FDA requires the company to complete confirmatory trials in the post-approval period, and may withdraw approval of the drug if no benefit is shown in these phase 4 trials.
However, between 1996 and 2003, only 6 of 23 cancer drugs have gone through such post-marketing trials, and the FDA has not withdrawn approval for any of the 23 drugs. Furthermore, many of these drugs cost thousands of dollars per treatment and have low response rates, yet the FDA has not required companies to better define the target populations for their use.10
The problem with using surrogate endpoints in short trials has been demonstrated in cardiovascular medicine with the experience using antiarrhythmics to prevent premature ventricular contractions (later discovered to lead to increased mortality), inotropics to improve ejection fraction (no long-term benefit) and, most recently, with nesiritide (Natrecor) for heart failure. The latter drug was approved based on improved pulmonarycapillary wedge pressure and self-reported improvement in dyspnea. Subsequent research and analyses have shown increased rates of renal failure and death but only after hundreds of millions of dollars worth of treatments.11
Recommendations to make the system work
Given the controversy surrounding the release and marketing of medications that later have shown to cause problems, and given the array of real and perceived conflicts of interest in the approval process, the FDA has proposed establishing a Drug Safety Oversight Board that will include outside experts to review safety issues arising with new applications. Others have argued that such a board should be completely independent of the FDA so as to minimize conflicts of interest and potential agency interference with obtaining necessary information from the companies.
The FDA also recently announced a new rule to overhaul prescription drug labeling, information in package inserts, and some drug reference books. The labels are full of information but much of it is inconsequential or difficult to use for prescribing, and most physicians don’t read them. The new labels will list safety warnings, advise how to use the drug and dose it, and advise physicians on what patients should be told about the medication. The goal is to give physicians more useful information to make decisions about drug indications and dosing. While the new rule will not change the patient drug information sheets that pharmacists hand out, it may force consumer advertising to make clearer statements about medication risks. Finally, the rule will preempt state liability statutes, which upsets some trial lawyers and politicians.12
Outside experts have proposed additional ideas to improve the FDA’s work: eliminating the current drug company fees that support new drug reviews to minimize conflicts of interest, mandating post-approval studies to look for uncommon adverse events (phase 4 studies), providing more information on relative efficacy of new drugs compared with current medication treatment and attention to real clinical outcome end points, and prohibiting direct-to-consumer advertising in the first 3 years after a new drug is released.13
Considering the turmoil surrounding the FDA’s performance right now, it is very possible we will see some of these proposed changes come to pass. Given the importance of prescription medications, these changes will be very relevant to the practices of family physicians and their patients’ health.
CORRESPONDENCE
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Slater E. Today’s FDA. N Engl J Med 2005;352:293-297.
2. Curfman GD, Morrissey S, Drazen JM. Expression of concern: Bombardier et al., “Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis,” N Engl J Med 2000; 343:1520-8. Editorial. N Engl J Med 2005;353:2813-2814.
3. Harris G. Pfizer to pay $420 million in illegal marketing case. New York Times, May 14, 2004.
4. Markel H. Why America needs a strong FDA. JAMA 2005;294:2489-2491.
5. Kaiser Daily Health Policy Report. March 3, 2005. Available at: www.kaisernetwork.org/daily_reports/rep_index.cfm?hint=3&DR_ID=28455. Accessed on March 10, 2006.
6. Okie S. What ails the FDA. N Engl J Med 2005;352:1063-1066.
7. Berenson A. Drugs in ’05: Much promise, little payoff; just 20 new products are approved despite biotechnology’s hope. New York Times, Jan 11, 2006.
8. FDA’s review process for new drug applications. Office of Inspector General. March 2003. Available at: www.oig.hhs.gov/oei/reports/oei-01-01-00590.pdf. Accessed on March 10, 2006.
9. 65% of promised drug studies pending. Washington Post, March 4, 2006 (AP report)
10. Roberts T, Chabner B. Beyond fast track for drug approvals. N Engl J Med 2004;351:501-505.
11. Topol E. Nesiritide—not verified. N Engl J Med 2005;353:113-116.
12. Harris G. New drug label rule is intended to reduce medical errors. New York Times, January 19, 2006.
13. Ray W, Stein CM. Reform of drug regulation—beyond an independent drug-safety board. N Engl J Med 2006;354:194-201.
While often held in high esteem, the FDA has seen its reputation tarnished in recent years by adverse drug regulation experiences, apparent conflicts of interest, and problems with consistent agency leadership.
Examples of problems at the FDA
- The COX-2 inhibitor disaster that led to the withdrawal of rofecoxib (Vioxx) from the market due its association with adverse cardiovascular effects; a problem the company may have hidden from physicians and the public through manipulation of research reports.2
- The recent addition of black box warnings on selective serotonin reuptake inhibitor (SSRI) labels to warn of a potential association with suicidal behavior in adolescents after publicity that some FDA scientists were prevented from presenting this information to review panels.
- A plea bargain agreement in which Pfizer agreed to pay $430 million to resolve charges that Warner-Lambert, a company it took over, paid doctors to prescribe gabapentin (Neurontin) for off-label uses including bipolar disorder, attention deficit/hyperactivity disorder, and alcohol withdrawal seizures. Although physicians can prescribe drugs for whatever reason they choose, pharmaceutical companies are prohibited from promoting drugs for unapproved uses.3
- A lack of strong leadership with acting commissioners leading the agency 3 of the past 5 years. The last permanent commissioner resigned in September 2005 after 2 months in the position amidst accusations that he or his family had financial interests in some of the companies regulated by the agency.4
Given these and other negative events in conjunction with the dramatic increase in prescription drug use by Americans—up 60% in the past decade to 3.1 billion prescriptions in 2004 with reports of 375,000 adverse events—it’s not surprising that a recent survey showed the public wants a stronger FDA. In fact, two thirds support the creation of an independent oversight panel, 70% want the FDA to improve its gathering and reporting on possible harms of drugs and medical devices after their approval, and many believe that industry has too much influence over agency decisions.5
How the FDA performs its job matters very much to physicians, the public, and business. In recent months, outside experts and the FDA itself have proposed changes in how the agency handles new drug approvals that will affect all its stakeholders. Before reviewing those proposals, it’s worth understanding how the approval process works.
The Food and Drug Administration (FDA) is responsible for protecting the public health by assuring the safety and efficacy of human and veterinary drugs, biological products, medical devices, our nation’s food supply, cosmetics, and products that emit radiation—a list that accounts for at least 20% of consumer spending. It does this on a budget of about $1.7 billion and 10,000 employees. In comparison, the Department of Agriculture has a budget 50 times larger and a workforce 10 times larger.1
Speeding approval of new drugs
In the early 1990s, Congress responded to complaints about the FDA’s slow drug approval process, particularly regarding HIV drugs, by passing the Prescription Drug User Fee Act (PDUFA). The law mandated that pharmaceutical companies pay fees to the FDA that were then used by the agency to speed up the approval process by hiring more staff and adhering to a strict timetable for review. The PDUFA accomplished its goal; for instance, priority review drugs had their time to approval drop from a median of 14.9 months in 1993 to 6.7 months in 2003, and standard review drugs went from 27.2 months to 23.1 months during the same period.6 Ironically, in spite of the quicker approval process, in 2005, pharmaceutical companies had a record low number of FDA drug approvals—only 20 as opposed to 36 in 2004.7
As quality improvement experts say, however, you often get exactly the results your system was built for. Thus, it is not surprising that in recent years some FDA scientists have complained of increasing time pressures to perform reviews, increased pressure to approve drugs, and inability to communicate directly with the companies about the drugs they were reviewing so as to clarify study designs and data analyses.8
In addition, many observers believe that having the industry support the FDA budget (about $300 million/year) presents a potential conflict of interest for the agency, particularly in the current pro-business climate of the Bush administration.
Given these reports as well as the adverse drug safety events that have occurred recently, Congress and independent scientists are now calling for more attention to safety and less focus on the approval timetable.
How the drug approval process works
Companies obtain FDA approval for human trials after promising results from animal trials. Human trials begin with phase 1 trials, which are small studies looking at safety issues in healthy volunteers. Phase 2 studies are trials of safety and efficacy (how well the drug works) in patients with the target condition. If these prove successful, phase 3 studies are undertaken, which include at least 2 large randomized control trials of safety and efficacy. After these studies, which may involve 2000 to 5000 patients at most, the FDA can grant approval for the drug to be sold to the public.
The agency may require a company to conduct additional post-marketing studies as a condition of drug approval. These so-called phase 4 studies are often designed to identify uncommon adverse events or further investigate a drug’s effectiveness. Unfortunately, completion of many of these studies is either significantly delayed or never happens. For instance, as of September 2005, the FDA reported that 65% of the 1231 “post-marketing” studies that companies had pledged to carry out were still pending and that many of these had not even been started.9
After the drug is released, the agency may collect post-marketing data on adverse events and ask the companies to participate in this effort. But no further studies can be required of the company.
Major complaints about phase 3 studies are 1) they are relatively small and often study patients who differ in terms of demographics and extent of disease from those who end up using the medicine; 2) they are placebo-controlled trials rather than comparison trials between the new drug and the current standard therapy; and 3) the FDA increasingly uses surrogate endpoints to judge efficacy, such as tumor shrinkage for cancer drugs or LDL cholesterol decrease for lipid therapy, instead of definitive outcomes like morbidity and mortality. To the extent these surrogate endpoints strongly correlate with major outcomes, this can appropriately speed up the approval process, but the value of surrogate markers is not always clear. These characteristics of phase 3 studies often lead to approval of many “me-too” drugs without the information necessary to decide whether they are better than current standard therapy.
Deficiencies in using surrogate markers
A particular problem with surrogate markers occurs with the review of higher-priority drugs. For these, the FDA may grant provisional approval on the basis of a surrogate measure of clinical benefit shown in a single, uncontrolled trial as long as the treatment addresses an unmet need for a serious medical illness. In return, the FDA requires the company to complete confirmatory trials in the post-approval period, and may withdraw approval of the drug if no benefit is shown in these phase 4 trials.
However, between 1996 and 2003, only 6 of 23 cancer drugs have gone through such post-marketing trials, and the FDA has not withdrawn approval for any of the 23 drugs. Furthermore, many of these drugs cost thousands of dollars per treatment and have low response rates, yet the FDA has not required companies to better define the target populations for their use.10
The problem with using surrogate endpoints in short trials has been demonstrated in cardiovascular medicine with the experience using antiarrhythmics to prevent premature ventricular contractions (later discovered to lead to increased mortality), inotropics to improve ejection fraction (no long-term benefit) and, most recently, with nesiritide (Natrecor) for heart failure. The latter drug was approved based on improved pulmonarycapillary wedge pressure and self-reported improvement in dyspnea. Subsequent research and analyses have shown increased rates of renal failure and death but only after hundreds of millions of dollars worth of treatments.11
Recommendations to make the system work
Given the controversy surrounding the release and marketing of medications that later have shown to cause problems, and given the array of real and perceived conflicts of interest in the approval process, the FDA has proposed establishing a Drug Safety Oversight Board that will include outside experts to review safety issues arising with new applications. Others have argued that such a board should be completely independent of the FDA so as to minimize conflicts of interest and potential agency interference with obtaining necessary information from the companies.
The FDA also recently announced a new rule to overhaul prescription drug labeling, information in package inserts, and some drug reference books. The labels are full of information but much of it is inconsequential or difficult to use for prescribing, and most physicians don’t read them. The new labels will list safety warnings, advise how to use the drug and dose it, and advise physicians on what patients should be told about the medication. The goal is to give physicians more useful information to make decisions about drug indications and dosing. While the new rule will not change the patient drug information sheets that pharmacists hand out, it may force consumer advertising to make clearer statements about medication risks. Finally, the rule will preempt state liability statutes, which upsets some trial lawyers and politicians.12
Outside experts have proposed additional ideas to improve the FDA’s work: eliminating the current drug company fees that support new drug reviews to minimize conflicts of interest, mandating post-approval studies to look for uncommon adverse events (phase 4 studies), providing more information on relative efficacy of new drugs compared with current medication treatment and attention to real clinical outcome end points, and prohibiting direct-to-consumer advertising in the first 3 years after a new drug is released.13
Considering the turmoil surrounding the FDA’s performance right now, it is very possible we will see some of these proposed changes come to pass. Given the importance of prescription medications, these changes will be very relevant to the practices of family physicians and their patients’ health.
CORRESPONDENCE
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
While often held in high esteem, the FDA has seen its reputation tarnished in recent years by adverse drug regulation experiences, apparent conflicts of interest, and problems with consistent agency leadership.
Examples of problems at the FDA
- The COX-2 inhibitor disaster that led to the withdrawal of rofecoxib (Vioxx) from the market due its association with adverse cardiovascular effects; a problem the company may have hidden from physicians and the public through manipulation of research reports.2
- The recent addition of black box warnings on selective serotonin reuptake inhibitor (SSRI) labels to warn of a potential association with suicidal behavior in adolescents after publicity that some FDA scientists were prevented from presenting this information to review panels.
- A plea bargain agreement in which Pfizer agreed to pay $430 million to resolve charges that Warner-Lambert, a company it took over, paid doctors to prescribe gabapentin (Neurontin) for off-label uses including bipolar disorder, attention deficit/hyperactivity disorder, and alcohol withdrawal seizures. Although physicians can prescribe drugs for whatever reason they choose, pharmaceutical companies are prohibited from promoting drugs for unapproved uses.3
- A lack of strong leadership with acting commissioners leading the agency 3 of the past 5 years. The last permanent commissioner resigned in September 2005 after 2 months in the position amidst accusations that he or his family had financial interests in some of the companies regulated by the agency.4
Given these and other negative events in conjunction with the dramatic increase in prescription drug use by Americans—up 60% in the past decade to 3.1 billion prescriptions in 2004 with reports of 375,000 adverse events—it’s not surprising that a recent survey showed the public wants a stronger FDA. In fact, two thirds support the creation of an independent oversight panel, 70% want the FDA to improve its gathering and reporting on possible harms of drugs and medical devices after their approval, and many believe that industry has too much influence over agency decisions.5
How the FDA performs its job matters very much to physicians, the public, and business. In recent months, outside experts and the FDA itself have proposed changes in how the agency handles new drug approvals that will affect all its stakeholders. Before reviewing those proposals, it’s worth understanding how the approval process works.
The Food and Drug Administration (FDA) is responsible for protecting the public health by assuring the safety and efficacy of human and veterinary drugs, biological products, medical devices, our nation’s food supply, cosmetics, and products that emit radiation—a list that accounts for at least 20% of consumer spending. It does this on a budget of about $1.7 billion and 10,000 employees. In comparison, the Department of Agriculture has a budget 50 times larger and a workforce 10 times larger.1
Speeding approval of new drugs
In the early 1990s, Congress responded to complaints about the FDA’s slow drug approval process, particularly regarding HIV drugs, by passing the Prescription Drug User Fee Act (PDUFA). The law mandated that pharmaceutical companies pay fees to the FDA that were then used by the agency to speed up the approval process by hiring more staff and adhering to a strict timetable for review. The PDUFA accomplished its goal; for instance, priority review drugs had their time to approval drop from a median of 14.9 months in 1993 to 6.7 months in 2003, and standard review drugs went from 27.2 months to 23.1 months during the same period.6 Ironically, in spite of the quicker approval process, in 2005, pharmaceutical companies had a record low number of FDA drug approvals—only 20 as opposed to 36 in 2004.7
As quality improvement experts say, however, you often get exactly the results your system was built for. Thus, it is not surprising that in recent years some FDA scientists have complained of increasing time pressures to perform reviews, increased pressure to approve drugs, and inability to communicate directly with the companies about the drugs they were reviewing so as to clarify study designs and data analyses.8
In addition, many observers believe that having the industry support the FDA budget (about $300 million/year) presents a potential conflict of interest for the agency, particularly in the current pro-business climate of the Bush administration.
Given these reports as well as the adverse drug safety events that have occurred recently, Congress and independent scientists are now calling for more attention to safety and less focus on the approval timetable.
How the drug approval process works
Companies obtain FDA approval for human trials after promising results from animal trials. Human trials begin with phase 1 trials, which are small studies looking at safety issues in healthy volunteers. Phase 2 studies are trials of safety and efficacy (how well the drug works) in patients with the target condition. If these prove successful, phase 3 studies are undertaken, which include at least 2 large randomized control trials of safety and efficacy. After these studies, which may involve 2000 to 5000 patients at most, the FDA can grant approval for the drug to be sold to the public.
The agency may require a company to conduct additional post-marketing studies as a condition of drug approval. These so-called phase 4 studies are often designed to identify uncommon adverse events or further investigate a drug’s effectiveness. Unfortunately, completion of many of these studies is either significantly delayed or never happens. For instance, as of September 2005, the FDA reported that 65% of the 1231 “post-marketing” studies that companies had pledged to carry out were still pending and that many of these had not even been started.9
After the drug is released, the agency may collect post-marketing data on adverse events and ask the companies to participate in this effort. But no further studies can be required of the company.
Major complaints about phase 3 studies are 1) they are relatively small and often study patients who differ in terms of demographics and extent of disease from those who end up using the medicine; 2) they are placebo-controlled trials rather than comparison trials between the new drug and the current standard therapy; and 3) the FDA increasingly uses surrogate endpoints to judge efficacy, such as tumor shrinkage for cancer drugs or LDL cholesterol decrease for lipid therapy, instead of definitive outcomes like morbidity and mortality. To the extent these surrogate endpoints strongly correlate with major outcomes, this can appropriately speed up the approval process, but the value of surrogate markers is not always clear. These characteristics of phase 3 studies often lead to approval of many “me-too” drugs without the information necessary to decide whether they are better than current standard therapy.
Deficiencies in using surrogate markers
A particular problem with surrogate markers occurs with the review of higher-priority drugs. For these, the FDA may grant provisional approval on the basis of a surrogate measure of clinical benefit shown in a single, uncontrolled trial as long as the treatment addresses an unmet need for a serious medical illness. In return, the FDA requires the company to complete confirmatory trials in the post-approval period, and may withdraw approval of the drug if no benefit is shown in these phase 4 trials.
However, between 1996 and 2003, only 6 of 23 cancer drugs have gone through such post-marketing trials, and the FDA has not withdrawn approval for any of the 23 drugs. Furthermore, many of these drugs cost thousands of dollars per treatment and have low response rates, yet the FDA has not required companies to better define the target populations for their use.10
The problem with using surrogate endpoints in short trials has been demonstrated in cardiovascular medicine with the experience using antiarrhythmics to prevent premature ventricular contractions (later discovered to lead to increased mortality), inotropics to improve ejection fraction (no long-term benefit) and, most recently, with nesiritide (Natrecor) for heart failure. The latter drug was approved based on improved pulmonarycapillary wedge pressure and self-reported improvement in dyspnea. Subsequent research and analyses have shown increased rates of renal failure and death but only after hundreds of millions of dollars worth of treatments.11
Recommendations to make the system work
Given the controversy surrounding the release and marketing of medications that later have shown to cause problems, and given the array of real and perceived conflicts of interest in the approval process, the FDA has proposed establishing a Drug Safety Oversight Board that will include outside experts to review safety issues arising with new applications. Others have argued that such a board should be completely independent of the FDA so as to minimize conflicts of interest and potential agency interference with obtaining necessary information from the companies.
The FDA also recently announced a new rule to overhaul prescription drug labeling, information in package inserts, and some drug reference books. The labels are full of information but much of it is inconsequential or difficult to use for prescribing, and most physicians don’t read them. The new labels will list safety warnings, advise how to use the drug and dose it, and advise physicians on what patients should be told about the medication. The goal is to give physicians more useful information to make decisions about drug indications and dosing. While the new rule will not change the patient drug information sheets that pharmacists hand out, it may force consumer advertising to make clearer statements about medication risks. Finally, the rule will preempt state liability statutes, which upsets some trial lawyers and politicians.12
Outside experts have proposed additional ideas to improve the FDA’s work: eliminating the current drug company fees that support new drug reviews to minimize conflicts of interest, mandating post-approval studies to look for uncommon adverse events (phase 4 studies), providing more information on relative efficacy of new drugs compared with current medication treatment and attention to real clinical outcome end points, and prohibiting direct-to-consumer advertising in the first 3 years after a new drug is released.13
Considering the turmoil surrounding the FDA’s performance right now, it is very possible we will see some of these proposed changes come to pass. Given the importance of prescription medications, these changes will be very relevant to the practices of family physicians and their patients’ health.
CORRESPONDENCE
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Slater E. Today’s FDA. N Engl J Med 2005;352:293-297.
2. Curfman GD, Morrissey S, Drazen JM. Expression of concern: Bombardier et al., “Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis,” N Engl J Med 2000; 343:1520-8. Editorial. N Engl J Med 2005;353:2813-2814.
