User login
ORLANDO – Adding rituximab to standard induction chemotherapy in adults with precursor B-cell acute lymphoblastic leukemia (B-ALL) appears to improve event-free survival, but four doses are insufficient, according to the first analysis from the randomized, phase 3 UKALL14 trial.
The findings also suggest that the significant event-free survival (EFS) benefit of adding 16-18 doses of rituximab in B-ALL patients, as demonstrated in “the recent and very important” GRAALL-2005/R study, may be generalizable to B-precursor ALL patients regardless of Philadelphia (Ph) chromosome status or CD20-positive expression level, Adele K. Fielding, MBBS, PhD, reported at the annual meeting of the American Society of Hematology.
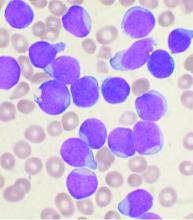
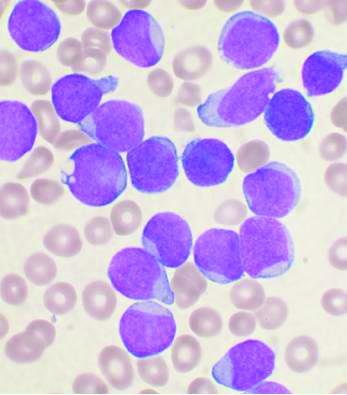
Unlike GRAALL-2005/R (NCT00327678), which included only patients with greater than 20% of ALL blasts expressing CD20 and with Ph-negative ALL, UKALL14 (NCT01085617) included B-ALL patients regardless of Ph chromosome status or CD20 expression level, explained Dr. Fielding of the Cancer Institute, University College London.
Overall, EFS rates among patients in the UKALL14 study at a median follow-up of 40.5 months were 41.9% in 288 patients randomized to receive standard-of-care chemotherapy (SOC), and 48.7% among 289 randomized to receive SOC plus rituximab, but the difference was not statistically significant (hazard ratio, 0.88; P = .28), she said.
“Likewise there was a nonsignificant improvement in 3-year event-free survival and in median event-free survival in the rituximab arms, but these differences did not meet our predetermined criteria,” she added.
Similarly, the overall survival findings showed slight, but non–statistically significant improvement in the rituximab arms (HR, 0.9; P = .39). The 3-year and median overall survival outcomes appeared to favor rituximab, but “this was not the magnitude of benefit that we were seeking in our study,” she said.
However, while a preplanned subgroup analysis by cytogenetic and other risk groups, as well as by cell surface CD20 expression, did not reveal any significant interactions for EFS, they did show that the percentage of blasts expressing CD20 was a strong independent poor prognostic factor.
A cutoff of 11.6%, compared with the 20% typically used, was found to be ideal based on the Youden Index, which determines the best balance between sensitivity and specificity.
“Interestingly, in addition to this, we did not find any impact of CD20 expression on response to rituximab,” Dr. Fielding noted.
Further, outcomes analyses by post–induction treatment assignment showed that, in patients who received myeloablative allogeneic stem cell transplant, “there was a large and statistically significant benefit to [adding rituximab], she said.
Landmark analysis showed an EFS hazard ratio of 0.48 at the time of transplant (P = .037), she said, noting that the SOC and SOC plus rituximab arms were well matched among this subset of patients.
The difference appeared to relate to relapse risk (HR, .38), but on an intention-to-treat analysis including all patients under age 40 years, the difference was “no longer quite so pronounced.”
“We do not understand the biological basis for this finding,” Dr. Fielding said, noting that it wasn’t explained by differences in graft-versus-host disease or infection. “This difference was not apparent in patients who received or were intended to receive reduced-intensity allogeneic conditioning.”
A multivariable analysis did not show a significant treatment effect, but did show “the same trend toward a better outcome in the rituximab arm,” she added.
UKALL14 subjects were adults aged 25-65 years with de novo ALL, regardless of Ph status or cell surface CD20 expression, who were recruited from 70 centers in the United Kingdom between December 2010 and July 2017. Those randomized to standard of care received a standard four-drug induction after a steroid prephase – with or without four doses of rituximab.
After a second induction, patients underwent risk assessment; low-risk patients were treated on the SOC arm and received high-dose methotrexate and additional pegylated asparaginase followed by four cycles of consolidation therapy. This was followed by 2 years of maintenance treatment.
High-risk patients with a sibling or fully matched unrelated donor available underwent allogeneic stem cell transplant, with those aged 40 years and younger receiving myeloablative conditioning and those over 40 years receiving reduced-intensity conditioning.
Most patients in the SOC plus rituximab arm received all four doses of rituximab, and the treatment arms were well-balanced with respect to risk characteristics, Dr. Fielding said, adding that no differences were noted in adverse events or mortality between the arms.