3. Harris G. Pfizer to pay $420 million in illegal marketing case. New York Times, May 14, 2004.
4. Markel H. Why America needs a strong FDA. JAMA 2005;294:2489-2491.
5. Kaiser Daily Health Policy Report. March 3, 2005. Available at: www.kaisernetwork.org/daily_reports/rep_index.cfm?hint=3&DR_ID=28455. Accessed on March 10, 2006.
6. Okie S. What ails the FDA. N Engl J Med 2005;352:1063-1066.
7. Berenson A. Drugs in ’05: Much promise, little payoff; just 20 new products are approved despite biotechnology’s hope. New York Times, Jan 11, 2006.
8. FDA’s review process for new drug applications. Office of Inspector General. March 2003. Available at: www.oig.hhs.gov/oei/reports/oei-01-01-00590.pdf. Accessed on March 10, 2006.
9. 65% of promised drug studies pending. Washington Post, March 4, 2006 (AP report)
10. Roberts T, Chabner B. Beyond fast track for drug approvals. N Engl J Med 2004;351:501-505.
11. Topol E. Nesiritide—not verified. N Engl J Med 2005;353:113-116.
12. Harris G. New drug label rule is intended to reduce medical errors. New York Times, January 19, 2006.
13. Ray W, Stein CM. Reform of drug regulation—beyond an independent drug-safety board. N Engl J Med 2006;354:194-201.
1. Slater E. Today’s FDA. N Engl J Med 2005;352:293-297.
2. Curfman GD, Morrissey S, Drazen JM. Expression of concern: Bombardier et al., “Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis,” N Engl J Med 2000; 343:1520-8. Editorial. N Engl J Med 2005;353:2813-2814.
3. Harris G. Pfizer to pay $420 million in illegal marketing case. New York Times, May 14, 2004.
4. Markel H. Why America needs a strong FDA. JAMA 2005;294:2489-2491.
5. Kaiser Daily Health Policy Report. March 3, 2005. Available at: www.kaisernetwork.org/daily_reports/rep_index.cfm?hint=3&DR_ID=28455. Accessed on March 10, 2006.
6. Okie S. What ails the FDA. N Engl J Med 2005;352:1063-1066.
7. Berenson A. Drugs in ’05: Much promise, little payoff; just 20 new products are approved despite biotechnology’s hope. New York Times, Jan 11, 2006.
8. FDA’s review process for new drug applications. Office of Inspector General. March 2003. Available at: www.oig.hhs.gov/oei/reports/oei-01-01-00590.pdf. Accessed on March 10, 2006.
9. 65% of promised drug studies pending. Washington Post, March 4, 2006 (AP report)
10. Roberts T, Chabner B. Beyond fast track for drug approvals. N Engl J Med 2004;351:501-505.
11. Topol E. Nesiritide—not verified. N Engl J Med 2005;353:113-116.
12. Harris G. New drug label rule is intended to reduce medical errors. New York Times, January 19, 2006.
13. Ray W, Stein CM. Reform of drug regulation—beyond an independent drug-safety board. N Engl J Med 2006;354:194-201.
Medicare prescription drug bill: Resources to inform and equip your patients
On November 15, 2005, enrollment opened for Medicare’s new prescription drug program. You have most likely been asked by patients for help in understanding the complexity of the options. No small task. Though there is a lot to disagree with in the way the Medicare drug benefit has been crafted, it’s what we now have and many beneficiaries can benefit from it. In addition to knowing which agencies in your communities serve seniors, you can better equip your elderly patients to make decisions by becoming familiar with web sites and other resources listed in this article.
Confusion reigns
By May 15, 2006, when the initial enrollment period ends, the government predicts that over 29 million of 43 million eligible beneficiaries will have signed up for the new benefit, with another 9 to 10 million beneficiaries maintaining their drug coverage through an employer-sponsored plan (FIGURE 1).
Whether these predictions come to pass is an open question. A Kaiser Family Foundation survey from late October 2005 found that 60% of senior citizens did not understand the benefit and 50% thought it would not help them. Of those surveyed, 43% did not know whether they would enroll, 37% said they would not enroll, and only 20% said they would definitely enroll.
One problem for seniors is that unlike the traditional Medicare program in which there are only 2 choices—whether to sign up for the traditional fee-for-service plan or a managed care plan—the new drug plan is administered by a large number of private plans that cover different medications and charge different prices for them. Seniors who said they understood the drug benefit (a minority) were more likely to view the program favorably. Perhaps most relevant for physicians is that seniors said they would likely turn to the Medicare program (33%), their personal doctor (32%), or their pharmacist (25%) for assistance.1
A quick review
Readers may recall that the prescription drug benefit portion of the Medicare Modernization Act of 2003 includes a premium (current national average of $32/month), an annual deductible, copays, and the infamous “donut hole” (FIGURE 2).2
With the program beginning in January 2006, however, there is more to keep in mind than the costs to beneficiaries: for example, who will be offering the benefit, what medications will be covered (formularies), and the effect on dual-eligibles (beneficiaries covered by both Medicare and Medicaid), so-called “Medigap” policyholders, and low-income beneficiaries.
FIGURE 1
Estimated Medicare prescription drug benefit participation, 2006
* “Others” not enrolled includes federal retirees with drug coverage through FEHBP or TRICARE, and those who lack drug coverage.
† “Other low-income” includes non-dual-eligibles with incomes <150% FPL.
Source: HHS OACT, MMA final rule, January 2005.
FIGURE 2
Standard Medicare prescription drug benefit, 2006
* Annual amount based on $32.20 national average beneficiary premium (CMS, August 2005)
Source: Kaiser Family Foundation illustration of standard Medicare drug benefit described in the Medicare Modernization Act of 2003
Who offers the plans
Medicare beneficiaries can obtain the drug benefit in either of 2 ways: through a stand-alone prescription drug plan (PDP) that covers only drugs (with the usual medical benefits obtained through the traditional Medicare program), or through a Medicare Advantage plan (MA) that is essentially a managed care plan, providing drug coverage and medical benefits in place of the traditional Medicare program.
The Bush administration has long promoted MA plans as a way to better control Medicare costs, even though the federal government currently spends more per MA beneficiary than for beneficiaries in traditional Medicare. MA plans can offer additional benefits and adjust premiums to attract customers—drug benefits for a lower premium, vision benefits, and dental benefits. However, there may be limits on using providers outside the MA plan’s network of physicians and hospitals.3
Drug plans must cover at least 2 drugs in each therapeutic class approved by Medicare, but they may use tiered cost-sharing (eg, generics and brand-name drugs in different tiers) and other management tools as long as they meet the minimum requirements of the overall bill. The decision by the White House and Congress to approve the privatization of the drug benefit has led to the current situation in which multiple private plans are competing for enrollment in each geographic region and offering different drugs for different prices. For instance, in my county, there are 7 MA plans and 43 PDP plans being offered. This makes the system overly complex and confusing. In addition, the Medicare Modernization Act allows plans to increase the copays or even end coverage of specific drugs with 60 days notice.
Dual eligibles and low-income beneficiaries
The Medicare Modernization Act provided additional assistance to persons of limited means—those currently covered by Medicare and Medicaid plans who receive their medications through Medicaid (the dual-eligibles) and those who have limited income and resources but are only covered by Medicare. The former group will automatically be enrolled into PDPs if they do not sign up on their own, and they will pay reduced fees for their medications. The states, in turn, will reimburse the federal government for the drug cost savings gained by their Medicaid programs.
Other low-income individuals may also be eligible for drug benefit subsidies based on their income and assets (TABLE). Clearly, the Medicare Modernization Act offers significant drug benefits to beneficiaries of limited means. The Centers for Medicare and Medicaid Services (CMS) projects that 10.9 million beneficiaries will receive low-income subsidies out of 14.5 million eligible.
TABLE
Medicare prescription drug benefit subsidies for low-income beneficiaries, 2006
| LOW-INCOME SUBSIDY LEVEL | PREMIUM | MONTHLY DEDUCTIBLE | ANNUAL COPAYMENTS |
|---|---|---|---|
| Full-benefit dual eligibles Income <100% of poverty ($9750 individual; $12,830/couple) | $0 | $0 | $1/generic, $3/brand-name; no copays after total drug spending reaches $5100 |
| Full-benefit dual eligibles Income ≥100% of poverty | $0 | $0 | $2/generic, $5/brand-name; no copays after total drug spending reaches $5100 |
| Institutionalized full-benefit dual eligibles | $0 | $0 | No copays |
| Individuals with income <135% of poverty ($12,920 individuals, $17,321/couple) and assets <$6000/individual; $9000/couple | $0 | $0 | $2/generic, $5/brand-name; no copays after total drug spending reaches $5100 |
| Individuals with income 135%–150% of poverty ($12,920–$14,355 individuals, $17,321–$19,245/couple) and assets <$10,000/individual, $20,000/couple | Sliding scale up to $32.20* | $50 | 15% of total costs up to $5100; $2/generic, $5/brand-name thereafter |
| Note: Poverty-level dollar amounts are for 2005. Additional assests of up to $1500/individual and $3000/couple for funeral or burial expenses are permitted. *$32.20 is the national monthly Part D base beneficiary premium for 2006. | |||
| Source: Kaiser Family Foundation summary of Medicare prescription drug benefit low-income subsidies in 2006. | |||
Medigap and employer-sponsored plans
Many current beneficiaries have Medigap insurance policies, which cover part or all of the financial holes in the traditional Medicare plan—eg, deductibles, copays, and other benefits such as drug coverage. Beginning in January 2006, new policies that include drug coverage can no longer be issued. Policyholders can keep their current Medigap policies that cover medications; however, these are generally not considered equivalent to the new coverage. In addition, Medicare will provide subsidies to employers to encourage them to continue any current retiree plans that provide drug coverage comparable to the new plans.4
Enrollment
While the new drug plans start on January 1, 2006, the initial enrollment period runs until May 15, 2006. Beneficiaries who enroll after that time and do not currently have drug coverage as good as the new Medicare drug benefit will pay a higher premium equal to 1% of the average monthly premium for each month they delay enrollment. Those who enroll may change plans one time between December 31, 2005 and May 15, 2006. After May 15, the next enrollment period will be from November 15 to December 31, 2006. Any enrollee can change plans during that time.
In order to assist beneficiaries in making a decision about whether to enroll in a Medicare drug plan and which to choose, the federal government, assisted by a number of medical organizations (such as the AAFP) and nonprofits like the local Area Agencies on Aging, is providing seniors with information in a variety of formats. Beneficiaries should all have received a booklet, “Medicare and You,” in October 2005. There is a 24-hour telephone help line, 1-800-MEDICARE, that has automated answers and can provide access to a real person.
Finally, there is the Internet: www.medicare.gov. While 3 of 4 seniors have never been online, this is the best method to locate available plans in your area, find out which specific medications are included in each plan, and try to compare costs.5 For many seniors, it will be worth asking family members, friends, or community agencies for help in navigating the web site and the information it contains.
CORRESPONDENCE
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Kaiser Family Foundation news release. Available at: www.kff.org/kaiserpolls/med111005nr.cfm. Accessed on November 21, 2005.
2. Henley E. What the new Medicare prescription drug bill may mean for providers and patients. J Fam Pract 2004;53:389-392.
3. Fuhrmans V, Lueck S. Insurers sweeten health plans for seniors. Wall Street Journal, November 8, 2005.
4. The Medicare Prescription Drug Benefit. Kaiser Family Foundation. Available at: www.kff.org/medicare/7044-02.cfm. Accessed on December 4, 2005.
5. Glendinning D. Patients look to doctors for help on Medicare drug plans. AMA News, December 5, 2005.
On November 15, 2005, enrollment opened for Medicare’s new prescription drug program. You have most likely been asked by patients for help in understanding the complexity of the options. No small task. Though there is a lot to disagree with in the way the Medicare drug benefit has been crafted, it’s what we now have and many beneficiaries can benefit from it. In addition to knowing which agencies in your communities serve seniors, you can better equip your elderly patients to make decisions by becoming familiar with web sites and other resources listed in this article.
Confusion reigns
By May 15, 2006, when the initial enrollment period ends, the government predicts that over 29 million of 43 million eligible beneficiaries will have signed up for the new benefit, with another 9 to 10 million beneficiaries maintaining their drug coverage through an employer-sponsored plan (FIGURE 1).
Whether these predictions come to pass is an open question. A Kaiser Family Foundation survey from late October 2005 found that 60% of senior citizens did not understand the benefit and 50% thought it would not help them. Of those surveyed, 43% did not know whether they would enroll, 37% said they would not enroll, and only 20% said they would definitely enroll.
One problem for seniors is that unlike the traditional Medicare program in which there are only 2 choices—whether to sign up for the traditional fee-for-service plan or a managed care plan—the new drug plan is administered by a large number of private plans that cover different medications and charge different prices for them. Seniors who said they understood the drug benefit (a minority) were more likely to view the program favorably. Perhaps most relevant for physicians is that seniors said they would likely turn to the Medicare program (33%), their personal doctor (32%), or their pharmacist (25%) for assistance.1
A quick review
Readers may recall that the prescription drug benefit portion of the Medicare Modernization Act of 2003 includes a premium (current national average of $32/month), an annual deductible, copays, and the infamous “donut hole” (FIGURE 2).2
With the program beginning in January 2006, however, there is more to keep in mind than the costs to beneficiaries: for example, who will be offering the benefit, what medications will be covered (formularies), and the effect on dual-eligibles (beneficiaries covered by both Medicare and Medicaid), so-called “Medigap” policyholders, and low-income beneficiaries.
FIGURE 1
Estimated Medicare prescription drug benefit participation, 2006
* “Others” not enrolled includes federal retirees with drug coverage through FEHBP or TRICARE, and those who lack drug coverage.
† “Other low-income” includes non-dual-eligibles with incomes <150% FPL.
Source: HHS OACT, MMA final rule, January 2005.
FIGURE 2
Standard Medicare prescription drug benefit, 2006
* Annual amount based on $32.20 national average beneficiary premium (CMS, August 2005)
Source: Kaiser Family Foundation illustration of standard Medicare drug benefit described in the Medicare Modernization Act of 2003
Who offers the plans
Medicare beneficiaries can obtain the drug benefit in either of 2 ways: through a stand-alone prescription drug plan (PDP) that covers only drugs (with the usual medical benefits obtained through the traditional Medicare program), or through a Medicare Advantage plan (MA) that is essentially a managed care plan, providing drug coverage and medical benefits in place of the traditional Medicare program.
The Bush administration has long promoted MA plans as a way to better control Medicare costs, even though the federal government currently spends more per MA beneficiary than for beneficiaries in traditional Medicare. MA plans can offer additional benefits and adjust premiums to attract customers—drug benefits for a lower premium, vision benefits, and dental benefits. However, there may be limits on using providers outside the MA plan’s network of physicians and hospitals.3
Drug plans must cover at least 2 drugs in each therapeutic class approved by Medicare, but they may use tiered cost-sharing (eg, generics and brand-name drugs in different tiers) and other management tools as long as they meet the minimum requirements of the overall bill. The decision by the White House and Congress to approve the privatization of the drug benefit has led to the current situation in which multiple private plans are competing for enrollment in each geographic region and offering different drugs for different prices. For instance, in my county, there are 7 MA plans and 43 PDP plans being offered. This makes the system overly complex and confusing. In addition, the Medicare Modernization Act allows plans to increase the copays or even end coverage of specific drugs with 60 days notice.
Dual eligibles and low-income beneficiaries
The Medicare Modernization Act provided additional assistance to persons of limited means—those currently covered by Medicare and Medicaid plans who receive their medications through Medicaid (the dual-eligibles) and those who have limited income and resources but are only covered by Medicare. The former group will automatically be enrolled into PDPs if they do not sign up on their own, and they will pay reduced fees for their medications. The states, in turn, will reimburse the federal government for the drug cost savings gained by their Medicaid programs.
Other low-income individuals may also be eligible for drug benefit subsidies based on their income and assets (TABLE). Clearly, the Medicare Modernization Act offers significant drug benefits to beneficiaries of limited means. The Centers for Medicare and Medicaid Services (CMS) projects that 10.9 million beneficiaries will receive low-income subsidies out of 14.5 million eligible.
TABLE
Medicare prescription drug benefit subsidies for low-income beneficiaries, 2006
| LOW-INCOME SUBSIDY LEVEL | PREMIUM | MONTHLY DEDUCTIBLE | ANNUAL COPAYMENTS |
|---|---|---|---|
| Full-benefit dual eligibles Income <100% of poverty ($9750 individual; $12,830/couple) | $0 | $0 | $1/generic, $3/brand-name; no copays after total drug spending reaches $5100 |
| Full-benefit dual eligibles Income ≥100% of poverty | $0 | $0 | $2/generic, $5/brand-name; no copays after total drug spending reaches $5100 |
| Institutionalized full-benefit dual eligibles | $0 | $0 | No copays |
| Individuals with income <135% of poverty ($12,920 individuals, $17,321/couple) and assets <$6000/individual; $9000/couple | $0 | $0 | $2/generic, $5/brand-name; no copays after total drug spending reaches $5100 |
| Individuals with income 135%–150% of poverty ($12,920–$14,355 individuals, $17,321–$19,245/couple) and assets <$10,000/individual, $20,000/couple | Sliding scale up to $32.20* | $50 | 15% of total costs up to $5100; $2/generic, $5/brand-name thereafter |
| Note: Poverty-level dollar amounts are for 2005. Additional assests of up to $1500/individual and $3000/couple for funeral or burial expenses are permitted. *$32.20 is the national monthly Part D base beneficiary premium for 2006. | |||
| Source: Kaiser Family Foundation summary of Medicare prescription drug benefit low-income subsidies in 2006. | |||
Medigap and employer-sponsored plans
Many current beneficiaries have Medigap insurance policies, which cover part or all of the financial holes in the traditional Medicare plan—eg, deductibles, copays, and other benefits such as drug coverage. Beginning in January 2006, new policies that include drug coverage can no longer be issued. Policyholders can keep their current Medigap policies that cover medications; however, these are generally not considered equivalent to the new coverage. In addition, Medicare will provide subsidies to employers to encourage them to continue any current retiree plans that provide drug coverage comparable to the new plans.4
Enrollment
While the new drug plans start on January 1, 2006, the initial enrollment period runs until May 15, 2006. Beneficiaries who enroll after that time and do not currently have drug coverage as good as the new Medicare drug benefit will pay a higher premium equal to 1% of the average monthly premium for each month they delay enrollment. Those who enroll may change plans one time between December 31, 2005 and May 15, 2006. After May 15, the next enrollment period will be from November 15 to December 31, 2006. Any enrollee can change plans during that time.
In order to assist beneficiaries in making a decision about whether to enroll in a Medicare drug plan and which to choose, the federal government, assisted by a number of medical organizations (such as the AAFP) and nonprofits like the local Area Agencies on Aging, is providing seniors with information in a variety of formats. Beneficiaries should all have received a booklet, “Medicare and You,” in October 2005. There is a 24-hour telephone help line, 1-800-MEDICARE, that has automated answers and can provide access to a real person.
Finally, there is the Internet: www.medicare.gov. While 3 of 4 seniors have never been online, this is the best method to locate available plans in your area, find out which specific medications are included in each plan, and try to compare costs.5 For many seniors, it will be worth asking family members, friends, or community agencies for help in navigating the web site and the information it contains.
CORRESPONDENCE
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
On November 15, 2005, enrollment opened for Medicare’s new prescription drug program. You have most likely been asked by patients for help in understanding the complexity of the options. No small task. Though there is a lot to disagree with in the way the Medicare drug benefit has been crafted, it’s what we now have and many beneficiaries can benefit from it. In addition to knowing which agencies in your communities serve seniors, you can better equip your elderly patients to make decisions by becoming familiar with web sites and other resources listed in this article.
Confusion reigns
By May 15, 2006, when the initial enrollment period ends, the government predicts that over 29 million of 43 million eligible beneficiaries will have signed up for the new benefit, with another 9 to 10 million beneficiaries maintaining their drug coverage through an employer-sponsored plan (FIGURE 1).
Whether these predictions come to pass is an open question. A Kaiser Family Foundation survey from late October 2005 found that 60% of senior citizens did not understand the benefit and 50% thought it would not help them. Of those surveyed, 43% did not know whether they would enroll, 37% said they would not enroll, and only 20% said they would definitely enroll.
One problem for seniors is that unlike the traditional Medicare program in which there are only 2 choices—whether to sign up for the traditional fee-for-service plan or a managed care plan—the new drug plan is administered by a large number of private plans that cover different medications and charge different prices for them. Seniors who said they understood the drug benefit (a minority) were more likely to view the program favorably. Perhaps most relevant for physicians is that seniors said they would likely turn to the Medicare program (33%), their personal doctor (32%), or their pharmacist (25%) for assistance.1
A quick review
Readers may recall that the prescription drug benefit portion of the Medicare Modernization Act of 2003 includes a premium (current national average of $32/month), an annual deductible, copays, and the infamous “donut hole” (FIGURE 2).2
With the program beginning in January 2006, however, there is more to keep in mind than the costs to beneficiaries: for example, who will be offering the benefit, what medications will be covered (formularies), and the effect on dual-eligibles (beneficiaries covered by both Medicare and Medicaid), so-called “Medigap” policyholders, and low-income beneficiaries.
FIGURE 1
Estimated Medicare prescription drug benefit participation, 2006
* “Others” not enrolled includes federal retirees with drug coverage through FEHBP or TRICARE, and those who lack drug coverage.
† “Other low-income” includes non-dual-eligibles with incomes <150% FPL.
Source: HHS OACT, MMA final rule, January 2005.
FIGURE 2
Standard Medicare prescription drug benefit, 2006
* Annual amount based on $32.20 national average beneficiary premium (CMS, August 2005)
Source: Kaiser Family Foundation illustration of standard Medicare drug benefit described in the Medicare Modernization Act of 2003
Who offers the plans
Medicare beneficiaries can obtain the drug benefit in either of 2 ways: through a stand-alone prescription drug plan (PDP) that covers only drugs (with the usual medical benefits obtained through the traditional Medicare program), or through a Medicare Advantage plan (MA) that is essentially a managed care plan, providing drug coverage and medical benefits in place of the traditional Medicare program.
The Bush administration has long promoted MA plans as a way to better control Medicare costs, even though the federal government currently spends more per MA beneficiary than for beneficiaries in traditional Medicare. MA plans can offer additional benefits and adjust premiums to attract customers—drug benefits for a lower premium, vision benefits, and dental benefits. However, there may be limits on using providers outside the MA plan’s network of physicians and hospitals.3
Drug plans must cover at least 2 drugs in each therapeutic class approved by Medicare, but they may use tiered cost-sharing (eg, generics and brand-name drugs in different tiers) and other management tools as long as they meet the minimum requirements of the overall bill. The decision by the White House and Congress to approve the privatization of the drug benefit has led to the current situation in which multiple private plans are competing for enrollment in each geographic region and offering different drugs for different prices. For instance, in my county, there are 7 MA plans and 43 PDP plans being offered. This makes the system overly complex and confusing. In addition, the Medicare Modernization Act allows plans to increase the copays or even end coverage of specific drugs with 60 days notice.
Dual eligibles and low-income beneficiaries
The Medicare Modernization Act provided additional assistance to persons of limited means—those currently covered by Medicare and Medicaid plans who receive their medications through Medicaid (the dual-eligibles) and those who have limited income and resources but are only covered by Medicare. The former group will automatically be enrolled into PDPs if they do not sign up on their own, and they will pay reduced fees for their medications. The states, in turn, will reimburse the federal government for the drug cost savings gained by their Medicaid programs.
Other low-income individuals may also be eligible for drug benefit subsidies based on their income and assets (TABLE). Clearly, the Medicare Modernization Act offers significant drug benefits to beneficiaries of limited means. The Centers for Medicare and Medicaid Services (CMS) projects that 10.9 million beneficiaries will receive low-income subsidies out of 14.5 million eligible.
TABLE
Medicare prescription drug benefit subsidies for low-income beneficiaries, 2006
| LOW-INCOME SUBSIDY LEVEL | PREMIUM | MONTHLY DEDUCTIBLE | ANNUAL COPAYMENTS |
|---|---|---|---|
| Full-benefit dual eligibles Income <100% of poverty ($9750 individual; $12,830/couple) | $0 | $0 | $1/generic, $3/brand-name; no copays after total drug spending reaches $5100 |
| Full-benefit dual eligibles Income ≥100% of poverty | $0 | $0 | $2/generic, $5/brand-name; no copays after total drug spending reaches $5100 |
| Institutionalized full-benefit dual eligibles | $0 | $0 | No copays |
| Individuals with income <135% of poverty ($12,920 individuals, $17,321/couple) and assets <$6000/individual; $9000/couple | $0 | $0 | $2/generic, $5/brand-name; no copays after total drug spending reaches $5100 |
| Individuals with income 135%–150% of poverty ($12,920–$14,355 individuals, $17,321–$19,245/couple) and assets <$10,000/individual, $20,000/couple | Sliding scale up to $32.20* | $50 | 15% of total costs up to $5100; $2/generic, $5/brand-name thereafter |
| Note: Poverty-level dollar amounts are for 2005. Additional assests of up to $1500/individual and $3000/couple for funeral or burial expenses are permitted. *$32.20 is the national monthly Part D base beneficiary premium for 2006. | |||
| Source: Kaiser Family Foundation summary of Medicare prescription drug benefit low-income subsidies in 2006. | |||
Medigap and employer-sponsored plans
Many current beneficiaries have Medigap insurance policies, which cover part or all of the financial holes in the traditional Medicare plan—eg, deductibles, copays, and other benefits such as drug coverage. Beginning in January 2006, new policies that include drug coverage can no longer be issued. Policyholders can keep their current Medigap policies that cover medications; however, these are generally not considered equivalent to the new coverage. In addition, Medicare will provide subsidies to employers to encourage them to continue any current retiree plans that provide drug coverage comparable to the new plans.4
Enrollment
While the new drug plans start on January 1, 2006, the initial enrollment period runs until May 15, 2006. Beneficiaries who enroll after that time and do not currently have drug coverage as good as the new Medicare drug benefit will pay a higher premium equal to 1% of the average monthly premium for each month they delay enrollment. Those who enroll may change plans one time between December 31, 2005 and May 15, 2006. After May 15, the next enrollment period will be from November 15 to December 31, 2006. Any enrollee can change plans during that time.
In order to assist beneficiaries in making a decision about whether to enroll in a Medicare drug plan and which to choose, the federal government, assisted by a number of medical organizations (such as the AAFP) and nonprofits like the local Area Agencies on Aging, is providing seniors with information in a variety of formats. Beneficiaries should all have received a booklet, “Medicare and You,” in October 2005. There is a 24-hour telephone help line, 1-800-MEDICARE, that has automated answers and can provide access to a real person.
Finally, there is the Internet: www.medicare.gov. While 3 of 4 seniors have never been online, this is the best method to locate available plans in your area, find out which specific medications are included in each plan, and try to compare costs.5 For many seniors, it will be worth asking family members, friends, or community agencies for help in navigating the web site and the information it contains.
CORRESPONDENCE
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Kaiser Family Foundation news release. Available at: www.kff.org/kaiserpolls/med111005nr.cfm. Accessed on November 21, 2005.
2. Henley E. What the new Medicare prescription drug bill may mean for providers and patients. J Fam Pract 2004;53:389-392.
3. Fuhrmans V, Lueck S. Insurers sweeten health plans for seniors. Wall Street Journal, November 8, 2005.
4. The Medicare Prescription Drug Benefit. Kaiser Family Foundation. Available at: www.kff.org/medicare/7044-02.cfm. Accessed on December 4, 2005.
5. Glendinning D. Patients look to doctors for help on Medicare drug plans. AMA News, December 5, 2005.
1. Kaiser Family Foundation news release. Available at: www.kff.org/kaiserpolls/med111005nr.cfm. Accessed on November 21, 2005.
2. Henley E. What the new Medicare prescription drug bill may mean for providers and patients. J Fam Pract 2004;53:389-392.
3. Fuhrmans V, Lueck S. Insurers sweeten health plans for seniors. Wall Street Journal, November 8, 2005.
4. The Medicare Prescription Drug Benefit. Kaiser Family Foundation. Available at: www.kff.org/medicare/7044-02.cfm. Accessed on December 4, 2005.
5. Glendinning D. Patients look to doctors for help on Medicare drug plans. AMA News, December 5, 2005.
The uninsured: You can make a difference
Family physicians have always done what they can to care for those whose means of obtaining health care are inadequate. Such generosity has enriched, and literally saved, many persons’ lives. Sadly, though, the number of US citizens unable to pay for health care is increasing, and individual physician goodwill will not solve the problem. A comprehensive solution is needed, and that depends on restoring health to the currently paralyzed national political process.
If you are inclined to become more involved in pursuing efforts to address the issue in political, social, and educational spheres, you will find help in this article, which summarizes the problem and reviews health policy options being considered and their implications for our patients. You will also find resources through which you can make your voice heard.
Scope of the problem
The US is the only country in the developed world without a national program for health insurance for all its citizens. Though we spend over $1.7 trillion on health care, the number of uninsured people in 2004 was about 46 million, almost 16% of the population. In 2000, 40 million people (14%) were uninsured.1 The premise of consumer-driven care is that patients who pay more of the true cost of their care will become more prudent purchasers of health services.
Critics argue that increasing out-of-pocket costs (deductibles and co-pays) acts as a deterrent to seeking appropriate care, particularly for the less well off; that wealthy and healthy people will purchase these policies, thus increasing the costs of caring for those left in the traditional insurance system; and that such a system preserves the inefficient and wasteful private insurance industry.
Government as the single payer, not employer, of health care. Proponents argue that administrative cost savings in such a system (no or minimal private health insurance) will more than cover the cost of care for the uninsured.
Web resources for information on the uninsured and other health policy topics:
Kaiser Family Foundation: www.kff.org
Cover the Uninsured campaign: www.covertheuninsured.org
Health policy for students and faculty: www.KaiserEdu.org
Physicians for a National Health Plan (single-payer advocates): www.pnhp.org
Opponents of this idea argue it will lead to long lines for specialty care (read: Britain and Canada) and stifle innovation. Considering the US spends almost 50% more of its GDP on health care than does Canada and almost double what Britain spends, it seems unlikely our experience will parallel those countries. Ironically, in a system such as Canada’s, where most care is delivered by private practice physicians, there is almost certainly less administrative burden on physicians than they currently experience in the US.
A system of health care vouchers as recently proposed by Emanuel and Fuchs. Their plan would preserve the private insurance system, phase out Medicare, Medicaid, and employer-based insurance, include Federal oversight of the benefit package and technology assessment, and be funded by an earmarked value-added tax. It attempts to achieve administrative savings and a more equitable system of care, while not challenging the current substantial role of the private insurance sector. Pay-for-performance: What can you expect?”, J Fam Pract 2005; 54[7]:609) exemplify this effort. Certainly, efforts at improving care quality are worthwhile as our health system has too much practice variability as well as over- and underutilization of care. However, it is difficult to believe that such efforts will control costs enough to allow for the expansion of health insurance rates.
Where to go from here
Given past failures at expanding health care to all, many observers are resigned to the incrementalist approach such as expanding small business insurance by pooling risk, perhaps controlling costs through consumer-driven care and pay-for-performance programs, and dealing more effectively with malpractice problems (high premiums and defensive medicine). Most would agree, however, that such efforts will not make a serious dent in the uninsured problem.
The continual increase in the number of uninsured in a country as wealthy as ours is a national tragedy and is tremendously frustrating, particularly for family physicians who constantly see the negative health effects. Physicians need to become better informed about health policy and its implications for our patients and more actively work in political, social, and educational spheres to help move the political process out of its current paralysis to address the uninsured problem.
For information on the uninsured and other health policy topics, and for adding your voice to the debate, see Web resources.
CORRESPONDING AUTHOR
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Finkelstein J. 45.8 million now uninsured. AMA News, September 19 2005;1-2.
2. Rhoades J. The uninsured in America, 2004. Statistical Brief #83. Available at: www.meps.ahrq.gov/papers/st83/stat83.pdf.
3. Hoffman C, Wang M. Health Insurance Coverage in America. 2002 Update. Washington, DC: Kaiser Commission on Medicaid and the Uninsured; 2002.
4. Finkelstein J. Many workers lack insurance, report shows. AMA News, May 16, 2005;5.-
5. Gabel J, Claxton G, Gil I, et al. Health benefits in 2004: four years of double-digit premium increases take their toll on coverage. Health Aff (Millwood) 2004;23:200-209.
6. Institute of Medicine. Care without Coverage: Too Little, Too Late. Washington, DC: National Academies Press; 2002.
7. Himmelstein D, Warren E, Thorn D, Woolhandler Sl. Health Aff (Millwood) 2005;W5 63-73.Feb 2, 2005.
8. Paying a premium: The added cost of care for the uninsured. A Report from Families USA. June 2005. Available at: www.familiesusa.org/site/PageServer?pagename=Paing_a_Premium_Findings. Accessed on October 5, 2005.
9. Mongan JJ, Lee TH. Do we really want broad access to health care? N Engl J Med 2005;352:1260-1263.
10. Emanuel EJ, Fuchs VR. Health care vouchers—a proposal for universal coverage. N Engl J Med 2005;352:1255-1260.
Family physicians have always done what they can to care for those whose means of obtaining health care are inadequate. Such generosity has enriched, and literally saved, many persons’ lives. Sadly, though, the number of US citizens unable to pay for health care is increasing, and individual physician goodwill will not solve the problem. A comprehensive solution is needed, and that depends on restoring health to the currently paralyzed national political process.
If you are inclined to become more involved in pursuing efforts to address the issue in political, social, and educational spheres, you will find help in this article, which summarizes the problem and reviews health policy options being considered and their implications for our patients. You will also find resources through which you can make your voice heard.
Scope of the problem
The US is the only country in the developed world without a national program for health insurance for all its citizens. Though we spend over $1.7 trillion on health care, the number of uninsured people in 2004 was about 46 million, almost 16% of the population. In 2000, 40 million people (14%) were uninsured.1 The premise of consumer-driven care is that patients who pay more of the true cost of their care will become more prudent purchasers of health services.
Critics argue that increasing out-of-pocket costs (deductibles and co-pays) acts as a deterrent to seeking appropriate care, particularly for the less well off; that wealthy and healthy people will purchase these policies, thus increasing the costs of caring for those left in the traditional insurance system; and that such a system preserves the inefficient and wasteful private insurance industry.
Government as the single payer, not employer, of health care. Proponents argue that administrative cost savings in such a system (no or minimal private health insurance) will more than cover the cost of care for the uninsured.
Web resources for information on the uninsured and other health policy topics:
Kaiser Family Foundation: www.kff.org
Cover the Uninsured campaign: www.covertheuninsured.org
Health policy for students and faculty: www.KaiserEdu.org
Physicians for a National Health Plan (single-payer advocates): www.pnhp.org
Opponents of this idea argue it will lead to long lines for specialty care (read: Britain and Canada) and stifle innovation. Considering the US spends almost 50% more of its GDP on health care than does Canada and almost double what Britain spends, it seems unlikely our experience will parallel those countries. Ironically, in a system such as Canada’s, where most care is delivered by private practice physicians, there is almost certainly less administrative burden on physicians than they currently experience in the US.
A system of health care vouchers as recently proposed by Emanuel and Fuchs. Their plan would preserve the private insurance system, phase out Medicare, Medicaid, and employer-based insurance, include Federal oversight of the benefit package and technology assessment, and be funded by an earmarked value-added tax. It attempts to achieve administrative savings and a more equitable system of care, while not challenging the current substantial role of the private insurance sector. Pay-for-performance: What can you expect?”, J Fam Pract 2005; 54[7]:609) exemplify this effort. Certainly, efforts at improving care quality are worthwhile as our health system has too much practice variability as well as over- and underutilization of care. However, it is difficult to believe that such efforts will control costs enough to allow for the expansion of health insurance rates.
Where to go from here
Given past failures at expanding health care to all, many observers are resigned to the incrementalist approach such as expanding small business insurance by pooling risk, perhaps controlling costs through consumer-driven care and pay-for-performance programs, and dealing more effectively with malpractice problems (high premiums and defensive medicine). Most would agree, however, that such efforts will not make a serious dent in the uninsured problem.
The continual increase in the number of uninsured in a country as wealthy as ours is a national tragedy and is tremendously frustrating, particularly for family physicians who constantly see the negative health effects. Physicians need to become better informed about health policy and its implications for our patients and more actively work in political, social, and educational spheres to help move the political process out of its current paralysis to address the uninsured problem.
For information on the uninsured and other health policy topics, and for adding your voice to the debate, see Web resources.
CORRESPONDING AUTHOR
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
Family physicians have always done what they can to care for those whose means of obtaining health care are inadequate. Such generosity has enriched, and literally saved, many persons’ lives. Sadly, though, the number of US citizens unable to pay for health care is increasing, and individual physician goodwill will not solve the problem. A comprehensive solution is needed, and that depends on restoring health to the currently paralyzed national political process.
If you are inclined to become more involved in pursuing efforts to address the issue in political, social, and educational spheres, you will find help in this article, which summarizes the problem and reviews health policy options being considered and their implications for our patients. You will also find resources through which you can make your voice heard.
Scope of the problem
The US is the only country in the developed world without a national program for health insurance for all its citizens. Though we spend over $1.7 trillion on health care, the number of uninsured people in 2004 was about 46 million, almost 16% of the population. In 2000, 40 million people (14%) were uninsured.1 The premise of consumer-driven care is that patients who pay more of the true cost of their care will become more prudent purchasers of health services.
Critics argue that increasing out-of-pocket costs (deductibles and co-pays) acts as a deterrent to seeking appropriate care, particularly for the less well off; that wealthy and healthy people will purchase these policies, thus increasing the costs of caring for those left in the traditional insurance system; and that such a system preserves the inefficient and wasteful private insurance industry.
Government as the single payer, not employer, of health care. Proponents argue that administrative cost savings in such a system (no or minimal private health insurance) will more than cover the cost of care for the uninsured.
Web resources for information on the uninsured and other health policy topics:
Kaiser Family Foundation: www.kff.org
Cover the Uninsured campaign: www.covertheuninsured.org
Health policy for students and faculty: www.KaiserEdu.org
Physicians for a National Health Plan (single-payer advocates): www.pnhp.org
Opponents of this idea argue it will lead to long lines for specialty care (read: Britain and Canada) and stifle innovation. Considering the US spends almost 50% more of its GDP on health care than does Canada and almost double what Britain spends, it seems unlikely our experience will parallel those countries. Ironically, in a system such as Canada’s, where most care is delivered by private practice physicians, there is almost certainly less administrative burden on physicians than they currently experience in the US.
A system of health care vouchers as recently proposed by Emanuel and Fuchs. Their plan would preserve the private insurance system, phase out Medicare, Medicaid, and employer-based insurance, include Federal oversight of the benefit package and technology assessment, and be funded by an earmarked value-added tax. It attempts to achieve administrative savings and a more equitable system of care, while not challenging the current substantial role of the private insurance sector. Pay-for-performance: What can you expect?”, J Fam Pract 2005; 54[7]:609) exemplify this effort. Certainly, efforts at improving care quality are worthwhile as our health system has too much practice variability as well as over- and underutilization of care. However, it is difficult to believe that such efforts will control costs enough to allow for the expansion of health insurance rates.
Where to go from here
Given past failures at expanding health care to all, many observers are resigned to the incrementalist approach such as expanding small business insurance by pooling risk, perhaps controlling costs through consumer-driven care and pay-for-performance programs, and dealing more effectively with malpractice problems (high premiums and defensive medicine). Most would agree, however, that such efforts will not make a serious dent in the uninsured problem.
The continual increase in the number of uninsured in a country as wealthy as ours is a national tragedy and is tremendously frustrating, particularly for family physicians who constantly see the negative health effects. Physicians need to become better informed about health policy and its implications for our patients and more actively work in political, social, and educational spheres to help move the political process out of its current paralysis to address the uninsured problem.
For information on the uninsured and other health policy topics, and for adding your voice to the debate, see Web resources.
CORRESPONDING AUTHOR
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Finkelstein J. 45.8 million now uninsured. AMA News, September 19 2005;1-2.
2. Rhoades J. The uninsured in America, 2004. Statistical Brief #83. Available at: www.meps.ahrq.gov/papers/st83/stat83.pdf.
3. Hoffman C, Wang M. Health Insurance Coverage in America. 2002 Update. Washington, DC: Kaiser Commission on Medicaid and the Uninsured; 2002.
4. Finkelstein J. Many workers lack insurance, report shows. AMA News, May 16, 2005;5.-
5. Gabel J, Claxton G, Gil I, et al. Health benefits in 2004: four years of double-digit premium increases take their toll on coverage. Health Aff (Millwood) 2004;23:200-209.
6. Institute of Medicine. Care without Coverage: Too Little, Too Late. Washington, DC: National Academies Press; 2002.
7. Himmelstein D, Warren E, Thorn D, Woolhandler Sl. Health Aff (Millwood) 2005;W5 63-73.Feb 2, 2005.
8. Paying a premium: The added cost of care for the uninsured. A Report from Families USA. June 2005. Available at: www.familiesusa.org/site/PageServer?pagename=Paing_a_Premium_Findings. Accessed on October 5, 2005.