There is strong rationale for studying rituximab in ALL, she noted. For example, rituximab is safe to add to chemotherapy, and it has potential relevance at any level of CD20 expression, she said, explaining the basis for the study. Indeed, the findings support its use in this setting.
“Rituximab benefits patients with ALL,” she said. “But in our hands, four doses is insufficient to realize the full benefit.”
Dr. Fielding is a consultant for Amgen, Novartis, Pfizer, and Incyte.
SOURCE: Marks D et al. ASH 2019, Abstract 739.
ORLANDO – Adding rituximab to standard induction chemotherapy in adults with precursor B-cell acute lymphoblastic leukemia (B-ALL) appears to improve event-free survival, but four doses are insufficient, according to the first analysis from the randomized, phase 3 UKALL14 trial.
The findings also suggest that the significant event-free survival (EFS) benefit of adding 16-18 doses of rituximab in B-ALL patients, as demonstrated in “the recent and very important” GRAALL-2005/R study, may be generalizable to B-precursor ALL patients regardless of Philadelphia (Ph) chromosome status or CD20-positive expression level, Adele K. Fielding, MBBS, PhD, reported at the annual meeting of the American Society of Hematology.
Unlike GRAALL-2005/R (NCT00327678), which included only patients with greater than 20% of ALL blasts expressing CD20 and with Ph-negative ALL, UKALL14 (NCT01085617) included B-ALL patients regardless of Ph chromosome status or CD20 expression level, explained Dr. Fielding of the Cancer Institute, University College London.
Overall, EFS rates among patients in the UKALL14 study at a median follow-up of 40.5 months were 41.9% in 288 patients randomized to receive standard-of-care chemotherapy (SOC), and 48.7% among 289 randomized to receive SOC plus rituximab, but the difference was not statistically significant (hazard ratio, 0.88; P = .28), she said.
“Likewise there was a nonsignificant improvement in 3-year event-free survival and in median event-free survival in the rituximab arms, but these differences did not meet our predetermined criteria,” she added.
Similarly, the overall survival findings showed slight, but non–statistically significant improvement in the rituximab arms (HR, 0.9; P = .39). The 3-year and median overall survival outcomes appeared to favor rituximab, but “this was not the magnitude of benefit that we were seeking in our study,” she said.
However, while a preplanned subgroup analysis by cytogenetic and other risk groups, as well as by cell surface CD20 expression, did not reveal any significant interactions for EFS, they did show that the percentage of blasts expressing CD20 was a strong independent poor prognostic factor.
A cutoff of 11.6%, compared with the 20% typically used, was found to be ideal based on the Youden Index, which determines the best balance between sensitivity and specificity.
“Interestingly, in addition to this, we did not find any impact of CD20 expression on response to rituximab,” Dr. Fielding noted.
Further, outcomes analyses by post–induction treatment assignment showed that, in patients who received myeloablative allogeneic stem cell transplant, “there was a large and statistically significant benefit to [adding rituximab], she said.
Landmark analysis showed an EFS hazard ratio of 0.48 at the time of transplant (P = .037), she said, noting that the SOC and SOC plus rituximab arms were well matched among this subset of patients.
The difference appeared to relate to relapse risk (HR, .38), but on an intention-to-treat analysis including all patients under age 40 years, the difference was “no longer quite so pronounced.”
“We do not understand the biological basis for this finding,” Dr. Fielding said, noting that it wasn’t explained by differences in graft-versus-host disease or infection. “This difference was not apparent in patients who received or were intended to receive reduced-intensity allogeneic conditioning.”
A multivariable analysis did not show a significant treatment effect, but did show “the same trend toward a better outcome in the rituximab arm,” she added.
UKALL14 subjects were adults aged 25-65 years with de novo ALL, regardless of Ph status or cell surface CD20 expression, who were recruited from 70 centers in the United Kingdom between December 2010 and July 2017. Those randomized to standard of care received a standard four-drug induction after a steroid prephase – with or without four doses of rituximab.
After a second induction, patients underwent risk assessment; low-risk patients were treated on the SOC arm and received high-dose methotrexate and additional pegylated asparaginase followed by four cycles of consolidation therapy. This was followed by 2 years of maintenance treatment.
High-risk patients with a sibling or fully matched unrelated donor available underwent allogeneic stem cell transplant, with those aged 40 years and younger receiving myeloablative conditioning and those over 40 years receiving reduced-intensity conditioning.
Most patients in the SOC plus rituximab arm received all four doses of rituximab, and the treatment arms were well-balanced with respect to risk characteristics, Dr. Fielding said, adding that no differences were noted in adverse events or mortality between the arms.
There is strong rationale for studying rituximab in ALL, she noted. For example, rituximab is safe to add to chemotherapy, and it has potential relevance at any level of CD20 expression, she said, explaining the basis for the study. Indeed, the findings support its use in this setting.
“Rituximab benefits patients with ALL,” she said. “But in our hands, four doses is insufficient to realize the full benefit.”