9. Mongan JJ, Lee TH. Do we really want broad access to health care? N Engl J Med 2005;352:1260-1263.
10. Emanuel EJ, Fuchs VR. Health care vouchers—a proposal for universal coverage. N Engl J Med 2005;352:1255-1260.
1. Finkelstein J. 45.8 million now uninsured. AMA News, September 19 2005;1-2.
2. Rhoades J. The uninsured in America, 2004. Statistical Brief #83. Available at: www.meps.ahrq.gov/papers/st83/stat83.pdf.
3. Hoffman C, Wang M. Health Insurance Coverage in America. 2002 Update. Washington, DC: Kaiser Commission on Medicaid and the Uninsured; 2002.
4. Finkelstein J. Many workers lack insurance, report shows. AMA News, May 16, 2005;5.-
5. Gabel J, Claxton G, Gil I, et al. Health benefits in 2004: four years of double-digit premium increases take their toll on coverage. Health Aff (Millwood) 2004;23:200-209.
6. Institute of Medicine. Care without Coverage: Too Little, Too Late. Washington, DC: National Academies Press; 2002.
7. Himmelstein D, Warren E, Thorn D, Woolhandler Sl. Health Aff (Millwood) 2005;W5 63-73.Feb 2, 2005.
8. Paying a premium: The added cost of care for the uninsured. A Report from Families USA. June 2005. Available at: www.familiesusa.org/site/PageServer?pagename=Paing_a_Premium_Findings. Accessed on October 5, 2005.
9. Mongan JJ, Lee TH. Do we really want broad access to health care? N Engl J Med 2005;352:1260-1263.
10. Emanuel EJ, Fuchs VR. Health care vouchers—a proposal for universal coverage. N Engl J Med 2005;352:1255-1260.
Pay-for-performance: What can you expect?
Pay-for-performance programs (P4P) are spreading. Medicare has committed to a national P4P demonstration project, a large employer group has initiated its own program, and the American Medical Association (AMA) has published principles it will use to assess such programs. The American Academy of Family Physicians (AAFP) published its own criteria last year. What are the characteristics of P4P programs, private and public examples, and benefits and risks of their use?
How does it work?
Pay-for-performance refers to financial-incentive programs that pay bonuses to participants (physicians, physician groups, health plans, or hospitals) that make progress, or attain specific benchmarks, in quality and efficiency. Alternatively, P4P programs may create different tiers of providers based on quality standards, and then give patients financial incentives (such as lower co-payments) to use one tier instead of another. This latter mechanism is currently the subject of a nasty argument between the Barnes Jewish health system in St. Louis and United Healthcare.
Goals may be clinical or nonclinical. Clinical goals usually measure processes of care (eg, measurement of hemoglobin A1C and lipids in persons with diabetes, use of beta-blockers and aspirin after myocardial infarction, anti-inflammatory medications for chronic asthma, or appropriate cancer screening). However, of late there has been movement toward using intermediate out-come measures, such as control of hypertension and blood sugar, and long-term outcomes such as mortality, morbidity, and quality of life. Nonclinical goals include implementing such information technology as electronic health records, or improving access to care and patient satisfaction.
How prevalent is P4P? A national survey conducted by Med-Vantage, a health informatics company, in November 2004, identified 84 programs—covering 39 million beneficiaries—that had some P4P characteristics.
They found P4P programs expanding from primary care providers to specialist involvement, from HMOs to PPOs, and from annual bonuses to tiered fee schedules. They also reported an emphasis on using the National Commission for Quality Assurance (NCQA) measures as performance goals, rewarding information technology adoption, and increasing involvement of the Center for Medicare and Medicaid Services (CMS).
P4P programs surveyed reported quality improvement as the #1 reason for their programs, validity of the data as their #1 concern, and early provider involvement and use of standardized measures as the main recommendations for new programs.1
National programs and how they might affect you
MedPAC and providers stress information technology. The Medicare Payment Advisory Commission (MedPAC), which makes recommendations on provider payments to CMS, announced in its 2005 annual report that Medicare should begin paying all physicians differently based on how they perform. MedPAC envisions rewarding the use of information technology such as electronic health records first, and later adding measures for quality outcome.2
Almost simultaneously with this recommendation, CMS announced that 10 large physician group practices would participate in a new P4P Medicare demonstration project. These practices hope to improve quality and lower Medicare costs (by focusing on disease management strategies and information technology), and in return, CMS will return a portion of the savings to them. Initially, CMS will base the majority of bonus payments on financial savings rather than quality improvement; this has led to concern that costs are the primary driver of the program.3
Premier Hospital Quality Incentive focusing on 5 clinical areas. CMS also sponsors the Premier Hospital Quality Incentive Demonstration, a P4P program that tracks performance for 5 common clinical conditions at 270 participating hospitals. The program rewards high performers from a bonus pool of $7 million per year over a 3-year period. In May 2005, Mark McClellan, MD, PhD, the director of CMS, announced improvement in all 5 areas (acute myocardial infarction care, coronary artery bypass graft surgery, care for congestive heart failure, hip and knee replacement surgery, and pneumonia care) in the first year of the project.4
Bridge to Excellence encourages more patient involvement. A national private sector response to the P4P movement has been Bridge to Excellence (BTE), a nonprofit organization whose board represents employers, providers, and health plans (emphasis on the employers) with major funding from large companies. It was created in response to the Institute of Medicine’s 2001 report, Crossing the Quality Chasm, which included a recommendation to redesign the way providers are paid to encourage quality improvement (TABLE 1).
TABLE 1
Bridges to Excellence key principles
|
BTE has developed several P4P programs in cooperation with the NCQA. Physician Office Link pays physician’s offices up to $50 per year for each patient covered by a participating employer or plan. NCQA criteria include the use of clinical information systems, education to promote patient self-management, a quality improvement system, and programs to care for patients with chronic disease.
Diabetes Care Link rewards physicians who meet NCQA standards for its Diabetes Physician Recognition program with up to $80 for each patient with diabetes covered by the employer or health plan sponsor.
Cardiac Care Link rewards physicians who qualify for NCQA’s Heart/Stroke Recognition Program with up to $160 for each covered patient with cardiac disease. Physicians must submit data on blood pressure, lipid testing, antithrombotic use, and smoking cessation. Physicians qualify for the bonus based on a combination of process measures (performing tests/screenings) and outcome measures (eg, appropriate LDL level, aspirin use).5 The program started with about a dozen employers in just a few areas (Cincinnati, Massachusetts, and upstate New York). In March 2005, BTE announced that coalitions in 3 additional states (Illinois, Colorado, and Arkansas) are working with employers to license and launch BTE-related incentive projects later this year.6
NCQA. As the leader in accrediting managed care organizations, the nonprofit NCQA is often thought of as the expert in developing reliable performance measures. For almost 15 years, the NCQA has been refining its Health Plan Employer Data and Information Set (HEDIS) as a means for evaluating health plans. Many physicians have had their care reviewed as part of having contracts with managed care companies that apply for NCQA accreditation. The NCQA’s longstanding commitment to the development of reliable performance measures and the involvement of multiple health system stakeholders in its work has given them a great deal of national credibility.
Concerns
While embracing the quality improvement movement, major physician organizations have been cautious in their support of current P4P programs. Both the AAFP and AMA guidelines emphasize the need to focus on quality rather than cost reduction, involve physicians in program design, use evidence-based and statistically valid performance measures, reward both performance improvement and attainment of predetermined targets, and use new money for incentive payments rather than reducing existing payments to physicians (TABLE 2).7,8
TABLE 2
AMA principles to evaluate P4P programs
|
Benefits and risks
The hope is that P4P will change physician and systems behavior to improve quality and patient safety. It may be that such changes will also reduce costs, although it is certainly true that additional resources will be needed initially to help implement the technology expected to make such improvement more likely. Proponents hope that incentive payments and improved information systems will also lead to improved population management: caring for an entire practice, not just the patient who comes to the office. Disease registries and electronic health records are envisioned as 2 of the keys to making this happen.9
Why success will not be easy. One hurdle will be the difficulty of providing sufficient incentives to individual physicians or small group practices that deal with numerous insurers. One company’s P4P program may not matter much to a physician who cares for only a small number of that company’s patients. Groups of health plans and purchasers will need to cooperate in developing a common set of measures and incentives to use in a P4P program. Such cooperation will not be easy to accomplish.
Another challenge will be to identify the right number and type of measures to engage providers and actually improve care.
Then there are the financing issues—will additional money be made available for positive incentives, or will there be a revenue-neutral system in which some providers get more money while others less? This is an issue that greatly concerns the AAFP and AMA.
Finally, how will P4P affect physicians who care for the underserved and socioeconomically disadvantaged. These patients are often more difficult to care for than those with adequate healthcare coverage, and often require more intensive use of resources—all of which may limit the ability of their providers to qualify for incentive payments through P4P programs. This could lead to the unintended consequence of physicians reducing the number of underserved patients they care for.
One way to address this problem would be to improve risk-adjustment methods to compensate for the increased difficulty in providing high-quality care to certain kinds of patients. Development of such methods will likely be difficult to implement. Another way would be to evaluate providers based on their quality improvement over time rather than establishing minimum targets that have to be met to qualify for any incentive payments.
P4P is likely to expand
The recent entrance of CMS into P4P programs as well as the interest coming from large employers makes it likely that P4P will continue to expand. While paying more for higher-quality care makes sense and should save money in the long run, the constraint on resources currently available from the government and private insurers to reward higher performers as well as fund improvements necessary to ensure better care make it probable that there will be increased tension between P4P as a quality-improvement vs a cost-savings effort.
CORRESPONDING AUTHOR
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Med-Vantage, Inc. Provider Pay-for-performance incentive programs: 2004 national study results. Available at: www.medvantageinc.com/Pdf/MV_2004_P4P_National_ Study_Results-Exec_Summary.pdf. Accessed on May 25, 2005.
2. Glendinning D. AMA: Medicare pay-for-performance must be voluntary and not punitive. AMNews, March 21, 2005.
3. Glendinning D. Medicare tests pay-for-performance. AMNews, February 21, 2005.
4. Centers for Medicare and Medicare Services. Medicare pay-for-performance demonstration shows significant quality of care improvement at participating hospitals. Medicare news release, May 3, 2005. Available at: www.cms.hhs.gov/media/press/release.asp?Counter= 1441. Accessed on May 25, 2005.
5. National Committee for Quality Assurance. Bridges to excellence. Available at: www.ncqa.org/Programs/bridgestoexcellence/index.htm. Accessed on May 25, 2005.
6. National Committee for Quality Assurance. Physicians, business, government and industry embrace common strategy to improve health care: pay-for-performance. Available at: www.ncqa.org/Communications/News/BTE_P4P2005.htm. Accessed on May 25, 2005.
7. American Academy of Family Physicians. Pay-for-per-formance. Available at: http://www.aafp.org/x30307.xml. Accessed on May 25, 2005.
8. American Medical Association. Quality, fairness must be paramount in pay-for-performance. Available at: www.ama-assn.org/ama/pub/category/14774.html. Accessed on May 25, 2005.
9. Epstein A, Lee T, Hamel M. Paying physicians for high-quality care. N Engl J Med 2004;350:406-410.
Pay-for-performance programs (P4P) are spreading. Medicare has committed to a national P4P demonstration project, a large employer group has initiated its own program, and the American Medical Association (AMA) has published principles it will use to assess such programs. The American Academy of Family Physicians (AAFP) published its own criteria last year. What are the characteristics of P4P programs, private and public examples, and benefits and risks of their use?
How does it work?
Pay-for-performance refers to financial-incentive programs that pay bonuses to participants (physicians, physician groups, health plans, or hospitals) that make progress, or attain specific benchmarks, in quality and efficiency. Alternatively, P4P programs may create different tiers of providers based on quality standards, and then give patients financial incentives (such as lower co-payments) to use one tier instead of another. This latter mechanism is currently the subject of a nasty argument between the Barnes Jewish health system in St. Louis and United Healthcare.
Goals may be clinical or nonclinical. Clinical goals usually measure processes of care (eg, measurement of hemoglobin A1C and lipids in persons with diabetes, use of beta-blockers and aspirin after myocardial infarction, anti-inflammatory medications for chronic asthma, or appropriate cancer screening). However, of late there has been movement toward using intermediate out-come measures, such as control of hypertension and blood sugar, and long-term outcomes such as mortality, morbidity, and quality of life. Nonclinical goals include implementing such information technology as electronic health records, or improving access to care and patient satisfaction.
How prevalent is P4P? A national survey conducted by Med-Vantage, a health informatics company, in November 2004, identified 84 programs—covering 39 million beneficiaries—that had some P4P characteristics.
They found P4P programs expanding from primary care providers to specialist involvement, from HMOs to PPOs, and from annual bonuses to tiered fee schedules. They also reported an emphasis on using the National Commission for Quality Assurance (NCQA) measures as performance goals, rewarding information technology adoption, and increasing involvement of the Center for Medicare and Medicaid Services (CMS).
P4P programs surveyed reported quality improvement as the #1 reason for their programs, validity of the data as their #1 concern, and early provider involvement and use of standardized measures as the main recommendations for new programs.1
National programs and how they might affect you
MedPAC and providers stress information technology. The Medicare Payment Advisory Commission (MedPAC), which makes recommendations on provider payments to CMS, announced in its 2005 annual report that Medicare should begin paying all physicians differently based on how they perform. MedPAC envisions rewarding the use of information technology such as electronic health records first, and later adding measures for quality outcome.2
Almost simultaneously with this recommendation, CMS announced that 10 large physician group practices would participate in a new P4P Medicare demonstration project. These practices hope to improve quality and lower Medicare costs (by focusing on disease management strategies and information technology), and in return, CMS will return a portion of the savings to them. Initially, CMS will base the majority of bonus payments on financial savings rather than quality improvement; this has led to concern that costs are the primary driver of the program.3
Premier Hospital Quality Incentive focusing on 5 clinical areas. CMS also sponsors the Premier Hospital Quality Incentive Demonstration, a P4P program that tracks performance for 5 common clinical conditions at 270 participating hospitals. The program rewards high performers from a bonus pool of $7 million per year over a 3-year period. In May 2005, Mark McClellan, MD, PhD, the director of CMS, announced improvement in all 5 areas (acute myocardial infarction care, coronary artery bypass graft surgery, care for congestive heart failure, hip and knee replacement surgery, and pneumonia care) in the first year of the project.4
Bridge to Excellence encourages more patient involvement. A national private sector response to the P4P movement has been Bridge to Excellence (BTE), a nonprofit organization whose board represents employers, providers, and health plans (emphasis on the employers) with major funding from large companies. It was created in response to the Institute of Medicine’s 2001 report, Crossing the Quality Chasm, which included a recommendation to redesign the way providers are paid to encourage quality improvement (TABLE 1).
TABLE 1
Bridges to Excellence key principles
|
BTE has developed several P4P programs in cooperation with the NCQA. Physician Office Link pays physician’s offices up to $50 per year for each patient covered by a participating employer or plan. NCQA criteria include the use of clinical information systems, education to promote patient self-management, a quality improvement system, and programs to care for patients with chronic disease.
Diabetes Care Link rewards physicians who meet NCQA standards for its Diabetes Physician Recognition program with up to $80 for each patient with diabetes covered by the employer or health plan sponsor.
Cardiac Care Link rewards physicians who qualify for NCQA’s Heart/Stroke Recognition Program with up to $160 for each covered patient with cardiac disease. Physicians must submit data on blood pressure, lipid testing, antithrombotic use, and smoking cessation. Physicians qualify for the bonus based on a combination of process measures (performing tests/screenings) and outcome measures (eg, appropriate LDL level, aspirin use).5 The program started with about a dozen employers in just a few areas (Cincinnati, Massachusetts, and upstate New York). In March 2005, BTE announced that coalitions in 3 additional states (Illinois, Colorado, and Arkansas) are working with employers to license and launch BTE-related incentive projects later this year.6
NCQA. As the leader in accrediting managed care organizations, the nonprofit NCQA is often thought of as the expert in developing reliable performance measures. For almost 15 years, the NCQA has been refining its Health Plan Employer Data and Information Set (HEDIS) as a means for evaluating health plans. Many physicians have had their care reviewed as part of having contracts with managed care companies that apply for NCQA accreditation. The NCQA’s longstanding commitment to the development of reliable performance measures and the involvement of multiple health system stakeholders in its work has given them a great deal of national credibility.
Concerns
While embracing the quality improvement movement, major physician organizations have been cautious in their support of current P4P programs. Both the AAFP and AMA guidelines emphasize the need to focus on quality rather than cost reduction, involve physicians in program design, use evidence-based and statistically valid performance measures, reward both performance improvement and attainment of predetermined targets, and use new money for incentive payments rather than reducing existing payments to physicians (TABLE 2).7,8
TABLE 2
AMA principles to evaluate P4P programs
|
Benefits and risks
The hope is that P4P will change physician and systems behavior to improve quality and patient safety. It may be that such changes will also reduce costs, although it is certainly true that additional resources will be needed initially to help implement the technology expected to make such improvement more likely. Proponents hope that incentive payments and improved information systems will also lead to improved population management: caring for an entire practice, not just the patient who comes to the office. Disease registries and electronic health records are envisioned as 2 of the keys to making this happen.9
Why success will not be easy. One hurdle will be the difficulty of providing sufficient incentives to individual physicians or small group practices that deal with numerous insurers. One company’s P4P program may not matter much to a physician who cares for only a small number of that company’s patients. Groups of health plans and purchasers will need to cooperate in developing a common set of measures and incentives to use in a P4P program. Such cooperation will not be easy to accomplish.
Another challenge will be to identify the right number and type of measures to engage providers and actually improve care.
Then there are the financing issues—will additional money be made available for positive incentives, or will there be a revenue-neutral system in which some providers get more money while others less? This is an issue that greatly concerns the AAFP and AMA.
Finally, how will P4P affect physicians who care for the underserved and socioeconomically disadvantaged. These patients are often more difficult to care for than those with adequate healthcare coverage, and often require more intensive use of resources—all of which may limit the ability of their providers to qualify for incentive payments through P4P programs. This could lead to the unintended consequence of physicians reducing the number of underserved patients they care for.
One way to address this problem would be to improve risk-adjustment methods to compensate for the increased difficulty in providing high-quality care to certain kinds of patients. Development of such methods will likely be difficult to implement. Another way would be to evaluate providers based on their quality improvement over time rather than establishing minimum targets that have to be met to qualify for any incentive payments.
P4P is likely to expand
The recent entrance of CMS into P4P programs as well as the interest coming from large employers makes it likely that P4P will continue to expand. While paying more for higher-quality care makes sense and should save money in the long run, the constraint on resources currently available from the government and private insurers to reward higher performers as well as fund improvements necessary to ensure better care make it probable that there will be increased tension between P4P as a quality-improvement vs a cost-savings effort.
CORRESPONDING AUTHOR
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
Pay-for-performance programs (P4P) are spreading. Medicare has committed to a national P4P demonstration project, a large employer group has initiated its own program, and the American Medical Association (AMA) has published principles it will use to assess such programs. The American Academy of Family Physicians (AAFP) published its own criteria last year. What are the characteristics of P4P programs, private and public examples, and benefits and risks of their use?
How does it work?
Pay-for-performance refers to financial-incentive programs that pay bonuses to participants (physicians, physician groups, health plans, or hospitals) that make progress, or attain specific benchmarks, in quality and efficiency. Alternatively, P4P programs may create different tiers of providers based on quality standards, and then give patients financial incentives (such as lower co-payments) to use one tier instead of another. This latter mechanism is currently the subject of a nasty argument between the Barnes Jewish health system in St. Louis and United Healthcare.
Goals may be clinical or nonclinical. Clinical goals usually measure processes of care (eg, measurement of hemoglobin A1C and lipids in persons with diabetes, use of beta-blockers and aspirin after myocardial infarction, anti-inflammatory medications for chronic asthma, or appropriate cancer screening). However, of late there has been movement toward using intermediate out-come measures, such as control of hypertension and blood sugar, and long-term outcomes such as mortality, morbidity, and quality of life. Nonclinical goals include implementing such information technology as electronic health records, or improving access to care and patient satisfaction.
How prevalent is P4P? A national survey conducted by Med-Vantage, a health informatics company, in November 2004, identified 84 programs—covering 39 million beneficiaries—that had some P4P characteristics.
They found P4P programs expanding from primary care providers to specialist involvement, from HMOs to PPOs, and from annual bonuses to tiered fee schedules. They also reported an emphasis on using the National Commission for Quality Assurance (NCQA) measures as performance goals, rewarding information technology adoption, and increasing involvement of the Center for Medicare and Medicaid Services (CMS).
P4P programs surveyed reported quality improvement as the #1 reason for their programs, validity of the data as their #1 concern, and early provider involvement and use of standardized measures as the main recommendations for new programs.1
National programs and how they might affect you
MedPAC and providers stress information technology. The Medicare Payment Advisory Commission (MedPAC), which makes recommendations on provider payments to CMS, announced in its 2005 annual report that Medicare should begin paying all physicians differently based on how they perform. MedPAC envisions rewarding the use of information technology such as electronic health records first, and later adding measures for quality outcome.2
Almost simultaneously with this recommendation, CMS announced that 10 large physician group practices would participate in a new P4P Medicare demonstration project. These practices hope to improve quality and lower Medicare costs (by focusing on disease management strategies and information technology), and in return, CMS will return a portion of the savings to them. Initially, CMS will base the majority of bonus payments on financial savings rather than quality improvement; this has led to concern that costs are the primary driver of the program.3
Premier Hospital Quality Incentive focusing on 5 clinical areas. CMS also sponsors the Premier Hospital Quality Incentive Demonstration, a P4P program that tracks performance for 5 common clinical conditions at 270 participating hospitals. The program rewards high performers from a bonus pool of $7 million per year over a 3-year period. In May 2005, Mark McClellan, MD, PhD, the director of CMS, announced improvement in all 5 areas (acute myocardial infarction care, coronary artery bypass graft surgery, care for congestive heart failure, hip and knee replacement surgery, and pneumonia care) in the first year of the project.4
Bridge to Excellence encourages more patient involvement. A national private sector response to the P4P movement has been Bridge to Excellence (BTE), a nonprofit organization whose board represents employers, providers, and health plans (emphasis on the employers) with major funding from large companies. It was created in response to the Institute of Medicine’s 2001 report, Crossing the Quality Chasm, which included a recommendation to redesign the way providers are paid to encourage quality improvement (TABLE 1).
TABLE 1
Bridges to Excellence key principles
|
BTE has developed several P4P programs in cooperation with the NCQA. Physician Office Link pays physician’s offices up to $50 per year for each patient covered by a participating employer or plan. NCQA criteria include the use of clinical information systems, education to promote patient self-management, a quality improvement system, and programs to care for patients with chronic disease.
Diabetes Care Link rewards physicians who meet NCQA standards for its Diabetes Physician Recognition program with up to $80 for each patient with diabetes covered by the employer or health plan sponsor.
Cardiac Care Link rewards physicians who qualify for NCQA’s Heart/Stroke Recognition Program with up to $160 for each covered patient with cardiac disease. Physicians must submit data on blood pressure, lipid testing, antithrombotic use, and smoking cessation. Physicians qualify for the bonus based on a combination of process measures (performing tests/screenings) and outcome measures (eg, appropriate LDL level, aspirin use).5 The program started with about a dozen employers in just a few areas (Cincinnati, Massachusetts, and upstate New York). In March 2005, BTE announced that coalitions in 3 additional states (Illinois, Colorado, and Arkansas) are working with employers to license and launch BTE-related incentive projects later this year.6
NCQA. As the leader in accrediting managed care organizations, the nonprofit NCQA is often thought of as the expert in developing reliable performance measures. For almost 15 years, the NCQA has been refining its Health Plan Employer Data and Information Set (HEDIS) as a means for evaluating health plans. Many physicians have had their care reviewed as part of having contracts with managed care companies that apply for NCQA accreditation. The NCQA’s longstanding commitment to the development of reliable performance measures and the involvement of multiple health system stakeholders in its work has given them a great deal of national credibility.
Concerns
While embracing the quality improvement movement, major physician organizations have been cautious in their support of current P4P programs. Both the AAFP and AMA guidelines emphasize the need to focus on quality rather than cost reduction, involve physicians in program design, use evidence-based and statistically valid performance measures, reward both performance improvement and attainment of predetermined targets, and use new money for incentive payments rather than reducing existing payments to physicians (TABLE 2).7,8
TABLE 2
AMA principles to evaluate P4P programs
|
Benefits and risks
The hope is that P4P will change physician and systems behavior to improve quality and patient safety. It may be that such changes will also reduce costs, although it is certainly true that additional resources will be needed initially to help implement the technology expected to make such improvement more likely. Proponents hope that incentive payments and improved information systems will also lead to improved population management: caring for an entire practice, not just the patient who comes to the office. Disease registries and electronic health records are envisioned as 2 of the keys to making this happen.9
Why success will not be easy. One hurdle will be the difficulty of providing sufficient incentives to individual physicians or small group practices that deal with numerous insurers. One company’s P4P program may not matter much to a physician who cares for only a small number of that company’s patients. Groups of health plans and purchasers will need to cooperate in developing a common set of measures and incentives to use in a P4P program. Such cooperation will not be easy to accomplish.
Another challenge will be to identify the right number and type of measures to engage providers and actually improve care.
Then there are the financing issues—will additional money be made available for positive incentives, or will there be a revenue-neutral system in which some providers get more money while others less? This is an issue that greatly concerns the AAFP and AMA.
Finally, how will P4P affect physicians who care for the underserved and socioeconomically disadvantaged. These patients are often more difficult to care for than those with adequate healthcare coverage, and often require more intensive use of resources—all of which may limit the ability of their providers to qualify for incentive payments through P4P programs. This could lead to the unintended consequence of physicians reducing the number of underserved patients they care for.
One way to address this problem would be to improve risk-adjustment methods to compensate for the increased difficulty in providing high-quality care to certain kinds of patients. Development of such methods will likely be difficult to implement. Another way would be to evaluate providers based on their quality improvement over time rather than establishing minimum targets that have to be met to qualify for any incentive payments.
P4P is likely to expand
The recent entrance of CMS into P4P programs as well as the interest coming from large employers makes it likely that P4P will continue to expand. While paying more for higher-quality care makes sense and should save money in the long run, the constraint on resources currently available from the government and private insurers to reward higher performers as well as fund improvements necessary to ensure better care make it probable that there will be increased tension between P4P as a quality-improvement vs a cost-savings effort.
CORRESPONDING AUTHOR
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Med-Vantage, Inc. Provider Pay-for-performance incentive programs: 2004 national study results. Available at: www.medvantageinc.com/Pdf/MV_2004_P4P_National_ Study_Results-Exec_Summary.pdf. Accessed on May 25, 2005.
2. Glendinning D. AMA: Medicare pay-for-performance must be voluntary and not punitive. AMNews, March 21, 2005.
3. Glendinning D. Medicare tests pay-for-performance. AMNews, February 21, 2005.
4. Centers for Medicare and Medicare Services. Medicare pay-for-performance demonstration shows significant quality of care improvement at participating hospitals. Medicare news release, May 3, 2005. Available at: www.cms.hhs.gov/media/press/release.asp?Counter= 1441. Accessed on May 25, 2005.
5. National Committee for Quality Assurance. Bridges to excellence. Available at: www.ncqa.org/Programs/bridgestoexcellence/index.htm. Accessed on May 25, 2005.
6. National Committee for Quality Assurance. Physicians, business, government and industry embrace common strategy to improve health care: pay-for-performance. Available at: www.ncqa.org/Communications/News/BTE_P4P2005.htm. Accessed on May 25, 2005.
7. American Academy of Family Physicians. Pay-for-per-formance. Available at: http://www.aafp.org/x30307.xml. Accessed on May 25, 2005.
8. American Medical Association. Quality, fairness must be paramount in pay-for-performance. Available at: www.ama-assn.org/ama/pub/category/14774.html. Accessed on May 25, 2005.
9. Epstein A, Lee T, Hamel M. Paying physicians for high-quality care. N Engl J Med 2004;350:406-410.
1. Med-Vantage, Inc. Provider Pay-for-performance incentive programs: 2004 national study results. Available at: www.medvantageinc.com/Pdf/MV_2004_P4P_National_ Study_Results-Exec_Summary.pdf. Accessed on May 25, 2005.
2. Glendinning D. AMA: Medicare pay-for-performance must be voluntary and not punitive. AMNews, March 21, 2005.
3. Glendinning D. Medicare tests pay-for-performance. AMNews, February 21, 2005.
4. Centers for Medicare and Medicare Services. Medicare pay-for-performance demonstration shows significant quality of care improvement at participating hospitals. Medicare news release, May 3, 2005. Available at: www.cms.hhs.gov/media/press/release.asp?Counter= 1441. Accessed on May 25, 2005.
5. National Committee for Quality Assurance. Bridges to excellence. Available at: www.ncqa.org/Programs/bridgestoexcellence/index.htm. Accessed on May 25, 2005.
6. National Committee for Quality Assurance. Physicians, business, government and industry embrace common strategy to improve health care: pay-for-performance. Available at: www.ncqa.org/Communications/News/BTE_P4P2005.htm. Accessed on May 25, 2005.
7. American Academy of Family Physicians. Pay-for-per-formance. Available at: http://www.aafp.org/x30307.xml. Accessed on May 25, 2005.
8. American Medical Association. Quality, fairness must be paramount in pay-for-performance. Available at: www.ama-assn.org/ama/pub/category/14774.html. Accessed on May 25, 2005.
9. Epstein A, Lee T, Hamel M. Paying physicians for high-quality care. N Engl J Med 2004;350:406-410.
The growing threat of avian influenza
In the first half of 2004, an estimated 200 million poultry birds across Asia died or were destroyed in an effort to control a widespread outbreak of avian influenza.1 Though such outbreaks have occurred before, this one, primarily in China and Southeast Asia, demonstrated increased pathogenicity in poultry, increased resistance to environmental controls, and an expanded range of mammalian hosts.
Lest anyone conclude this is a problem affecting only the birds and the people who raise them, clinical and laboratory evidence since 1997 point to a series of human cases of avian flu (TABLE 1), most of which were associated with outbreaks of the disease in poultry.2 The change in characteristics of the current outbreak in birds in Asia combined with increased knowledge of the characteristics of human influenza have many scientists and public health officials increasingly concerned that we may be watching the unfolding of the next great flu pandemic. In this article, I describe how this might happen and what we can attempt to prevent it.
TABLE 1
Avian influenza infection in humans
| Hong Kong (1997): | 18 people hospitalized with 6 deaths |
| China and Hong Kong (1999): | 2 cases in children who recovered |
| Virginia (2002): | 1 person with serologic evidence of avian flu |
| China and Hong Kong (2003): | 2 adults cases with one death |
| Netherlands (2003): | 89 human cases with 1 death; most cases were of conjunctivitis, some with flu symptoms. The antiviral drug oseltamivir was used to help control spread |
| Canada (2004): | Human infections among poultry workers consisting of eye infections |
| Asia (since January 2004): | 55 cases with 42 deaths in Vietnam, Thailand, and Cambodia |
Human influenza pandemics
A flu pandemic is a global outbreak of disease among people after the emergence of a new influenza A virus. Three great pandemics occurred in the 20th century, all spreading worldwide within 1 year.
- Spanish flu (1918–1919): 20 to 50 million people died, more than 500,000 in the US. Nearly half of those dying were young, healthy adults.
- Asian flu (1957–1958): Caused about 70,000 deaths in the US.
- Hong Kong flu (1968–1969): Caused about 34,000 deaths in the US.
Both the Asian and Hong Kong flus resulted from a mixing of a human and avian influenza virus; the Spanish flu may have resulted from a mutation in a purely avian virus.3
How avian influenza viruses spread
Avian influenza A viruses vary greatly, owing to the myriad combinations of their 15 hemagglutinins and 9 neuraminidases. The viruses are widespread in migratory birds and waterfowl and are usually of low pathogenicity. Water birds, in particular, act as hosts for influenza viruses, carrying them in their intestines and then shedding them.
Wild bird hosts do not usually get sick, but they can spread influenza to other birds. For instance, there have been 16 outbreaks of H5 and H7 influenza in US poultry since 1997. Usually, such events are from low-pathogenic avian viruses that cause little illness in affected chickens. When highly pathogenic viruses cause outbreaks, 90% to 100% of affected poultry can die. This has been happening in the current Asian outbreaks.
In the past, isolating poultry, culling (destroying) infected flocks, and vaccinating poultry eventually quelled the outbreaks. Such measures have not worked this time, and scientists think it likely that H5N1 infection among birds has become endemic to the region—ie, Cambodia, China, Indonesia, Malaysia, Thailand, and Vietnam.4
Rethinking how humans become infected
Since humans have distinct receptors for human viruses, as do birds for avian viruses, it was thought that an intermediate host—the pig, with both receptors—was necessary to allow the mixing of the viruses and subsequent human infection with avian influenza. However, recent outbreaks in poultry with proven spread of highly pathogenic virus from chickens directly to humans have challenged this theory (TABLE 1). While the mortality rate so far has been very high, this rate may turn out to be overstated as milder, nonfatal cases of human avian flu are discovered.
New research has raised additional concerns: ducks infected with H5N1 are now shedding virus for longer times while remaining asymptomatic; pigs in China and tigers in Thailand have been infected with H5 virus; and experiments with house cats in the Netherlands have demonstrated they could be infected with H5. These findings are troubling because the reassortment of avian viruses is more likely to occur when they are able to infect multiple species. Moreover, the infection in humans from Vietnam has shown resistance to the older antiviral drugs, amantadine and rimantadine, leaving oseltamivir and zanamivir as the antivirals likely effective against avian influenza A H5N1.4
Fear of human-to-human transmission
Direct spread of avian influenza from poultry to humans resulting in a high fatality rate is of course a major concern. What worries scientists and public health officials more, however, is the increasing risk of person-to-person transmission as a result of a change in the viral genome. Genomic variation could occur if avian virus genetic material mixes with that of a human virus in an intermediate host such as the pig or in a patient infected simultaneously with both avian and human influenza strains; or it could occur with spontaneous mutation of an avian virus. A recent report provided strong evidence of avian influenza that spread from the index patient to her mother and aunt (both the 11-year old girl and her mother died). No further spread occurred, suggesting the infection resulted from a purely avian virus with no human virus involved.5
How effective will preventive measures be?
The current danger to people from avian influenza has been recognized sooner than the threats that preceded previous influenza pandemics, which burst upon the world with little warning. Lessons from the severe acute respiratory syndrome (SARS) epidemic in 2003, advances in science and public health surveillance, and cooperation among countries and organizations such as the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) have allowed us to observe the emergence of avian influenza in rural Asia and consider its wider implications. CDC’s response has focused on enhanced surveillance and laboratory testing for human and avian influenza in the US and Asia and work on vaccine development with WHO and the National Institutes of Health (TABLE 2). CDC has not recommended avoiding travel to any of the involved countries, but has released guidelines for travel to affected areas (TABLE 3).6
In the event of an avian flu outbreak in humans, there will be questions about the best public health response, the use of antiviral agents, and infection control in health care settings. The SARS outbreak was substantially controlled through the use of the traditional public health measures of isolation (separating ill or infected people from others) and quarantine (separating people exposed to infected people from others in order to prevent the further spread of the infection). Whether such measures would be successful with a highly contagious viral infection like influenza is debatable. Stohr of WHO has outlined a research agenda that includes study of hospital infection control practices, vaccine clinical immunogenicity, early interventions such as use of antivirals or vaccine to slow the spread of an emerging pandemic virus, the role of animal and bird species in influenza virus development, and risk assessment.7
TABLE 2
CDC response to avian influenza
|
TABLE 3
General precautions for travel to countries with avian influenza outbreaks*
|
| * As of February 4, 2005 this was directed at travelers to Vietnam only; see reference #6 for complete details. |
Awareness the best defense now
If avian influenza makes the leap to person-to-person transmission, family physicians across the globe will be at the forefront of diagnosis and treatment. Though no immediate actions are necessary, we all must follow developments and support the work of health departments at improving their ability to monitor emerging outbreaks. The interconnectedness of global health is well exemplified in the concern about avian flu and the efforts to prevent an influenza pandemic.
Correspondence
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Altman L. UN agency to urge vaccinations to health avian flu. New York Times, July 30, 2004.
2. World Health Organization. Disease surveillance. Cumulative number of confirmed cases of human avian influenza. WHO Communicable. Available at: www.who.int/csr/disease/avian_influenza/country/cas es_table_2005_02_02/en/. Accessed on April 10. 2005.
3. Monto A. The threat of an avian influenza pandemic. New Engl J Med 2005;352:323-325.
4. Centers for Disease Control and Prevention (CDC) Avian Influenza home page. Available at: www.cdc.gov/flu/avian/index.htm. Accessed on April 10, 2005.
5. Ungchusak K, Auewarakul P, Dowell S, et al. Probable person-person transmission of avian influenza (H5N1). New Engl J Med 2005;352:333-340.
6. CDC Asian Influenza, Vietnam, Notice to Travelers. Updated February 4, 2005. Available at: www.cdc.gov/travel/other/avian_flu_vietnam_2005_tra velers.htm. Accessed on April 10, 2005.
7. Stohr K. Avian influenza and pandemics. New Engl J Med 2005;352:405-407.
In the first half of 2004, an estimated 200 million poultry birds across Asia died or were destroyed in an effort to control a widespread outbreak of avian influenza.1 Though such outbreaks have occurred before, this one, primarily in China and Southeast Asia, demonstrated increased pathogenicity in poultry, increased resistance to environmental controls, and an expanded range of mammalian hosts.
Lest anyone conclude this is a problem affecting only the birds and the people who raise them, clinical and laboratory evidence since 1997 point to a series of human cases of avian flu (TABLE 1), most of which were associated with outbreaks of the disease in poultry.2 The change in characteristics of the current outbreak in birds in Asia combined with increased knowledge of the characteristics of human influenza have many scientists and public health officials increasingly concerned that we may be watching the unfolding of the next great flu pandemic. In this article, I describe how this might happen and what we can attempt to prevent it.
TABLE 1
Avian influenza infection in humans
| Hong Kong (1997): | 18 people hospitalized with 6 deaths |
| China and Hong Kong (1999): | 2 cases in children who recovered |
| Virginia (2002): | 1 person with serologic evidence of avian flu |
| China and Hong Kong (2003): | 2 adults cases with one death |
| Netherlands (2003): | 89 human cases with 1 death; most cases were of conjunctivitis, some with flu symptoms. The antiviral drug oseltamivir was used to help control spread |
| Canada (2004): | Human infections among poultry workers consisting of eye infections |
| Asia (since January 2004): | 55 cases with 42 deaths in Vietnam, Thailand, and Cambodia |
Human influenza pandemics
A flu pandemic is a global outbreak of disease among people after the emergence of a new influenza A virus. Three great pandemics occurred in the 20th century, all spreading worldwide within 1 year.
- Spanish flu (1918–1919): 20 to 50 million people died, more than 500,000 in the US. Nearly half of those dying were young, healthy adults.
- Asian flu (1957–1958): Caused about 70,000 deaths in the US.
- Hong Kong flu (1968–1969): Caused about 34,000 deaths in the US.
Both the Asian and Hong Kong flus resulted from a mixing of a human and avian influenza virus; the Spanish flu may have resulted from a mutation in a purely avian virus.3
How avian influenza viruses spread
Avian influenza A viruses vary greatly, owing to the myriad combinations of their 15 hemagglutinins and 9 neuraminidases. The viruses are widespread in migratory birds and waterfowl and are usually of low pathogenicity. Water birds, in particular, act as hosts for influenza viruses, carrying them in their intestines and then shedding them.
Wild bird hosts do not usually get sick, but they can spread influenza to other birds. For instance, there have been 16 outbreaks of H5 and H7 influenza in US poultry since 1997. Usually, such events are from low-pathogenic avian viruses that cause little illness in affected chickens. When highly pathogenic viruses cause outbreaks, 90% to 100% of affected poultry can die. This has been happening in the current Asian outbreaks.
In the past, isolating poultry, culling (destroying) infected flocks, and vaccinating poultry eventually quelled the outbreaks. Such measures have not worked this time, and scientists think it likely that H5N1 infection among birds has become endemic to the region—ie, Cambodia, China, Indonesia, Malaysia, Thailand, and Vietnam.4
Rethinking how humans become infected
Since humans have distinct receptors for human viruses, as do birds for avian viruses, it was thought that an intermediate host—the pig, with both receptors—was necessary to allow the mixing of the viruses and subsequent human infection with avian influenza. However, recent outbreaks in poultry with proven spread of highly pathogenic virus from chickens directly to humans have challenged this theory (TABLE 1). While the mortality rate so far has been very high, this rate may turn out to be overstated as milder, nonfatal cases of human avian flu are discovered.