Dr. Fielding is a consultant for Amgen, Novartis, Pfizer, and Incyte.
SOURCE: Marks D et al. ASH 2019, Abstract 739.
ORLANDO – Adding rituximab to standard induction chemotherapy in adults with precursor B-cell acute lymphoblastic leukemia (B-ALL) appears to improve event-free survival, but four doses are insufficient, according to the first analysis from the randomized, phase 3 UKALL14 trial.
The findings also suggest that the significant event-free survival (EFS) benefit of adding 16-18 doses of rituximab in B-ALL patients, as demonstrated in “the recent and very important” GRAALL-2005/R study, may be generalizable to B-precursor ALL patients regardless of Philadelphia (Ph) chromosome status or CD20-positive expression level, Adele K. Fielding, MBBS, PhD, reported at the annual meeting of the American Society of Hematology.
Unlike GRAALL-2005/R (NCT00327678), which included only patients with greater than 20% of ALL blasts expressing CD20 and with Ph-negative ALL, UKALL14 (NCT01085617) included B-ALL patients regardless of Ph chromosome status or CD20 expression level, explained Dr. Fielding of the Cancer Institute, University College London.
Overall, EFS rates among patients in the UKALL14 study at a median follow-up of 40.5 months were 41.9% in 288 patients randomized to receive standard-of-care chemotherapy (SOC), and 48.7% among 289 randomized to receive SOC plus rituximab, but the difference was not statistically significant (hazard ratio, 0.88; P = .28), she said.
“Likewise there was a nonsignificant improvement in 3-year event-free survival and in median event-free survival in the rituximab arms, but these differences did not meet our predetermined criteria,” she added.
Similarly, the overall survival findings showed slight, but non–statistically significant improvement in the rituximab arms (HR, 0.9; P = .39). The 3-year and median overall survival outcomes appeared to favor rituximab, but “this was not the magnitude of benefit that we were seeking in our study,” she said.
However, while a preplanned subgroup analysis by cytogenetic and other risk groups, as well as by cell surface CD20 expression, did not reveal any significant interactions for EFS, they did show that the percentage of blasts expressing CD20 was a strong independent poor prognostic factor.
A cutoff of 11.6%, compared with the 20% typically used, was found to be ideal based on the Youden Index, which determines the best balance between sensitivity and specificity.
“Interestingly, in addition to this, we did not find any impact of CD20 expression on response to rituximab,” Dr. Fielding noted.
Further, outcomes analyses by post–induction treatment assignment showed that, in patients who received myeloablative allogeneic stem cell transplant, “there was a large and statistically significant benefit to [adding rituximab], she said.
Landmark analysis showed an EFS hazard ratio of 0.48 at the time of transplant (P = .037), she said, noting that the SOC and SOC plus rituximab arms were well matched among this subset of patients.
The difference appeared to relate to relapse risk (HR, .38), but on an intention-to-treat analysis including all patients under age 40 years, the difference was “no longer quite so pronounced.”
“We do not understand the biological basis for this finding,” Dr. Fielding said, noting that it wasn’t explained by differences in graft-versus-host disease or infection. “This difference was not apparent in patients who received or were intended to receive reduced-intensity allogeneic conditioning.”
A multivariable analysis did not show a significant treatment effect, but did show “the same trend toward a better outcome in the rituximab arm,” she added.
UKALL14 subjects were adults aged 25-65 years with de novo ALL, regardless of Ph status or cell surface CD20 expression, who were recruited from 70 centers in the United Kingdom between December 2010 and July 2017. Those randomized to standard of care received a standard four-drug induction after a steroid prephase – with or without four doses of rituximab.
After a second induction, patients underwent risk assessment; low-risk patients were treated on the SOC arm and received high-dose methotrexate and additional pegylated asparaginase followed by four cycles of consolidation therapy. This was followed by 2 years of maintenance treatment.
High-risk patients with a sibling or fully matched unrelated donor available underwent allogeneic stem cell transplant, with those aged 40 years and younger receiving myeloablative conditioning and those over 40 years receiving reduced-intensity conditioning.
Most patients in the SOC plus rituximab arm received all four doses of rituximab, and the treatment arms were well-balanced with respect to risk characteristics, Dr. Fielding said, adding that no differences were noted in adverse events or mortality between the arms.
There is strong rationale for studying rituximab in ALL, she noted. For example, rituximab is safe to add to chemotherapy, and it has potential relevance at any level of CD20 expression, she said, explaining the basis for the study. Indeed, the findings support its use in this setting.
“Rituximab benefits patients with ALL,” she said. “But in our hands, four doses is insufficient to realize the full benefit.”
Dr. Fielding is a consultant for Amgen, Novartis, Pfizer, and Incyte.
SOURCE: Marks D et al. ASH 2019, Abstract 739.
REPORTING FROM ASH 2019