New research has raised additional concerns: ducks infected with H5N1 are now shedding virus for longer times while remaining asymptomatic; pigs in China and tigers in Thailand have been infected with H5 virus; and experiments with house cats in the Netherlands have demonstrated they could be infected with H5. These findings are troubling because the reassortment of avian viruses is more likely to occur when they are able to infect multiple species. Moreover, the infection in humans from Vietnam has shown resistance to the older antiviral drugs, amantadine and rimantadine, leaving oseltamivir and zanamivir as the antivirals likely effective against avian influenza A H5N1.4
Fear of human-to-human transmission
Direct spread of avian influenza from poultry to humans resulting in a high fatality rate is of course a major concern. What worries scientists and public health officials more, however, is the increasing risk of person-to-person transmission as a result of a change in the viral genome. Genomic variation could occur if avian virus genetic material mixes with that of a human virus in an intermediate host such as the pig or in a patient infected simultaneously with both avian and human influenza strains; or it could occur with spontaneous mutation of an avian virus. A recent report provided strong evidence of avian influenza that spread from the index patient to her mother and aunt (both the 11-year old girl and her mother died). No further spread occurred, suggesting the infection resulted from a purely avian virus with no human virus involved.5
How effective will preventive measures be?
The current danger to people from avian influenza has been recognized sooner than the threats that preceded previous influenza pandemics, which burst upon the world with little warning. Lessons from the severe acute respiratory syndrome (SARS) epidemic in 2003, advances in science and public health surveillance, and cooperation among countries and organizations such as the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) have allowed us to observe the emergence of avian influenza in rural Asia and consider its wider implications. CDC’s response has focused on enhanced surveillance and laboratory testing for human and avian influenza in the US and Asia and work on vaccine development with WHO and the National Institutes of Health (TABLE 2). CDC has not recommended avoiding travel to any of the involved countries, but has released guidelines for travel to affected areas (TABLE 3).6
In the event of an avian flu outbreak in humans, there will be questions about the best public health response, the use of antiviral agents, and infection control in health care settings. The SARS outbreak was substantially controlled through the use of the traditional public health measures of isolation (separating ill or infected people from others) and quarantine (separating people exposed to infected people from others in order to prevent the further spread of the infection). Whether such measures would be successful with a highly contagious viral infection like influenza is debatable. Stohr of WHO has outlined a research agenda that includes study of hospital infection control practices, vaccine clinical immunogenicity, early interventions such as use of antivirals or vaccine to slow the spread of an emerging pandemic virus, the role of animal and bird species in influenza virus development, and risk assessment.7
TABLE 2
CDC response to avian influenza
|
TABLE 3
General precautions for travel to countries with avian influenza outbreaks*
|
| * As of February 4, 2005 this was directed at travelers to Vietnam only; see reference #6 for complete details. |
Awareness the best defense now
If avian influenza makes the leap to person-to-person transmission, family physicians across the globe will be at the forefront of diagnosis and treatment. Though no immediate actions are necessary, we all must follow developments and support the work of health departments at improving their ability to monitor emerging outbreaks. The interconnectedness of global health is well exemplified in the concern about avian flu and the efforts to prevent an influenza pandemic.
Correspondence
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
In the first half of 2004, an estimated 200 million poultry birds across Asia died or were destroyed in an effort to control a widespread outbreak of avian influenza.1 Though such outbreaks have occurred before, this one, primarily in China and Southeast Asia, demonstrated increased pathogenicity in poultry, increased resistance to environmental controls, and an expanded range of mammalian hosts.
Lest anyone conclude this is a problem affecting only the birds and the people who raise them, clinical and laboratory evidence since 1997 point to a series of human cases of avian flu (TABLE 1), most of which were associated with outbreaks of the disease in poultry.2 The change in characteristics of the current outbreak in birds in Asia combined with increased knowledge of the characteristics of human influenza have many scientists and public health officials increasingly concerned that we may be watching the unfolding of the next great flu pandemic. In this article, I describe how this might happen and what we can attempt to prevent it.
TABLE 1
Avian influenza infection in humans
| Hong Kong (1997): | 18 people hospitalized with 6 deaths |
| China and Hong Kong (1999): | 2 cases in children who recovered |
| Virginia (2002): | 1 person with serologic evidence of avian flu |
| China and Hong Kong (2003): | 2 adults cases with one death |
| Netherlands (2003): | 89 human cases with 1 death; most cases were of conjunctivitis, some with flu symptoms. The antiviral drug oseltamivir was used to help control spread |
| Canada (2004): | Human infections among poultry workers consisting of eye infections |
| Asia (since January 2004): | 55 cases with 42 deaths in Vietnam, Thailand, and Cambodia |
Human influenza pandemics
A flu pandemic is a global outbreak of disease among people after the emergence of a new influenza A virus. Three great pandemics occurred in the 20th century, all spreading worldwide within 1 year.
- Spanish flu (1918–1919): 20 to 50 million people died, more than 500,000 in the US. Nearly half of those dying were young, healthy adults.
- Asian flu (1957–1958): Caused about 70,000 deaths in the US.
- Hong Kong flu (1968–1969): Caused about 34,000 deaths in the US.
Both the Asian and Hong Kong flus resulted from a mixing of a human and avian influenza virus; the Spanish flu may have resulted from a mutation in a purely avian virus.3
How avian influenza viruses spread
Avian influenza A viruses vary greatly, owing to the myriad combinations of their 15 hemagglutinins and 9 neuraminidases. The viruses are widespread in migratory birds and waterfowl and are usually of low pathogenicity. Water birds, in particular, act as hosts for influenza viruses, carrying them in their intestines and then shedding them.
Wild bird hosts do not usually get sick, but they can spread influenza to other birds. For instance, there have been 16 outbreaks of H5 and H7 influenza in US poultry since 1997. Usually, such events are from low-pathogenic avian viruses that cause little illness in affected chickens. When highly pathogenic viruses cause outbreaks, 90% to 100% of affected poultry can die. This has been happening in the current Asian outbreaks.
In the past, isolating poultry, culling (destroying) infected flocks, and vaccinating poultry eventually quelled the outbreaks. Such measures have not worked this time, and scientists think it likely that H5N1 infection among birds has become endemic to the region—ie, Cambodia, China, Indonesia, Malaysia, Thailand, and Vietnam.4
Rethinking how humans become infected
Since humans have distinct receptors for human viruses, as do birds for avian viruses, it was thought that an intermediate host—the pig, with both receptors—was necessary to allow the mixing of the viruses and subsequent human infection with avian influenza. However, recent outbreaks in poultry with proven spread of highly pathogenic virus from chickens directly to humans have challenged this theory (TABLE 1). While the mortality rate so far has been very high, this rate may turn out to be overstated as milder, nonfatal cases of human avian flu are discovered.
New research has raised additional concerns: ducks infected with H5N1 are now shedding virus for longer times while remaining asymptomatic; pigs in China and tigers in Thailand have been infected with H5 virus; and experiments with house cats in the Netherlands have demonstrated they could be infected with H5. These findings are troubling because the reassortment of avian viruses is more likely to occur when they are able to infect multiple species. Moreover, the infection in humans from Vietnam has shown resistance to the older antiviral drugs, amantadine and rimantadine, leaving oseltamivir and zanamivir as the antivirals likely effective against avian influenza A H5N1.4
Fear of human-to-human transmission
Direct spread of avian influenza from poultry to humans resulting in a high fatality rate is of course a major concern. What worries scientists and public health officials more, however, is the increasing risk of person-to-person transmission as a result of a change in the viral genome. Genomic variation could occur if avian virus genetic material mixes with that of a human virus in an intermediate host such as the pig or in a patient infected simultaneously with both avian and human influenza strains; or it could occur with spontaneous mutation of an avian virus. A recent report provided strong evidence of avian influenza that spread from the index patient to her mother and aunt (both the 11-year old girl and her mother died). No further spread occurred, suggesting the infection resulted from a purely avian virus with no human virus involved.5
How effective will preventive measures be?
The current danger to people from avian influenza has been recognized sooner than the threats that preceded previous influenza pandemics, which burst upon the world with little warning. Lessons from the severe acute respiratory syndrome (SARS) epidemic in 2003, advances in science and public health surveillance, and cooperation among countries and organizations such as the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) have allowed us to observe the emergence of avian influenza in rural Asia and consider its wider implications. CDC’s response has focused on enhanced surveillance and laboratory testing for human and avian influenza in the US and Asia and work on vaccine development with WHO and the National Institutes of Health (TABLE 2). CDC has not recommended avoiding travel to any of the involved countries, but has released guidelines for travel to affected areas (TABLE 3).6
In the event of an avian flu outbreak in humans, there will be questions about the best public health response, the use of antiviral agents, and infection control in health care settings. The SARS outbreak was substantially controlled through the use of the traditional public health measures of isolation (separating ill or infected people from others) and quarantine (separating people exposed to infected people from others in order to prevent the further spread of the infection). Whether such measures would be successful with a highly contagious viral infection like influenza is debatable. Stohr of WHO has outlined a research agenda that includes study of hospital infection control practices, vaccine clinical immunogenicity, early interventions such as use of antivirals or vaccine to slow the spread of an emerging pandemic virus, the role of animal and bird species in influenza virus development, and risk assessment.7
TABLE 2
CDC response to avian influenza
|
TABLE 3
General precautions for travel to countries with avian influenza outbreaks*
|
| * As of February 4, 2005 this was directed at travelers to Vietnam only; see reference #6 for complete details. |
Awareness the best defense now
If avian influenza makes the leap to person-to-person transmission, family physicians across the globe will be at the forefront of diagnosis and treatment. Though no immediate actions are necessary, we all must follow developments and support the work of health departments at improving their ability to monitor emerging outbreaks. The interconnectedness of global health is well exemplified in the concern about avian flu and the efforts to prevent an influenza pandemic.
Correspondence
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Altman L. UN agency to urge vaccinations to health avian flu. New York Times, July 30, 2004.
2. World Health Organization. Disease surveillance. Cumulative number of confirmed cases of human avian influenza. WHO Communicable. Available at: www.who.int/csr/disease/avian_influenza/country/cas es_table_2005_02_02/en/. Accessed on April 10. 2005.
3. Monto A. The threat of an avian influenza pandemic. New Engl J Med 2005;352:323-325.
4. Centers for Disease Control and Prevention (CDC) Avian Influenza home page. Available at: www.cdc.gov/flu/avian/index.htm. Accessed on April 10, 2005.
5. Ungchusak K, Auewarakul P, Dowell S, et al. Probable person-person transmission of avian influenza (H5N1). New Engl J Med 2005;352:333-340.
6. CDC Asian Influenza, Vietnam, Notice to Travelers. Updated February 4, 2005. Available at: www.cdc.gov/travel/other/avian_flu_vietnam_2005_tra velers.htm. Accessed on April 10, 2005.
7. Stohr K. Avian influenza and pandemics. New Engl J Med 2005;352:405-407.
1. Altman L. UN agency to urge vaccinations to health avian flu. New York Times, July 30, 2004.
2. World Health Organization. Disease surveillance. Cumulative number of confirmed cases of human avian influenza. WHO Communicable. Available at: www.who.int/csr/disease/avian_influenza/country/cas es_table_2005_02_02/en/. Accessed on April 10. 2005.
3. Monto A. The threat of an avian influenza pandemic. New Engl J Med 2005;352:323-325.
4. Centers for Disease Control and Prevention (CDC) Avian Influenza home page. Available at: www.cdc.gov/flu/avian/index.htm. Accessed on April 10, 2005.
5. Ungchusak K, Auewarakul P, Dowell S, et al. Probable person-person transmission of avian influenza (H5N1). New Engl J Med 2005;352:333-340.
6. CDC Asian Influenza, Vietnam, Notice to Travelers. Updated February 4, 2005. Available at: www.cdc.gov/travel/other/avian_flu_vietnam_2005_tra velers.htm. Accessed on April 10, 2005.
7. Stohr K. Avian influenza and pandemics. New Engl J Med 2005;352:405-407.
Consumer-directed health care: One step forward, two steps back?
The way to manage rising health care costs—espoused by some analysts and the current administration—is to give consumers greater control over health care decisions through a concept termed consumer-directed health care (CDHC). Sounds good. But how would the likely implications really play out?
CDHC in a nutshell
Herzlinger, a CDHC proponent from the Harvard Business School, describes the concept’s key principles:
- Insurers and providers freely design and price their services to offer good value for the money.
- Consumers receive excellent information about the costs, quality, and scope of services, so they can make better health care purchasing decisions.
- Consumers buy health insurance plans, sometimes with employer funds, knowing their full costs so they can obtain good value for the money.1
In the US, CDHC combines a high-deductible health plan (HDHP) with a health savings account (HSA). In 2004, the consulting firm Mercer estimated that just 1% of all covered employees were in consumer-directed health plans, but 26% of all employers were likely to offer a CDHP within the next 2 years.
Recently, Humana introduced a health plan that could be combined with an HSA. UnitedHealth Group bought Definity Health, which specializes in HSAs. Blue Cross/Blue Shield announced it would offer HSA-compatible health plans nationwide by 2006, and Kaiser said it would do the same by 2005.2 The American Medical Association has made the provision of HSAs a key part of its health policy agenda.3
High-deductible health plans
An HDHP is a health insurance policy with a minimum deductible of $1050 for self or $2100 for family coverage. The minimum deductible amount will likely increase yearly. The policy’s annual out-of-pocket expenses, including deductibles and co-pays, cannot exceed $5000 for self or $10,000 for family coverage (Table 1).
The program may offer medical services through the variety of managed care options such as health maintenance organization (HMO), preferred provider organization (PPO), or point of service plans with in-network and out-of-network providers. Persons using in-net-work options save money by receiving price discounts on services.
Companies may offer HDHPs with no deductible for preventive services (eg, physicals, immunizations, screening tests, prenatal and well child care) and higher deductibles and co-pays for using out-of-network providers.4
TABLE 1
Allowable limits on HDHP and HSA accounts
| High-deductible health plan (HDHP) | |
| Minimum deductible: | |
| Individual | $1050 |
| Family | $2100 |
| Maximum out-of-pocket spending | |
| Individual | $5000 |
| Family | $10,000 |
| Health savings account (HSA) | |
| Maximum annual contribution | |
| Whichever is lesser: the HDHP deductible, or | |
| Individual | $2600 |
| Family | $5150 |
Health savings account: how it works
An HSA is a tax-exempt personal savings account used to pay for qualified medical expenses. Think of it as an IRA for health. Legislation to establish HSAs was included in the Medicare Prescription Drug Bill of 2003.
To set up an HSA, a consumer must have an HDHP, have no other health insurance, and be ineligible for Medicare. Individuals can sign up on their own through insurance companies (including the American Medical Association insurance company) or banks, or may be offered an HSA option through their employer. Contributions to the account can be made by individuals, by an employer, or both. If made by the individual, contributions are tax exempt; if by the employer, they are not taxable as income to the employee.
The maximum deposit that can be made in 2005 is the lesser of either the HDHP deductible or $2650 for the individual or $5250 for family coverage. These amounts will be indexed to inflation yearly. Individuals aged 55 to 65 can make catch-up contributions. Once eligible for Medicare, you can no longer contribute to an HSA.4,5
Funds in an HSA are usually controlled by the individual who sets up the account. Withdrawals are tax-exempt if used for qualified medical expenses. If used for other expenses, withdrawals are taxed and subject to a tax penalty. Monies in an HSA can accrue tax-exempt savings from investments (stocks, bonds, etc) and be rolled over each year with no maximum cap. Since the individual owns the account, it is portable. If a person moves, the account moves too. However, contributions to an HSA must stop if the person is no longer enrolled in an HDHP.
After age 65, a person may continue to use an HSA for medical expenses or to pay insurance premiums like Medicare Part B and Medicare HMOs, or the funds can be taxed and used for non-medical expenses. In addition to the usual services covered in a traditional health plan, the list of qualified medical expenses is quite extensive (Table 2). Cosmetic surgery is generally not a qualified expense. The general goal is to have enough funds in the HSA to cover all medical expenses before the deductible in the health insurance plan is met.4,5
TABLE 2
Qualified CDHC medical coverage beyond traditional services
| Certain alternative medicine therapies |
| Substance abuse therapy |
| Ambulance service |
| Medical equipment and home remodeling related to medical requirements |
| Reproductive health services |
| Vision, hearing aides, and dental care |
| Certain health insurance premium costs |
| Long-term care |
| Medications and home oxygen |
| Mental health services |
| Source: Internal Revenue Service Publication 502. Available at: http://www.irs.gov/publications/p502/ar02.html#d0e516. Accessed on February 1, 2005. |
Public opinion generally unfavorable
A recent survey by the Kaiser Family Foundation found that 73% of respondents with employer-sponsored insurance had an unfavorable view of a health plan that combined an HDHP with an HSA, and 78% said they would feel vulnerable to high medical bills with this type of coverage.6
Iimplications of cdhc
Advocates of CDHC believe the financial disincentives of co-pays and high deductibles will encourage consumers to reduce their use of marginal services and to seek lower-cost, higher quality providers. They cite early studies showing CDHC participants decreased their use of certain medical services while increasing their use of preventive services and maintaining a balance in their HSA from one year to the next.1
Opponents of CDHC emphasize research that shows patients who pay more of their health care bills consume less care, including essential care. The RAND study of the 1970s confirmed that greater cost-sharing by patients reduced the chance they would receive effective medical care. This was particularly so for low-income patients. A recent study showed that increased medication cost-sharing led patients to stop using important drugs like statins and ACE inhibitors.7
How accessible/usable are health data?
Herzlinger cites informed consumer choices as a strength of the CDHC concept. However, the amount of information on cost and quality of health care is limited, albeit growing. More worrisome perhaps, there is little evidence that most patients can use this kind of information to make good health care decisions.
Who would benefit, who would not?
Another concern is that HDHPs and HSAs will more likely appeal to healthier, well-off people who can take full advantage of the tax incentives and more readily fund their accounts. If a significant number of consumers in this group moves toward CDHC plans, it would leave more unhealthy people in the traditional insurance system. This, in turn, would lead insurers to increase premiums for those less healthy consumers, thus making their insurance more expensive and, ironically, increasing the numbers of uninsured. CDHC plans may also appeal to the uninsured and those who have difficulty paying the usual health insurance premiums. This group is likely to have more difficulty fully funding their HSAs and, consequently, they will need to pay more of their deductible costs out-of-pocket, which can create a disincentive to seek needed care.
In an alternative analysis of CDHC, Robinson sees HSA products representing an evolution from collective insurance in which those in good health help finance the care of unhealthy enrollees with high expenditures (traditional health insurance with its “use it or lose it” design) to one in which unspent balances are retained by healthy enrollees rather than diverted to pay for the care of others (an HSA account with its “use it or save it” design).
In this scenario, healthy (and often well-off) consumers are favored by low-premium, high-deductible products. The savings are also financially protected from chronically ill users who would pay more in deductibles and coinsurance. The negative consequence is further diminishment of the already fragile social pooling effect of the current health insurance system and the potential for increasing the plight of the uninsured and underinsured.8
While it is likely that CDHC will attract more participants, it remains to be seen whether the public will support the concept if reports start appearing of significant numbers of patients refusing recommended services when faced with high deductibles and large out-of-pocket costs. CDHC will likely look attractive to healthy and well-off consumers, but its ability to control costs and improve quality in our already stressed health care system is suspect.
Correspondence
Eric Henley, MD, MPH, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Herzlinger R, Parsa-Parsi R. Consumer-driven health care. JAMA 2004;292:1213-1220.
2. Vogt K. Doctors looking at details as interest in HSAs grows. American Medical News, December 20, 2004.
3. Health savings accounts at a glance American Medical Association web site. November 2004. Available at: www.ama-assn.org/ama1/pub/upload/mm/363/hsabrochure.pdf. Accessed on February 1, 2005.
4. High deductible health plans with health savings accounts Office of Personnel Management web site. Available at: www.opm.gov/hsa/faq.asp. Accessed February 1, 2005.
5. US Department of the Treasury Health savings accounts. Available at: http://www.ustreas.gov/offices/public-affairs/hsa/. Accessed on February 1, 2005.
6. Kaiser Family Foundation. Kaiser health poll report, September/October 2004. Available at www.kff.org/healthpollreport/Oct_2004/13.cfm. Accessed February 1, 2005.
7. Davis K. Consumer-directed health care: Will it improve health system performance? Health Services Research 2004;39:1219-1233.
8. Robinson J. Reinvention of health insurance in the consumer era. JAMA 2004;291:1880-1886.
The way to manage rising health care costs—espoused by some analysts and the current administration—is to give consumers greater control over health care decisions through a concept termed consumer-directed health care (CDHC). Sounds good. But how would the likely implications really play out?
CDHC in a nutshell
Herzlinger, a CDHC proponent from the Harvard Business School, describes the concept’s key principles:
- Insurers and providers freely design and price their services to offer good value for the money.
- Consumers receive excellent information about the costs, quality, and scope of services, so they can make better health care purchasing decisions.
- Consumers buy health insurance plans, sometimes with employer funds, knowing their full costs so they can obtain good value for the money.1
In the US, CDHC combines a high-deductible health plan (HDHP) with a health savings account (HSA). In 2004, the consulting firm Mercer estimated that just 1% of all covered employees were in consumer-directed health plans, but 26% of all employers were likely to offer a CDHP within the next 2 years.
Recently, Humana introduced a health plan that could be combined with an HSA. UnitedHealth Group bought Definity Health, which specializes in HSAs. Blue Cross/Blue Shield announced it would offer HSA-compatible health plans nationwide by 2006, and Kaiser said it would do the same by 2005.2 The American Medical Association has made the provision of HSAs a key part of its health policy agenda.3
High-deductible health plans
An HDHP is a health insurance policy with a minimum deductible of $1050 for self or $2100 for family coverage. The minimum deductible amount will likely increase yearly. The policy’s annual out-of-pocket expenses, including deductibles and co-pays, cannot exceed $5000 for self or $10,000 for family coverage (Table 1).
The program may offer medical services through the variety of managed care options such as health maintenance organization (HMO), preferred provider organization (PPO), or point of service plans with in-network and out-of-network providers. Persons using in-net-work options save money by receiving price discounts on services.
Companies may offer HDHPs with no deductible for preventive services (eg, physicals, immunizations, screening tests, prenatal and well child care) and higher deductibles and co-pays for using out-of-network providers.4
TABLE 1
Allowable limits on HDHP and HSA accounts
| High-deductible health plan (HDHP) | |
| Minimum deductible: | |
| Individual | $1050 |
| Family | $2100 |
| Maximum out-of-pocket spending | |
| Individual | $5000 |
| Family | $10,000 |
| Health savings account (HSA) | |
| Maximum annual contribution | |
| Whichever is lesser: the HDHP deductible, or | |
| Individual | $2600 |
| Family | $5150 |
Health savings account: how it works
An HSA is a tax-exempt personal savings account used to pay for qualified medical expenses. Think of it as an IRA for health. Legislation to establish HSAs was included in the Medicare Prescription Drug Bill of 2003.
To set up an HSA, a consumer must have an HDHP, have no other health insurance, and be ineligible for Medicare. Individuals can sign up on their own through insurance companies (including the American Medical Association insurance company) or banks, or may be offered an HSA option through their employer. Contributions to the account can be made by individuals, by an employer, or both. If made by the individual, contributions are tax exempt; if by the employer, they are not taxable as income to the employee.
The maximum deposit that can be made in 2005 is the lesser of either the HDHP deductible or $2650 for the individual or $5250 for family coverage. These amounts will be indexed to inflation yearly. Individuals aged 55 to 65 can make catch-up contributions. Once eligible for Medicare, you can no longer contribute to an HSA.4,5
Funds in an HSA are usually controlled by the individual who sets up the account. Withdrawals are tax-exempt if used for qualified medical expenses. If used for other expenses, withdrawals are taxed and subject to a tax penalty. Monies in an HSA can accrue tax-exempt savings from investments (stocks, bonds, etc) and be rolled over each year with no maximum cap. Since the individual owns the account, it is portable. If a person moves, the account moves too. However, contributions to an HSA must stop if the person is no longer enrolled in an HDHP.
After age 65, a person may continue to use an HSA for medical expenses or to pay insurance premiums like Medicare Part B and Medicare HMOs, or the funds can be taxed and used for non-medical expenses. In addition to the usual services covered in a traditional health plan, the list of qualified medical expenses is quite extensive (Table 2). Cosmetic surgery is generally not a qualified expense. The general goal is to have enough funds in the HSA to cover all medical expenses before the deductible in the health insurance plan is met.4,5
TABLE 2
Qualified CDHC medical coverage beyond traditional services
| Certain alternative medicine therapies |
| Substance abuse therapy |
| Ambulance service |
| Medical equipment and home remodeling related to medical requirements |
| Reproductive health services |
| Vision, hearing aides, and dental care |
| Certain health insurance premium costs |
| Long-term care |
| Medications and home oxygen |
| Mental health services |
| Source: Internal Revenue Service Publication 502. Available at: http://www.irs.gov/publications/p502/ar02.html#d0e516. Accessed on February 1, 2005. |
Public opinion generally unfavorable
A recent survey by the Kaiser Family Foundation found that 73% of respondents with employer-sponsored insurance had an unfavorable view of a health plan that combined an HDHP with an HSA, and 78% said they would feel vulnerable to high medical bills with this type of coverage.6
Iimplications of cdhc
Advocates of CDHC believe the financial disincentives of co-pays and high deductibles will encourage consumers to reduce their use of marginal services and to seek lower-cost, higher quality providers. They cite early studies showing CDHC participants decreased their use of certain medical services while increasing their use of preventive services and maintaining a balance in their HSA from one year to the next.1
Opponents of CDHC emphasize research that shows patients who pay more of their health care bills consume less care, including essential care. The RAND study of the 1970s confirmed that greater cost-sharing by patients reduced the chance they would receive effective medical care. This was particularly so for low-income patients. A recent study showed that increased medication cost-sharing led patients to stop using important drugs like statins and ACE inhibitors.7
How accessible/usable are health data?
Herzlinger cites informed consumer choices as a strength of the CDHC concept. However, the amount of information on cost and quality of health care is limited, albeit growing. More worrisome perhaps, there is little evidence that most patients can use this kind of information to make good health care decisions.
Who would benefit, who would not?
Another concern is that HDHPs and HSAs will more likely appeal to healthier, well-off people who can take full advantage of the tax incentives and more readily fund their accounts. If a significant number of consumers in this group moves toward CDHC plans, it would leave more unhealthy people in the traditional insurance system. This, in turn, would lead insurers to increase premiums for those less healthy consumers, thus making their insurance more expensive and, ironically, increasing the numbers of uninsured. CDHC plans may also appeal to the uninsured and those who have difficulty paying the usual health insurance premiums. This group is likely to have more difficulty fully funding their HSAs and, consequently, they will need to pay more of their deductible costs out-of-pocket, which can create a disincentive to seek needed care.
In an alternative analysis of CDHC, Robinson sees HSA products representing an evolution from collective insurance in which those in good health help finance the care of unhealthy enrollees with high expenditures (traditional health insurance with its “use it or lose it” design) to one in which unspent balances are retained by healthy enrollees rather than diverted to pay for the care of others (an HSA account with its “use it or save it” design).
In this scenario, healthy (and often well-off) consumers are favored by low-premium, high-deductible products. The savings are also financially protected from chronically ill users who would pay more in deductibles and coinsurance. The negative consequence is further diminishment of the already fragile social pooling effect of the current health insurance system and the potential for increasing the plight of the uninsured and underinsured.8
While it is likely that CDHC will attract more participants, it remains to be seen whether the public will support the concept if reports start appearing of significant numbers of patients refusing recommended services when faced with high deductibles and large out-of-pocket costs. CDHC will likely look attractive to healthy and well-off consumers, but its ability to control costs and improve quality in our already stressed health care system is suspect.
Correspondence
Eric Henley, MD, MPH, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
The way to manage rising health care costs—espoused by some analysts and the current administration—is to give consumers greater control over health care decisions through a concept termed consumer-directed health care (CDHC). Sounds good. But how would the likely implications really play out?
CDHC in a nutshell
Herzlinger, a CDHC proponent from the Harvard Business School, describes the concept’s key principles:
- Insurers and providers freely design and price their services to offer good value for the money.
- Consumers receive excellent information about the costs, quality, and scope of services, so they can make better health care purchasing decisions.
- Consumers buy health insurance plans, sometimes with employer funds, knowing their full costs so they can obtain good value for the money.1
In the US, CDHC combines a high-deductible health plan (HDHP) with a health savings account (HSA). In 2004, the consulting firm Mercer estimated that just 1% of all covered employees were in consumer-directed health plans, but 26% of all employers were likely to offer a CDHP within the next 2 years.
Recently, Humana introduced a health plan that could be combined with an HSA. UnitedHealth Group bought Definity Health, which specializes in HSAs. Blue Cross/Blue Shield announced it would offer HSA-compatible health plans nationwide by 2006, and Kaiser said it would do the same by 2005.2 The American Medical Association has made the provision of HSAs a key part of its health policy agenda.3
High-deductible health plans
An HDHP is a health insurance policy with a minimum deductible of $1050 for self or $2100 for family coverage. The minimum deductible amount will likely increase yearly. The policy’s annual out-of-pocket expenses, including deductibles and co-pays, cannot exceed $5000 for self or $10,000 for family coverage (Table 1).
The program may offer medical services through the variety of managed care options such as health maintenance organization (HMO), preferred provider organization (PPO), or point of service plans with in-network and out-of-network providers. Persons using in-net-work options save money by receiving price discounts on services.
Companies may offer HDHPs with no deductible for preventive services (eg, physicals, immunizations, screening tests, prenatal and well child care) and higher deductibles and co-pays for using out-of-network providers.4
TABLE 1
Allowable limits on HDHP and HSA accounts
| High-deductible health plan (HDHP) | |
| Minimum deductible: | |
| Individual | $1050 |
| Family | $2100 |
| Maximum out-of-pocket spending | |
| Individual | $5000 |
| Family | $10,000 |
| Health savings account (HSA) | |
| Maximum annual contribution | |
| Whichever is lesser: the HDHP deductible, or | |
| Individual | $2600 |
| Family | $5150 |
Health savings account: how it works
An HSA is a tax-exempt personal savings account used to pay for qualified medical expenses. Think of it as an IRA for health. Legislation to establish HSAs was included in the Medicare Prescription Drug Bill of 2003.
To set up an HSA, a consumer must have an HDHP, have no other health insurance, and be ineligible for Medicare. Individuals can sign up on their own through insurance companies (including the American Medical Association insurance company) or banks, or may be offered an HSA option through their employer. Contributions to the account can be made by individuals, by an employer, or both. If made by the individual, contributions are tax exempt; if by the employer, they are not taxable as income to the employee.
The maximum deposit that can be made in 2005 is the lesser of either the HDHP deductible or $2650 for the individual or $5250 for family coverage. These amounts will be indexed to inflation yearly. Individuals aged 55 to 65 can make catch-up contributions. Once eligible for Medicare, you can no longer contribute to an HSA.4,5
Funds in an HSA are usually controlled by the individual who sets up the account. Withdrawals are tax-exempt if used for qualified medical expenses. If used for other expenses, withdrawals are taxed and subject to a tax penalty. Monies in an HSA can accrue tax-exempt savings from investments (stocks, bonds, etc) and be rolled over each year with no maximum cap. Since the individual owns the account, it is portable. If a person moves, the account moves too. However, contributions to an HSA must stop if the person is no longer enrolled in an HDHP.
After age 65, a person may continue to use an HSA for medical expenses or to pay insurance premiums like Medicare Part B and Medicare HMOs, or the funds can be taxed and used for non-medical expenses. In addition to the usual services covered in a traditional health plan, the list of qualified medical expenses is quite extensive (Table 2). Cosmetic surgery is generally not a qualified expense. The general goal is to have enough funds in the HSA to cover all medical expenses before the deductible in the health insurance plan is met.4,5
TABLE 2
Qualified CDHC medical coverage beyond traditional services
| Certain alternative medicine therapies |
| Substance abuse therapy |
| Ambulance service |
| Medical equipment and home remodeling related to medical requirements |
| Reproductive health services |
| Vision, hearing aides, and dental care |
| Certain health insurance premium costs |
| Long-term care |
| Medications and home oxygen |
| Mental health services |
| Source: Internal Revenue Service Publication 502. Available at: http://www.irs.gov/publications/p502/ar02.html#d0e516. Accessed on February 1, 2005. |
Public opinion generally unfavorable
A recent survey by the Kaiser Family Foundation found that 73% of respondents with employer-sponsored insurance had an unfavorable view of a health plan that combined an HDHP with an HSA, and 78% said they would feel vulnerable to high medical bills with this type of coverage.6
Iimplications of cdhc
Advocates of CDHC believe the financial disincentives of co-pays and high deductibles will encourage consumers to reduce their use of marginal services and to seek lower-cost, higher quality providers. They cite early studies showing CDHC participants decreased their use of certain medical services while increasing their use of preventive services and maintaining a balance in their HSA from one year to the next.1
Opponents of CDHC emphasize research that shows patients who pay more of their health care bills consume less care, including essential care. The RAND study of the 1970s confirmed that greater cost-sharing by patients reduced the chance they would receive effective medical care. This was particularly so for low-income patients. A recent study showed that increased medication cost-sharing led patients to stop using important drugs like statins and ACE inhibitors.7
How accessible/usable are health data?
Herzlinger cites informed consumer choices as a strength of the CDHC concept. However, the amount of information on cost and quality of health care is limited, albeit growing. More worrisome perhaps, there is little evidence that most patients can use this kind of information to make good health care decisions.
Who would benefit, who would not?
Another concern is that HDHPs and HSAs will more likely appeal to healthier, well-off people who can take full advantage of the tax incentives and more readily fund their accounts. If a significant number of consumers in this group moves toward CDHC plans, it would leave more unhealthy people in the traditional insurance system. This, in turn, would lead insurers to increase premiums for those less healthy consumers, thus making their insurance more expensive and, ironically, increasing the numbers of uninsured. CDHC plans may also appeal to the uninsured and those who have difficulty paying the usual health insurance premiums. This group is likely to have more difficulty fully funding their HSAs and, consequently, they will need to pay more of their deductible costs out-of-pocket, which can create a disincentive to seek needed care.
In an alternative analysis of CDHC, Robinson sees HSA products representing an evolution from collective insurance in which those in good health help finance the care of unhealthy enrollees with high expenditures (traditional health insurance with its “use it or lose it” design) to one in which unspent balances are retained by healthy enrollees rather than diverted to pay for the care of others (an HSA account with its “use it or save it” design).
In this scenario, healthy (and often well-off) consumers are favored by low-premium, high-deductible products. The savings are also financially protected from chronically ill users who would pay more in deductibles and coinsurance. The negative consequence is further diminishment of the already fragile social pooling effect of the current health insurance system and the potential for increasing the plight of the uninsured and underinsured.8
While it is likely that CDHC will attract more participants, it remains to be seen whether the public will support the concept if reports start appearing of significant numbers of patients refusing recommended services when faced with high deductibles and large out-of-pocket costs. CDHC will likely look attractive to healthy and well-off consumers, but its ability to control costs and improve quality in our already stressed health care system is suspect.
Correspondence
Eric Henley, MD, MPH, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Herzlinger R, Parsa-Parsi R. Consumer-driven health care. JAMA 2004;292:1213-1220.
2. Vogt K. Doctors looking at details as interest in HSAs grows. American Medical News, December 20, 2004.
3. Health savings accounts at a glance American Medical Association web site. November 2004. Available at: www.ama-assn.org/ama1/pub/upload/mm/363/hsabrochure.pdf. Accessed on February 1, 2005.
4. High deductible health plans with health savings accounts Office of Personnel Management web site. Available at: www.opm.gov/hsa/faq.asp. Accessed February 1, 2005.
5. US Department of the Treasury Health savings accounts. Available at: http://www.ustreas.gov/offices/public-affairs/hsa/. Accessed on February 1, 2005.
6. Kaiser Family Foundation. Kaiser health poll report, September/October 2004. Available at www.kff.org/healthpollreport/Oct_2004/13.cfm. Accessed February 1, 2005.
7. Davis K. Consumer-directed health care: Will it improve health system performance? Health Services Research 2004;39:1219-1233.
8. Robinson J. Reinvention of health insurance in the consumer era. JAMA 2004;291:1880-1886.
1. Herzlinger R, Parsa-Parsi R. Consumer-driven health care. JAMA 2004;292:1213-1220.
2. Vogt K. Doctors looking at details as interest in HSAs grows. American Medical News, December 20, 2004.
3. Health savings accounts at a glance American Medical Association web site. November 2004. Available at: www.ama-assn.org/ama1/pub/upload/mm/363/hsabrochure.pdf. Accessed on February 1, 2005.
4. High deductible health plans with health savings accounts Office of Personnel Management web site. Available at: www.opm.gov/hsa/faq.asp. Accessed February 1, 2005.
5. US Department of the Treasury Health savings accounts. Available at: http://www.ustreas.gov/offices/public-affairs/hsa/. Accessed on February 1, 2005.
6. Kaiser Family Foundation. Kaiser health poll report, September/October 2004. Available at www.kff.org/healthpollreport/Oct_2004/13.cfm. Accessed February 1, 2005.
7. Davis K. Consumer-directed health care: Will it improve health system performance? Health Services Research 2004;39:1219-1233.
8. Robinson J. Reinvention of health insurance in the consumer era. JAMA 2004;291:1880-1886.
Acting on synergies between clinic and community strategies to improve preventive medicine
From their days in training through their years of practice, family physicians emphasize preventive medicine. They counsel patients on diet and exercise, safe sex, and substance abuse; screen for early detection of cancer (eg, cervical, breast, colon, prostate); and administer chemo- and immunoprophylaxis (daily aspirin, vaccinations).
Though physicians are accustomed to caring for individuals, adopting a population perspective—considering the practice’s patient panel or even the larger community—is a logical extension of one’s daily practice. The public’s health benefits from parallel efforts in risk assessment and community-wide prevention. And, as we propose here, awareness of the similarities between the 2 areas of endeavor strengthens both.
Assessing risk for the individual
Risk factors are characteristics of a person that increase the likelihood of becoming diseased. Obvious risk factors include physical traits and laboratory values such as obesity, high cholesterol levels, and high blood pressure, and behaviors such as smoking and binge drinking. Other risk factors include demographic traits (age, race/ethnicity, gender, income), environmental influences (occupation, geographic location), and system issues (insurance status, usual source of care). Though risk factors may not cause disease, their presence can increase the probability that disease will eventually develop.
The degree to which a risk factor may influence disease development can be calculated with 2 measures.
Absolute risk is the difference between the incidence of disease in the group exposed to a risk factor and the incidence in the group not exposed to the factor.
Relative risk is the extent to which persons exposed to a risk are likely to develop the disease compared with those not exposed (see Calculating relative risk). Relative risk is more useful for judging a factor’s strength of disease causality, but it does not necessarily indicate the magnitude of risk for a population. With an uncommon disease, for instance, the relative risk of disease from an exposure may be large but the absolute risk may be small.
Relative risk—the likelihood that those exposed to a risk factor for disease will become diseased, compared with those not exposed to the factor—is calculated as follows:
Relative risk = (a/a+b) / (c/c+d)
The incidence of disease among those exposed (a divided by a+b) divided by the indicence of disease among those not exposed (c divided by c+d).
For more on relative risk, see “Relative risks and odds ratios: What’s the difference?,” in the February 2004 JFP.
Assessing risk for a population
Just as absolute and relative risk help quantify an individual’s susceptibility to disease, the population attributable risk helps gauge the level of risk to a community. The calculation takes into account disease incidence as well as how often the population is exposed to a related risk factor. This measure can be particularly influential in health policy decisions such as how to spend scarce resources—illustrated in the following example.2
Individual and population risks are determined in part by the prevalence of a risk factor. Consider the risk of death in the case of hypertension. For an individual, the risk of death is greater with severe hypertension than with mild hypertension. However, mild hypertension is quite common; severe hypertension is not. Therefore, the population attributable risk of hypertension (the number of extra deaths in the population attributable to hypertension) is greater for those with mild hypertension, even though the risk to an individual is greatest when hypertension is severe. This suggests that more lives can be saved by treating lots of people who have mild hypertension than by treating the much smaller number of people with severe hypertension—a concept called the “prevention paradox,” and one that underlies a number of national prevention efforts.
Synergies between individual and community prevention
Individual and community prevention strategies both have merit. For example, public health smoking cessation campaigns have been effective at the population level. At the same time, physician efforts to promote smoking cessation through office counseling sessions are effective with individuals. Individual physician efforts become population efforts if thousands of physicians each day provide cessation counseling to their patients. Public health campaigns help accomplish this by reinforcing the importance of cessation efforts including those delivered by practicing physicians.
Another excellent example of this type of reinforcing synergy is the work of the past decade to address hyperlipidemia as a risk factor for heart disease. In this effort, physician screening and treatment of patients has been both promoted and complemented by national media campaigns, local health fairs, and ongoing research, to the point where many individuals now are familiar with cholesterol and want to know their own lipid values.
Drawing on both arenas to prioritize prevention strategies
The federal government has supported the development of several types of evidence-based prevention guidelines. Family physicians are familiar with the US Preventive Service Task Force Guide to Clinical Preventive Services,3 but may be less familiar with the more recently developed Guide to Community Preventive Services.4 For a comprehensive continuum of preventive care, both guides can be used in combination. Using both sets of recommendations can help physicians to prioritize prevention strategies for individuals, office patient populations, and communities.
The Community Guide is a federally sponsored initiative producing evidenced-based recommendations for health promotion and disease prevention from a population-based perspective sponsored by the Centers for Disease Control and Prevention (Table 1). Like the Guide to Clinical Preventive Services, the independent task force that oversees the Community Guide develops evidence-based reviews of potential interventions and translates the findings into recommendations that can be used by policy makers, public health entities, and health systems.
TABLE 1
Topics covered in the Guide to Community Preventive Services
|
Actionable recommendations for practitioners
Though the Community Guide takes a population-based approach to prevention (eg, recommendations for policy strategies, mass media campaigns, and school health programs), a number of its recommendations focus on the health care system and are directly applicable to practicing family physicians. For instance:
- Vaccination: a section on provider-based interventions such as reminder systems, standing orders for adults, and provider feedback strategies
- Diabetes: a section on disease management strategies
- Tobacco: a section on improving the delivery of cessation services
- Cancer: a section on improving screening for specific diseases.
The topic reviews grade the strength of each intervention (strong evidence, sufficient evidence, or insufficient evidence), provide a 1-page summary of the recommendations, and have links to longer papers that review the specific evidence.
Complementary web support
The website also includes an excellent 2-page summary of types of activities family doctors can undertake, with links to specific directions for implementing prevention activities. Included are patient and provider reminder systems, disease and case management, and use of standing orders.4
The recommendations in both the Clinical and Community Guides that address common conditions provide especially strong “roadmaps” for focusing on and addressing health promotion and disease prevention. By using both guides, physicians can develop a range of effective, evidence-based approaches for practice. For example, Table 2 presents evidence-based recommendations concerning smoking. These recommendations fall across a range of preventive interventions.
Producing successful initiatives
Using the guides as a reference, the family physician could initiate screening of patients for smoking status in the practice and then implement one or more of the recommended strategies listed in Table 2. Possibilities include adding a provider reminder system, delivering brief counseling to quit smoking, prescribing cessation medications, and coupling these efforts with advocacy for development of a media campaign in the community. Family physicians who undertake community prevention efforts outside the office setting are likely to improve their success by partnering with other community health and social service professionals to implement agreed upon prevention interventions.5
As more topics are developed for the Community Guide, family physicians will likely find more reasons to refer to it as a companion to the Clinical Guide. Using the 2 together will enhance the benefit gained from using each alone in a way that is analogous to the added health benefits obtained when the traditional health care system works more closely with the public health system.
TABLE 2
Intervention strategies in the clinical and community guides
| Intervention strategy recommendation | Where it’s found | Where it’s to be used |
|---|---|---|
| Screening patients for tobacco use | Clinical Guide | Office |
| Provider gives brief advise to quit to patients who use tobacco | Clinical Guide | Office |
| Provider counseling to patients on tobacco cessation | Clinical Guide | Office |
| Self-help education materials for patients who use tobacco | Clinical Guide | Office |
| Pharmacologic treatment for tobacco and dependence | Clinical Guide | Office |
| Provider reminder systems | Community Guide | Office |
| Multicomponent clinical program (provider reminder + education) | Community Guide | Office |
| Patient-oriented interventions (telephone support; sliding fee scale) | Community Guide | Office |
| Policies, regulations, and laws (smoking ban and restrictions) | Community Guide | Community |
| Mass media campaigns | Community Guide | Community |
| Sources: Adapted from USPSTF, Guide to Clinical Preventive Services, 19963; | ||
| USPSTF, Guide to Community Preventive Services, 2000.4 | ||
Correspondence
Eric Henley, MD, MPH, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Turabian JL, Perez-Franco B. Cual es el sentido de la educacion para la salud y las actividades “communitaria” en atencion primaria? Aten Primaria. 1998;22:662-666.
2. Fletcher R, Fletcher S, Wagner E. Clinical Epidemiology: The Essentials. Philadelphia, Pa: Williams and Wilkins; 1996.
3. US Preventive Services Task Force. Guide to Clinical Preventive Services. Available at: www.ahcpr.gov/clinic/cpsix.htm
4. US Preventive Services Task Force. Guide to Community Preventive Services. Available at: www.thecommunityguide.org.
5. Peters K, Elster A. Population based medicine: Roadmaps to clinical practice. Chicago, Ill: American Medical Association, 2002.
From their days in training through their years of practice, family physicians emphasize preventive medicine. They counsel patients on diet and exercise, safe sex, and substance abuse; screen for early detection of cancer (eg, cervical, breast, colon, prostate); and administer chemo- and immunoprophylaxis (daily aspirin, vaccinations).
Though physicians are accustomed to caring for individuals, adopting a population perspective—considering the practice’s patient panel or even the larger community—is a logical extension of one’s daily practice. The public’s health benefits from parallel efforts in risk assessment and community-wide prevention. And, as we propose here, awareness of the similarities between the 2 areas of endeavor strengthens both.
Assessing risk for the individual
Risk factors are characteristics of a person that increase the likelihood of becoming diseased. Obvious risk factors include physical traits and laboratory values such as obesity, high cholesterol levels, and high blood pressure, and behaviors such as smoking and binge drinking. Other risk factors include demographic traits (age, race/ethnicity, gender, income), environmental influences (occupation, geographic location), and system issues (insurance status, usual source of care). Though risk factors may not cause disease, their presence can increase the probability that disease will eventually develop.
The degree to which a risk factor may influence disease development can be calculated with 2 measures.
Absolute risk is the difference between the incidence of disease in the group exposed to a risk factor and the incidence in the group not exposed to the factor.
Relative risk is the extent to which persons exposed to a risk are likely to develop the disease compared with those not exposed (see Calculating relative risk). Relative risk is more useful for judging a factor’s strength of disease causality, but it does not necessarily indicate the magnitude of risk for a population. With an uncommon disease, for instance, the relative risk of disease from an exposure may be large but the absolute risk may be small.
Relative risk—the likelihood that those exposed to a risk factor for disease will become diseased, compared with those not exposed to the factor—is calculated as follows:
Relative risk = (a/a+b) / (c/c+d)
The incidence of disease among those exposed (a divided by a+b) divided by the indicence of disease among those not exposed (c divided by c+d).
For more on relative risk, see “Relative risks and odds ratios: What’s the difference?,” in the February 2004 JFP.
Assessing risk for a population
Just as absolute and relative risk help quantify an individual’s susceptibility to disease, the population attributable risk helps gauge the level of risk to a community. The calculation takes into account disease incidence as well as how often the population is exposed to a related risk factor. This measure can be particularly influential in health policy decisions such as how to spend scarce resources—illustrated in the following example.2
Individual and population risks are determined in part by the prevalence of a risk factor. Consider the risk of death in the case of hypertension. For an individual, the risk of death is greater with severe hypertension than with mild hypertension. However, mild hypertension is quite common; severe hypertension is not. Therefore, the population attributable risk of hypertension (the number of extra deaths in the population attributable to hypertension) is greater for those with mild hypertension, even though the risk to an individual is greatest when hypertension is severe. This suggests that more lives can be saved by treating lots of people who have mild hypertension than by treating the much smaller number of people with severe hypertension—a concept called the “prevention paradox,” and one that underlies a number of national prevention efforts.
Synergies between individual and community prevention
Individual and community prevention strategies both have merit. For example, public health smoking cessation campaigns have been effective at the population level. At the same time, physician efforts to promote smoking cessation through office counseling sessions are effective with individuals. Individual physician efforts become population efforts if thousands of physicians each day provide cessation counseling to their patients. Public health campaigns help accomplish this by reinforcing the importance of cessation efforts including those delivered by practicing physicians.
Another excellent example of this type of reinforcing synergy is the work of the past decade to address hyperlipidemia as a risk factor for heart disease. In this effort, physician screening and treatment of patients has been both promoted and complemented by national media campaigns, local health fairs, and ongoing research, to the point where many individuals now are familiar with cholesterol and want to know their own lipid values.
Drawing on both arenas to prioritize prevention strategies
The federal government has supported the development of several types of evidence-based prevention guidelines. Family physicians are familiar with the US Preventive Service Task Force Guide to Clinical Preventive Services,3 but may be less familiar with the more recently developed Guide to Community Preventive Services.4 For a comprehensive continuum of preventive care, both guides can be used in combination. Using both sets of recommendations can help physicians to prioritize prevention strategies for individuals, office patient populations, and communities.
The Community Guide is a federally sponsored initiative producing evidenced-based recommendations for health promotion and disease prevention from a population-based perspective sponsored by the Centers for Disease Control and Prevention (Table 1). Like the Guide to Clinical Preventive Services, the independent task force that oversees the Community Guide develops evidence-based reviews of potential interventions and translates the findings into recommendations that can be used by policy makers, public health entities, and health systems.
TABLE 1
Topics covered in the Guide to Community Preventive Services
|
Actionable recommendations for practitioners
Though the Community Guide takes a population-based approach to prevention (eg, recommendations for policy strategies, mass media campaigns, and school health programs), a number of its recommendations focus on the health care system and are directly applicable to practicing family physicians. For instance:
- Vaccination: a section on provider-based interventions such as reminder systems, standing orders for adults, and provider feedback strategies
- Diabetes: a section on disease management strategies
- Tobacco: a section on improving the delivery of cessation services
- Cancer: a section on improving screening for specific diseases.
The topic reviews grade the strength of each intervention (strong evidence, sufficient evidence, or insufficient evidence), provide a 1-page summary of the recommendations, and have links to longer papers that review the specific evidence.
Complementary web support
The website also includes an excellent 2-page summary of types of activities family doctors can undertake, with links to specific directions for implementing prevention activities. Included are patient and provider reminder systems, disease and case management, and use of standing orders.4
The recommendations in both the Clinical and Community Guides that address common conditions provide especially strong “roadmaps” for focusing on and addressing health promotion and disease prevention. By using both guides, physicians can develop a range of effective, evidence-based approaches for practice. For example, Table 2 presents evidence-based recommendations concerning smoking. These recommendations fall across a range of preventive interventions.
Producing successful initiatives
Using the guides as a reference, the family physician could initiate screening of patients for smoking status in the practice and then implement one or more of the recommended strategies listed in Table 2. Possibilities include adding a provider reminder system, delivering brief counseling to quit smoking, prescribing cessation medications, and coupling these efforts with advocacy for development of a media campaign in the community. Family physicians who undertake community prevention efforts outside the office setting are likely to improve their success by partnering with other community health and social service professionals to implement agreed upon prevention interventions.5
As more topics are developed for the Community Guide, family physicians will likely find more reasons to refer to it as a companion to the Clinical Guide. Using the 2 together will enhance the benefit gained from using each alone in a way that is analogous to the added health benefits obtained when the traditional health care system works more closely with the public health system.
TABLE 2
Intervention strategies in the clinical and community guides
| Intervention strategy recommendation | Where it’s found | Where it’s to be used |
|---|---|---|
| Screening patients for tobacco use | Clinical Guide | Office |
| Provider gives brief advise to quit to patients who use tobacco | Clinical Guide | Office |
| Provider counseling to patients on tobacco cessation | Clinical Guide | Office |
| Self-help education materials for patients who use tobacco | Clinical Guide | Office |
| Pharmacologic treatment for tobacco and dependence | Clinical Guide | Office |
| Provider reminder systems | Community Guide | Office |
| Multicomponent clinical program (provider reminder + education) | Community Guide | Office |
| Patient-oriented interventions (telephone support; sliding fee scale) | Community Guide | Office |
| Policies, regulations, and laws (smoking ban and restrictions) | Community Guide | Community |
| Mass media campaigns | Community Guide | Community |
| Sources: Adapted from USPSTF, Guide to Clinical Preventive Services, 19963; | ||
| USPSTF, Guide to Community Preventive Services, 2000.4 | ||
Correspondence
Eric Henley, MD, MPH, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
From their days in training through their years of practice, family physicians emphasize preventive medicine. They counsel patients on diet and exercise, safe sex, and substance abuse; screen for early detection of cancer (eg, cervical, breast, colon, prostate); and administer chemo- and immunoprophylaxis (daily aspirin, vaccinations).
Though physicians are accustomed to caring for individuals, adopting a population perspective—considering the practice’s patient panel or even the larger community—is a logical extension of one’s daily practice. The public’s health benefits from parallel efforts in risk assessment and community-wide prevention. And, as we propose here, awareness of the similarities between the 2 areas of endeavor strengthens both.
Assessing risk for the individual
Risk factors are characteristics of a person that increase the likelihood of becoming diseased. Obvious risk factors include physical traits and laboratory values such as obesity, high cholesterol levels, and high blood pressure, and behaviors such as smoking and binge drinking. Other risk factors include demographic traits (age, race/ethnicity, gender, income), environmental influences (occupation, geographic location), and system issues (insurance status, usual source of care). Though risk factors may not cause disease, their presence can increase the probability that disease will eventually develop.
The degree to which a risk factor may influence disease development can be calculated with 2 measures.
Absolute risk is the difference between the incidence of disease in the group exposed to a risk factor and the incidence in the group not exposed to the factor.
Relative risk is the extent to which persons exposed to a risk are likely to develop the disease compared with those not exposed (see Calculating relative risk). Relative risk is more useful for judging a factor’s strength of disease causality, but it does not necessarily indicate the magnitude of risk for a population. With an uncommon disease, for instance, the relative risk of disease from an exposure may be large but the absolute risk may be small.
Relative risk—the likelihood that those exposed to a risk factor for disease will become diseased, compared with those not exposed to the factor—is calculated as follows:
Relative risk = (a/a+b) / (c/c+d)
The incidence of disease among those exposed (a divided by a+b) divided by the indicence of disease among those not exposed (c divided by c+d).
For more on relative risk, see “Relative risks and odds ratios: What’s the difference?,” in the February 2004 JFP.
Assessing risk for a population
Just as absolute and relative risk help quantify an individual’s susceptibility to disease, the population attributable risk helps gauge the level of risk to a community. The calculation takes into account disease incidence as well as how often the population is exposed to a related risk factor. This measure can be particularly influential in health policy decisions such as how to spend scarce resources—illustrated in the following example.2
Individual and population risks are determined in part by the prevalence of a risk factor. Consider the risk of death in the case of hypertension. For an individual, the risk of death is greater with severe hypertension than with mild hypertension. However, mild hypertension is quite common; severe hypertension is not. Therefore, the population attributable risk of hypertension (the number of extra deaths in the population attributable to hypertension) is greater for those with mild hypertension, even though the risk to an individual is greatest when hypertension is severe. This suggests that more lives can be saved by treating lots of people who have mild hypertension than by treating the much smaller number of people with severe hypertension—a concept called the “prevention paradox,” and one that underlies a number of national prevention efforts.
Synergies between individual and community prevention
Individual and community prevention strategies both have merit. For example, public health smoking cessation campaigns have been effective at the population level. At the same time, physician efforts to promote smoking cessation through office counseling sessions are effective with individuals. Individual physician efforts become population efforts if thousands of physicians each day provide cessation counseling to their patients. Public health campaigns help accomplish this by reinforcing the importance of cessation efforts including those delivered by practicing physicians.
Another excellent example of this type of reinforcing synergy is the work of the past decade to address hyperlipidemia as a risk factor for heart disease. In this effort, physician screening and treatment of patients has been both promoted and complemented by national media campaigns, local health fairs, and ongoing research, to the point where many individuals now are familiar with cholesterol and want to know their own lipid values.
Drawing on both arenas to prioritize prevention strategies
The federal government has supported the development of several types of evidence-based prevention guidelines. Family physicians are familiar with the US Preventive Service Task Force Guide to Clinical Preventive Services,3 but may be less familiar with the more recently developed Guide to Community Preventive Services.4 For a comprehensive continuum of preventive care, both guides can be used in combination. Using both sets of recommendations can help physicians to prioritize prevention strategies for individuals, office patient populations, and communities.
The Community Guide is a federally sponsored initiative producing evidenced-based recommendations for health promotion and disease prevention from a population-based perspective sponsored by the Centers for Disease Control and Prevention (Table 1). Like the Guide to Clinical Preventive Services, the independent task force that oversees the Community Guide develops evidence-based reviews of potential interventions and translates the findings into recommendations that can be used by policy makers, public health entities, and health systems.
TABLE 1
Topics covered in the Guide to Community Preventive Services
|
Actionable recommendations for practitioners
Though the Community Guide takes a population-based approach to prevention (eg, recommendations for policy strategies, mass media campaigns, and school health programs), a number of its recommendations focus on the health care system and are directly applicable to practicing family physicians. For instance:
- Vaccination: a section on provider-based interventions such as reminder systems, standing orders for adults, and provider feedback strategies
- Diabetes: a section on disease management strategies
- Tobacco: a section on improving the delivery of cessation services
- Cancer: a section on improving screening for specific diseases.
The topic reviews grade the strength of each intervention (strong evidence, sufficient evidence, or insufficient evidence), provide a 1-page summary of the recommendations, and have links to longer papers that review the specific evidence.
Complementary web support
The website also includes an excellent 2-page summary of types of activities family doctors can undertake, with links to specific directions for implementing prevention activities. Included are patient and provider reminder systems, disease and case management, and use of standing orders.4
The recommendations in both the Clinical and Community Guides that address common conditions provide especially strong “roadmaps” for focusing on and addressing health promotion and disease prevention. By using both guides, physicians can develop a range of effective, evidence-based approaches for practice. For example, Table 2 presents evidence-based recommendations concerning smoking. These recommendations fall across a range of preventive interventions.
Producing successful initiatives
Using the guides as a reference, the family physician could initiate screening of patients for smoking status in the practice and then implement one or more of the recommended strategies listed in Table 2. Possibilities include adding a provider reminder system, delivering brief counseling to quit smoking, prescribing cessation medications, and coupling these efforts with advocacy for development of a media campaign in the community. Family physicians who undertake community prevention efforts outside the office setting are likely to improve their success by partnering with other community health and social service professionals to implement agreed upon prevention interventions.5
As more topics are developed for the Community Guide, family physicians will likely find more reasons to refer to it as a companion to the Clinical Guide. Using the 2 together will enhance the benefit gained from using each alone in a way that is analogous to the added health benefits obtained when the traditional health care system works more closely with the public health system.
TABLE 2
Intervention strategies in the clinical and community guides
| Intervention strategy recommendation | Where it’s found | Where it’s to be used |
|---|---|---|
| Screening patients for tobacco use | Clinical Guide | Office |
| Provider gives brief advise to quit to patients who use tobacco | Clinical Guide | Office |
| Provider counseling to patients on tobacco cessation | Clinical Guide | Office |
| Self-help education materials for patients who use tobacco | Clinical Guide | Office |
| Pharmacologic treatment for tobacco and dependence | Clinical Guide | Office |
| Provider reminder systems | Community Guide | Office |
| Multicomponent clinical program (provider reminder + education) | Community Guide | Office |
| Patient-oriented interventions (telephone support; sliding fee scale) | Community Guide | Office |
| Policies, regulations, and laws (smoking ban and restrictions) | Community Guide | Community |
| Mass media campaigns | Community Guide | Community |
| Sources: Adapted from USPSTF, Guide to Clinical Preventive Services, 19963; | ||
| USPSTF, Guide to Community Preventive Services, 2000.4 | ||
Correspondence
Eric Henley, MD, MPH, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Turabian JL, Perez-Franco B. Cual es el sentido de la educacion para la salud y las actividades “communitaria” en atencion primaria? Aten Primaria. 1998;22:662-666.
2. Fletcher R, Fletcher S, Wagner E. Clinical Epidemiology: The Essentials. Philadelphia, Pa: Williams and Wilkins; 1996.
3. US Preventive Services Task Force. Guide to Clinical Preventive Services. Available at: www.ahcpr.gov/clinic/cpsix.htm
4. US Preventive Services Task Force. Guide to Community Preventive Services. Available at: www.thecommunityguide.org.
5. Peters K, Elster A. Population based medicine: Roadmaps to clinical practice. Chicago, Ill: American Medical Association, 2002.
1. Turabian JL, Perez-Franco B. Cual es el sentido de la educacion para la salud y las actividades “communitaria” en atencion primaria? Aten Primaria. 1998;22:662-666.
2. Fletcher R, Fletcher S, Wagner E. Clinical Epidemiology: The Essentials. Philadelphia, Pa: Williams and Wilkins; 1996.
3. US Preventive Services Task Force. Guide to Clinical Preventive Services. Available at: www.ahcpr.gov/clinic/cpsix.htm
4. US Preventive Services Task Force. Guide to Community Preventive Services. Available at: www.thecommunityguide.org.
5. Peters K, Elster A. Population based medicine: Roadmaps to clinical practice. Chicago, Ill: American Medical Association, 2002.